Structural heart disease (SHD) is at the cutting edge of innovation in cardiovascular disease with a focus on the Importance of the “heart team” (1,2). Development of the heart team, with a broad range of specialty and subspecialty members, has resulted in the creation of a new subspecialty within cardiology and anesthesiology, namely interventional imaging for SHD interventions (3). New and advanced skills in echocardiography and computed tomography (CT) imaging form the current backbone of this specialty, but other modalities are expected to contribute in the future. Because interventional echocardiographers are crucial to the technical success of many complex SHD procedures, they must possess specialized knowledge sets as well as advanced image acquisition and interpretive skills.
Training program guidelines, standards, credentialing, and board examinations for echocardiography have been developed and adopted (4), with the 2019 American College of Cardiology (ACC)/American Heart Association (AHA)/American Society of Echocardiography (ASE) Advanced Training Statement (ATS) on Echocardiography (5) alluding to the specialized training that this new field might require. In addition, the recent 2019 American Association for Thoracic Surgery (AATS)/ACC/ASE/Society for Cardiovascular Angiography and Interventions (SCAI)/Society of Thoracic Surgeons (STS) Expert Consensus Systems of Care Document for valvular heart disease (5) identifies the need for an echocardiographer with expertise in valve disease and transcatheter and surgical interventions for all levels of care; it also specifically identifies the need for an interventional echocardiographer to provide imaging guidance for transcatheter and intraoperative procedures for comprehensive Level I care.
A more granular look at the core competencies that define the interventional imager would help define this subspecialty and justify focused additional training. This document is a consensus statement of expert cardiologists and anesthesiologists in the United States and Europe who are currently performing structural heart interventions; it is not a guideline and has not been endorsed by professional organizations such as the ACC, the AHA, the SCAI, the ASE, or the Society of Cardiovascular Anesthesiologists. Herein the authors propose core competencies for the interventional echocardiographer and a framework for acquiring these competencies, either during a structured training program or “on the job” outside a traditional training program. Importantly, physicians wishing to pursue competence in interventional echocardiography after fellowship should adhere to the same rigorous standards as those completing training within a structured fellowship. Defining the minimum standards for competency within the field of SHD poses challenges as new devices and procedures continue to be developed and imaging technology advances. This document should therefore be expected to change as the field evolves. Finally, this document only outlines general principles that we believe are applicable to most centers performing SHD imaging, and it is not meant to comprehensively address the nuances and logistics of every health system format around the world.
DEFINING THE INTERVENTIONAL ECHOCARDIOGRAPHY TRAINING PATHWAY
Given the current expansion of subspecialties within cardiology, professionals worldwide, including fellows-in-training, have been required to structure their training according to their perceived professional interests. For example, in the United States, multimodality imaging has become an important goal for fellows-in-training interested in imaging, and Core Cardiovascular Training Statement 4 Task Force documents (4,6) suggest that Level II competency in 2 imaging modalities could be achieved during the standard 3-year cardiovascular fellowship depending on the trainees’ career goals and use of elective rotations. However, training in >2 imaging modalities or achieving Level III competency will likely require additional training. This additional training should be obtained through programs equipped with the faculty, facilities, case volume, and educational infrastructure necessary to accomplish competency in numerous modalities (6). Training for the SHD imager should thus occur in the framework of an Intersocietal Accreditation Commission-accredited echocardiography laboratory with supervision provided by Level III, SHD-trained and National Board of Echocardiography (NBE)-certified imagers, or equivalent, depending on national guidelines and requirements.
Because of the different knowledge base and skill set required of the interventional echocardiographer, the consensus of the writing group is that this subspecialty within echocardiography also requires special training in appropriate institutions, which may be different from the centers offering traditional training. To distinguish advanced echocardiography training from interventional echocardiography-focused training, this document uses the designations III and III-SHD, respectively. Given the differences in knowledge and skills required for this new subspecialty, as well as the focused training required within the SHD interventional field, there might be multiple competencies in which the Level III and Level III-SHD trainees may overlap (Central Illustration).
CENTRAL ILLUSTRATION. Proposed Training Pathways for Advanced Cardiovascular Training in Echocardiography.
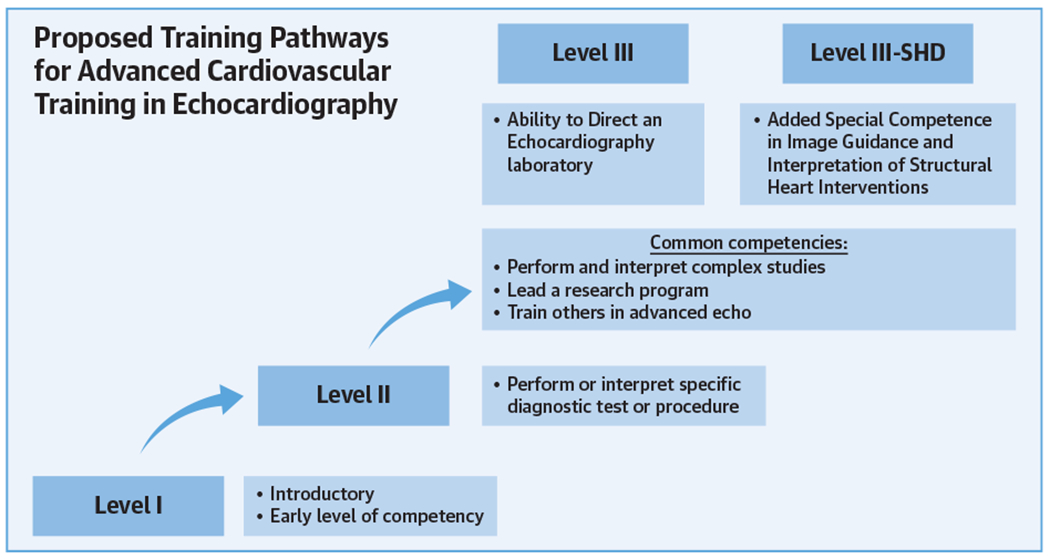
After achieving Level II training, trainees may seek to fulfill Level III or Level III-structural heart disease (SHD) training. Many competencies are shared by both pathways; however, Level III-SHD training requires focusing procedural volumes within structural heart disease interventions.
The recently published ATS document (7) already highlights several specific competencies that an interventional imager should achieve during training. Within this document, we describe additional competencies that should be achieved during training in SHD and specific knowledge-based competencies that could be omitted to ensure that the total duration of training is not adversely affected. In addition, we propose the minimum training requirements that might be required for optimal performance in this new subspecialty.
CORE COMPETENCY COMPONENTS OF TRAINING IN INTERVENTIONAL ECHOCARDIOGRAPHY
The core competency components of training specific to Level III-SHD echocardiography include medical knowledge (Table 1) and patient care and procedural skills (Table 2). In keeping with the premise that Level III-SHD training should not be simplified to signify added competencies only, it is just as important to recognize the Level III competencies that are not generally regarded as central to the performance of interventional echocardiography. Although it is reasonable to debate the merits of any subtracted specific medical knowledge competency, it is clear that the demonstrated knowledge expected to lead an academic medical center echocardiography laboratory will be in some way different from the demonstrated medical knowledge and technical skills expected of those who perform (and teach) interventional echocardiography for highly complex interventions.
TABLE 1.
Medical Knowledge Competency Domain for Level III-Structural Heart Disease Training in Echocardiography
| General competencies |
| 1. Level II medical knowledge including but not limited to: physical principles of ultrasound and the instrumentation used to obtain images; appropriate use criteria; limitations and potential artifacts of the echocardiographic examination; standard views for a comprehensive transthoracic and transesophageal echocardiogram; techniques to quantify cardiac chamber sizes and function; use of echocardiographic and Doppler data to evaluate native and prosthetic valve function and diseases; echocardiographic findings of pericardial disease; characteristic findings of basic and complex/post-operative adult congenital heart disease; principles and applications of 3D echocardiography |
| 2. Know the cardiac anatomy relevant to specific device implantation |
| 3. Know the nonstandard views (transthoracic and transesophageal) for assessing cardiac structures, including but not limited to: complex native and prosthetic valve function and diseases; left atrial appendage anatomy; interatrial septal anatomy |
| 4. Know the appropriate utilization of 3D imaging, including knowledge of the imaging views and quantitation necessary for pre-procedural planning and post-procedural hemodynamic assessment of structural intervention cardiac function (including ventricular and valvular function). |
| 5. Know the standard and nonstandard imaging of post-procedural complications. |
| Device and procedural competency |
| 6. Know the details of device design and the protocols for device implantation |
| 7. Know the correlation between invasive cardiovascular pressure measurements and echocardiographic parameters in structural heart disease. Know the limitations of echocardiographic and invasive measurement methods to reconcile discrepancies |
| 8. Know the appropriate utilization of 3D imaging, including knowledge of the imaging views required for intraprocedural guidance of structural valvular transcatheter procedures (including aortic, mitral, tricuspid, and pulmonic valve procedures) |
| 9. Know the appropriate utilization of 3D imaging, including knowledge of the imaging views required for intra-procedural guidance of structural nonvalvular procedures (including LAA occlusion and septal closure devices) |
| 10. Know the appropriate imaging views and modalities required for rapid assessment of hemodynamically unstable patients, including but not limited to the assessment of volume status, valvular function, ventricular function, and tamponade physiology |
| 11. Know the standard and nonstandard imaging of intraprocedural complications |
| Multimodality imaging |
| 12. Know the appropriate use of multiple imaging modalities for valvular and nonvalvular heart disease, including but not limited to: intracardiac echocardiography, computed tomography, and cardiac magnetic resonance imaging |
Evaluation tools include conference presentation, direct observation, and in-training examination.
3D = 3-dimensional; LAA = left atrial appendage.
TABLE 2.
Patient Care and Procedural Skills Competency Domain for Level III-Structural Heart Disease Training in Echocardiography
| 1. Skill to perform and interpret a basic and comprehensive transthoracic echocardiographic examination as per Level II training, including 2-dimensional and 3D imaging and advanced quantitation of valvular heart disease severity |
| 2. Skill to perform and interpret a basic and comprehensive transesophageal echocardiographic examination as per Level II training, including 2-dimensional and 3D imaging and advanced quantitation of valvular heart disease severity |
| 3. Skill to supervise and interpret stress echocardiograms performed to assess patients with valvular heart disease |
| 4. Skill to perform and interpret echocardiographic imaging for pre-procedural planning and post-procedural assessment of cardiac function (including ventricular and valvular function). |
| 5. Skill to perform and interpret rapid assessment of echocardiographic imaging for intra-procedural guidance of structural valvular transcatheter procedures (including aortic, mitral, tricuspid, and pulmonic valve procedures) |
| 6. Skill to perform and interpret rapid assessment of echocardiographic imaging for intraprocedural guidance of structural nonvalvular procedures (including LAA occlusion and septal closure devices) |
| 7. Skill to perform and interpret rapid assessment of hemodynamically unstable (undifferentiated shock) patients including but not limited to the assessment of volume status, valvular function, ventricular function, and tamponade physiology |
| 8. Skill to perform, render and interpret basic and advanced 3D echocardiography during guidance of procedures and/or surgery |
| 9. Skill to incorporate risk/benefit, safety, and cost considerations in the use of ultrasound techniques and structural interventions |
| 10. Skill to communicate effectively in the interventional environment |
Evaluation tools include direct observation, logbook, and simulation.
Abbreviations as in Table 1.
MEDICAL KNOWLEDGE.
As described in Table 1, required medical knowledge for interventional echocardiographers extends beyond familiarity with devices and includes imaging acquisition and analysis protocols. Interventional echocardiographers must effectively communicate with their interventional colleagues, which may require the development of site-specific terminology, particularly for anatomic localization. Given the important role of 3-dimensional (3D) echocardiography, interventional echocardiographers must know all pertinent aspects that must be acquired to provide pre-, intra-, and post-procedural assessments. They must know how to perform online and offline 3D reconstruction and quantitative analyses that may be device-specific, and they should be familiar with the imaging information available from and typically acquired with other imaging modalities, particularly CT imaging (8). Importantly, they must know the complications that can occur during procedures, their hemodynamic manifestations and echocardiographic appearance, and the most efficient ways to demonstrate these findings (9,10).
PATIENT CARE AND PROCEDURAL SKILLS.
The interventional echocardiographer should acquire all Level II patient care and procedural skills in echocardiography. These include the ability to perform and interpret basic and comprehensive transthoracic echocardiography (TTE) as well as transesophageal echocardiography (TEE) studies. Acquisition of these studies, and not just interpretation, is a major difference compared with the Level II requirement because the interventional imager will typically perform these tests without the assistance of a sonographer. Thus, accruing first-hand experience and hands-on procedure volumes (numbers) over and above the minimum recommended for Level II competency will be very helpful for candidates contemplating a career in SHD imaging.
Advanced imaging.
In addition to standard interpretation, the interventional imager should be knowledgeable in advanced quantitation of valvular heart disease. This should include the ability to supervise and interpret stress testing for valvular heart disease (11). Although a Level II echocardiographer should be able to perform a comprehensive TTE and TEE, Level III-SHD pre-procedural echocardiograms for planning require an understanding of the possible surgical or interventional device therapies that might be appropriate for a specific patient anatomy. Depending on the particular intervention, additional views or analysis may be required. Similarly, the post-procedural assessment and/or performance of studies to evaluate device function requires a comprehensive understanding of device appearance, intended position, and function. Familiarity with the complications of each procedure is essential for follow-up imaging. Because of the inherent risks of interventional procedures, the imager should be able to acquire and interpret images rapidly and to effectively communicate these findings to the interventionalists. In particular, expeditious evaluation of intraprocedural hemodynamic instability is critical to the outcome of procedures. Although routine checklists can be used, the competent interventional imager should be able to triage based on specific clinical scenarios.
A growing number of procedures have begun to rely on intracardiac echocardiography (ICE) to supplement or, at times, supplant TEE imaging (12–14). This stems from the inherent difficulties in TEE imaging “around” large guide catheters, the narrow anatomic targets, and the high precision required for device positioning. Currently, there is no standardization of ICE imaging or operator requirements, resulting in a lack of general expertise and understanding of training requirements. However, as the need for adjunctive intraprocedural imaging grows, addressing these gaps will become a priority.
Interventional suite protocols.
Interventional echocardiographers will be spending a significant proportion of their time in the cardiac interventional or hybrid suite instead of the echocardiography laboratory. They need to understand and practice both standard infection prevention protocols and radiation safety protocols for the patient and themselves. Due to their close proximity to the x-ray equipment during most cases, the echocardiographer could receive some of the highest doses of radiation (15). Ideally, the imagers should participate in the same radiation safety courses provided to interventional cardiology fellows at the beginning of their training to understand how to minimize risk. Interventional echocardiographers should work with the catheterization laboratory or operating room staff to ensure they have the necessary protective gear (fitted lead aprons, thyroid shield, and lead glasses, as well as a lead shield between the table and them) to minimize exposure. Studies have shown that simple implementation of additional shields reduced radiation exposure to the interventional echocardiographer by 82% (15). Radiation badges should be worn at all times to document individual exposure. Institutions should support the Program for Keeping Occupational Radiation Exposures to Levels as Low as Reasonably Achievable (ALARA) should provide quarterly reports of radiation exposure to the imager. In those cases where exposure is above the ALAR I levels, safety precautions should be reviewed to ensure appropriate conduct.
Communication.
Interventional echocardiographers must have the skills to work with the interventional cardiologist, surgeon, and anesthesiologist to determine the most appropriate anesthetic approach given the intraprocedural imaging needs for each individual case. For example, heart team review of the pre-operative imaging for a routine transcatheter aortic valve replacement (TAVR) case, including both echocardiography and CT scans, may reveal high-risk anatomic features (i.e., low-lying left main coronary artery or bulky leaflet/annular calcium) for which TEE imaging is required to ensure optimal outcomes. These cases may require general anesthesia. Conversely, if there is a good quality pre-operative echocardiogram or CT scan with no high-risk features, the team may determine that the risks of general anesthesia in an elderly frail patient with poor biventricular function or chronic obstructive pulmonary disease exceed the benefits of this approach. These procedures should be performed with monitored anesthetic care. In addition, some cases may require general anesthesia for factors unrelated to imaging, which should also be communicated and understood before the procedure because it may change the imaging modality used.
Integration into the heart team.
The importance of imager participation in the heart team discussions before the intervention cannot be overemphasized. By having the imager be engaged, pre-operative imaging results can be reviewed by the team, especially when the severity of disease is being established. The heart team meeting is an ideal forum in which to review all studies of a particular patient to determine the best course of action. The contribution of a well-trained echocardiographer who understands the guidelines and can also assess image quality is invaluable. In many cases, additional imaging is needed, and the presence of the interventional echocardiographer will ensure that the appropriate study is performed.
During interventions, interventional echocardiographers should be considered part of the procedural team rather than simply a colleague from the echocardiography laboratory coming to assist in the case or an anesthesiologist present intraoperatively. Therefore, interventional echocardiographers require the skills to work collaboratively with the staff in the electrophysiology or catheterization laboratory or operating room to ensure optimal procedural flow. To optimize intraprocedural results, it is crucial that team members have mutual respect and trust, which are often developed through shared decision-making and open communication and common procedural terminology. Sharing imaging screens so that both interventionalist and imager see all available imaging simultaneously may also help foster bidirectional communication.
Finally, an important aspect of communication among the team members is to have an understanding of the goals for the procedure for a given patient. For example, in a younger, relatively healthy patient with good anatomy for an edge-to-edge mitral valve repair, the goal would be to reduce the mitral regurgitation to less than mild, whereas in an older frail patient with poor valve anatomy (16), the goal would be to reduce the leak from torrential to moderate. These discussions should take place before starting the intervention, and there should be consensus among the team regarding the expectations for individual patients.
It is not feasible for all imagers in training to have expertise in every procedure because the field continues to evolve and expand. Imagers will require continuing education after training. In some instances, they may need to bring in outside proctors and visit other sites to gain additional experience with particular devices. This is especially true when a site starts performing new procedures. Often, the interventionalist must attend a training course before initiating a new structural procedure with a new device. It is critical that the imager be a part of this training process to ensure that the team functions smoothly. Indeed, many device companies already insist that the imager participates in the training delivered to a site team for a new structural procedure or device, as they are aware that full involvement of the imaging team is crucial to ensure procedural success.
MINIMUM PROCEDURAL VOLUME FOR SHD IMAGERS
There are no robust data that outline the number of procedures that an echocardiographer needs to train before becoming competent in SHD imaging. Moreover, technology and commensurate imaging requirements are changing rapidly and call for new skills, often developed over a small number of patients undergoing these advanced procedures. In the absence of learning curve data, arriving at a consensus on the suggested minimum numbers of procedures to achieve SHD competence requires a challenging compromise between what is achievable in centers performing low volumes of structural interventions and what is considered the minimum requirement to achieve a safe level of proficiency and competence in uncomplicated cases. As more data become available on the learning curve for various procedures, which for imaging intensive procedures would be similar for both interventionalists and imagers, defining minimum requirements may be possible. Importantly, for trainees to move from being considered proficient to having achieved a level of expertise that would allow them to support more complex cases, the number of supervised examinations would necessarily be higher. The minimal procedural volumes for Level III-SHD echocardiography competency has been suggested in the 2019 ACC/AHA/ASE ATS and is reproduced in Table 3.
TABLE 3.
Minimum Procedural Volume Typically Necessary for the Development and Demonstration of Level III-Structural Heart Disease
| Procedure/Technical Skill | Number* | |
|---|---|---|
| Echocardiographic guidance of interventional procedures, which includes: | 75 | |
| Structural valvular intervention† | 30 | |
| Transseptal catheterization guidance | 10 | |
| Percutaneous closure of septal defects/perivalvular leaks | 15 | |
| Alcohol septal ablation | 10 | |
| Placement of devices to exclude the left atrial appendage | 10 | |
| Ventricular assist device placement and assessment | 20 | |
| Intraoperative transesophageal echocardiography,† which includes: | 75 | |
| surgical valve repair or replacement | 50 | |
| Intracardiac echocardiography | 10 | |
From Wiegers et al. (7).
Numbers are based on consensus and intended as general guidance based on the educational needs and progress of typical Level III echocardiography trainees. Competency to perform each procedure must be based on evaluation by the supervising echocardiography laboratory director and may exceed or be below the threshold number shown in this table.
The Level III trainee should successfully complete both right-sided and left-sided procedures if the goal is to obtain competency in the full range of structural heart disease interventions.
Training should occur within cardiology departments with established SHD programs that perform a reasonable volume of most types of structural interventions (e.g., TAVR, edge-to-edge mitral valve repair, left atrial appendage occlusion, patent forman ovale/atrial septal defect closure). The components of a functioning heart team and SHD program have been previously described (17–19). In addition, operator (interventionalist) and institutional volumes for most transcatheter procedures have been described in recent multisocietal documents for each procedure (20,21) as well as the 2018 AATS/ACC/SCAI/ STS Expert Consensus Systems of Care Document for TAVR (22) and the 2019 ATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document for valvular heart disease (5). These documents should provide some insight into the training requirements for the interventional imager. For TAVR (20), the proceduralist should possess previous experience at an active TAVR site and have participated in at least 100 transfemoral TAVR cases with at least 50 cases as the primary operator. Established programs should perform at least 50 TAVRs per year or 100 TAVRs over 2 years and maintain an STS/ACC TVT Registry score above the bottom 10% for metrics. Because TAVR is typically guided primarily by fluoroscopy, there are a growing number of TAVRs performed under conscious sedation with intraprocedural TTE instead of TEE (23). It is recognized that in many countries, TTE is mainly performed by sonographers. Therefore, competency numbers may refer to the interpretation of peri-procedure and post-procedure TTE examinations by interventional echocardiographers/imaging cardiologists, while also recognizing it is unlikely that TTE examinations will be performed by anesthesiologists. Thus, the consensus recommendation for the SHD imager includes 50 comprehensive TTE studies performed or interpreted during or after SHD intervention. The utility of TEE imaging during complex cases (24), however, continues to warrant experience in TEE guidance of TAVR and should be included in the recommended ≥75 comprehensive echocardiographic studies for guidance of interventional procedures (Table 3).
For transcatheter mitral valve procedures (21), the institutional volume of catheterizations should be at least 1000 per year with at least 400 percutaneous coronary interventions per year with a surgical program performing at least 25 mitral valve procedures per year, of which at least 10 must be mitral valve repairs. The interventionalists’ volume should be at least 50 structural procedures per year (including atrial septal defect/patent forman ovale and transseptal punctures) with suitable training on the specific devices to be used. Access to the left heart by transseptal puncture is used in a variety of other SHD interventions, including mitral edge-to-edge repair, mitral valve-in-valve (or valve-in-ring, valve-in-mitral annular calcium), and left atrial appendage occlusion. The SHD imager thus should acquire experience in ≥25 TEE-guided transseptal punctures for access to the left heart, preferably for a variety of indications (25) as indicated in Table 3. In addition, among the ≥75 comprehensive TEE studies for guidance of interventional procedures, it is the consensus of this writing group that at least 10 should be guidance of mitral leaflet coaptation devices (i.e., edge-to-edge repair). Finally, the ATS document recommends a minimum of 10 procedures at a training facility familiar with the use of ICE for competency in ICE imaging. However, given the lack of societal guidelines for standardization of ICE imaging or operator requirements, what constitutes adequate training for this imaging modality remains hypothetical.
TRAINING PROGRAM REQUIREMENTS
The interventional imaging trainee should be under the supervision of an experienced interventional echocardiographer who has been performing unsupervised structural imaging for a minimum of 5 years with volumes similar to that of the experienced proceduralist: at least 100 TAVR and 15 transcatheter mitral procedures. The duration of training will depend on the level of exposure to structural cases that the trainee receives. This, in turn, will depend on the volume of cases undertaken in the center where the training is being delivered as well as the number of trainees being trained at any one time. In a center performing a minimum of 200 structural interventions per year, it should be possible for the trainee to achieve experience in the requisite number of individual interventions in 6 months. Whereas, if the center volume is ~100 cases a year, it is likely to take a minimum of 9 months to achieve the recommended case numbers. At the time of writing, in the United States, there are ~600 sites performing TAVR with a site average of ~100 cases per year (ranging from a few cases to >400, with most sites performing ~60 per year). For percutaneous edge-to-edge mitral valve repair, ~300 sites are currently performing this procedure, with a previous study of the STS/ACC TVT Registry reporting a site average of 6 cases over the 10-month study period (interquartile range: 4 to 10 cases; range: 1 to 58 cases) (26). Because the suggested minimum number of TAVR and mitral valve repair procedures may not be performed at any single site, some training programs may not be able to fulfill the minimum requirements for Level III-SHD training of the interventional imager over the course of 1 year. As the need for trained interventional imagers increases, it is possible that the learning curve could be shortened with the use of simulation training. Prat et al. (27) showed that with the use of computerized TEE simulation training, competency for hemodynamic assessment of ventilated patients in the intensive care unit was achieved after an average of 32.5 ± 10 supervised studies in the control group compared with only 13.6 ± 8.5 in the group trained using simulators (p < 0.0001). Matyal et al. (28) showed that with simulator TEE training, the novice and more advanced trainee had no significant differences in image capture success rates.
Peri-procedural TEE training requirements are common to both anesthesiologists and cardiologists. Although the intraprocedural imager can draw on previous experience performing routine TEE, to achieve the skills for rapid acquisition, interpretation, and communications, as well as the required breadth of experience across the range of structural interventions that are currently performed, it is recommended that the trainee performs and interprets a minimum of 75 peri-procedural TEEs under expert supervision with at least one-third of these cases involving imaging to access the left atrium and a minimum of 10 transcatheter edge-to-edge mitral valve repair cases. The 2019 Expert Consensus Systems of Care document (5) recommends that existing sites perform at least 50 TAVRs per year or 100 TAVRs over 2 years; however, the recent STS/TVT database suggests that ~40% of TAVRs are being performed without general anesthesia. Thus, depending on the volume of TEE-imaged procedures at a given site, a longer period of advanced training may be required.
The range of peri-procedural complications that can occur during structural cases is large; however, given the expertise that now goes into case selection and procedural planning, complications are rare, and it is possible that a trainee exposed to the numbers of cases recommended in this document may not see many, if any, complications. It is therefore recommended that centers providing structural echo training maintain a library of peri-procedural echo cases with complications and that trainees review those cases with their supervisors. These case reviews should be logged, and the supervisor should attest to the fact that the trainee is independently able to recognize the complications.
It is important to realize that the amount of training time or the number of procedures performed cannot ensure competency or proficiency. Thus, it is the opinion of the writing group that to help ensure competency, a certification process must be established that would include not only a program director statement of competency and completion of training requirements but also a standardized evaluation.
POST-FELLOWSHIP TRAINING PATHWAYS
TRAINING PATHWAYS FOR CARDIOLOGISTS.
Evolution of SHD interventions typifies an innovation that is often disruptive, unpredictable, and precedes regulation. It is not the first time that techniques/ therapies have been introduced into clinical practice without guidelines for their use (29). Interventional cardiology, laparoscopic surgery, and TEE are a few of the many examples of procedures that had widespread clinical adoption prior to societal guidelines for their use. The major challenge in this situation is whether novel techniques should be identified as a ubiquitous skill set to the existing level of training or be recognized as another level of expertise requiring independent recognition and certification.
It is a bigger challenge to address the educational needs of fully trained practitioners (30). In the absence of pathways for training in imaging for structural heart interventions, a large number of practicing physicians are at risk of being disenfranchised. In addition, there will not be enough “qualified” imagers to guide structural procedures. There are fiscal, logistic, and administrative constraints/consequences for practicing physicians to become retrained and re-certified. Ideally, such programs should cause minimal hardship or professional disruption. Besides being comprehensive, multifaceted, and practical in their scope, these programs should address these practical concerns and avoid untoward consequences for those who want to enhance their skills. The new Expert Consensus Systems of Care Document: Operator and Institutional Recommendations and Requirements for Transcatheter Aortic Valve Replacement (20) suggests that for interventionalists, a pathway could include a formal proctorship wherein an established interventional cardiologist or cardiac surgeon participates in an established TAVR program under the tutelage of an experienced team. It is the opinion of the writing committee that for any training program to be widely adopted, there should be personal, institutional, and societal levels of commitment to the importance of image guidance for structural heart interventions.
Personal commitment.
Practicing cardiologists who want to be recognized as “interventional echocardiographers” have to demonstrate a personal level of commitment to improve their education and training. They have to commit to the concept of continuous learning and actively seek available opportunities to educate and train themselves.
Institutional commitment.
Health care institutions/departments have to recognize the improved patient safety, quality of care, and fiscal value of providing opportunities for skill enhancement for their staff. In this context, it is important to provide multimodal educational resources (print/web-based), expert interaction, and logistic and administrative support for those who wish to enhance their skill level. Once achieved, there should also be mechanisms to credential and recognize this expertise.
Societal commitment.
Professional organizations and societies should develop guidelines, educational curricula, and training symposia for an ongoing training program in the form of continuing medical education activities. With the availability of high-fidelity haptic simulators, it is now possible to acquire a level of familiarity with instruments and practice basic psychomotor skills. Complex tasks can now be mastered without any consequences of failure or risk to the patient. These opportunities should be available with minimal fiscal and logistic constraints to those practitioners who wish to enhance their expertise. Finally, independent certification criteria should be developed that are inclusive and have pathways/mechanisms for certification for practicing cardiologists.
TRAINING PATHWAYS FOR ANESTHESIOLOGISTS.
Proficiency in peri-operative TEE for anesthesiologists has evolved from a desirable skill in the 1990s to an expected competency in the last decade (31). With establishment of an accreditation system of cardiac anesthesia fellowship training, peri-operative TEE training has become more organized, with a defined number of TEEs required to be performed and interpreted by a fellow during training. Although the total number has been determined, the nature of these TEE examinations has not been specified, and the scope of imaging exposure is expected to be broad and comprehensive.
Similar to their involvement in cardiac surgery, cardiac anesthesiologists also often provide anesthetic and echocardiography services for SHD interventions. As with a cardiovascular fellow seeking Level III-SHD training, cardiac anesthesiologists performing the imaging guidance for SHD interventions must have a firm understanding of basic imaging skills along with an additional knowledge base and skills that are additive requirements to cardiac anesthesiology Level II training. Therefore, it is the opinion of the writing committee that a baseline proficiency in peri-operative TEE should be a prerequisite to establish competence in providing imaging guidance for structural heart interventions for cardiac anesthesia fellows.
The Accreditation Council for Graduate Medical Education expects the fellows enrolled in cardiac anesthesia fellowship programs to meet the requirements for board certification by the NBE for special competence in advanced peri-operative TEE. Cardiac anesthesia fellows are required to perform at least 300 TEE examinations, of which 150 examinations must be personally performed and interpreted (Figure 1A). Guidance for exposure required to determine competence in procedural imaging for structural heart interventions can be drawn from the Core Cardiovascular Training Statement 4 guidelines for cardiology fellows. Because anesthesiologists are generally not involved in screening or post-procedure follow-up echocardiography, structural heart TEE requirements for cardiac anesthesia fellows should be peri-procedural TEE examinations only. It is recommended that to be identified as competent in structural heart imaging, cardiac anesthesia fellows should participate in at least 75 structural heart cases, of which at least 40 must be personally performed, while the remainder may be interpreted with a supervising echocardiographer to meet the requirement of a total of 75 examinations (Figure 1B). These examinations can be included in or exceed the 300 required to meet NBE certification criteria.
FIGURE 1. Proposed Training Requirements for Anesthesiologist.
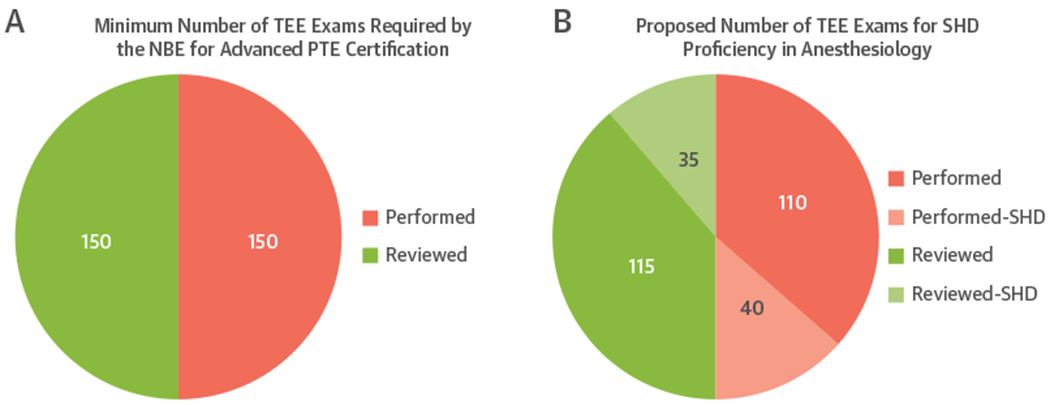
(A) Minimum number of transesophageal echocardiograms (TEEs) required by the National Board of Echocardiography (NBE) for Advanced Peri-operative Transesophageal Echocardiography (PTE) certification. (B) Breakdown of the recommended 75 specific structural heart disease (SHD) studies (shaded): the pink shaded section shows the studies performed (n = 40), and the green shaded section shows the additional cases reviewed (n = 35).
The importance of acquisition of proficiency in structural heart imaging for cardiac anesthesiologists has been acknowledged (29). However, due to the ever-increasing scope, education and training for imaging in structural heart interventions will require continuous updating. Besides providing hands-on training and ensuring possession of basic psychomotor skills, it is also expected that fellowship programs will introduce formal didactics and curricula based on SHD interventions. These educational materials should include but not be limited to access to print and online educational resources, data repositories for imaging review, conferences, and interaction with experts with opportunities for in-service/orientation with new technology and procedures. It is the opinion of the writing committee that competence in structural heart imaging, whether for the Level III-SHD cardiology trainee or the cardiac anesthesia fellow, should require a specific letter of verification by the program director acknowledging that the trainee performed the requisite number of examinations. Cardiac anesthesia fellowship programs will possibly have to be individually accredited as structural heart training programs, a first step to introduction of a certification in structural heart imaging for anesthesiologists.
SUMMARY
Interventional echocardiography is a new subspecialty within cardiology and anesthesiology that requires advanced skills in performing and interpreting echocardiographic studies, as well as guiding interventional procedures. For the cardiology fellow in training interested in this subspecialty, the training pathway could diverge from the traditional pathway after Level II training is achieved. Level III-SHD minimum procedural numbers do not differ significantly from those of Level III; however, the emphasis on imaging for valvular heart disease and SHD interventions distinguishes this training from the traditional pathway. Importantly, this consensus document may provide a starting point for discussions of more official professional society position documents and credentialing pathways.
REFERENCES
- 1.Holmes DR Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–54. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252–89. [DOI] [PubMed] [Google Scholar]
- 3.Hahn RT. The new paradigm for the management of valvular heart disease: the multidisciplinary heart team. J Am Soc Echocardiogr 2011;24:A28. [DOI] [PubMed] [Google Scholar]
- 4.Ryan T, Berlacher K, Lindner JR, Mankad SV, Rose GA, Wang A. COCATS 4 Task Force 5: training in echocardiography. J Am Coll Cardiol 2015;65: 1786–99. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura RA, O’Gara PT, Bavaria JE, et al. 2019 AATS/ACC/ASE/SCAI/STS Expert Consensus Systems of Care Document: a proposal to optimize care for patients with valvular heart disease: a joint report of the American Association for Thoracic Surgery, American College of Cardiology, American Society of Echocardiography, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2019;73:2609–35. [DOI] [PubMed] [Google Scholar]
- 6.Narula J, Chandrashekhar YS, Dilsizian V, et al. COCATS 4 Task Force 4: training in multimodality imaging. J Am Coll Cardiol 2015;65:1778–85. [DOI] [PubMed] [Google Scholar]
- 7.Wiegers SE, Ryan T, Arrighi JA, et al. 2019 ACC/AHA/ASE Advanced Training Statement on Echocardiography (Revision of the 2003 ACC/AHA Clinical Competence Statement on Echocardiography): a report of the ACC Competency Management Committee. J Am Coll Cardiol 2019;74: 377–402. [DOI] [PubMed] [Google Scholar]
- 8.Leipsic J, Nørgaard BL, Khalique O, et al. Core competencies in cardiac CT for imaging structural heart disease interventions: an expert consensus statement. J Am Coll Cardiol Img 2019;12:2555–9. [DOI] [PubMed] [Google Scholar]
- 9.Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for multimodality imaging in valvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;70: 1647–72. [DOI] [PubMed] [Google Scholar]
- 10.Doherty JU, Kort S, Mehran R, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 appropriate use criteria for multimodality imaging in the assessment of cardiac structure and function in nonvalvular heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2019;73:488–516. [DOI] [PubMed] [Google Scholar]
- 11.Lancellotti P, Pellikka PA, Budts W, et al. The clinical use of stress echocardiography in non-ischaemic heart disease: recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:101–38. [DOI] [PubMed] [Google Scholar]
- 12.Alkhouli M, Hijazi ZM, Holmes DR Jr., Rihal CS, Wiegers SE Intracardiac echocardiography in structural heart disease interventions. J Am Coll Cardiol Intv 2018;11:2133–47. [DOI] [PubMed] [Google Scholar]
- 13.Bartel T, Muller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J 2014;35: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alqahtani F, Bhirud A, Aljohani S, et al. Intracardiac versus transesophageal echocardiography to guide transcatheter closure of interatrial communications: nationwide trend and comparative analysis. J Interv Cardiol 2017;30:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowhurst JA, Scalia GM, Whitby M, et al. Radiation exposure of operators performing transesophageal echocardiography during percutaneous structural cardiac interventions. J Am Coll Cardiol 2018;71:1246–54. [DOI] [PubMed] [Google Scholar]
- 16.Hahn RT. Transcathether valve replacement and valve repair: review of procedures and intraprocedural echocardiographic imaging. Circ Res 2016;119:341–56. [DOI] [PubMed] [Google Scholar]
- 17.Holmes DR Jr., Rich JB, Zoghbi WA, Mack MJ.The heart team of cardiovascular care. J Am Coll Cardiol 2013;61:903–7. [DOI] [PubMed] [Google Scholar]
- 18.O’Gara PT, Grayburn PA, Badhwar V, et al. 2017 ACC expert consensus decision pathway on the management of mitral regurgitation: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70:2421–49. [DOI] [PubMed] [Google Scholar]
- 19.Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2017;69:1313–46. [DOI] [PubMed] [Google Scholar]
- 20.Bavaria JE, Tommaso CL, Brindis RG, et al. 2018 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter aortic valve replacement: a joint report of the American Association for Thoracic Surgery, the American College of Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2019;73:340–74. [DOI] [PubMed] [Google Scholar]
- 21.Tommaso CL, Fullerton DA, Feldman T, et al. SCAI/AATS/ACC/STS operator and institutional requirements for transcatheter valve repair and replacement. Part II. Mitral valve. J Am Coll Cardiol 2014;64:1515–26. [DOI] [PubMed] [Google Scholar]
- 22.Hijazi ZM, Ruiz CE, Zahn E, et al. SCAI/AATS/ACC/STS operator and institutional requirements for transcatheter valve repair and replacement, part III: pulmonic valve. J Am Coll Cardiol 2015;65:2556–63. [DOI] [PubMed] [Google Scholar]
- 23.Grover FL, Vemulapalli S, Carroll JD, et al. 2016 Annual report of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol 2017;69:1215–30. [DOI] [PubMed] [Google Scholar]
- 24.Hahn RT, Nicoara A, Kapadia S, Svensson L, Martin R. Echocardiographic imaging for transcatheter aortic valve replacement. J Am Soc Echocardiogr 2018;31:405–33. [DOI] [PubMed] [Google Scholar]
- 25.Alkhouli M, Rihal CS, Holmes DR Jr. Transseptal techniques for emerging structural heart interventions. J Am Coll Cardiol Intv 2016;9: 2465–80. [DOI] [PubMed] [Google Scholar]
- 26.Sorajja P, Mack M, Vemulapalli S, et al. Initial experience with commercial transcatheter mitral valve repair in the United States. J Am Coll Cardiol 2016;67:1129–40. [DOI] [PubMed] [Google Scholar]
- 27.Prat G, Charron C, Repesse X, et al. The use of computerized echocardiographic simulation improves the learning curve for transesophageal hemodynamic assessment in critically ill patients. Annals Intens Care 2016;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matyal R, Mahmood F, Knio ZO, et al. Evaluation of the quality of transesophageal echocardiography images and verification of proficiency. Echo Res Pract 2018;5:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmood F Predicting the future by creating it: Let us drive the change and not be its victim. J Cardiothorac Vasc Anesth 2017;31:166–8. [DOI] [PubMed] [Google Scholar]
- 30.Matyal R, Mitchell JD, Mahmood F, et al. Faculty-focused perioperative ultrasound training program: a single-center experience. J Cardiothorac Vasc Anesth 2019;33:1037–43. [DOI] [PubMed] [Google Scholar]
- 31.Mahmood F, Matyal R, Skubas N, et al. Perioperative ultrasound training in anesthesiology: a call to action. Anesth Analg 2016;122: 1794–804. [DOI] [PubMed] [Google Scholar]


