SUMMARY
Our immune system has evolved to protect us from pathogens and maintain homeostasis through localization in diverse tissue sites throughout the body. Immune responses are orchestrated by T cells, which direct pathogen clearance at the infection site and establish tissue resident memory T cells (TRM) for protection immunity. Here, we discuss how tissue immune responses are influenced by various stressors (e.g., metabolic, environmental, aging) that are rapidly changing due to climate fluctuations and globalization. We propose potential strategies for targeting tissue immunity to mitigate future pathogenic and environmental challenges, and areas of investigation that can elucidate mechanisms for adapting and restoring homeostasis.
INTRODUCTION
Human survival depends on the ability to protect ourselves from exposure to the diverse types of foreign substances in the environment that we breathe in and the food we eat -- containing a multitude of antigens in the form of microorganisms, nutrients, airborne particles, pollutants, and toxins. The immune system is critical to preserving the body (i.e., the self) amidst these different invaders. Immune cells develop in specialized lymphoid organs, but function and are maintained in tissue sites throughout the body. In addition to its critical function in host defense against pathogens, the immune system maintains homeostasis through immunosurveillance, sensing metabolic changes, and controlling inflammation due to environmental insults. Studies in mouse models and humans have revealed how immune cells are compartmentalized into tissue sites, and the key role of site-directed immunity in long-term memory, protective immunity, and homeostasis. These studies provide a new framework for studying the immune response based on tissue localization and for addressing future challenges to human health in the form of emerging pathogens and major environmental stressors due to climate change.
Immune cells can be subdivided into innate or adaptive based on their recognition properties and functional roles. Innate immune cells such as macrophages, dendritic cells (DC), and granulocytes exhibit broad recognition for molecules expressed by microorganisms, or which are released during tissue injury. Innate cells—both resident in and recruited to tissues--comprise the early responders to pathogen encounter in situ. Adaptive immunity, by definition, develops as a result of antigen encounter, and is mediated by B and T lymphocytes, collectively expressing antigen-specific receptors of diverse specificities. T cells develop in the thymus as distinct lineages of CD4+ (T-helper) and CD8+ (Cytotoxic) T cells which seed secondary lymphoid organs (spleen, lymph node), where they become activated by DC presenting antigen. Activated CD4+ and CD8+T cells differentiate to effector cells and migrate to tissue sites of infection for directing pathogen clearance and lysis of infected cells. Pathogen-specific B cells also become activated in lymphoid sites where they interact with CD4+T helper cells for differentiation to antibody-producing plasma cells; circulating antibodies subsequently bind to pathogens, marking them for destruction. Together, these innate and adaptive immune processes mediate pathogen removal, with T cells playing pivotal roles in the initiation and functional regulation of these cellular and humoral immune responses.
A key feature of the adaptive immunity is immunological memory maintained by pathogen-specific memory T and B cells, and antibodies in plasma. Memory T cells derive from activated or effector T cells generated during the initial immune response, and consist of non-circulating tissue-resident memory T cells (TRM) retained in diverse tissue sites, and circulating, tissue surveilling memory T cells (Szabo et al., 2019). Humoral immunity is maintained as memory B cells and long-lived plasma cells—both largely confined to lymphoid organs (for a review, see (Weisel and Shlomchik, 2017)). The establishment of immune memory is essential for protective immunity. While antibodies can provide direct protection through pathogen neutralization, T cells exhibit much broader and diverse roles in protective immunity. TRM, in particular coordinate immune surveillance, protection, and homeostasis, and exhibit tissue-specific adaptations (Szabo et al., 2019). TRM are also the major adaptive immune cells in barrier sites throughout most of adult life (Kumar et al., 2018; Senda et al., 2019; Thome et al., 2014), suggesting that they play major roles in controlling immunity over a lifetime.
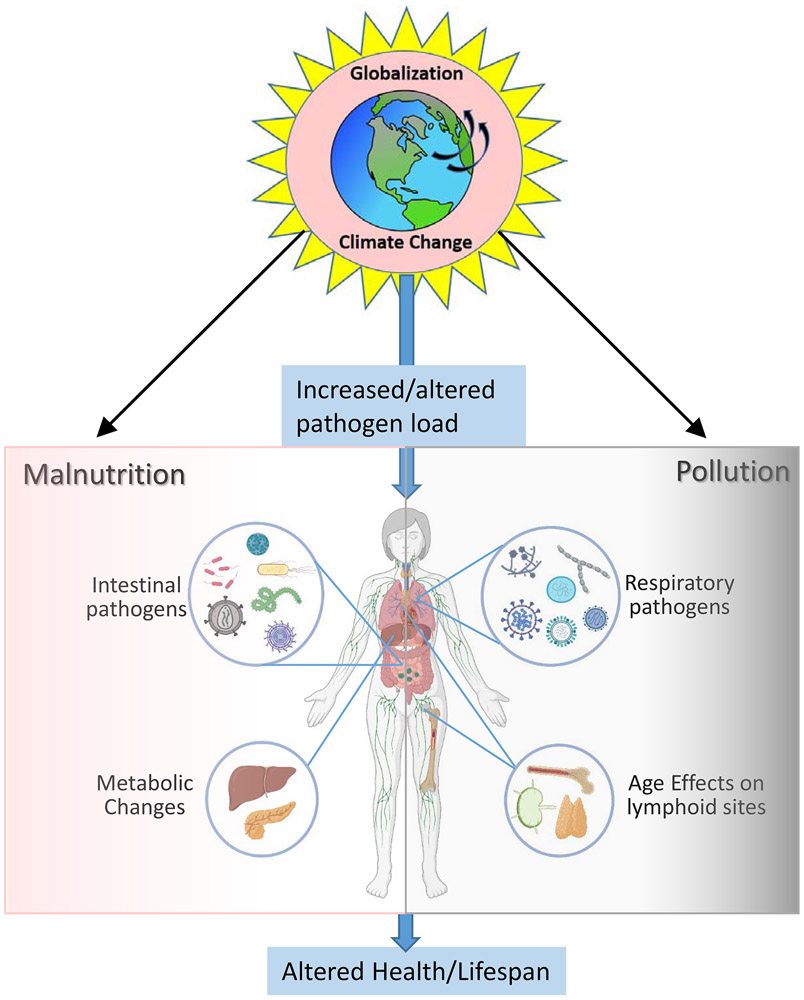
The broad anatomic organization of the immune system enables protection from diverse pathogens and environmental challenges. Currently, our world is undergoing a rapid transformation on multiple levels with major consequences for our immune system (Figure 1). Global climate change and globalization itself has resulted in an increased number, variety, and dissemination of pathogens, particularly those infecting the respiratory and gastrointestinal (GI) tract (Figure 1). As the current COVID-19 pandemic has demonstrated, we remain highly vulnerable to infectious disease caused by novel pathogens; ensuring the health and protection of future populations requires that we plan for their emergence. Climate change is also causing dramatic alterations to global fauna and flora, with major impacts on the food supply, nutrient availability, and air quality due to pollution and environmental toxins, with direct impacts on the sites of infectious challenges (Figure 1). These metabolic challenges will impact the immune system, and particularly adaptive immunity that develops over time and age, along with age-associated alterations (Figure 1). In this review, we will discuss how the anatomic organization of the immune system is critical to host defense, the impact of nutritional alterations and diminishing air quality on immune system function, and strategies to modulate and fortify localized immunity to protect current and future generations.
Figure 1. Overview of how our changing environment potentially impacts our immune system.
Climate change and globalization has led to an increase in pathogen load, diversity, and transmissibility which provide increasing challenges to the immune system. Diagram shows the multiple bacterial, viral and fungal pathogens encountered through the respiratory and oral-gastrointestinal tract that can spread throughout the population. These pathogens require mobilization and persistence of immune responses at these sites to prevent their infection and dissemination throughout the body. Aging imposes further changes on the immune system, which diminishes protective capacity to new pathogens. The changing global environment imposes changes in the food supply leading to malnutrition, systemic metabolic changes and metabolic disease (left), along with worsening air quality due to pollutants and toxins we breathe (right). The cumulative effects of all of these stresses results in worse overall health of the population and reduced lifespan.
Tissue Compartmentalization of the human immune system
A key feature of the immune system is its organization in multiple anatomic sites. While blood is the most readily sampled site for investigations of human immune cells, studies from the past decade using tissue samples obtained from surgical explants and more recently, from human organ donors (non-diseased sites obtained at the time of organ acquisition for transplantation (Thome et al., 2014)), have generated an evolving spatial atlas of human immune cell composition (for reviews, see (Poon and Farber, 2020; Szabo et al., 2019)). Results based on analysis of hundreds of donors reveal tissue-intrinsic compositions of innate and adaptive immune cells that are conserved between individuals (Figure 2) (Dogra et al., 2020; Granot et al., 2017; Kumar et al., 2018; Kumar et al., 2017; Thome et al., 2014; Weisberg et al., 2019; Weisel et al., 2020). Innate immune cells including DC, Innate lymphoid cells such as NK cells, exhibit tissue-specific subset compositions in lung, intestines, lymphoid organs (BM, spleen LN) and blood which do not vary with age (Dogra et al., 2020; Granot et al., 2017). Lymphocytes—both T and B cells—persist as tissue resident and circulating subsets with the frequency and phenotype specific to the tissue site (Kumar et al., 2017; Szabo et al., 2019; Thome et al., 2014; Weisel et al., 2020). TRM predominate in mucosal and exocrine sites, and are also found in lymphoid organs (bone marrow, spleen, LN), while circulating memory T cells and naïve T cells are in blood and lymphoid sites (Kumar et al., 2018). Memory B cells can also persist as tissue resident populations in intestines and are found in lymphoid sites, while naïve B cells predominate in blood (Weisel et al., 2020). Together these studies indicate that the human immune system is anatomically localized in diverse sites in a tissue-specific manner.
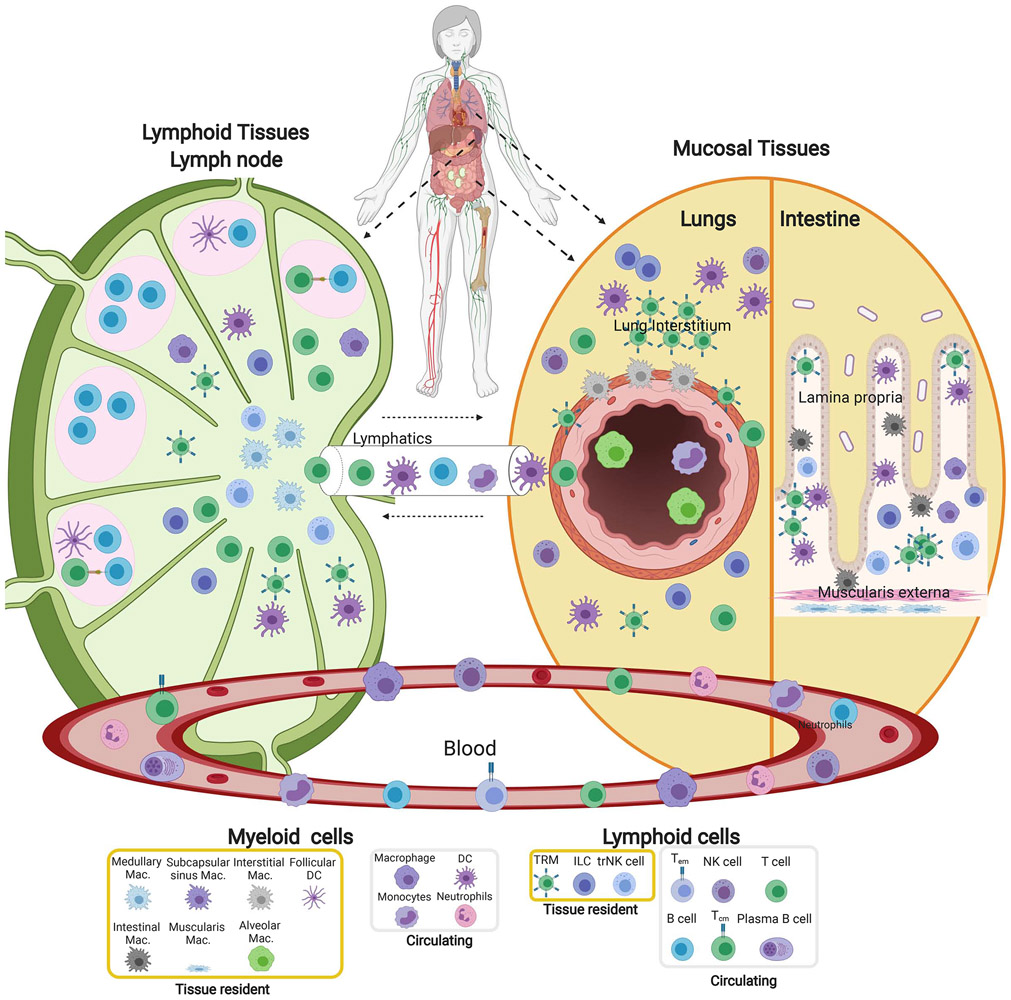
Figure 2: Compartmentalization of immune cells in blood and tissue sites.
The immune system is organized in multiple tissue sites throughout the body; the distribution and composition of immune cells are specific to the tissue sites. Diagram depicts the structural organization and composition of immune cells in mucosal sites (right, lungs and intestines), a lymph node as a representative secondary lymphoid organ (left) and their distinct localization relative to the major epithelial cells and structures of each site. Mucosal sites contain macrophages and tissue resident T cells in the epithelial layers (interstitium) of airways and intestinal villi. In the lymph node, B cells are situated in follicles along with DC surrounded by T cell areas and distinct macrophage subsets in T cell areas. Germinal centers within follicles are the sites of T-B cell interactions and differentiation of B cells to antibody-secreting cells. Also depicted are the major conduits to circulation (Blood, lymphatics) and circulating immune cells. The identity of individual cell subsets is indicated in the legend at bottom.
These site-specific immune cell compositions reflect their distinct localization within tissue niches (Figure 2). Imaging studies are beginning to elucidate immune cell niches within human lymphoid and mucosal tissues. In mucosal sites, tissue resident cells such as macrophages and TRM localize near airways and in intraepithelial layers of the lung (Pizzolla et al., 2018; Snyder and Farber, 2019), while in intestines, they are situated in the lamina propria and intraepithelial regions (Mowat and Agace, 2014) (Figure 2). In secondary lymphoid organs where immune responses are initiated, lymphocytes are organized into follicles which are sites of interaction of T follicular-helper (Tfh) cells with B cells, and germinal centers where B cell differentiation and antibody class switching occur (Figure 2). Human lymph nodes contain memory T cells and TRM along with DC essential for priming T cells (Granot et al., 2017; Miron et al., 2018) (Figure 2). This specialization of immunity within tissues likely evolved to promote optimal, tissue-adapted protection to the pathogens which typically invade these sites.
Role of TRM in protection to pathogens
Within tissues, there is increasing evidence from mice that TRM coordinate localized protective responses to multiple pathogens in diverse sites particularly to virus infections (recently reviewed in (Paik and Farber, 2020)). TRM can also coordinate clearance of multiple pathogen types including bacteria (Sakai et al., 2014), fungal pathogens (Park et al., 2018), and parasites (e.g., malaria and Leishmania) (Fernandez-Ruiz et al., 2016; Glennie et al., 2015) in the different sites (lung, skin, liver) where they invade. In humans, TRM-specific for pathogens tend to be maintained at the infection site; TRM-specific for influenza are found in lungs (Pizzolla et al., 2018; Turner et al., 2014), hepatitis in the liver (Pallett et al., 2017), and multiple systemic pathogens in the bone marrow (Okhrimenko et al., 2014). Together these studies and the overall predominance of TRM in human tissues point to major roles for TRM in coordinating tissue protection.
Establishment of TRM occurs when antigen-stimulated T cells at the primary infection site (e.g., lung, intestine) encounter inductive signals released by the local tissue that enable stable residence in the tissue and prevent egress. (Romagnoli et al., 2017; Turner et al., 2014). The signals for TRM generation can also originate in the tissue-associated LN, due to the cytokine environment , including a role for TGF-b in licensing T cell differentiation to TRM (Mani et al., 2019). Recognition of cognate antigen also drives TRM establishment, while non-specific infiltrating T cells entering the tissue during an infection are not retained (Hirai et al., 2021). TRM maintained in diverse tissue sites exhibit a core signature involving expression of integrins (CD103,CD49a), chemokine receptors (e.g., CXCR6) and downregulation of molecules that trigger egress (CCR7, S1PR1), to anchor them in the tissue (Kumar et al., 2017; Mackay and Kallies, 2017). TRM also develop tissue-specific adaptations in their phenotype and function in certain sites, which may enable their long-term residency (Szabo et al., 2019).
Upon pathogen re-exposure at a protected tissue site, TRM-driven secondary responses involve robust and rapid expansion of TRM in situ, immediate cytokine production, and recruitment of additional immune cells (Beura et al., 2018; Paik and Farber, 2021). TRM can produce multiple pro-inflammatory cytokines; however, TRM expression multiple inhibitory molecules to potentially limit their capacity for collateral tissue damage (Kumar et al., 2017; Pallett et al., 2017). This dual functional nature of TRM and their position in tissues suggests an important role in tissue homeostasis as an important are for future studies.
Intrinsic challenges for immunity: the role of aging
The global population is aging, with numbers of elderly individuals expected to double in the next 40 years. Aging is generally associated with a decline in functional adaptive immunity to previously and newly encountered pathogens including diminished responses to infection and vaccines (reviewed in (Goronzy and Weyand, 2013)). A striking example of age-related differences in protective immunity is observed with influenza (Czaja et al., 2019) and human coronavirus infection with case mortality rates of near 20% for the elderly; the underlying causes likely include age-related changes in both innate (e.g. type I interferon activity) and adaptive immune responses (Channappanavar and Perlman, 2020).
Protection from new pathogens requires a functional and diverse naïve T cell pool. In children, naive T cells are generated from early T cell progenitors in the thymus, which begins to atrophy in puberty with thymopoiesis at negligible levels after age 40 (Haynes et al., 2000; Thome et al., 2016). This reduced production of naïve T cells in adulthood occurs concomitant with an accumulation of memory T cells with antigen exposure (Kumar et al., 2018). The T cell compartment is the elderly therefore, contains few naïve T cells, and the majority are clonally expanded memory T cells (Qi et al., 2014). Studies in humans and non-human primates show that the declining frequency of naïve T cells with age, along with age-specific functional impairments, correlates with a diminished magnitude and efficacy of primary immune responses (Briceno et al., 2016; Cicin-Sain et al., 2010). This quantitative and qualitative deterioration of the naïve T cell compartment is associated with atrophy and dysfunction of secondary lymphoid tissues (Becklund et al., 2016; Senda et al., 2019), resulting in reduced lymphoid-mediated surveillance of tissues. Together, these factors significantly compromise the ability of the elderly to respond to new pathogens, such as SARS-CoV-2.
TRM established in human tissues such as intestines and lungs exhibit stable frequencies from late childhood throughout many decades of adult life (Senda et al., 2019; Thome et al., 2014), suggesting long-term maintenance. It is not known, however, whether TRM are maintained in a quiescent state or are continually replenished, and these are important aspects for defining the durability of human memory responses. Whether TRM can exhibit crossreactivity to newly encountered antigens, as suggested from studies of memory T cells in blood (Su et al., 2013) will be important to define, to assess whether tissue memory can exhibit broad protection. The functional capacity of long-lived TRM in humans is also unclear. Recent mouse studies have shown that aging can be associated with dysregulated TRM leading to chronic lung disease and decreased immune protection from lung pathogens (Goplen et al., 2020). Whether these functional impairments derive from a lack of influx of new TRM primed in associated LN is possible mechanism to explore in future studies. Immunosenescence may act in concert with other environmental stressors which exacerbate tissue damage at the sites where protection is required.
Extrinsic challenges to human immunity: the global pandemic of malnutrition
The combined forces of increasing globalization and climate change have altered many aspects of our environment, including the type and extent of nutrients available to feed the world. Currently, there is a rapidly escalating worldwide pandemic of malnutrition (Swinburn et al., 2019). Shifts away from agrarian economies, increased urbanization, and industrialization of the global food supply has increased access to inexpensive highly processed foods that are energy dense and low in dietary fiber (Swinburn et al., 2019). These changes culminate in high prevalence of obesity particularly in high income countries. Conversely, climate change is contributing to food insecurity in low and middle income countries who are at increased risk for undernutrition (Swinburn et al., 2019) (Figure 3).
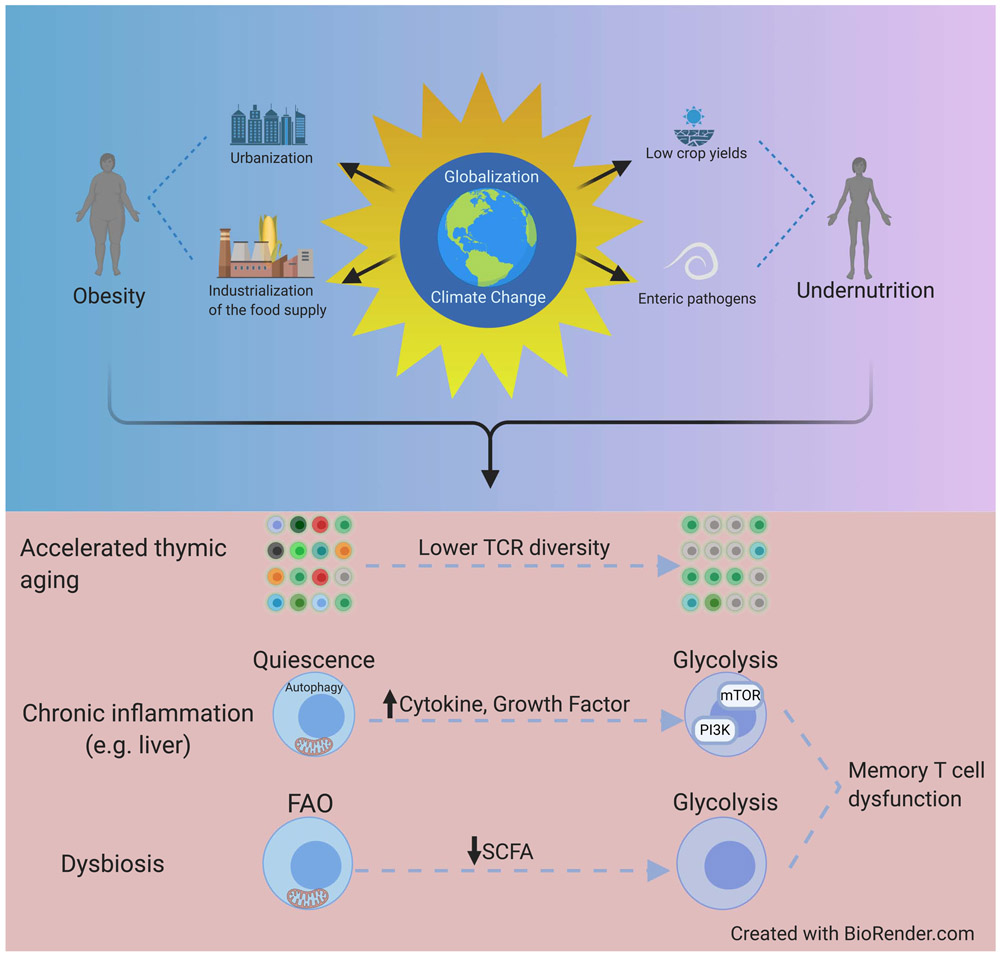
Figure 3: Impact of globalization on the metabolic function of immune cells.
Top: Schematic depicts how consequences of globalization and global warming contribute to the global pandemic of human malnutrition encompassing both obesity and undernutrition. Bottom: Major biological impacts of these changes and their impacts on immune system function. Both undernutrition and obesity can cause accelerated thymic atrophy leading to decreased naïve T cells and T cell receptor diversity, along with chronic inflammation and increased cytokine signaling. Diminished memory T cell function due to malnutrition arises from enhanced mTOR activation and glycolytic flux. Dysbiosis reduces production of short chain fatty acid (SCFA) metabolites which TRM require for their maintenance.
There is evidence that all forms of malnutrition – undernutrition, specific micronutrient deficiency and obesity - are associated with impaired immune protection (Figure 3). Undernutrition in children is associated with increased mortality risk, mostly attributable to common infectious diseases (Rytter et al., 2014). Obesity, the more common form of malnutrition in high-income countries, is also associated with increased infection-related morbidity and mortality. Epidemiological data collected during the 2009 H1N1 influenza pandemic and ongoing SARS-CoV-2 pandemic have established that obesity is a risk factor for severe disease (Stefan et al., 2020; Van Kerkhove et al., 2011). The mechanisms linking different forms of malnutrition to immune impairments remain incompletely defined; however, several recent studies have shed light on how metabolism controls immune responses and how immune protection may be influenced by various forms of nutrient stress (Figure 3).
Malnutrition synergizes with aging to impair primary immune responses
As described above, a source of naïve T cells is essential for efficacious responses to new pathogens. Studies in humans and mouse models have established that, independent of age, both undernutrition and obesity are associated with accelerated thymic atrophy, resulting in decreased thymic output of naïve T cells (Bourke et al., 2016). Such ‘accelerated thymic aging’ is associated with decreased T cell receptor diversity within the naïve T cell pool and decreased abundance of naïve relative to memory T cells (Yang et al., 2009) (Figure 3). For an aging world population that is becoming increasingly malnourished, this synergy of aging and malnutrition to deplete the naïve T cell pool may be particularly problematic in generating adequate immune responses to future pandemic pathogens.
Maternal malnutrition can also induce heritable epigenetic changes with the potential to alter the course of immune development of individuals prior to conception. Studies in animal models have established that maternal nutritional status shapes DNA methylation at multiple genetic loci in offspring leading to heritable phenotypic changes that can persist for generations (Hardikar et al., 2015). Accordingly, studies of women and children conceived during periods of famine have identified epigenetic modifications in genes controlling immune function (Dominguez-Salas et al., 2014). The functional consequences of nutritionally driven alterations in the immune epigenome have not been fully defined. In a future where mothers and children will be increasingly affected by malnutrition and nutrient stress, a more complete understanding of how maternal nutrition shapes immune function will help future generations better cope with infectious challenges.
Metabolic control of immunological memory
Both intracellular and systemic metabolism play a major role in shaping protective immunological memory. Metabolic dysfunction associated with obesity and undernutrition is associated with impaired formation and maintenance of protective memory responses to diverse pathogens including influenza and tuberculosis (Hoang et al., 2015; Karlsson et al., 2010). Metabolic reprogramming plays a central role in the consolidation of a primary immune response into effective T cell memory and in the adaptation of TRM to their tissue niche (Pan et al., 2017; Pearce et al., 2009). Thus, development and persistence of memory responses are susceptible to nutrient stress. During T cell activation, cytokine and TCR-driven glycolytic flux provide bioenergetic needs for effector differentiation and proliferation. Resolution of the immune response leads to cessation of TCR, cytokine and growth factor stimulation, which produces metabolic stress by abruptly interrupting nutrient supply, and leads to contraction of the effector response (Geltink et al., 2018). Formation of long-lived immunological memory requires metabolic reprogramming to upregulate mitochondrial fatty acid oxidation which helps to maintain T cell survival and functional potential in the absence of ongoing glycolytic stimulation (O'Sullivan et al., 2014) (Figure 3). Fatty acid metabolism therefore plays a central role in maintenance of immunological memory throughout life.
The persistence of protective immune cells in tissue barrier sites critically depends on nutrient availability. Long term survival of memory T cells in non-lymphoid barrier tissues requires additional specialized adaptations to these distinct metabolic niches. Lung TRM residing in airway epithelium show an epigenetic and transcriptional signature of nutrient deprivation which promote apoptosis, suggesting that nutrient stress may limit the persistence of TRM in some non-lymphoid barrier sites (Hayward et al., 2020). Maintenance of functional potential and survival in the TRM niche thus requires expression of metabolic programs that promote extraction of fatty acids from the glucose-poor microenvironment as a metabolic fuel source (Pan et al., 2017). Understanding mechanisms for metabolic adaptation to microenvironments with variations in nutrient availability will be important for promoting tissue protection in different metabolic states.
The quality and quantity of fatty acids needed for memory T cell function is shaped by both diet and the microbiome. For example, fermentation of dietary fiber by gut microbiota produces short chain fatty acids which promote memory T cell formation and maintenance (Bachem et al., 2019) (Figure 3). Lack of intestinal microbiota in germ-free mice leads to deficient memory CD8+T cell-mediated recall responses, while dietary fiber supplementation enhances metabolic fitness and functional recall of memory CD8+T cells (Bachem et al., 2019). Moreover, metabolites produced by gut microbiota can enter systemic circulation and influence immune protection at distant barrier sites such as lung. Dietary fiber supplementation was shown to enhance T cell-mediated clearance of primary influenza infection by enhancing fatty acid availability and improving metabolic fitness of pulmonary effector T cells (Trompette et al., 2018). Thus, diet composition may influence the development and maintenance of protective immunity through interactions with metabolites generated by the microbiome.
Signaling pathways linking metabolism and immunity
Appropriate cellular adaptations to fluctuations in energy balance are essential for maintenance of protective immunity. Signaling pathways for cellular growth and metabolism converge on the mechanistic target of rapamycin (mTOR) - the catalytic subunit of the mTORC1 and mTORC2 kinase complexes. Inhibition of the mTOR pathway leads to a state of metabolic quiescence (Saxton and Sabatini, 2017). In the context of immune memory, mTOR inhibition can enhance establishment of memory responses during pathogen challenge to a systemic virus (Araki et al., 2009). However, the role of mTOR in TRM formation is unclear and may differ from systemic memory responses (Sowell et al., 2014). Transient caloric restriction (CR) and induction of autophagy (lysosome-mediated recycling of cytoplasmic contents) are other factors that have been shown to promote metabolic quiescence and lead to enhanced protective immunity (Mannick et al., 2014). Induction of autophagy may also contribute to functional maintenance of human TRM (Swadling et al., 2020). By contrast, chronic over-nutrition leads to mTOR hyper-activation and suppression of autophagy resulting in detrimental effects on immune memory (Liu and Sabatini, 2020) (Figure 3).
In addition to effects of metabolism and energy balance on systemic immune function, localized tissue immunity in certain nutrient sensing organs may be distinctly impacted by disorders of under and over-nutrition. Nutrient-sensing organs such as pancreas, adipose tissue and liver, harbor significant populations of resident immune cells including macrophages and lymphocytes (Misumi et al., 2019; Obstfeld et al., 2010; Weisberg et al., 2019; Weisberg et al., 2003), and these show chronic overproduction of pro-inflammatory cytokines and dysfunctional antigen specific responses in obesity (Weisberg et al., 2003). These site specific impacts of metabolic disease on resident immune cells may increase susceptibility to pathogen challenge and inflammatory disease-- important areas for future studies.
The importance of nutrient availability and metabolic adaptations for establishment and persistence of memory T cells suggest that diet, energy balance and metabolic disease states likely shape immune protection. Global economic forces have led to major shifts in how humans obtain and consume food. There remains profound inequality in nutrition across the globe; malnutrition due to undernutrition and food insecurity on the one hand, and increased consumption of processed and unhealthy foods on the other, is increasing in prevalence across the globe (Swinburn et al., 2019). Efforts to mitigate climate change are likely to result in reduced raising of livestock, increased vegetarianism, and consumption of different types of artificial and processed protein sources (Fresan and Sabate, 2019); such changes will likely have beneficial effects on reducing obesity and in improving our immunological fitness.
Environmental changes that shape tissue specific immune responses
In addition to intrinsic influences of age and metabolic state, the environment in which we live also has direct effects on the immune system, particularly in localized responses. Declining air quality due to pollution is a major threat to human health, being directly responsible for over four million deaths each year (Cohen et al., 2017), with disproportional effects with increased age (Lelieveld et al., 2020). Several sources lead to air pollution including motor vehicles, mines, oil refineries, residential heating, and wildfires. Health concerns related to air quality mainly originate from particulate matter (PM) inhalation. PM comprise a heterogeneous mix of hydrocarbons and black carbon (Salim et al., 2014). PMs are identified according to their diameter as PM10 (<10 micron) and PM2.5 (<2.5 micron) (Salim et al., 2014). As PM10 tends localize in the upper respiratory tract within large airways, while PM2.5 can deposit throughout the lung tissue, including lower respiratory tract, leading to multiple respiratory diseases. Due to its small size, PM2.5 can also enter the bloodstream and deposit in multiple peripheral tissues, with potential effects on immune dysregulation, leading to cardiovascular or gastrointestinal diseases (Guarnieri and Balmes, 2014).
Tissue specific impacts of environmental toxins on barrier sites
The lung is the major site for PM and associated toxin exposures. A number of studies have demonstrated adverse immune effects of PMs on the immune system, through inducing inflammation and exacerbating disease progression after infection (Brandt et al., 2015; Matthews et al., 2016; O'Driscoll et al., 2018) (Figure 4). Macrophages exposed to PM particulates lead to ROS production, with increased oxidative stress in the tissue (Li et al., 2008). Polycyclic aromatic hydrocarbons (PAHs) are found in PMs and are ligands for the aryl hydrocarbon receptor (AHR) expressed by T cells (O'Driscoll et al., 2018). AHR engagement on CD4+T cells signals for differentiation and propagation of Th17-type effector cells producing the pro-inflammatory cytokine IL-17 (Veldhoen et al., 2008), and can impair responses to respiratory infections such as influenza (Burke et al., 2019). PM2.5 exposure is also implicated in worsened progression of SARS-CoV-2 infection (Engin et al., 2020). Therefore, pollution can worsen immune protection to respiratory pathogens through multiple mechanisms.
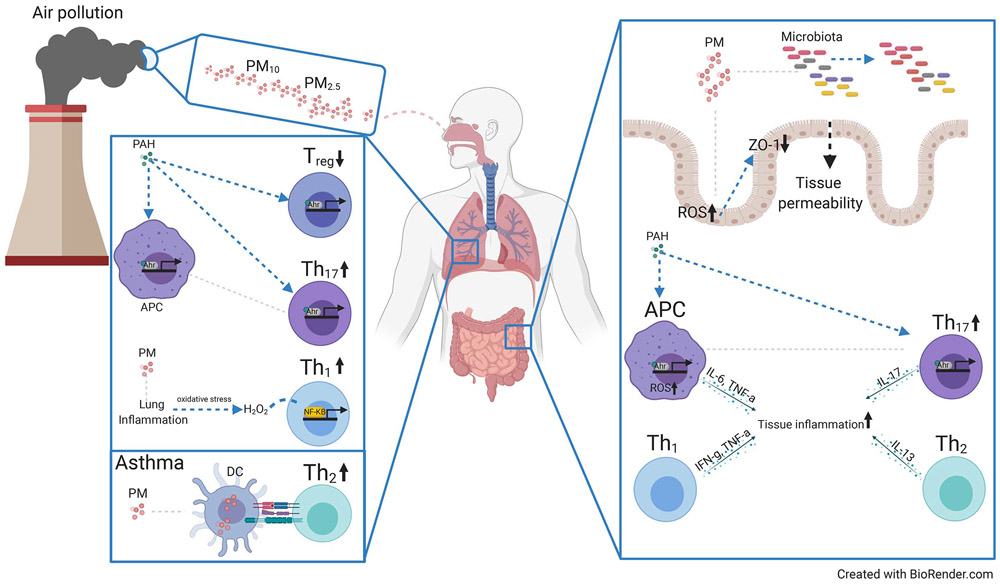
Figure 4. Air pollution effects on mucosal T cell function and homeostasis.
Schematic showing effects of particulate matter (PM) derived from air pollutants on the lung and intestinal immune system. The smaller sized PM2.5 can seed the lung tissue and activate several inflammatory pathways, via uptake by DC and other antigen-presenting cells (APC) which promote proinflammatory cytokine production by T cells. Polyaromatic hydrocarbons (PAH) are a component of PM which bind the aryl hydrocarbon receptor (AHR) expressed by T cells to promote Th17 and inhibit Treg generation. Allergic asthma can be directly triggered by PM or exacerbated due to PM promoting Th2 responses. Right: Schematic showing effects of PM on the intestinal immune system. PM accumulation in the gut tissue alters the microbiota and upregulates reactive oxygen species (ROS) production in the epithelial cells, increasing intestinal permeability and oxidative stress in the tissue. PAH induces AHR activation in APCs and Th17 cells in the gut, increasing local inflammation.
PM exposure also is well known for its effects in inducing or exacerbating allergic asthma, characterized by inflammation of the airways and airway hyperresponsiveness following inhalation of allergens. Allergic Asthma is mediated by the infiltration of type 2 effector CD4+ T cells (Th2) producing IL-4, 5 and 13 to the lung tissue (Cohn et al., 2004). As PMs deposit to the lung tissue, they can exacerbate asthma through different mechanisms. PM-loaded DCs can directly promote pro-inflammatory cytokine secretion by lung CD4+T cells (Matthews et al., 2016), while AHR signaling promotes inflammatory Th17 cells in the lung (Harb et al., 2020) (Figure 4). Allergen exposure generates CD4+TRM (Hondowicz et al., 2016; Turner et al., 2018) which can persist and may perpetuate asthma responses. During allergen challenge, CD4+TRM induce a rapid inflammatory response leading to airway hyperresponsiveness (Turner et al., 2018). PM exposure further exacerbates the local asthma response by promoting memory CD4+T cell-mediated lung pathology (Brandt et al., 2015) (Figure 4).
The gastrointestinal (GI) tract can also be exposed to PMs in multiple ways. Inhaled PMs can be transported to the gut tissue through mucociliary transport (Beamish et al., 2011) or PMs can be ingested through contaminated water (Salim et al., 2014). PM deposition first induces ROS in the epithelial cells of the gut lining, which downregulates tight junction proteins, leading to intestinal permeability (Mutlu et al., 2018). Moreover, PM deposition in the gut tissue was shown to induce alteration in the microbiome composition (Li et al., 2017). Such direct effects on gut physiology by PM exposure can lead to the upregulation of genes for pro-inflammatory cytokine (IFNG, IL17) and genes related to T cell migration (CCR7, and CCl19) (Kish et al., 2013), leading to alterations in the homeostatic or protective environment of intestines (Figure 4).
Preserving tissue immunity amidst declining air quality
Globalization along with climate change is associated with worsening air quality in many parts of the world. Global warming, resulting in hotter weather, and more arid regions has triggered a disturbing and devastating increase in wildfires and widespread dissemination of particulates. These changes result in higher concentrations of environmental toxins—all with impacts on the immune response. Given the skewed Th17 response in the presence of toxins which engage AHR, pharmacologic modulators of the IL-17 pathways that are currently in development for autoimmune and inflammatory diseases (Bianchi and Rogge, 2019) could be adapted for normalizing immune responses in regions with high pollution levels. Conversely, alleviating lung-localized Th2 responses would be an important therapeutic strategy for improving immune protection and lessening asthma. New technologies involving inhibition of TH2 response through by RNA silencing (siRNA) of the transcription factor GATA3 delivered by nanoparticles to facilitate their uptake by inhalation (Keil et al., 2020), are promising approaches for modulating local responses. Use of probiotics to skew the inflammatory process also shows potential in reducing Th2-mediated skewing (Fujimura et al., 2014). Future studies to develop lung-localized therapeutics which preserve lung immune homeostasis amidst declining air quality will be highly beneficial for future health.
While there is a wide range of research on the effect of ambient air pollution on the lung, lymph nodes are intricately associated with the lung, and act to filter and surveil lung antigens. In cases of high environmental PM levels, the lung-associated LN can be repositories for black particulates (Devarajan et al., 2018). Myeloid cells that phagocytose PMs migrate to the other tissues, such as lymph node, through the lymphatics. However, how immune cells with PMs that migrate to lung LNs alter the immune response is not well known. The impact of PM on the interaction of immune response between the tissue and associated LN are important areas for future investigation, particularly for responses to emerging viruses, where lymphoid organs are essential for initiating primary immunity.
Fortifying tissue immunity for future challenges
As the immune response is the first and, in many cases, the only defense we have to foreign substances, how can we modulate the immune response to adapt to multiple environmental challenges compounded by aging of the population? The changing global landscape with regard to climate and increased globalization has created potential for emergence of new pathogens and their rapid spread over the globe, as most recently and tragically manifested in the current COVID-19 pandemic. Given their central role in situ for protective responses, TRM are promising targets for establishing protection in vaccines. The lungs and intestines are key mucosal and barrier sites with majority TRM populations that are entry and infection sites for diverse pathogens, many with high pathogenicity and transmission rates. A plethora of harmful viral (e.g., SARS and MERS Co-V, highly pathogenic influenza strains) and bacterial (mycobacteria, tularemia) respiratory pathogens are transmitted by aerosol, while enteric pathogens (norovirus, pathogenic E. coli, salmonella) are readily spread through contact and contaminated food. We propose that targeting immunity at the local sites of infection in vaccines that establish TRM-based protection, or through non-specific enhancement of immunity could be beneficial for protecting against current and future pathogen threats (Figure 5).
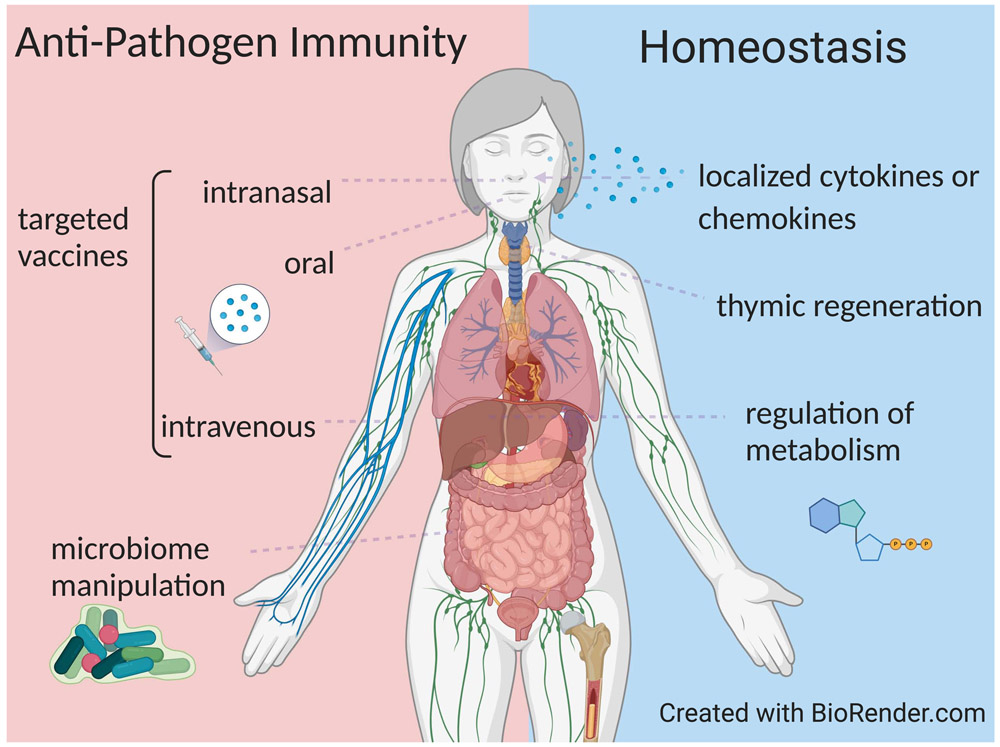
Figure 5. Strategies for fortifying tissue immunity to pathogens and during homeostasis.
Left: Diagram shows the different site-specific strategies that are showing efficacy in animal models and human trials for promoting tissue-resident memory T cell responses and protective immunity in situ. Right: Non-specific strategies that modulate or enhance immunity at specific sites, in the absence of pathogen challenge, and could serve to fortify tissue responses in advance of new pathogens, and also to address specific immune dysregulations that occurs during aging, from malnutrition, and due to environmental toxins.
Vaccine approaches for establishing TRM could be particularly beneficial for pathogens which undergo rapid mutation, like influenza, and therefore evade antibody-mediated protection which is the protective correlate for most of the current vaccines. Studies in animal models have established the feasibility and efficacy of targeted vaccination to generate protective TRM for respiratory and enteric pathogens (Figure 5). The use of site-specific immunization can directly prime for TRM in the desired site as shown in animal models. In the lung, intranasal (i.n.) vaccination of mice with the live-attenuated influenza vaccine (LAIV) formulation (as given to humans) generated protective TRM to heterosubtypic influenza challenge (Zens et al., 2016). For protection in the GI tract, oral administration of live-attenuated vaccine formulations for rotavirus and poliovirus show high (80-90%) efficacy and stimulate both mucosal and systemic immunity (Czerkinsky and Holmgren, 2015). Intravenous (i.v.) administration can have the benefit of targeting immunity in highly vascularized tissues, such as the liver and lung. Vaccination of malaria sporozoites i.v. generated CD8+TRM in the liver and protection to liver stage infection (Epstein et al., 2011), and shows promising efficacy in human trials (Ishizuka et al., 2016). Similarly, i.v. administration of BCG to non-human primates generated lung CD4+TRM and prevented development of tuberculosis to mycobacterial challenge (Darrah et al., 2020). Together, these studies in animal models and human trials indicate that vaccination which targets the infection site can be highly protective, particularly for pathogens where conventional vaccine approaches have failed.
Vaccination requires identifying pathogen-specific immunogens, which for future emerging pathogens cannot be known. Potential strategies for boosting overall immunity in specific sites would be desirable, by administering factors for non-specific immune cell activation and recruitment, or through disruption of tissue homeostasis (Figure 5). In mouse studies, adjunctive chemokine administration to vaginal mucosa in conjunction with vaccination can pull in various immune cells and create an inflammatory environment thereby enhancing antigen-specific tissue immunity (Shin and Iwasaki, 2012). Recently, less expensive and more readily available pharmacologic agents, including aminoglycoside antibiotics and neomycin, have been identified that can similarly induce local chemokine expression in vaginal mucosa to recruit T cells and enhance protection (Gopinath et al., 2020). Further studies are warranted to determine if such approaches are effective in humans and could be used prophylactically to enhance immunity in barrier sites. Methods to enhance tissue immunity through manipulation of the GI microbiome have also shown promising potential in mouse models. (Becattini et al., 2020; Gopinath et al., 2020). Future research efforts to understand how tissue immune cells regulate homeostasis will be essential for designing new approaches for fortifying site-specific immunity.
Addressing the underlying pathways of age and malnutrition-related immune system stress might provide additional strategies to fortify immunity if such interventions are found to be safe and can be made broadly available. In order to increase the potential of older individuals to respond to new pathogens, a source of additional naïve T cells is needed, by cellular therapy or by rejuvenation of thymopoiesis (Figure 5). LN can serve as specific reservoirs for naïve T cell maintenance in older individuals (Thome et al., 2016), and could potentially serve as sources of naïve pathogen-specific T cells for adoptive cellular therapy for anti-viral immunity (Keller et al., 2020). For promoting thymopoiesis, cytokines and growth factors can directly act on thymic epithelial cells or thymocyte precursors to promote T cell development (Velardi et al., 2020). Enhancing immunity in an aging population will continue to be a major challenge for human health.
Targeting metabolism using dietary interventions and modulation of specific pathways such as mTOR and autophagy warrant further investigation as potential means to fortify the immune system against the stress of malnutrition (Figure 5). Inhibiting the mTORC1 complex in a clinical trial reduced overall infections in the elderly (Mannick et al., 2018), and may serve to enhance immunity overall. Moreover, studies in humans and mice show that induction of autophagy can reverse some age-related functional defects in memory T cells (Alsaleh et al., 2020). Together, strategies for site-and age-warrant further investigation for enhancing immune protection in vulnerable populations.
Concluding remarks
The human immune system has evolved to defend against pathogen challenge in a changing and unpredictable environment. The organization of immune cells with diverse anatomic sites throughout the body, provides tissue-adapted and localized protection. In particular, tissue-localized responses mediated by antigen-specific TRM can provide immediate protection at the entry point for pathogens, thus preserving tissue integrity and homeostasis. However, the rapid changes in our environment triggered by the accelerating rise of global temperatures as well as economic globalization are challenging the capacity of the human immune system to adapt. Aging and chronic over- and under-nutrition are synergistically decimating the diversity of the human T cell armamentarium while stunting the development of immunological memory. Metabolic disease from malnutrition as well as marked increases in air pollution also directly impact the content and functionality of tissue resident immune cells that serve as sentinels against pathogen invaders in vital organs. Ongoing global efforts to mitigate human-induced environmental impacts and malnutrition worldwide are essential given the current rapid trajectory of global climate and socioeconomic change. In light of the near certainty that novel pathogens will continue to challenge our populations in the near future, it is also essential to amplify our efforts to understand exactly how these forces impact the immune system and devise tools to better fortify human immunity against an uncertain future.
Acknowledgements
S.P.W. is supported by NIH K08 DK122130; B.B.U. is supported by NIH T32HL105323 and D.L.F. is supported by NIH grants AI128949, AI06697, AI100119, AI150680 and HL145547.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alsaleh G, Panse I, Swadling L, Zhang H, Richter FC, Meyer A, Lord J, Barnes E, Klenerman P, Green C, et al. (2020). Autophagy in T cells from aged donors is maintained by spermidine and correlates with function and vaccine responses. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, and Ahmed R (2009). mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, Whitney PG, Fernandez-Ruiz D, Dahling S, Kastenmuller W, et al. (2019). Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8(+) T Cells. Immunity 51, 285–297 e285. [DOI] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, and Wine E (2011). Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis 5, 279–286. [DOI] [PubMed] [Google Scholar]
- Becattini S, Littmann ER, Seok R, Amoretti L, Fontana E, Wright R, Gjonbalaj M, Leiner IM, Plitas G, Hohl TM, et al. (2020). Enhancing mucosal immunity by transient microbiota depletion. Nat Commun 11, 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becklund BR, Purton JF, Ramsey C, Favre S, Vogt TK, Martin CE, Spasova DS, Sarkisyan G, LeRoy E, Tan JT, et al. (2016). The aged lymphoid tissue environment fails to support naive T cell homeostasis. Sci Rep 6, 30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beura LK, Mitchell JS, Thompson EA, Schenkel JM, Mohammed J, Wijeyesinghe S, Fonseca R, Burbach BJ, Hickman HD, Vezys V, et al. (2018). Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 19, 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, and Rogge L (2019). The IL-23/IL-17 pathway in human chronic inflammatory diseases-new insight from genetics and targeted therapies. Genes Immun 20, 415–425. [DOI] [PubMed] [Google Scholar]
- Bourke CD, Berkley JA, and Prendergast AJ (2016). Immune Dysfunction as a Cause and Consequence of Malnutrition. Trends Immunol 37, 386–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, Biagini Myers JM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, LeMasters GK, Bernstein DI, Lockey JE, LeCras TD, et al. (2015). Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J Allergy Clin Immunol 136, 295–303 e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceno O, Lissina A, Wanke K, Afonso G, von Braun A, Ragon K, Miquel T, Gostick E, Papagno L, Stiasny K, et al. (2016). Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell 15, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke CG, Myers JR, Boule LA, Post CM, Brookes PS, and Lawrence BP (2019). Early life exposures shape the CD4(+) T cell transcriptome, influencing proliferation, differentiation, and mitochondrial dynamics later in life. Sci Rep 9, 11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R, and Perlman S (2020). Age-related susceptibility to coronavirus infections: role of impaired and dysregulated host immunity. J Clin Invest 130, 6204–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L, Smyk-Pearson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, et al. (2010). Loss of naive T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol 184, 6739–6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, et al. (2017). Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L, Elias JA, and Chupp GL (2004). Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol 22, 789–815. [DOI] [PubMed] [Google Scholar]
- Czaja CA, Miller L, Alden N, Wald HL, Cummings CN, Rolfes MA, Anderson EJ, Bennett NM, Billing LM, Chai SJ, et al. (2019). Age-Related Differences in Hospitalization Rates, Clinical Presentation, and Outcomes Among Older Adults Hospitalized With Influenza-U.S. Influenza Hospitalization Surveillance Network (FluSurv-NET). Open Forum Infect Dis 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerkinsky C, and Holmgren J (2015). Vaccines against enteric infections for the developing world. Philos Trans R Soc Lond B Biol Sci 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH 2nd, Hughes TK, Pokkali S, Swanson PA 2nd, Grant NL, Rodgers MA, et al. (2020). Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 577, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devarajan SR, Zarrin-Khameh N, and Alapat P (2018). Black lungs and big nodes: A case of airway anthracosis with bronchial anthracofibrosis. Respir Med Case Rep 25, 9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, Kubota M, Matsumoto R, Thapa P, Szabo PA, et al. (2020). Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 180, 749–763 e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Salas P, Moore SE, Baker MS, Bergen AW, Cox SE, Dyer RA, Fulford AJ, Guan Y, Laritsky E, Silver MJ, et al. (2014). Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat Commun 5, 3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin AB, Engin ED, and Engin A (2020). The effect of environmental pollution on immune evasion checkpoints of SARS-CoV-2. Environ Toxicol Pharmacol 81, 103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JE, Tewari K, Lyke KE, Sim BK, Billingsley PF, Laurens MB, Gunasekera A, Chakravarty S, James ER, Sedegah M, et al. (2011). Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 334, 475–480. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, et al. (2016). Liver-Resident Memory CD8+ T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity 45, 889–902. [DOI] [PubMed] [Google Scholar]
- Fresan U, and Sabate J (2019). Vegetarian Diets: Planetary Health and Its Alignment with Human Health. Adv Nutr 10, S380–S388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, Boushey HA, Zoratti E, Ownby D, Lukacs NW, et al. (2014). House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A 111, 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geltink RIK, Kyle RL, and Pearce EL (2018). Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 36, 461–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, and Scott P (2015). Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med 212, 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S, Lu P, and Iwasaki A (2020). Cutting Edge: The Use of Topical Aminoglycosides as an Effective Pull in "Prime and Pull" Vaccine Strategy. J Immunol 204, 1703–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goplen NP, Wu Y, Son YM, Li C, Wang Z, Cheon IS, Jiang L, Zhu B, Ayasoufi K, Chini EN, et al. (2020). Tissue-resident CD8(+) T cells drive age-associated chronic lung sequelae after viral pneumonia. Sci Immunol 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, and Weyand CM (2013). Understanding immunosenescence to improve responses to vaccines. Nat Immunol 14, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot T, Senda T, Carpenter DJ, Matsuoka N, Weiner J, Gordon CL, Miron M, Kumar BV, Griesemer A, Ho SH, et al. (2017). Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 46, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M, and Balmes JR (2014). Outdoor air pollution and asthma. Lancet 383, 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb H, Stephen-Victor E, Crestani E, Benamar M, Massoud A, Cui Y, Charbonnier LM, Arbag S, Baris S, Cunnigham A, et al. (2020). A regulatory T cell Notch4-GDF15 axis licenses tissue inflammation in asthma. Nat Immunol 21, 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardikar AA, Satoor SN, Karandikar MS, Joglekar MV, Puranik AS, Wong W, Kumar S, Limaye A, Bhat DS, Januszewski AS, et al. (2015). Multigenerational Undernutrition Increases Susceptibility to Obesity and Diabetes that Is Not Reversed after Dietary Recuperation. Cell Metab 22, 312–319. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Markert ML, Sempowski GD, Patel DD, and Hale LP (2000). The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol 18, 529–560. [DOI] [PubMed] [Google Scholar]
- Hayward SL, Scharer CD, Cartwright EK, Takamura S, Li ZT, Boss JM, and Kohlmeier JE (2020). Environmental cues regulate epigenetic reprogramming of airway-resident memory CD8(+) T cells. Nat Immunol 21, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai T, Yang Y, Zenke Y, Li H, Chaudhri VK, De La Cruz Diaz JS, Zhou PY, Nguyen BA, Bartholin L, Workman CJ, et al. (2021). Competition for Active TGFbeta Cytokine Allows for Selective Retention of Antigen-Specific Tissue- Resident Memory T Cells in the Epidermal Niche. Immunity 54, 84–98 e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T, Agger EM, Cassidy JP, Christensen JP, and Andersen P (2015). Protein energy malnutrition during vaccination has limited influence on vaccine efficacy but abolishes immunity if administered during Mycobacterium tuberculosis infection. Infect Immun 83, 2118–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, et al. (2016). Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity 44, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka AS, Lyke KE, DeZure A, Berry AA, Richie TL, Mendoza FH, Enama ME, Gordon IJ, Chang LJ, Sarwar UN, et al. (2016). Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 22, 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EA, Sheridan PA, and Beck MA (2010). Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 184, 3127–3133. [DOI] [PubMed] [Google Scholar]
- Keil TWM, Baldassi D, and Merkel OM (2020). T-cell targeted pulmonary siRNA delivery for the treatment of asthma. Wiley Interdiscip Rev Nanomed Nanobiotechnol 12, e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MD, Harris KM, Jensen-Wachspress MA, Kankate V, Lang H, Lazarski CA, Durkee-Shock JR, Lee PH, Chaudhry K, Webber K, et al. (2020). SARS-CoV-2 specific T-cells Are Rapidly Expanded for Therapeutic Use and Target Conserved Regions of Membrane Protein. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Ganzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, et al. (2013). Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One 8, e62220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Connors TJ, and Farber DL (2018). Human T Cell Development, Localization, and Function throughout Life. Immunity 48, 202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho SH, Lerner H, et al. (2017). Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Rep 20, 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Pozzer A, Poschl U, Fnais M, Haines A, and Munzel T (2020). Loss of life expectancy from air pollution compared to other risk factors: a worldwide perspective. Cardiovasc Res 116, 1910–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Xia T, and Nel AE (2008). The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 44, 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Yang J, Saffari A, Jacobs J, Baek KI, Hough G, Larauche MH, Ma J, Jen N, Moussaoui N, et al. (2017). Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci Rep 7, 42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, and Sabatini DM (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, and Kallies A (2017). Transcriptional Regulation of Tissue-Resident Lymphocytes. Trends Immunol 38, 94–103. [DOI] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Aijo T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-beta to precondition naive CD8(+) T cells for tissue-resident memory fate. Science 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, et al. (2014). mTOR inhibition improves immune function in the elderly. Sci Transl Med 6, 268ra179. [DOI] [PubMed] [Google Scholar]
- Mannick JB, Morris M, Hockey HP, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, et al. (2018). TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med 10. [DOI] [PubMed] [Google Scholar]
- Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, and Lee TH (2016). Urban Particulate Matter-Activated Human Dendritic Cells Induce the Expansion of Potent Inflammatory Th1, Th2, and Th17 Effector Cells. Am J Respir Cell Mol Biol 54, 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron M, Kumar BV, Meng W, Granot T, Carpenter DJ, Senda T, Chen D, Rosenfeld AM, Zhang B, Lerner H, et al. (2018). Human Lymph Nodes Maintain TCF-1(hi) Memory T Cells with High Functional Potential and Clonal Diversity throughout Life. J Immunol 201, 2132–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi I, Starmer J, Uchimura T, Beck MA, Magnuson T, and Whitmire JK (2019). Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep 27, 514–524 e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat AM, and Agace WW (2014). Regional specialization within the intestinal immune system. Nat Rev Immunol 14, 667–685. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazici C, Soberanes S, Hamanaka RB, Nigdelioglu R, Meliton AY, Ghio AJ, et al. (2018). Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut 240, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Driscoll CA, Gallo ME, Hoffmann EJ, Fechner JH, Schauer JJ, Bradfield CA, and Mezrich JD (2018). Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS One 13, e0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. (2014). Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, and Ferrante AW Jr. (2010). C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 59, 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhrimenko A, Grun JR, Westendorf K, Fang Z, Reinke S, von Roth P, Wassilew G, Kuhl AA, Kudernatsch R, Demski S, et al. (2014). Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc Natl Acad Sci U S A 111, 9229–9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DH, and Farber DL (2020). Anti-viral protective capacity of tissue resident memory T cells. Curr Opin Virol 46, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DH, and Farber DL (2021). Influenza infection fortifies local lymph nodes to promote lung-resident heterosubtypic immunity. J Exp Med 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallett LJ, Davies J, Colbeck EJ, Robertson F, Hansi N, Easom NJW, Burton AR, Stegmann KA, Schurich A, Swadling L, et al. (2017). IL-2high tissue-resident T cells in the human liver: Sentinels for hepatotropic infection. J Exp Med 214, 1567–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O'Malley JT, Gehad A, Teague JE, et al. (2017). Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CO, Fu X, Jiang X, Pan Y, Teague JE, Collins N, Tian T, O'Malley JT, Emerson RO, Kim JH, et al. (2018). Staged development of long-lived T-cell receptor alphabeta TH17 resident memory T-cell population to Candida albicans after skin infection. J Allergy Clin Immunol 142, 647–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, and Choi Y (2009). Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzolla A, Nguyen TH, Sant S, Jaffar J, Loudovaris T, Mannering SI, Thomas PG, Westall GP, Kedzierska K, and Wakim LM (2018). Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J Clin Invest 128, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MML, and Farber DL (2020). The Whole Body as the System in Systems Immunology. iScience 23, 101509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Liu Y, Cheng Y, Glanville J, Zhang D, Lee JY, Olshen RA, Weyand CM, Boyd SD, and Goronzy JJ (2014). Diversity and clonal selection in the human T-cell repertoire. Proc Natl Acad Sci U S A 111, 13139–13144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli PA, Fu HH, Qiu Z, Khairallah C, Pham QM, Puddington L, Khanna KM, Lefrancois L, and Sheridan BS (2017). Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol 10, 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytter MJ, Kolte L, Briend A, Friis H, and Christensen VB (2014). The immune system in children with malnutrition--a systematic review. PLoS One 9, e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, and Barber DL (2014). Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. J Immunol 192, 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim SY, Kaplan GG, and Madsen KL (2014). Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes 5, 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton RA, and Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senda T, Dogra P, Granot T, Furuhashi K, Snyder ME, Carpenter DJ, Szabo PA, Thapa P, Miron M, and Farber DL (2019). Microanatomical dissection of human intestinal T-cell immunity reveals site-specific changes in gut-associated lymphoid tissues over life. Mucosal Immunol 12, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, and Iwasaki A (2012). A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature 491, 463–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder ME, and Farber DL (2019). Human lung tissue resident memory T cells in health and disease. Curr Opin Immunol 59, 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell RT, Rogozinska M, Nelson CE, Vezys V, and Marzo AL (2014). Cutting edge: generation of effector cells that localize to mucosal tissues and form resident memory CD8 T cells is controlled by mTOR. J Immunol 193, 2067–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan N, Birkenfeld AL, Schulze MB, and Ludwig DS (2020). Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol 16, 341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, and Davis MM (2013). Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity 38, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadling L, Pallett LJ, Diniz MO, Baker JM, Amin OE, Stegmann KA, Burton AR, Schmidt NM, Jeffery-Smith A, Zakeri N, et al. (2020). Human Liver Memory CD8(+) T Cells Use Autophagy for Tissue Residence. Cell Rep 30, 687–698 e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Kraak VI, Allender S, Atkins VJ, Baker PI, Bogard JR, Brinsden H, Calvillo A, De Schutter O, Devarajan R, et al. (2019). The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 393, 791–846. [DOI] [PubMed] [Google Scholar]
- Szabo PA, Miron M, and Farber DL (2019). Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Grinshpun B, Kumar BV, Kubota M, Ohmura Y, Lerner H, Sempowski GD, Shen Y, and Farber DL (2016). Longterm maintenance of human naive T cells through in situ homeostasis in lymphoid tissue sites. Sci Immunol 1, aah6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, and Farber DL (2014). Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, and Marsland BJ (2018). Dietary Fiber Confers Protection against Flu by Shaping Ly6c(−) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity 48, 992–1005 e1008. [DOI] [PubMed] [Google Scholar]
- Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, and Farber DL (2014). Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 7, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Goldklang M, Cvetkovski F, Paik D, Trischler J, Barahona J, Cao M, Dave R, Tanna N, D'Armiento JM, et al. (2018). Biased Generation and In Situ Activation of Lung Tissue-Resident Memory CD4 T Cells in the Pathogenesis of Allergic Asthma. J Immunol 200, 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, et al. (2011). Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med 8, e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardi E, Tsai JJ, and van den Brink MRM (2020). T cell regeneration after immunological injury. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, and Stockinger B (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109. [DOI] [PubMed] [Google Scholar]
- Watzky M, de Dieuleveult M, Letessier A, Saint-Ruf C, and Miotto B (2020). Assessing the consequences of environmental exposures on the expression of the human receptor and proteases involved in SARS-CoV-2 cell-entry. Environ Res, 110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Carpenter DJ, Chait M, Dogra P, Gartrell-Corrado RD, Chen AX, Campbell S, Liu W, Saraf P, Snyder ME, et al. (2019). Tissue-Resident Memory T Cells Mediate Immune Homeostasis in the Human Pancreas through the PD-1/PD-L1 Pathway. Cell Rep 29, 3916–3932 e3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, and Ferrante AW Jr. (2003). Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112, 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisel F, and Shlomchik M (2017). Memory B Cells of Mice and Humans. Annu Rev Immunol 35, 255–284. [DOI] [PubMed] [Google Scholar]
- Weisel NM, Weisel FJ, Farber DL, Borghesi LA, Shen Y, Ma W, Luning Prak NT, and Shlomchik MJ (2020). Comprehensive analysis of B cell compartments across the human body reveals novel subsets and a gut resident memory phenotype. Blood In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Youm YH, Vandanmagsar B, Rood J, Kumar KG, Butler AA, and Dixit VD (2009). Obesity accelerates thymic aging. Blood 114, 3803–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zens KD, Chen J-K, and Farber DL (2016). Vaccine-Generated Lung Tissue-Resident Memory T cells Provide Heterosubtypic Protection to Influenza Infection. J Clin Invest Insight 1 e85832. [DOI] [PMC free article] [PubMed] [Google Scholar]