Abstract
Background:
Cardiovascular disease is the major cause of mortality in end stage renal disease (ESRD) patients on dialysis and myocardial infarction constitutes almost 20% of such deaths. We assessed the trends, characteristics and in-hospital outcomes in patients with ESRD.
Methods:
We used national inpatient sample (NIS) to identify patients with ESRD presenting with ST-segment elevation myocardial infarction (STEMI) for calendar years 2012–2016. Multiple logistic regression analysis and propensity matched data was used to compare outcomes for the purpose of our study.
Results:
Patients on dialysis who presented with STEMI were less likely to be treated with emergent reperfusion therapies including percutaneous coronary intervention, bypass graft surgery and thrombolytics with in first 24 h. In propensity-matched cohort, the mortality was nearly double in patients who have ESRD compared to patients without ESRD (29.7% vs. 15.9%, p < 0.01). In-patient morbidity such as utilization of tracheostomy, mechanical ventilation and feeding tubes was also more prevalent in propensity matched ESRD cohort. In multivariate regression analysis, ESRD remains a strong predictor of increased mortality in STEMI patients (OR 2.65, 95% CI, 2.57–2.75, p < 0.01).
Conclusion:
Our study showed low utilization of evidence-based prompt reperfusion therapies in ESRD patients with STEMI along with concomitant increased poor outcomes and resource utilization. Future research specifically targeting this extremely high-risk patient population is needed to identify the role of prompt reperfusion therapies in improving outcomes in these patients.
Keywords: End stage renal disease, ST elevation myocardial infarction, National in-patient sample database
1. Introduction
Cardiovascular disease continues to complicate the clinical course of end-stage renal disease patients on dialysis. Coronary artery disease (CAD) remains a significant co-morbidity in this specific patient cohort with myocardial infarction contributing to 20% of deaths [1]. This burden of CAD in end-stage renal disease (ESRD) patients is further projected to increase due to the aging population and increased burden of CAD associated morbidities. To date, there have been no prior studies reporting the trends in outcomes and utilization of reperfusion therapies in ST-elevation myocardial infarction (STEMI) patients with end-stage renal disease (ESRD) on dialysis. The goal of this study is to analyze the contemporary trends in utilization of evidence-based reperfusion therapy in STEMI patients on dialysis and to study the in-hospital outcomes of STEMI in this cohort of patients from a nationally representative United States population sample.
2. Methods
2.1. Study data
National inpatient sample (NIS) database is part of Healthcare Cost and Utilization Project (HCUP) databases and is sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS is the largest publicly available all-payer administrative claims-based database and contains information about patient discharges from 1000 hospitals in 45 states. Since 2012, the NIS was redesigned to include a sample of discharges from all hospitals participating in HCUP. These data are stratified to represent 20% of US inpatient hospitalizations across different hospitals and geographic regions (random sample). National estimates (NE) of the entire US hospitalized population were calculated using the Agency for Healthcare Research and Quality sampling and weighting method. Institutional review board approval and informed consent were not required for this study given the de-identified nature of the NIS database and public availability.
2.2. Study population
We analyzed NIS data from January 2012 to December 2016 using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes and International Classification of Diseases, 10th Revision, Clinical Modification ICD-10-CM codes (ICD-10-CM). All patients with STEMI who are 18 years and older were identified using ICD-9-CM code of 410 (excluding 410.7) & ICD-10-CM code of 121-22. The study population was then divided into two groups (ESRD vs no-ESRD). To account for potential confounding factors and selection bias, a propensity score-matching model was applied to match baseline characters (Table 1) using logistic regression for comparative outcomes analysis. A nearest neighbor 1:1 variable ratio, parallel, a balanced propensity-matching model was made using a caliper width of 0.2. All variables were matched within the set 0.2 SD level.
Table 1.
Baseline characteristic of the patients included in the analysis before and after propensity matching.
| Variable no. (%) | Unadjusted cohort | Adjusted cohort* | ||||
|---|---|---|---|---|---|---|
| No ESRD† 1,115,680 |
ESRD 27,355 |
p value | No ESRD† (25,055) |
ESRD (25,125) |
p value | |
| Age (mean [SD]) years | 65.1(14.01) | 66.8(12.4) | <0.01 | 66.9(13.9) | 66.74(12.4) | 0.13 |
| Female | 385,610(34.6) | 11,515(42.1) | <0.01 | 10,640(42.5) | 10,600(42.2) | 0.53 |
| Race | ||||||
| Caucasian | 811,990(77.6) | 13,580(51.8) | <0.01 | 13,540(54.0) | 13,130(52.3) | 0.01 |
| African American | 91,290(8.7) | 6570(25.1) | 6095(24.3) | 6285(25.0) | ||
| Hispanics | 76,905(7.3) | 3610(13.8) | 3280(13.1) | 3450(13.7) | ||
| Asian or Pacific Islander | 26,255(2.5) | 1235(4.7) | 1085(4.3) | 1145(4.6) | ||
| Native American | 5265(0.5) | 270(1.0) | 195(0.8) | 200(0.8) | ||
| AHRQ§ medical comorbidity | ||||||
| Anemia (deficiency) | 139,365(12.5) | 15,245(55.7) | <0.01 | 13,650(54.5) | 13,945(55.5) | 0.2 |
| Anemia (blood loss) | 7890(0.7) | 380(1.4) | <0.01 | 335(1.3) | 355(1.4) | 0.5 |
| Chronic pulmonary disease | 196,810(17.6) | 5765(21.1) | <0.01 | 5480(21.9) | 5355(21.3) | 0.5 |
| Congestive heart failure | 69,580(6.2) | 5310(19.4) | <0.01 | 4460(17.8) | 4855(19.3) | 0.01 |
| Coagulopathy | 67,125(6.0) | 3705(13.5) | <0.01 | 3290(13.1) | 3375(13.4) | 0.32 |
| Diabetes mellitus (complicated) | 68,160(6.1) | 8540(31.2) | <0.01 | 7310(29.2) | 7725(30.7) | 0.01 |
| Hypertension | 738,700(66.2) | 23,010(84.1) | <0.01 | 20,955(83.6) | 21,175(84.3) | 0.05 |
| Chronic liver disease | 17,820(1.6) | 1175(4.3) | <0.01 | 1015(4.1) | 1070(4.3) | 0.24 |
| Obesity | 158,850(14.2) | 3765(13.8) | 0.03 | 3585(14.3) | 3430(13.7) | 0.04 |
| Peripheral vascular disorders | 102,580(9.2) | 6450(23.6) | 5510(22.0) | 5780(23.0) | 0.01 | |
| Neurological disorders | 69,050(6.2) | 2610(9.5) | <0.01 | 2195(8.8) | 2400(9.6) | 0.01 |
| Paralysis | 21,935(2.0) | 1075(3.9) | <0.01 | 915(3.7) | 995(4.0) | 0.07 |
| Peptic ulcer disease | 1720(0.2) | 100(0.4) | <0.01 | 95(0.4) | 85(0.3) | 0.44 |
| Pulmonary circulation disorders | 12,575(1.1) | 1020(3.7) | <0.01 | 960(3.8) | 905(3.6) | 0.17 |
| Valvular disorder | 20,360(1.8) | 1585(5.8) | <0.01 | 1405(5.6) | 1440(5.7) | 0.55 |
| Weight loss | 40,435(3.6) | 2840(10.4) | <0.01 | 2325(9.3) | 2570(10.2) | <0.05 |
| Hospital location | ||||||
| Rural | 101,570(9.1) | 1910(7.0) | <0.01 | 1895(7.6) | 1725(6.9) | <0.05 |
| Urban non-teaching | 366,420(32.8) | 8890(32.5) | 8385(33.5) | 8390(33.4) | ||
| Urban teaching | 647,800(58.1) | 16,560(60.5) | 14,775(59.0) | 15,010(59.7) | ||
| Bed size of the hospital | ||||||
| Small | 148,910(13.3) | 3190(11.7) | <0.01 | 2795(11.2) | 2910(11.6) | 0.11 |
| Medium | 303,690(27.2) | 7780(28.4) | 7005(28.0) | 7135(28.4) | ||
| Large | 663,190(59.4) | 16,390(59.9) | 15,255(60.9) | 15,080(60.0) | ||
| Region | ||||||
| New England | 51,165(4.6) | 1080(3.9) | <0.01 | 1055(4.2) | 1025(4.1) | 0.01 |
| Mid-Atlantic | 142,940(12.8) | 3785(13.8) | 3635(14.5) | 3640(14.5) | ||
| East North Central | 182,945(16.4) | 3590(13.1) | 3405(13.6) | 3255(13.0) | ||
| West North Central | 84,715(7.6) | 1555(5.7) | 1150(4.6) | 1180(4.7) | ||
| South Atlantic | 224,635(20.1) | 5920(21.6) | 5365(21.4) | 5580(22.2) | ||
| East South Central | 94,950(8.5) | 1775(6.5) | 1870(7.5) | 1720(6.8) | ||
| West South Central | 122,345(11.0) | 3490(12.8) | 3420(13.6) | 3355(13.4) | ||
| Mountain | 71,825(6.4) | 1460(5.3) | 1175(4.7) | 1200(4.8) | ||
| Pacific | 140,270(12.6) | 4705(17.2) | 3980(15.9) | 4170(16.6) | ||
| Median household income percentile | ||||||
| 0–25th | 318,510(29.2) | 9915(37.0) | <0.01 | 9365(37.4) | 9440(37.6) | 0.5 |
| 26–50th | 297,590(27.3) | 6690(24.9) | 6250(24.9) | 6255(24.9) | ||
| 51–75th | 261,415(24.0) | 5665(21.1) | 5135(20.5) | 5230(20.8) | ||
| 76–100th | 213,750(19.6) | 4555(17.0) | 4305(17.2) | 4200(16.7) | ||
All variables were matched within 0.2 SD.
End stage renal disease.
Agency for Health Care Research and Quality.
2.3. Study endpoints
The primary endpoint of the study was in-hospital mortality. Secondary endpoints were (1) in-hospital morbidities (2) surrogates of severe disability and (3) cost of hospitalization and length of stay (Table 2).
Table 2.
Hospital encounter outcomes, resource utilization before and after propensity matching Abbreviations: ESRD; end stage renal disease, CABG; coronary artery bypass graft, PCI; percutaneous coronary intervention, pVADs; percutaneous left ventricular assist device, IABP; intra-aortic balloon pump.
| Variables no. (%) | Unmatched cohorts | Matched cohorts | ||||
|---|---|---|---|---|---|---|
| No ESRD (n = 1,115,790) |
ESRD (n = 27,360) |
p value | No ESRD (n = 25,055) |
ESRD (n = 25,125) |
p value | |
| In-hospital mortality | 126,285(11.3) | 8025(29.3) | <0.01 | 3980(15.9) | 7470(29.7) | <0.01 |
| Discharge disposition of surviving patients | ||||||
| Home | 695,635(70.3) | 7860(40.7) | <0.01 | 11,410(54.1) | 7235(410) | <0.01 |
| Short term hospital | 58,830(5.9) | 1970(10.2) | 1415(6.7) | 1740(9.9) | ||
| Another facility | 126,315(12.8) | 5850(30.3) | 4650(22.1) | 5375(30.4) | ||
| Home health | 99,165(10.0) | 3350(17.3) | 3355(15.9) | 3025(17.1) | ||
| In-hospital morbidities and procedures | ||||||
| PCI | 640,270(57.4) | 7010(25.6) | <0.01 | 10,410(41.5) | 6500(25.9) | <0.01 |
| CABG | 63,675(5.7) | 1255(4.6) | <0.01 | 1825(7.3) | 1110(4.4) | <0.01 |
| Thrombolytics | 19,045(1.7) | 435(1.6) | <0.01 | 385(1.5) | 420(1.7) | <0.01 |
| Thrombolytics at another facility (within 24 h) | 18,160(1.6) | 65(0.2) | <0.01 | 205(0.8) | 55(0.2) | |
| Vascular complications | 3990(0.4) | 150(0.5) | <0.01 | 100(0.4) | 130(0.5) | 0.05 |
| Tamponade | 3025(0.3) | 140(0.5) | <0.01 | 110(0.4) | 125(0.5) | 0.34 |
| Pericardiocentesis | 1825(0.2) | 115(0.4) | <0.01 | 70(0.3) | 105(0.4) | <0.01 |
| Cardiogenic shock | 122,970(110) | 4565(16.7) | <0.01 | 3475(13.9) | 4135(16.5) | <0.01 |
| pVADs | 8875(0.8) | 320(1.2) | <0.01 | 215(0.9) | 270(1.1) | <0.01 |
| IABP | 82,725(7.4) | 1895(6.9) | <0.01 | 2295(9.2) | 1735(6.9) | <0.01 |
| CPR | 45,425(4.1) | 3040(11.1) | <0.01 | 1550(6.2) | 2785(11.1) | <0.01 |
| Vasopressors use | 22,260(20) | 1050(3.8) | <0.01 | 815(3.3) | 990(3.9) | <0.01 |
| Stroke (after procedure) | 5105(0.5) | 130(0.5) | 0.67 | 110(0.4) | 110(0.4) | 0.99 |
| Severe disability surrogates | ||||||
| Mechanical ventilation | 144,265(12.9) | 8340(30.5) | <0.01 | 5285(21.1) | 7670(30.5) | <0.01 |
| PEG | 8750(0.5) | 520(0.7) | <0.01 | 345(1.4) | 480(1.9) | <0.01 |
| Trach | 10,265(0.9) | 895(3.3) | <0.01 | 495(20) | 775(3.1) | <0.01 |
| Resources utilization | ||||||
| Length of stay, mean (SD), days | 4.8(6.6) | 9.2(14.3) | <0.01 | 7.0(7.9) | 9.1(14.2) | <0.01 |
| Cost of hospitalization-mean (SD), $ | 93,181(117938) | 140,993(24217) | <0.01 | 115,941(136588) | 140,579(240251) | <0.01 |
Associated procedures and hospital morbidities were identified using ICD-9-CM and ICD-10-CM codes (Supplement). Flow chart of our study is shown in Fig. 1.
Fig. 1.
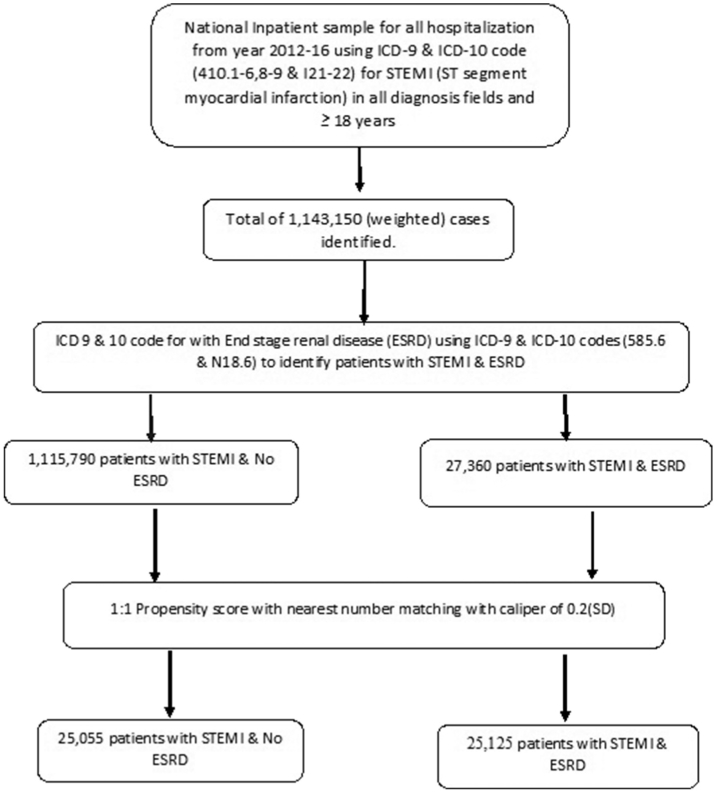
Flow sheet of our study.
2.4. Statistical analysis
Descriptive statistics were presented as frequencies with percentages for categorical variables and as means with standard deviations for continuous variables. Baseline characteristics were compared using a Pearson χ2 test and Fisher's exact test for categorical variables and independent samples t-test for continuous variables.
Initially, binomial logistic regression model was used to identify variables from demographic data (Table 1) that were significantly associated with patient mortality (p value < 0.10). These variables were then subsequently utilized in a multiple logistic regression model to identify predictors of mortality. A type I error rate of <0.05 was considered statistically significant. All statistical analyses were performed using statistical package for social science (SPSS) version 26 (IBM Corp.) and R, version 3.5 for propensity matching.
3. Results
A total of 1,143,035 patients with STEMI were identified. This included 27,355 (2.4%) of ESRD patients in the period between January 2012 to December 2016. Out of the total population, 397,125 (34.7%) were females, mean age was 65.1 ± 14.0, 825,570 (77.0%) were Caucasian, 91,290 (8.7%) were African American and 80,515 (7.5%) were Hispanics. Comparing ESRD patients to non-ESRD patients, PCI rate was lower in ESRD patients (25.6% vs 57.4%). ESRD patients had higher proportion of females (42.1% as compared to 34.6%, p < 0.01), had higher prevalence of Anemia (55.7% vs 12.5%; p < 0.01), diabetes mellitus (31.2% vs 6.1%; p < 0.01) and congestive heart failure (19.4% vs 6.2%; p < 0.01). The baseline characteristics of the two groups are shown in (Table 1).
3.1. Outcomes of unmatched cohort
Crude in-hospital mortality rate of the overall population was 134,310 (11.7%). The mortality was much higher in ESRD patients (29.3% vs 11.3%; p < 0.01). Patients with ESRD had higher proportion of in-hospital morbidities including Cardiopulmonary resuscitation (CPR) (11.1% vs 4.1%; p < 0.01), vasopressor use (2.0% vs 3.8%, p < 0.01) and mechanical ventilation (30.5% vs 12.9%; p < 0.01). Moreover, surrogates of severe disability (PEG, Tracheostomy and non-home discharges) were more frequent in the ESRD group, who also had longer hospitalization and higher cost of care (Table 2).
3.2. Outcomes of matched cohort
After propensity matching and adjusting for baseline demographics, clinical co-morbidities, region and hospital characteristics, the 2 groups were well matched (supp-1).
After propensity matching, mortality among the ESRD group was almost twice than in non-ESRD patients (29.7% vs 15.9%; p < 0.01). ESRD patients were also less likely to be treated with percutaneous coronary intervention (25.9% vs 41.5%; p < 0.01). Patients with ESRD also had higher proportion of major in-hospital complications including cardiogenic shock (16.5% vs 13.9%, p < 0.01) pericardial effusion requiring pericardiocentesis (0.4% vs 0.3%, p < 0.01), use of percutaneous ventricular assist device (1.1% vs 0.9%; p < 0.01) and cardiopulmonary resuscitation (11.1% vs 6.2%; p < 0.01) (Table 2). Moreover, surrogates of severe disability (PEG, tracheostomy and non-home discharges) were more frequent in the ESRD group. The mean cost of hospitalization (140,570$ vs 115,941$, p < 0.01) and length of stay (9.1 days vs7.0 days, p < 0.01) were higher in ESRD group as compared to non-ESRD group (Table 2).
3.3. Trends and predictors
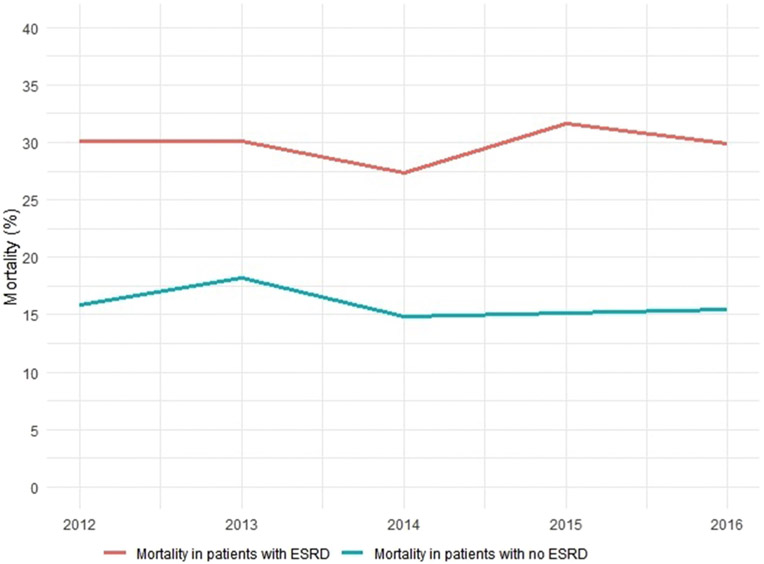
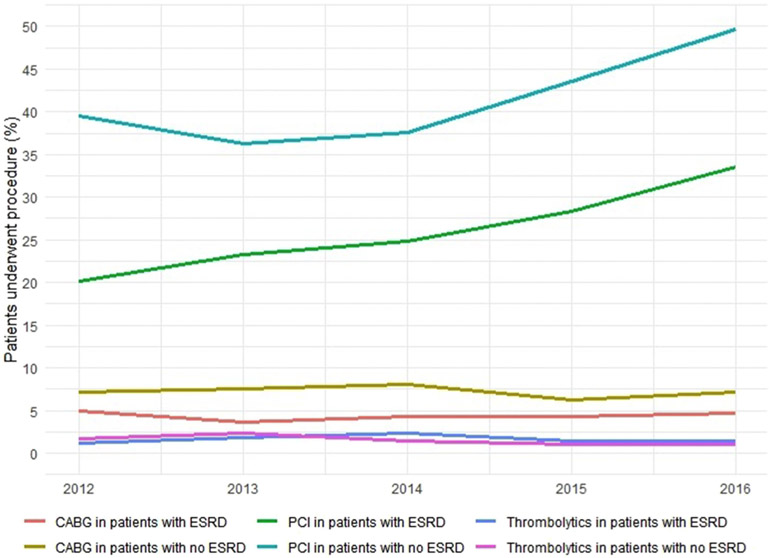
Mortality has remained the same over the years in both groups (Fig. 2). The mean length of stay has remained unchanged though the cost of stay has increased in both (Fig. 3A-B). Rates of PCI have increased for both groups (Fig. 4).
Fig. 2. Mortality trends in STEMI.
P<0.01 ESRD; End stage Renal disease, STEMI; ST segment elevation Myocardial Infarction
Fig. 3. Mean Length of stay in STEMI.
P<0.01 ESRD; End stage Renal disease, STEMI; ST segment elevation Myocardial Infarction
Fig. 4. Trends in Revascularization strategies in STEMI.
P<0.01 PCI; Percutaneous Coronary Intervention, CABG; Coronary Artery Bypass Grafting
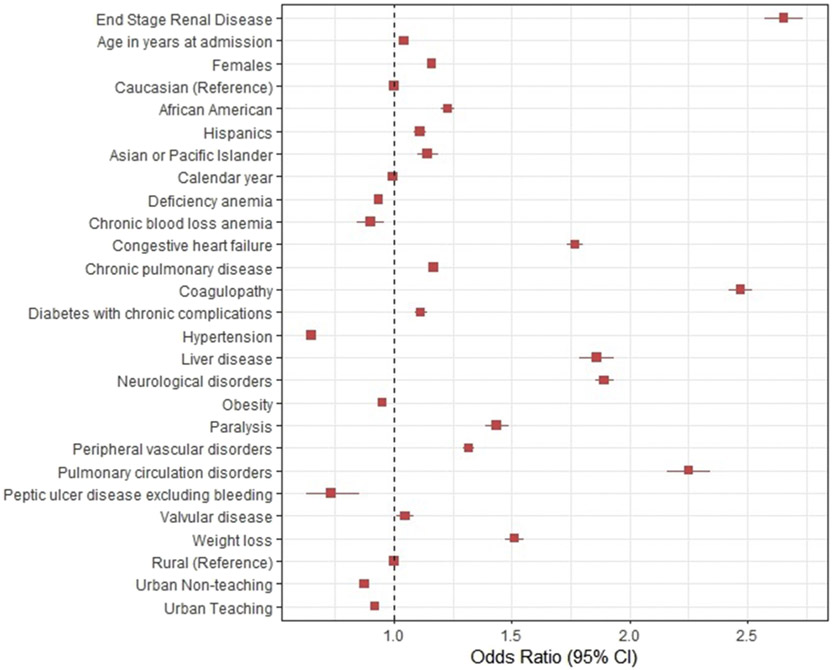
Predictors of mortality for patients With STEMI are shown in Fig. 5. ESRD was associated with higher odds of mortality (OR, 2.65 [95% CI, 2.57–2.75, p < 0.01]). Congestive heart failure (OR, 1.77 [95% CI, 1.73–1.81, p < 0.01), diabetes mellitus (OR, 1.11 [95% CI, 1.09–1.14, p < 0.01]) and coagulopathy (OR, 2.47[95% CI, 2.42–2.52, p < 0.01]) were associated with higher mortality.
Fig. 5. Predictors of mortality in ST-elevation myocardial infarction.
STEMI; ST segment elevation Myocardial Infarction, Multiple Logistic Regression model with stepwise entry (p<0.1)
4. Discussion
Cardiovascular disease remains the most common cause of death in patients with end-stage (ESRD) [1-3]. Both pre-existing ESRD and as a consequence of myocardial infarction is associated with poor clinical outcome [4]. Despite the significantly worse outcomes of STEMI in ESRD patients compared to their non ESRD counterparts, there is paucity of data on contemporary trends in management and outcomes of STEMI in this high-risk vulnerable patient population. Therefore, our aim was to use data from a national registry between 2012 and 2016 to evaluate the national prevalence, baseline characteristics of STEMI patients with ESRD and also to explore the rates of in-patient complications.
The main findings of our current study are: (1) Adjusted In-hospital mortality of STEMI among ESRD patients is almost twice that of patients without ESRD (30% vs 16%; p < 0.01). (2) mean length of stay in ESRD patients was significantly higher compared to patients without ESRD (9.2 days vs 4.8 days; p < 0.01). (3) Evidence-based management with reperfusion therapy which is the cornerstone of management of STEMI is utilized much less frequently in ESRD patients compared to those without ESRD. (4) ESRD is the strongest predictor of in-hospital mortality in patients with STEMI (OR, 2.65 [95% CI, 2.57–2.75, p < 0.01]) [5]. ESRD patients are more likely to suffer from major in-hospital complications including cardiogenic shock, use of percutaneous ventricular assist devices, pericardial effusion requiring pericardiocentesis, the requirement for mechanical ventilation and vasopressor use [6]. These patients are also less likely to be discharged home and more likely to be discharged to other short term care facilities or require home health care.
The progressive increase in cardiovascular risk with worsening estimated GFR is partly explained by factors associated with renal decline, including anemia, oxidative stress, derangements in calcium-phosphate homeostasis, inflammation, and conditions promoting coagulation, all of which are associated with accelerated atherosclerosis and endothelial dysfunction [5]. In one study by Varma R et al. anemia, hyperhomocysteinemia, hypoalbuminemia, increased troponin, increased oxidant stress, and decreased nitric oxide activity due to ESRD are assumed to be the factors that could contribute to increased CAD risk [6]. Moreover, comorbidities associated with ESRD like diabetes mellitus, hypertension and hypercholesterolemia can contribute increasingly towards adverse cardiovascular outcomes. In our study, we found increased rates of co-morbidities among ESRD patients which could possibly increase the chances of AMI. In our cohort of STEMI with ESRD, we found a significant proportion of diabetics (31%). This number is significantly higher than reported in (SWEDEHEART) registry, which has a diabetes rate between 25.8% in men and 28.2% in women [11]. Glycemic control optimization, especially in these patients, is important since ESRD and diabetes seem to have synergistic effects. In our study we found ESRD to be higher among females (42.1%) and our results are consistent with the (SWEDEHEART) registry where incidences of STEMI in female ESRD patients were higher compared to men. It is noteworthy to mention that two studies even marked ESRD in women as the most important and strong prognostic factor for STEMI compared with men [7,8].
Immediate reperfusion therapy with percutaneous coronary intervention is the standard of care in patients with STEMI [9-12]. Our study highlights the less frequent use of evidence-based reperfusion therapies and less aggressive management of ESRD patients with MI and this could possibly contribute to the higher risk of mortality and adverse outcomes. Our results can be supported by two studies which reported poorer outcomes in high-risk ESRD patients that less frequently used immediate evidence-based therapies for AMI including antiplatelet medications, beta-blockers, angiotensin-converting enzyme inhibitors and revascularization [13,14]. Similar outcomes were reported by one nationwide inpatient registry study by Brijesh Patel et al., though there was an increased use of PCI in during 2006–2012 among AMI patients, less life-saving procedures were performed in AMI patients with ESRD [15]. In another study by Chertow GM et al. poorer outcomes were observed for patients treated with medical therapy alone, hence PCI and CABG must be considered as therapeutic options in this patient population [16]. Therefore, more aggressive strategies for the diagnosis and treatment of AMI in patients with ESRD are needed. We found mortality rates almost twice more than non-ESRD patients and our results are consistent with previously published reports of increased mortality of AMI in (ESRD) patients' cohort [17-22].
The poor outcome of CKD patients with AMI would appear to be partially attributable to therapeutic nihilism (poor outcomes of CKD patients with AMI are at least partially attributable to therapeutic nihilism originating from fear of precipitating potential bleeding complications after institution of prompt reperfusion therapies and lack of clinical trial outcome data as ESRD patients were excluded from all AMI trials.) [23]. Moreover, Herzog et al. showed that dialysis patients with STEMI are less likely to present with chest pain and less likely to have ST-segment elevation on EKG and thus less likely to be accurately diagnosed with STEMI [24,25].
In our study, we observed that patients having ESRD had varied cardiovascular complications during hospitalization which includes cardiogenic shock, pericardial effusion, use of percutaneous ventricular assist device and cardiopulmonary resuscitation. These complications could possibly be attributed to multiple factors including a higher burden of comorbidities at baseline resulting in a sicker cohort of patients and due to undertreatment of AMI. This highlights the importance of multiple risk reduction measures in ESRD patients and maintaining a high index suspicion for STEMI given that these patients are less likely to have typical clinical and electrocardiographic manifestations of STEMI and also the importance of adherence to evidence-based prompt reperfusion therapies and other evidence-based therapies once STEMI is diagnosed.
Despite advances in management of STEMI and reduction of mortality from STEMI in the general population over the past two decades results of our study showing poor outcomes of STEMI in dialysis patients and corroborated by prior studies showing similar results, including higher in-hospital mortality and higher incidence of major complications including cardiogenic shock highlight the need to focus on a multi-pronged approach to improve clinical outcomes in this cohort of patients. However, based on their multiple comorbidities and severe clinical presentation there is a much higher risk of adverse outcomes; unfavorably affecting the risk-benefit ratio of coronary revascularization procedures. These further stress the importance of individualization, proper patient selection and patient-centric care management.
Our study has several limitations that need to be highlighted. First, NIS is an administrative claim-based database that uses ICD-9-CM and ICD-10-CM codes, which are prone to coding errors, however, the hard clinical endpoints used in this study such as revascularization, hospital mortality and discharge disposition are less prone to diagnostic and coding errors. Second, NIS collects data on in-patient discharges and each admission is registered as an independent event, it is, therefore, possible that one patient may have more than one admission in the same or subsequent years which may lead to duplicate registration of patients. Third, patients are not followed longitudinally in NIS so long-term outcomes could not be assessed from present dataset, nonetheless we believe our study gives useful insights into the significantly worse hospital outcomes and complications of STEMI in ESRD likely stemming at least partially from differences in acute management of STEMI in this cohort of patients and underutilization of reperfusion therapies.
In conclusion, this is the largest study examining the trends in treatment patterns and outcomes of acute myocardial infarction in patients with ESRD. ESRD patients are almost twice more likely to die from STEMI compared to their counterparts without ESRD, and after adjustment for confounding variables, ESRD is the strongest predictor of death among patients with STEMI. ESRD patients are also less likely to receive reperfusion therapy with either primary PCI or fibrinolysis compared to patients with normal renal function. We propose that future research should be directed towards improving the clinical outcomes of STEMI in dialysis patients especially with a focus on risk reduction and management of multiple co-morbidities.
Supplementary Material
Abbreviations:
- HCUP
Healthcare Cost and Utilization Project
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICD-10-CM
International Classification of Diseases, 10th Revision, Clinical Modification
- CAD
coronary artery disease
- PCI
percutaneous coronary intervention
- CABG
coronary bypass grating
- ESRD
end-stage renal disease
- STEMI
ST-segment elevation myocardial infarction
- NIS
national in-patient sample
- AHRQ
Agency for Healthcare Research and Quality
- CPR
cardiopulmonary resuscitation
- AMI
acute myocardial infarction
Footnotes
Declaration of competing interest
No disclosures.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.carrev.2020.05.004.
References
- [1].Marcello T, Natasha W, Bruce C, Andrew H, Chris R, Mei F, et al. Chronic kidney disease and mortality risk: a systemic review. J Am Soc Nephrol. 2006;17:2034–47. [DOI] [PubMed] [Google Scholar]
- [2].Wendy EB, Friedrich KP, Elizabeth AM, Robert AW. Causes of death in dialysis patients: racial and gender differences. J Am Soc Nephrol. 1994;5:1231–42. [DOI] [PubMed] [Google Scholar]
- [3].Al Wakeel JS, Mitwalli AH, Al Mohaya S, Abu-Aisha H, Tarif N, Malik GH, et al. Morbidity and mortality in ESRD patients on dialysis. Saudi J Kidney Dis Transpl. 2002; 13:473–7. [PubMed] [Google Scholar]
- [4].Amin AP, Spertus JA, Reid KJ, Lan X, Buchanan DM, Decker C, et al. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;160:1065–71. [DOI] [PubMed] [Google Scholar]
- [5].Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–95. [DOI] [PubMed] [Google Scholar]
- [6].Varma R, Garrick R, McClung J, Frishman WH. Chronic renal dysfunction as an independent risk factor for the development of cardiovascular disease. Cardiol Rev. 2005; 13:98–107. [DOI] [PubMed] [Google Scholar]
- [7].Sederholm Lawesson S, Todt T, Alfredsson J, et al. Gender difference in prevalence and prognostic impact of renal insufficiency in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Heart. 2011;97:308–14. [DOI] [PubMed] [Google Scholar]
- [8].Chen R, Kumar S, Timmis A, et al. Comparison of the relation between renal impairment, angiographic coronary artery disease, and long-term mortality in women versus men. Am J Cardiol. 2006;97:630–2. [DOI] [PubMed] [Google Scholar]
- [9].Keeley EC, Boura JA, Grines CL Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet (London, England). 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- [10].Zahn R, Schiele R, Schneider S, Gitt AK, Wienbergen H, Seidl K, et al. Decreasing hospital mortality between 1994 and 1998 in patients with acute myocardial infarction treated with primary angioplasty but not in patients treated with intravenous thrombolysis: results from the pooled data of the Maximal Individual Therapy in Acute Myocardial Infarction (MITRA) Registry and the Myocardial Infarction Registry (MIR). J Am Coll Cardiol. 2000;36:206–71. [DOI] [PubMed] [Google Scholar]
- [11].Weaver WD, Simes RJ, Betriu A, Grines CL Zijlstra F, Garcia E, et al. Comparison of primary coronary angioplasty and intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review. JAMA 1997;278:2093–8. [PubMed] [Google Scholar]
- [12].Anderson JL, Karagounis LA, Califf RM. Meta-analysis of five reported studies on the relation of early coronary patency grades with mortality and outcomes after acute myocardial infarction. Am J Cardiol. 1996. July 1;78:1–8. [DOI] [PubMed] [Google Scholar]
- [13].Berger AK, Duval S, Krumholz HM. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J Am Coll Cardiol. 2003;42:201–8. [DOI] [PubMed] [Google Scholar]
- [14].Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Cannon CP, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010; 121:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel Brijesh, Shah Mahek Dusaj Raman, Maynard Sharon, Patel Nainesh. Percutaneous coronary intervention and inpatient mortality in patients with advanced chronic kidney disease presenting with acute coronary syndrome. Proc (Bayl Univ Med Cent). 2017. October;30:400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chertow GM, Normand SL, Silva LR, McNeil BJ. Survival after acute myocardial infarction in patients with end-stage renal disease: results from the cooperative cardiovascular project. Am J Kidney Dis. 2000;35:1044–51. [DOI] [PubMed] [Google Scholar]
- [17].Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351: 1296–305. [DOI] [PubMed] [Google Scholar]
- [18].Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Szummer K, Lundman P, Jacobson SH, et al. Influence of renal function on the effects of early revascularization in non-ST-elevation myocardial infarction: data from the Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies (SWEDEHEART). Circulation. 2009;120:851–8. [DOI] [PubMed] [Google Scholar]
- [20].Herzog CA Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339:799–805. [DOI] [PubMed] [Google Scholar]
- [21].Wright RS, Reeder GS, Herzog CA Albright RC, Williams BA Dvorak DL et al. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–70. [DOI] [PubMed] [Google Scholar]
- [22].Cooper HA, Monge C, Panza JA. Patients with end-stage renal disease and acute myocardial infarction have poor short-term outcomes despite modern cardiac intensive care. Coron Artery Dis. 2008;19:231–5. [DOI] [PubMed] [Google Scholar]
- [23].Cardiovascular disease and dialysis patients: is therapeutic nihilism justified? In: Herzog CA editor. Seminars in dialysis. Wiley Online Library; 1999. [Google Scholar]
- [24].Herzog CA How to manage the renal patient with coronary heart disease: the agony and the ecstasy of opinion-based medicine. J Am Soc Nephrol. 2003;14:2556–72. [DOI] [PubMed] [Google Scholar]
- [25].Herzog CA Littrell K, Arko C, Frederick PD, Blaney M. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: a collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation. 2007;116:1465–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.