Abstract
The high rate of spreading of COVID-19 is attributed to airborne particles exhaled by infected but often asymptomatic individuals. In this review, the role of aerosols in SARS-CoV-2 coronavirus transmission is discussed from the biophysical perspective. The essential properties of the coronavirus virus transported inside aerosol droplets, their successive inhalation, and size-dependent deposition in the respiratory system are highlighted. The importance of face covers (respirators and masks) in the reduction of aerosol spreading is analyzed. Finally, the discussion of the physicochemical phenomena of the coronavirus entering the surface of lung liquids (bronchial mucus and pulmonary surfactant) is presented with a focus on a possible role of interfacial phenomena in pulmonary alveoli. Information given in this review should be important in understanding the essential biophysical conditions of COVID-19 infection via aerosol route as a prerequisite for effective strategies of respiratory tract protection, and possibly, indications for future treatments of the disease.
Keywords: Aerosol, Facemask, COVID-19 transmission, Particle-lung interactions, Pulmonary surfactant, Bronchial mucus
Graphical abstract
Introduction
Several types of coronaviruses (CoVs) have been identified during the last decades and many of them have caused various dysfunctions of the respiratory, gastric, and nervous systems [1,2]. Some diseases induced by CoVs have been of greater concern due to their substantial mortality, with SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome) as the most known but not exclusive examples [3, 4, 5]. The outbreak of the COVID-19 pandemic in the spring of 2020 and the necessity of searching for a protection, vaccine, and effective drugs for this new disease caused by the newly identified SARS-CoV-2 coronavirus, led to the urgent need for a deep understanding of various aspects of the virus properties, biological activity, and routes of transmission. A vast amount of data on this subject have been gathered and published in 2020, resulting in a kind of ‘information chaos’ created by a mix of essential with unimportant or even false conclusions
This review paper tries to organize and discuss selected aspects of this broad area, focusing mainly on the problems of airborne transmission of the CoV in the form of a colloidal system (aerosol), virus penetration to the respiratory system, and possible physicochemical interactions with the liquid layers of the lungs.
Physical and biochemical properties of coronaviruses
Coronaviruses (Coronaviridae family) are enveloped viruses of 120–160 nm in size, and they contain a large positive-sense single-strand RNA genome [4,6]. CoVs have a different outer structure than other known enveloped viruses (e.g., influenza [7,8]) due to the presence of spike glycoproteins, which form a kind of crown on the viral membrane [5]. The membrane-anchored spike glycoprotein of 180–220 kDa has a club-shaped trimeric form, where the N-terminal part (S1) contains the receptor-binding domain, and the C-terminal part (S2) has fusogenic activity [9,10]. Polysaccharide coating of the spikes allows to camouflage CoV against the host immune system [9]. The S2-induced fusion of CoV with a host cell is dependent on pH and differs from virus to virus, from neutral to slightly acidic (pH 6.5) [1]. The S2 part becomes active after cleavage of the spike protein by proteolytic enzymes of the host cell (e.g., furin, trypsin, cathepsins and others). The presence of these proteases on target cells determines whether CoVs enters the cells through fusion with the plasma membrane or rather via acidic pH-dependent endocytosis [1,2]. CoVs have an envelope with a lipid composition compatible with the cellular membranes, which facilitates their entry to the cells [1,5]. Interestingly, the viral envelope can resist the acidic environment of the stomach and the action of bile and lytic enzymes, and this allows CoVs to retain bioactivity in the gastrointestinal (GI) tract and induce GI symptoms [1].
The pathogenic activity of CoVs runs through a lysis or apoptosis of the infected cells. An occurrence of cytokine storms and accumulation of inflammatory cells (mainly: alveolar macrophages in the lung) have been observed during the peak of the SARS and COVID-19 diseases, and the virus also can replicate in the macrophages [1,11]. CoVs primarily infect the epithelial cells of the respiratory and gastrointestinal tracts, but it has been detected in the blood, lung, liver, and kidney, and secretions (stool and urine), which means that CoVs may induce systemic infection [1]. In the course of COVID-19, it is due to the fact that SARS-CoV-2 enters cells via the angiotensin-converting enzyme 2 (ACE2) receptor [12, 13, 14], and ACE2 is associated with the balanced mechanisms of the renin-angiotensin system responsible for the proper functions of the heart, kidney, vascular endothelium, liver, lung, pancreas, skeletal muscle, gonads, liver, placenta and brain [13]. Such interactions have been indirectly proven by long-term systemic effects of SARS-CoV-2 infection (‘long Covid’) [15,16].
Many of the physical and biochemical properties of CoV discussed above are essential for the proper recognition and assessment of coronavirus interactions on the lung surface that will be discussed later in the next parts paper.
Airborne transmission of COVID-19: dynamics of aerosol flow and deposition in the respiratory system
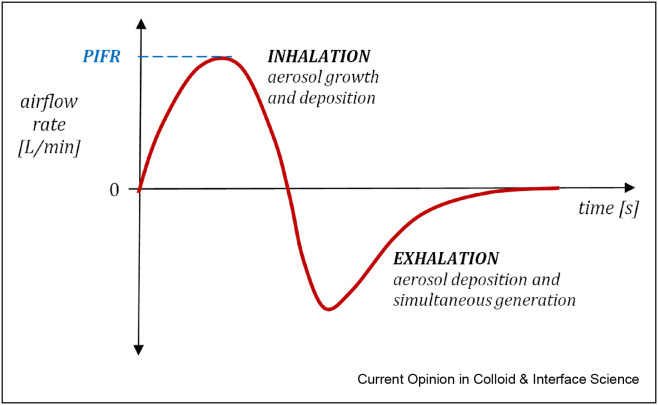
Airborne transmission is recognized as the main way of spreading viral infections in the population [8,17,18], including COVID-19 [19, 20, 21]. Inhalable aerosols must contain particles or liquid droplets smaller than ∼20 μm; however, only particles with a diameter below 5 μm (known as ‘fine particles’ [22]) can effectively penetrate to the lower parts of the respiratory system, i.e. the small bronchi and pulmonary alveoli. It may be mentioned that in regards to airborne transmission of pathogens by liquid aerosol particles, some authors use the terms ‘droplets’ for objects larger than 20 μm, and ‘droplet nuclei’ for objects smaller than 5 μm [23]. However, in this paper, all kinds of liquid particles suspended in the air will be called ‘droplets’. In general, the inhalability of large particles or droplets is restricted by their short residence time in the air outside the organism due to quick gravitational sedimentation. For instance, a 20 μm particle falls down with a speed above 1.2 cm/s, which means that it requires less than 3 min to reach the ground from a height of approximately 2 m. Inhalable particles that enter the body via mouth or nose during normal breathing undergo several physical mechanisms that cause their deposition on the surface in different parts of the respiratory tract [24]. The respiratory system may be considered a sequential filter, so lower airways may be reached only by particles that successfully penetrate through the earlier levels, i.e. the naso-oropharynx and trachea. An important feature of the aerosol flow during inhalation is unsteadiness. The flow rate profile starts and ends with zero value and achieves the maximum (PIFR – peak inspiratory flow rate) approximately in the middle of the inhalation phase. As seen from Figure 1 ., the exhalation in normal breathing is less symmetric and longer than inhalation. This figure also indicates additional phenomena (a possible particle growth and generation in the respiratory system), which will be discussed later.
Figure 1.
The typical airflow dynamics and aerosol behavior during each phase of breathing.
The contribution of forces acting on inhaled aerosol particles varies continuously in each phase of the breathing system. When aerosol gradually accelerates during inhalation towards PIFR, the inertial mechanism of deposition of large particles becomes predominant. Consequently, the majority of particles larger than 5 μm are effectively removed from the airstream already in the nose/mouth/throat (i.e. the upper airways), so they are absent in the aerosol that enters the bronchial tree. After passing the upper airways and trachea, the aerosol decelerates in bronchi due to the increase of the total cross-section area of the airways. Inertial deposition becomes unimportant in this region for two reasons: the lower airflow rate and the lack of heavy (large) particles already removed in the upper airways. Instead, the gravitational sedimentation starts to dominate for particles of 1–5 μm in size. Sedimentation is more effective here also due to increased particle residence time in the individual bronchial tubes and gradual reduction of their diameter (shorter sedimentation path). As a consequence, only a limited number of particles smaller than ∼3 μm can reach pulmonary alveoli and deposit there. The smallest submicrometer particles undergo Brownian motion that increases their deposition in the pulmonary region. This is also possible due to very slow air velocities (the creeping flow) and the short distance to the deposition surface since the average size of the alveolus is ∼200 μm [25]. Particles that are not deposited during inhalation can be captured in the respiratory system also during exhalation, and it has been demonstrated that a rapid change in the airflow direction (inhalation to exhalation) intensifies the deposition of ultrafine particles [26].
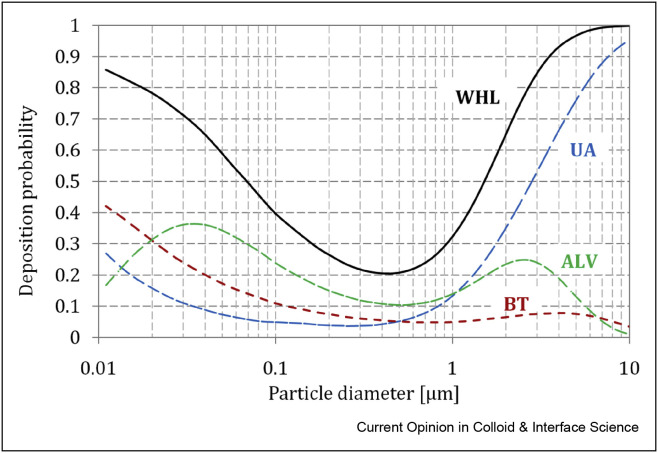
The size-dependent regional deposition probability of inhaled aerosols as a function of particle size is schematically shown in Figure 2 . These kinds of relationships have been measured during in vivo studies, and they can also be predicted by computational models [27]. It is seen that the total efficiency of deposition of fine particles in the lungs is below 100%, so many of these particles are exhausted to the surrounding during the exhalation phase. The exact numerical data of expected deposition of particles with different sizes in various lung regions depend on breathing dynamics, particle properties (density and shape, as quite often aerosol particles are not spherical [28]), and the actual geometry of the respiratory tract of a given individual. However, the graph shown in Figure 2 properly illustrates a general relationship.
Figure 2.
Probability (efficiency) of particle deposition in the respiratory system: ALV – alveolar (pulmonary) region, BT – bronchial tree, UA – upper airways (head), WHL – whole lung (total). Data calculated using MPDD model: Yeh-Schum symmetric lung geometry, Functional Lung Capacity, FRC = 2200 mL, Tidal Volume, TV = 500 mL, Breathing Rate, BR = 12 min−1 [27].
As in any filtration systemthere is a minimum of regional and total deposition for particles in the size range of 0.2–0.8 μm. According to the data presented in Figure 2, almost 75% of such particles are expected to be exhaled. The graph also shows that particles within size range 1–5 μm, which pass the upper airways, have a high probability of deposition in the bronchial tree and alveoli. This is why they are of great concern both in inhalation toxicology and aerosol therapy. Ultrafine particles smaller than 100 nm (i.e. nanoparticles), also have a high deposition probability in these regions. Such particles are usually neglected in inhalation drug delivery as practically absent in aerosols generated in medical inhalers [24]; however, nanoparticles are extremely important regarding the health effects of inhaled industrial and other anthropogenic aerosols [29∗∗, 30∗∗, 31]. This particle size range is also unimportant in the discussion of coronavirus aerosols as the virus itself is larger than 100 nm.
Let us indicate two additional effects that are important in the analysis of aerosol behavior in the respiratory system during inhalation–exhalation cycle (Figure 1):
-
1.
Aerosol particles may increase their size in the airways due to the condensation of water vapor (hygroscopic growth) and also due to coagulation/coalescence [32,33];
-
2.
Aerosol droplets can be generated in the respiratory system and emitted outside during exhalation [34,35].
The first factor suggests that even if aerosol particle or droplet has the initial size that corresponds to relatively poor deposition efficiency in the lungs, it can grow inside the respiratory tract to the final size that will result in the capturing of this particle. The second factor is of primary importance in the analysis of the transmission of COVID-19 by airborne particles, where SARS-CoV-2 coronavirus is contained in aerosol droplets is exhaled by an infected individual.
Indeed, it has been demonstrated that respiratory droplets are generated by breathing [34, 35∗∗, 36, 37]. Exhaled droplets are within the submicrometer size range so they are large enough to carry the ∼150 nm CoV particles out of the respiratory system. In the other situations, such as talking, coughing, or sneezing, droplets expelled from the mouth or nose are often larger (from several micrometers to almost 1–2 mm) and have a high velocity that allows them to be spread to a distance of a few meters [38, 39, 40, 41, 42, 43]. When face covers are worn, such large droplets will be largely stopped being expelled from them by inertial deposition. Despite ongoing discussion, wearing face masks or face covers is therefore a reasonable strategy to reduce spreading the COVID-19 infection in droplets expelled by infected individuals. This problem will be discussed in more detail in the next section. The comparison of published data regarding the size of aerosol droplets emitted during breathing, speaking, coughing, and sneezing is presented in Table 1 . It can be found that the data are dependent on the measuring technique (the limits of a particle in different size ranges). A comprehensive review on this subject was published by Gralton et al. [35].
Table 1.
Particle size of exhaled aerosol under variable breathing conditions by healthy and infected patients.
| Breathing maneuver | Particle size | Other essential data and remarks | Ref. |
|---|---|---|---|
| Mouth breathing, nose breathing, coughing, talking | The majority of particles are below 0.6 μm | Concentration of droplets ≤1 μm:
|
[34] |
| Breathing with different intensity and breath-holding | Modal value of droplet diameter ∼ 1 μm | Reduction of the number of exhaled droplets by breath-holding suggests that they are formed in bronchioles during inhalation (they sediment in the alveoli during breath-holding) | [44] |
| Breathing, vocalization, speech, cough | The majority of droplets are < 0.8 μm for all activities | Total droplet concentration:
|
[38] |
| Speaking, coughing | Median diameter (count): 13.5 μm cough 16.5 μm speech |
Droplet size distribution measured with interferometric Mie imaging Droplet concentration:
|
[45] |
| Speaking, coughing | Droplet size range: 10–100 μm | Measuring method: solid impaction and microscopy. The majority of droplets were 35–50 μm for speaking and 35–100 μm for coughing | [40] |
| Sneezing | Droplet size range 20–1000 μm (volume-based droplet size distribution | Unimodal or bimodal size distribution with volumetric mode diameter equal: 360 μm (unimodal) 72 and 386 μm (bi-modal) |
[41] |
| Breathing, speech, sustained vocalization, coughing | Droplets in the size range of 0.1–1000 μm | Generation of droplets in the lower respiratory tract (bronchial fluid film burst), larynx (voicing/coughing), and oral cavity (speech and coughing) results in trimodal droplet size distribution. Mode diameters:
|
[46] |
| Cough of patients with influenza: active and recovered | Droplet size range 0.35–10 μm, the majority < 3 μm (63% in the respirable size fraction) count median diameter: 0.63 μm | Number of droplets: 900–300 000 droplets per cough (active or recovered patients) | [47] |
| Oral and nasal breathing of various dynamics, speech with various loudness | 97% droplets < 1 μm | [48] | |
| Speech | Droplet size range: 0.05–10 μm; geometric mean diameter (number-based) ∼1 μm, regardless of voice amplitude | The number of emitted droplets increases with speech loudness from <100 dm−3 up to 300 dm−3 Vocalization activates laryngeal particle generation |
[39] |
| Cough and cough with covering (hand, tissue, surgical mask) | The majority of droplets <0.5 μm | Droplet concentration: up to 300 dm−3 No essential filtration effect of submicrometer particles by covering of mouth with hand, sleeve, tissue or surgical mask |
[49] |
| Breathing by patients infected with human rhinovirus (HRV) | 80% of exhaled droplets in 0.3–0.5 μm diameter range | Droplet concentration: up to 7200 dm−3 for exhalation with tidal volume. No HRV detected in collected breath samples |
[50] |
Strategies of protection of the respiratory tract against inhaled viruses
Personal protection equipment (PPE) is typically used to filter out particles from the air during inhalation to the lungs. However, in the COVID era the function of face masks has been extended to the role of reduction of the amount of aerosol expelled from the respiratory system. This is because the airborne transmission of the disease has been recognized as one of the major routes of infection that can be spread even by presymptomatic, asymptomatic, or low-symptomatic persons [20,51]. It has been explained by the fact that SARS-CoV-2, in contrast to other CoVs (e.g., SARS-CoV or MERS-CoV), exhibits high shedding in the upper respiratory tract already at the early stages of infection [52], which results in easy emission of the pathogen from the body. There is a growing body of evidence that face masks, independently of their actual filtration efficiency, can reduce both the total concentration of droplets expelled from the respiratory tract and modify the flow-field of exhaled aerosol in the vicinity of an infected person [53, 54∗, 55, 56]. Also, the recent research conducted in our laboratory (unpublished data) proved that various types of face masks and covers effectively change the flow direction of exhaled aerosol, which may help in reducing the exposure of a person in front of an infected individual Figure 3 . The reported preliminary study was done with the dummy and ultrafine aerosol (MMAD = 0.45 μm, GSD = 1.8), which is comparable to the aerosol emitted during normal breathing [37]. Visualization results demonstrated that the aerosol exhaled via the mouth or nose is only partially filtered out during the flow through the mask; however, its flow direction is significantly changed since it is exhausted mainly via the spaces of inadequate fit of the mask to the curvature of the face (mainly, close to the nose or cheeks – see arrows in Figure 3c, d, e). It is a benefit of using face covers since exhaled aerosol is deflected instead of being pushed toward regions in the front of the face (as in the case when no protection is worn, Figure 3a and b). High-efficient PPEs, e.g. class FFP1, FFP (=N95, shown in Figure 3c), or FFP3 respirators, are not obligatory for this function, and may not even be optimal if they are equipped with a low-resistant exhalation valve that allows an easy aerosol penetration outside the mask. Of course, such PPEs provide a significantly more effective filtration of inhaled aerosol particles/droplets that may carry CoVs as compared to less sophisticated face masks or covers but do not necessarily offer better protection against their exhalation. On the other hand, since the induction of the infection requires a minimal amount of viruses that can reach the target cells [8,23], even partial elimination of inhaled CoVs should reduce the risk of COVID-19 infection.
Figure 3.
The visualizations of the protective action of face masks: (a, b) - aerosol exhalation without the face mask (exhalation via nose or mouth), (c) - FFP2 (class N95) respirator; (d) – cotton face mask; (e) − surgical mask. The arrows show the main directions of aerosol penetration.
The problem of CoV persistence in the air was discussed by several authors who showed that aerosol could be spread in the air to a few meters distance depending on the external conditions and the way of aerosol exhalation (normal breathing, talking, laughing, singing, coughing, sneezing, etc) by an infected person wearing no face covering [57]. The droplets may evaporate in the air and form airborne particles composed of dry or partly dry CoVs [58]. Interestingly, the intense evaporation of solvent may inactivate viruses due to the increase of the concentration of salt and change in pH inside droplets [59]. It has also been discussed that surface-decorated partly hydrophobic enveloped CoVs accumulate on the droplet surface, which results in conformational changes of spike glycoproteins, probably reducing virus infectivity [60]. On the other hand, other results confirm that SARS-CoV-2 suspended in the air remains active for several hours [61].
In the case of high air humidity or short residence time of aerosol in this environment, the effect of evaporation decreases, so inhaled CoVs will still be present in the form of fine droplets. The probable rehydration of inhaled droplets inside the respiratory tract (with the relative humidity of ∼100%) has probably no effects on the biological activity of enveloped viruses [60] but should result in an increase of droplet diameter and deposition efficiency [ [33]].
Particles on the surface of the respiratory system: role of bronchial mucus and pulmonary surfactant in viral infections
As discussed earlier, inhaled particles or droplets are deposited in different levels of the respiratory system depending on their size, breathing dynamics, and other factors. Upon deposition, particles land on the thin aqueous layer that covers cells of the lung tissue. In the bronchial airways, this layer is formed by mucus, whereas in the pulmonary zone (alveoli), the liquid contains the pulmonary surfactant, PS. Each type of liquid blanket has its specific properties, and the initial interaction of deposits with the organism must involve certain physicochemical processes [30,62].
A viscous gel-like layer of mucus immobilizes deposited particles, which are then removed from the airways together with the mucus toward the throat. In addition to water (95%), bronchial mucus contains cross-linked mucin fibers, lipids (up to 5%), mineral salts (0.5%) salts, free proteins (1%), and other macromolecules [63,64]. The so-called ‘mucociliary escalator’ propels mucus, thanks to the motion of cilia of the bronchial cells. The cilia are immersed in a less viscous fluidic sublayer (periciliary liquid) located beneath non-Newtonian layer of mucus [65,66]. Since mucus forms a physical barrier for particles deposited in the bronchial tree, it probably retards the physical (diffusional) transfer of CoVs to the bronchial cells. The mucus layer is partly permeable for nanoparticles because of the noncontinuous microstructure [67], but ∼150 nm CoV is too large to diffuse quickly through the mucus blanket. This can explain why the clinical picture of COVID-19 is often related to the acute respiratory problems that develop not in the bronchial but the more distal, pulmonary (alveolar) lung regions. On the other hand, the viral infection also results in a secondary massive inflammation in the whole bronchial tree that requires medical treatment, often done by means of aerosol therapy, i.e. inhalations [68]. Protective properties of the mucus slow down the transport of inhaled medicines to the cells, i.e. reduces the therapeutic effectiveness of inhalations. This problem was observed, e.g., in gene therapy of cystic fibrosis, indicating that mucus is poorly penetrated by viral vectors [69]. This supports the hypothesis of the protective function of mucus against CoV during infection.
It is possible to control the rate of transport of inhaled medicinal particles through the mucus layer. Odziomek et al. [70] proposed a novel vehicle for aerosol drugs (e.g., anti-inflammatory or bronchodilating agent) in the form of a composite particle containing a mucus-thinning compound (e.g., N-acetylcysteine). The preparation of such powder particles with properties suitable for inhalations done with dry powder inhalers is challenging [71,72]; however, it was possible to obtain it and demonstrate an increase in the effective diffusion of model drugs through a mucus layer in vitro [73].
The overall picture of mass transfer processes is completely different when inhaled particles (including viruses) are deposited in the pulmonary region. The aqueous layer on the top of the cells contains PS composed of lipids (up to 90% by mass, mainly: phospholipids) and surfactant specific proteins (up to 10%: SP-A, SP-B, SP-C, and SP-D) [30,74,75]. Two of these proteins (SP–B and SP-C) are hydrophobic and show surface–active properties, while the other (SP-A and SP-D) are hydrophilic with no surface activity, however with an important role in the defense system of the pulmonary region [76]. Adsorption of surface-active components of PS on the air/liquid interface (ALI) reduces the surface tension in the system. It must be stressed that the whole system is dynamic, and so is the surface tension [30,77]. The dynamics of the system come directly from the breathing cycle, i.e. the repetitive expansion and contraction of the interfacial area of ALI in alveoli. The surface concentration of PS is continuously changing, inducing the oscillatory mass exchange of surfactant molecules between the interface and the underlying liquid. Since the time scale of variation in the surface area is in the order of a few seconds, i.e. fast comparing to the rate of molecular diffusion, the surface concentration of PS components is increased during exhalation and decreased during inhalation, Figure 4 . These changes are associated with the variations of the dynamic surface tension, which can be reduced to extra-low values (below 5 mN/m [75,78]) at the peak of exhalation when the interfacial area is at the minimum. It may be speculated that such a low surface tension comes from the equilibrium value and excess (or rather: deficit) value produced by mechanical factors. This is why the dilatational surface rheology should be considered in analyzing these dynamic phenomena [31,79, 80, 81∗].
Figure 4.
The schematic of dynamic interfacial phenomena in the alveoli pulmonary during breathing.
Physicochemical events related to particle deposition on the ALI have been analyzed quantitatively by Fiegel et al. [82] by extending the earlier hypothesis of Schürch et al. [83], who suggested particle wetting and sinking after contact with liquid in the presence of PS. This would provide the active mechanism of transport of deposited particles from the interface to the alveolar liquid, i.e. closer to the epithelial cells. Particles themselves may form an adsorption area for the surfactant molecules, which build a specific molecular layer over the particle surface [84,85].
A local depletion of the surfactant due to adsorption on particles disturbs the local surface concentration, which can be compensated in two ways: (i) by PS molecules that arrive at the ALI from the underneath liquid phase, and (ii) by a tangential flow (Marangoni effect) bringing PS molecules from the neighboring regions of the ALI due to the surface tension gradients [30,86], Figure 4. Dynamic Marangoni flows that are formed during breathing provide the mechanism of hydrodynamic removal of insoluble deposits from the alveolar ALI, which is believed to be an important PS function related to its surface properties [30,86,87]. It should be noted that several other vital physiological functions are attributed to the dynamic surface activity of the surfactant: (i) the minimization of the work of breathing, (ii) stabilizing the alveoli, (iii) maintaining a large gas transfer area, and (iv) preventing lung edema [88,89]. They prove the importance of physicochemical phenomena in this system and suggest possible physiological effects induced by PS interactions with deposited particles.
It has been recognized that the influence of deposits on interfacial processes in this system depends on particle size, shape, and surface properties. Nanostructured and nanoporous particles have the largest area/volume ratio and therefore induce stronger surface effects than nonporous micrometer-size particles. When a large number of inhaled particles land on the ALI of the pulmonary region, their direct physicochemical interactions with PS may alter the natural interfacial processes of this system [30,31,90]. It should be noted, however, that some deposits are fully or partly soluble in the pulmonary liquid, e.g. droplets of aqueous solutions or suspensions. After the deposition of droplets on the pulmonary surface, the compounds carried in them will be quickly released to the liquid beneath the interface, and the consecutive interactions with PS components take place inside this sublayer. Such a scenario may be expected, e.g. after inhalation of e-cigarette mist, where the primary effects are induced in the liquid subphase rather than directly in the air-exposed surfactant monolayer at ALI [91]. The situation should be similar when CoVs are deposited inside inhaled aqueous droplets.
It is known that PS components, mainly surfactant-specific proteins, have antimicrobial and antifungal functions [76,92], and they were also shown to exhibit antiviral activity [93,94]. This allowed putting a hypothesis of a possible role of PS in the protection against SARS-CoV-2, which was based on the observed detrimental effects caused by other active surfactants on enveloped viruses [95]; however, it has been also recognized that development of the disease in the respiratory system is associated with the rapid damage of alveolar type II cells (type II pneumocytes), which are responsible for the synthesis of PS. These cells are easily attacked because they have a high expression of ACE2, therefore serve as a basic target for SARS-CoV-2 [96]. As a result, COVID-19 quickly induces PS insufficiency in vivo, that is additionally amplified by surfactant inactivation by products of cell lysis as a common clinical picture of the acute respiratory distress syndrome, ARDS. Impaired function of PS leads to serious respiratory problems and a decrease in blood oxygenation, resulting in a life-threatening condition, often requiring artificial ventilation. ARDS is also frequently related to a physical blockage of gas exchange surface in the lungs by extensive foaming due to liquid accumulation in the alveoli and bronchioles, known as pulmonary edema [97]. In such a situation, all physiological functions (including the defensive ones) associated with the dynamic surface-active properties of PS at the ALI are destroyed. When the innate PS is inactivated, the supplementation of exogenous PS is a vital strategy needed to recover the pulmonary functions but also to provide enough surfactant to produce the postulated physicochemical activity against SARS-CoV-2 [95,98]. Several clinical trials are currently undertaken to test the applicability of the exogenous surfactants in the treatment of COVID-19 [99].
Regardless of the SARS-CoV-2 interactions at the cellular level, it may be interesting to take a look at possible purely physicochemical events associated with virus entrance onto the alveolar surface. As discussed earlier, inhaled CoV particles are typically contained inside droplets of saliva and mucus emitted from the respiratory tract of an infected person; however, other routes of airborne virus transmission have also been proposed, e.g. toilet water splashing [100], carrying CoV with the environmental dust [14], or generation by medical procedures, including oxygen and inhalation therapies [68]. Considering the most probable inhalation scenarios, the arrival of completely dry active virus particles to the surface of the lungs is unlikely. It is a quite different situation compared to the more often discussed deposition of inhaled natural or man-made dust microparticles or nanoparticles [28,31]. As schematically shown in Figure 5 , a droplet with SARS-CoV-2 deposited on the alveolar ALI will spread immediately and mix with the pulmonary fluid, which allows the virus to pass the air/liquid barrier formed by the surfactant more easily as compared to a dry particle that is captured on the interface [30]. This suggests that PS layer may not be an effective physical barrier for protecting the airway cells against SARS-CoV-2. One should also note that the virus size is comparable with the average depth of the alveolar fluid (∼100–200 nm [87,101,102]), which may facilitate a direct contact of SARS-CoV-2 with pneumocytes and quick biochemical effects at the cellular level. Such mechanism is typically absent upon deposition of biologically neutral dry dust nanoparticles.
Figure 5.
Postulated behavior of a droplet with CoV and dry particle after deposition in the lung fluids. (a) bronchial mucus: 1 – droplet landing, 2 – droplet spreading with CoV captured in the gel-like layer, then transported by ‘mucociliary escalator’; (b) pulmonary surfactant layer: 1’ – droplet landing, 2’ - droplet spreading with CoV translocation to the liquid subphase and the epithelium; (c) pulmonary surfactant layer: 1” – dry particle landing, 2” - longitudinal transport of floating particle by surface tension gradient ∇γ (U – the velocity of the interface and particle due to the Marangoni effect).
As discussed earlier, the hydrophobic bilayer of CoV is decorated by glycoproteins with a various degree of hydrophilicity in different fragments of their structure [103]. Transient interactions of CoV with the surfactant components may lead to localized interactions of various PS molecules (lipids, proteins) with the functional groups of the spike glycoprotein. By analogy to other particles deposited on the pulmonary surface [84, 104], this process may alter to some extent the activity of the CoVs. It has been recognized that the peculiar surface properties of the SARS-CoV-2 play a role in its adhesion to variable surfaces and different virus stability on these fomites [10]. Important factors determining CoV stability on solid surfaces are the surface charge and steric conformations; however, hydrogen bonding may also become effective in the presence of an aqueous phase. At this stage of knowledge, we cannot be sure if similar molecular interactions take place on the lung surface and if they can influence virus affinity to the pulmonary cells, which is determined mainly by biochemical mechanism (ACE2-signalling pathway).
Conclusions
One of the main routes of COVID-19 infection is the transmission of the SARS-CoV-2 virus in the form of aerosol that can enter directly to the surface of the respiratory system by inhalation. In this review, several aspects of this process have been discussed. The formation of aerosol particles in the respiratory system of infected, sometimes asymptomatic person during normal breathing, talking, coughing, or sneezing allows the virus to be expelled to the environment. The emitted droplets have various sizes and different velocities, but they can be eliminated and redirected by facial respirators, masks, and covers. Without nose and mouth coverings, droplets are extensively emitted to the environment, where they may settle down on various surfaces. Some of them, due to partial evaporation in the air, achieve the size that allows their persistence in the form of aerosol for some hours. Upon inhalation, different mechanisms of flow and deposition allow them to arrive at various levels of the respiratory system and land with size-dependent efficiency on the surface covered by protective liquid layers of the bronchial mucus or pulmonary surfactant. Interactions with these lung fluids can affect the fate and activity of the coronavirus in a given lung region. Recognition of these phenomena seems to be indispensable for understanding the process of COVID-19 transmission from the perspective of colloid and surface science. This knowledge should be of great value for the elaboration of new protective and therapeutic means against this disease.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The work was funded by IDUB against the COVID-19 project granted by Warsaw University of Technology under the program Excellence Initiative: Research University (IDUB).
This review comes from a themed issue on Hot Topic: COVID-19
Edited by Reinhard Miller and Libero Liggieri
References
- 1.Lai M.M.C. SARS Virus: the beginning of the unraveling of a new coronavirus. J Biomed Sci. 2003;10:664–675. doi: 10.1007/BF02256318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper demonstrates the mechanisms of spike protein in viral bioactivity that makes this protein a potential molecular target for vaccines and medicines against COVID-19.
- 3.Fouchier R.A.M., Hartwig N.G., Bestebroer T.M., Niemeyer B., de Jong J.C., Simon J.H., Osterhaus A.D.M.E. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci Unit States Am. 2004;101:6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Hoek L., Pyrc K., Jebbink M.F., et al. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woo P.C.Y., Huang Y., Lau S.K.P., Yuen K.-Y. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2:1804–1820. doi: 10.3390/v2081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr L.C., Tang J.W., van Mullekom J., Lakdawala S.S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface. 2019;16:20180298. doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber T.P., Stilianakis N.I. Inactivation of influenza A viruses in the environment and modes of transmission: a critical review. J Infect. 2008;57:361–373. doi: 10.1016/j.jinf.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y., Yang C., Xu X.-f., Xu W., Liu S.-w. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sinica. 2020;41:1141–1149. doi: 10.1038/s41401-020-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joonaki E., Hassanpouryouzband A., Heldt C.L., Areo O. Surface chemistry can unlock drivers of surface stability of SARS-CoV-2 in a variety of environmental conditions. Inside Chem. 2020;6:2135–2146. doi: 10.1016/j.chempr.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important data regarding physicochemical interactions of the coronavirus with various materials that influence virus activity.
- 11.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahat G. Covid-19 and the renin angiotensin system: implications for the older adults. J Nutr Health Aging. 2020;24:699–704. doi: 10.1007/s12603-020-1403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comunian S., Dongo D., Milani C., Palestini P. Air pollution and COVID-19: the role of particulate matter in the spread and increase of COVID-19's morbidity and mortality. Int J Environ Res Public Health. 2020;17:4487. doi: 10.3390/ijerph17124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley J.E. COVID-19 — the long road to recovery. J Nutr Health Aging. 2020;24:917–919. doi: 10.1007/s12603-020-1497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]; The paper describes the observed various systemic health effects of COVID-19 in recovered patients. This underlines the importance of protection against the infection even if direct symptoms are often mild.
- 16.Becker R.C. Anticipating the long-term cardiovascular effects of COVID-19. J Thromb Thrombolysis. 2020;50:512–524. doi: 10.1007/s11239-020-02266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicas M., Nazaroff W.W., Hubbard A. Towards understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Env Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection. Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 19.Asadi S., Bouvier N., Wexler A.S., Ristenpart W.D. The coronavirus pandemic and aerosols: does COVID-19 transmit via expiratory particles? Aerosol Sci Technol. 2020;54:635–638. doi: 10.1080/02786826.2020.1749229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. J Am Med Assoc. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prather K.A., Marr L.C., Schooley R.T., McDiarmid M.A., Wilson M.E., Milton D.K. Airborne transmission of SARS-CoV-2. Science. 2020;370:303–304. doi: 10.1126/science.abf0521. [DOI] [PubMed] [Google Scholar]
- Newman S. RDD Online-VCU; Richmond (USA): 2009. Respiratory drug delivery. Essential theory and practice. [Google Scholar]; A comprehensive source focused on various aspects of aerosols as carriers of medicines delivered via inhalation.
- 23.Killingley B., Nguyen-Van-Tam J. Routes of influenza transmission. Influenza Other Respir Viruses. 2013;7:42–51. doi: 10.1111/irv.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pirożyński M., Sosnowski T.R. Inhalation devices: from basic science to practical use, innovative vs generic products. Expert Opin. Drug Del. 2016;13:1559–1571. doi: 10.1080/17425247.2016.1198774. [DOI] [PubMed] [Google Scholar]
- 25.Ochs M., Nyengaard J.R., Jung A., et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120–124. doi: 10.1164/rccm.200308-1107OC. [DOI] [PubMed] [Google Scholar]
- 26.Sosnowski T.R., Moskal A., Gradoń L. Mechanims of aerosol particle deposition in the oro-pharynx under non-steady airflow. Ann Occup Hyg. 2007;51:19–25. doi: 10.1093/annhyg/mel072. [DOI] [PubMed] [Google Scholar]
- Multi-path particle deposition model. Available from: https://www.ara.com/mppd.; The webpage with the software that allows calculating the expected regional deposition of inhaled aerosol particles as a function of aerosol properties, inhalation pattern, and physiological parameters
- 28.Moskal A., Sosnowski T.R. In: Synthetic nano- and microfibers. Wagterveld M., et al., editors. Wetsus: European Centre of Excellence for Susutainable Water Technology; Leeuwarden (The Netherlands: 2020. Micro- and nanofibers: aerodynamics and physicochemical aspects; pp. 5–18. [Google Scholar]
- Maynard A.D., Kuempel E.D. Airborne nanostructured particles and occupational health. J Nanoparticle Res. 2005;7:587–614. [Google Scholar]; Paper discussing the sources and role of nanoparticles in inhalation toxicology.
- Sosnowski T.R. Particles on the lung surface – physicochemical and hydrodynamic effects. Curr Opin Colloid Interface Sci. 2018;36:1–9. [Google Scholar]; A review of surfactant functions related to the dynamic surface tension and liquid flows in the lungs, with the discussion of material-dependent and concentration-dependent inactivation of this phenomenon by inhaled particles.
- 31.Kondej D., Sosnowski T.R. Interfacial rheology for the assessment of potential health effects of inhaled carbon nanomaterials at variable breathing conditions. Sci Reports. 2020;10:14044. doi: 10.1038/s41598-020-70909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddrell A.E., Lewis D., Church T., Vehring R., Murnane D., Reid J.P. Pulmonary aerosol delivery and the importance of growth dynamics. Ther Deliv. 2017;8:1051–1061. doi: 10.4155/tde-2017-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]; The discussion of a possible increase in aerosol particle size in the respiratory tract due to humidity absorption that leads to changes in the regional deposition of inhaled particles.
- 33.Sosnowski T.R., Odziomek M. Particle size dynamics: towards a better understanding of electronic cigarette aerosol interactions with the respiratory system. Front Physiol. 2018;9:853. doi: 10.3389/fphys.2018.00853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1996;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Gralton J., Tovey E., McLaws M.-L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review properties of aerosol particles emitted from the respiratory system under various conditions.
- 36.Yan J., Grantham M., Pantelic J., Bueno de Mesquita P.J., Albert B., Liu F., Ehrman S., Milton D.K., EMIT Consortium Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc Nat Acad Sci. 2018;115:1081–1086. doi: 10.1073/pnas.1716561115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheuch G. Breathing is enough: for the spread of influenza virus and SARS-CoV-2 by breathing only. J Aerosol Med Pulm Drug Deliv. 2020;33:230–234. doi: 10.1089/jamp.2020.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morawska L., Johnson G.R., Ristovski Z.D., Hargreaves M., Mengersen K., Corbett S., Chao C.Y.H., Li Y., Katoshevski D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 2009;40:256–269. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asadi S., Wexler A.S., Cappa C.D., Barreda S., Bouvier N.M., Ristenpart W.D. Aerosol emission and superemission during human speech increase with voice loudness. Sci Reports. 2019;9:2348. doi: 10.1038/s41598-019-38808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie X., Li Y., Sun H., Liu L. Exhaled droplets due to talking and coughing. J R Soc Interface. 2009;6:S703–S714. doi: 10.1098/rsif.2009.0388.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Z.Y., Weng W.G., Huang Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J R Soc Interface. 2013;10:20130560. doi: 10.1098/rsif.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang J.W., Liebner T.J., Craven B.A., Settles G.S. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6:S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang J.W., Nicolle A.D., Klettner C.A., et al. Airflow dynamics of human jets: sneezing and breathing - potential sources of infectious aerosols. PloS One. 2013;8 doi: 10.1371/journal.pone.0059970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson G.R., Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv. 2009;22:229–237. doi: 10.1089/jamp.2008.0720. [DOI] [PubMed] [Google Scholar]
- 45.Chao C.Y.H., Wan M.P., Morawska L., et al. Characterization of expiratory air jets and droplet size distributions immediately at the mouth opening. J Aerosol Sci. 2009;40:123–133. doi: 10.1016/j.jaerosci.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson G.R., Morawska L., Ristovski Z.D., et al. Modality of human expired aerosol size distribution. J Aerosol Sci. 2011;42:839–851. [Google Scholar]
- 47.Lindsley W.G., Pearce T.A., Hudnall J.B., et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Envir Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zayas G., Chiang M.C., Wong E., et al. Cough aerosol in healthy participants: fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm Med. 2012;12:11. doi: 10.1186/1471-2466-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zayas G., Chiang M.C., Wong E., et al. Effectiveness of cough etiquette maneuvers in disrupting the chain of transmission of infectious respiratory diseases. BMC Publ Health. 2013;13:811. doi: 10.1186/1471-2458-13-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabian P., Brain J., Houseman A., Gern J., Milton D.K. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothe C., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Z., Harrich D., Li Z., Hu D., Li D. The unique features of SARS-CoV-2 transmission: comparison with SARS-CoV, MERS-CoV and 2009 H1N1 pandemic influenza virus. Rev Med Virol. 2020:e2171. doi: 10.1002/rmv.2171. [Online ahead of print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhtar J., Luna Garcia A., Saenz L., Kuravi S., Shu F., Kota K. Can face masks offer protection from airborne sneeze and cough droplets in close-up, face-to-face human interactions?—a quantitative study. Phys Fluids. 2020;32:127112. doi: 10.1063/5.0035072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähler C.J., Hain R. Fundamental protective mechanisms of face masks against droplet infections. J Aerosol Sci. 2020;148:105617. doi: 10.1016/j.jaerosci.2020.105617. [DOI] [PMC free article] [PubMed] [Google Scholar]; Up-to-date study and discussion of the role of various face covers and masks in protection against exhaled particles, including a proper fit to the face.
- 55.Leung N.H.L., Chu D.K.W., Schiu E.Y.C., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma S., Dhanak M., Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids. 2020;32 doi: 10.1063/5.0016018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dbouk T., Drikakis D. On coughing and airborne droplet transmission to humans. Phys Fluids. 2020;32 doi: 10.1063/5.0011960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dbouk T., Drikakis D. Weather impact on airborne coronavirus survival. Phys Fluids. 2020;32 doi: 10.1063/5.0024272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vejerano E.P., Marr L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J R Soc Interface. 2018;15:20170939. doi: 10.1098/rsif.2017.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discussion of the role of evaporation of exhaled droplets for the activity of pathogens contained inside.
- 60.Yang W., Marr L.C. Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol. 2012;78:6781–6788. doi: 10.1128/AEM.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Doremalen N., Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman E., Santini E. Lung surfactant-particles at fluid interfaces for toxicity assessments. Curr Opin Coll Interface Sci. 2019;39:24–39. [ ] [Google Scholar]; A comprehensive review related to identification of physicochemical interactions between particles and air-liquid interface in the presence of pulmonary surfactant.
- 63.Groo A.-C., Lagarce F. Mucus models to evaluate nanomedicines for diffusion. Drug Disc Today. 2014;19:1097–1108. doi: 10.1016/j.drudis.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 64.Netsomboon K., Bernkop-Schnürch A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur J Pharm Biopharm. 2016;98:76–89. doi: 10.1016/j.ejpb.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Smith D.J., Gaffney E.A., Blake J.R. A viscoelastic traction layer model of mucociliary transport. Bull Math Biol. 2007;69:289–327. doi: 10.1007/s11538-005-9036-x. [DOI] [PubMed] [Google Scholar]; The mathematical analysis of mucus transport in the bronchi, including predictions of clearance variability due to lung diseases.
- 66.van der Schans C.P. Bronchial mucus transport. Respir Care. 2007;52:1150–1156. [PubMed] [Google Scholar]
- Lai S.K., Wang Y.-Y., Wirtz D., Hanes J. Micro- and macrorheology of mucus. Adv Drug Del Rev. 2009;61:86–100. doi: 10.1016/j.addr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Essential review of the microstructure of mucus and its properties, including selective barrier functions.
- Fink J.B., Ehrmann S., Li J., et al. Reducing aerosol-related risk of transmission in the era of COVID-19: an interim guidance endorsed by the International Society of Aerosols in Medicine. J Aerosol Med Pulm Drug Deliv. 2020;33:300–304. doi: 10.1089/jamp.2020.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]; The expert opinion on the ways of minimization of bio-contamination by aerosols generated during medical and related procedures.
- 69.Hida K., Lai S.K., Suk J.S., Won S.Y., Boyle M.P., Hanes J. Common gene therapy viral vectors do not efficiently penetrate sputum from cystic fibrosis patients. PloS One. 2011;6 doi: 10.1371/journal.pone.0019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Odziomek M., Sosnowski T.R., Gradoń L. Conception, preparation and properties of functional carrier particles for pulmonary drug delivery. Int J Pharm. 2012;433:51–59. doi: 10.1016/j.ijpharm.2012.04.067. [DOI] [PubMed] [Google Scholar]
- 71.Gradoń L., Sosnowski T.R. Formation of particles for dry powder inhalers. Adv Powder Technol. 2014;25:43–55. [Google Scholar]
- 72.Kadota K., Sosnowski T.R., Tobita S., Tachibana I., Tse J., Uchiyama K., Tozuka Y. A particle technology approach toward designing dry-powder inhaler formulations for personalized medicine in respiratory diseases. Adv Powder Technol. 2020;31:219–226. [Google Scholar]
- 73.Odziomek M., Sosnowski T.R., Gradoń L. The influence of Functional Carrier Particles (FCPs) on the molecular transport rate through the reconstructed bronchial mucus - in vitro studies. Transp Porous Media. 2015;106:439–454. [Google Scholar]
- 74.Haagsman H.P., Diemel R.V. Surfactant-associated proteins: functions and structural variation. Comp Biochem Physiol Mol Integr Physiol. 2001;129:91–108. doi: 10.1016/s1095-6433(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Serrano A.G., Perez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids. 2006;141:105–118. doi: 10.1016/j.chemphyslip.2006.02.017. [DOI] [PubMed] [Google Scholar]; The review of the pulmonary composition and biophysical functions with the emphasis on the surfactant proteins and protein-lipid interactions.
- 76.Wright J.R. Pulmonary surfactant: a front line of lung host defense. J Clin Invest. 2003;111:1453–1455. doi: 10.1172/JCI18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bykov A.G., Loglio G., Miller R., Milyaeva O.Y., Michailov A.V., Noskov B.A. Dynamic properties and relaxation processes in surface layer of pulmonary surfactant solutions. Colloids Surf, A. 2019;573:14–21. [Google Scholar]
- 78.Zuo Y.Y., Possmayer F. How does pulmonary surfactant reduce surface tension to very low values? J Appl Physiol. 2007;102:1733–1734. doi: 10.1152/japplphysiol.00187.2007. [DOI] [PubMed] [Google Scholar]
- 79.Wüstneck R., Wüstneck N., Moser B., Karageorgieva V., Pison U. Surface dilatational behavior of pulmonary surfactant components spread on the surface of a pendant drop. 1. Dipalmitoyl phosphatidylcholine and surfactant protein C. Langmuir. 2002;18:1119–1124. [Google Scholar]
- 80.Moskal A., Sosnowski T.R. In: Practical aspects of chemical engineering. Ochowiak M., et al., editors. Springer Int. Publ. AG; Cham (Switzerland: 2018. Chemical engineering in biomedical problems – selected applications; pp. 307–318. [Google Scholar]
- Bykov A.G., Milyaeva O.Y., Isakov N.A., Michailov A.V., Loglio G., Miller R., Noskov B.A. Dynamic properties of adsorption layers of pulmonary surfactants. Influence of matter exchange with bulk phase. Colloids Surf, A. 2021;611:125851. [Google Scholar]; The paper discusses the kinetic factors required for the effective dynamic interfacial functions of the pulmonary surfactant.
- Fiegel J., Jina F., Hanes J., Stebe K. Wetting of a particle in a thin film. J Coll. Interface Sci. 2005;291:507–514. doi: 10.1016/j.jcis.2005.05.014. [DOI] [PubMed] [Google Scholar]; An elegant mathematical analysis of particle behavior at the air/liquid interface, including effects related to particles deposited in the lungs
- Schürch S., Gehr P., Im Hof V., Geiser M., Green F. Surfactant displaces particles toward the epithelium in airways and alveoli. Respiration Physiol. 1990;80:17–32. doi: 10.1016/0034-5687(90)90003-h. [DOI] [PubMed] [Google Scholar]; The first hypothesis of particle displacement from the liquid surface to the lung epithelium due to capillary effects.
- 84.Raesch S.S., Tenzer S., Storck W., et al. Proteomic and lipidomic analysis of nanoparticle corona upon contact with lung surfactant reveals differences in protein, but not lipid composition. ACS Nano. 2015;9:11872–11885. doi: 10.1021/acsnano.5b04215. [DOI] [PubMed] [Google Scholar]
- Garcia-Mouton C., Hidalgo A., Cruz A., Perez-Gil J. The Lord of the Lungs: the essential role of pulmonary surfactant upon inhalation of nanoparticles. Eur J Pharm Biopharm. 2019;144:230–243. doi: 10.1016/j.ejpb.2019.09.020. [DOI] [PubMed] [Google Scholar]; Up-to-date review of the role of pulmonary surfactant in nanoparticles interactions in the lungs.
- Gradoń L., Podgórski A. Hydrodynamical model of pulmonary clearance. Chem Eng Sci. 1989;44:741–749. [Google Scholar]; The first theoretical hypothesis of pulmonary surfactant function related to dynamic Marangoni effects on the lung surface.
- 87.Podgórski A., Gradoń L. An improved mathematical model of hydrodynamical self-cleansing of pulmonary alveoli. Ann Occup Hyg. 1993;37:347–365. doi: 10.1093/annhyg/37.4.347. [DOI] [PubMed] [Google Scholar]
- Notter R.H. CRC Press New York -Basel; 2000. Lung surfactants: basic science and clinical applications. [Google Scholar]; A comprehensive book on the lung surfactant viewed from a biological, physicochemical, and medical perspective.
- 89.Zuo Y.Y., Veldhuizen R.A.W., Neumann A.W., Petersen N.O., Possmayer F. Current perspectives in pulmonary surfactant — inhibition, enhancement and evaluation. Biochim Biophys Acta – Biomembranes. 2008;1778:1947–1977. doi: 10.1016/j.bbamem.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 90.Sosnowski T.R., Gradoń L., Podgórski A. Influence of insoluble aerosol deposits on the surface activity of the pulmonary surfactant: a possible mechanism of alveolar clearance retardation. Aerosol Sci Technol. 2000;32:52–60. [Google Scholar]
- 91.Sosnowski T.R., Jabłczyńska K., Odziomek M., Schlage W.K., Kuczaj A.K. Physicochemical studies of direct interactions between lung surfactant and components of electronic cigarettes liquid mixtures. Inhal Toxicol. 2018;30:159–168. doi: 10.1080/08958378.2018.1478916. [DOI] [PubMed] [Google Scholar]
- 92.Han S., Mallampalli R.K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann Am Thorac Soc. 2015;12:765–774. doi: 10.1513/AnnalsATS.201411-507FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Eijk M., Hillaire M.L.B., Rimmelzwaan G.F., et al. Enhanced antiviral activity of human surfactant protein d by site-specific engineering of the carbohydrate recognition domain. Front Immunol. 2019;22:2476. doi: 10.3389/fimmu.2019.02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Numata M., Mitchell J.R., Tipper J.L., Brand J.D., Trombley J.E., Nagashima Y., Kandasamy P., Chu H.W., Harrod K.S., Voelker D.R. Pulmonary surfactant lipids inhibit infections with the pandemic H1N1 influenza virus in several animal models. J Biol Chem. 2020;295:1704–1715. doi: 10.1074/jbc.RA119.012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano H. Pulmonary surfactant itself must be a strong defender against SARS-CoV-2. Med Hypoth. 2020;144:110020. doi: 10.1016/j.mehy.2020.110020. [DOI] [PMC free article] [PubMed] [Google Scholar]; A hypothesis regarding the role of the pulmonary surfactant in the development of the lung diseases caused by the coronavirus.
- 96.Hoseinzadeh E., Javan S., Farzadki M., Mohammadi G.F., Hossini H., Taghavif M. An updated mini-review on environmental route of the SARS-CoV-2 transmission. Ecotoxicol Environ Safety. 2020;202:111015. doi: 10.1016/j.ecoenv.2020.111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luisada A.A., Cardi L. Acute pulmonary edema; pathology, physiology and clinical management. Circulation. 1956;13:113–135. doi: 10.1161/01.cir.13.1.113. [DOI] [PubMed] [Google Scholar]
- 98.Ghati A., Dam P., Tasdemir D., et al. Exogenous pulmonary surfactant: a review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SP-A and SP-D as added clinical marker. Curr Opin Colloid Interface Sci. 2021;51:101413. doi: 10.1016/j.cocis.2020.101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clinicaltrials.gov. Curosurf® . 29 Nov 2020. Adult acute respiratory distress syndrome Due to COVID-19 (Caards-1) NCT04568018; London's exogenous surfactant Study for COVID19 (LESSCOVID) NCT04375735; surfactant-BL in adult acute respiratory distress syndrome Due to COVID-19 NCT04568018. Website: clinicaltrials.gov access. [Google Scholar]
- 100.Li Y., Wang J.X., Chen X. Can a toilet promote virus transmission? From a fluid dynamics perspective. Phys Fluids. 2020;32 doi: 10.1063/5.0013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Widdiecombie J. Airway and alveolar permeability and surface liquid thickness: theory. J Appl Physiol. 1997;82:3–12. doi: 10.1152/jappl.1997.82.1.3. [DOI] [PubMed] [Google Scholar]
- 102.Ochs M., Hegermann J., Lopez-Rodriguez E., Timm S., Nouailles G., Matuszak J., Simmons S., Witzenrath M., Kuebler W.M. On top of the alveolar epithelium: surfactant and the glycocalyx. Int J Mol Sci. 2020;21:3075. doi: 10.3390/ijms21093075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virology J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gasser M., Rothen-Rutishauser B., Krug H.F., et al. The adsorption of biomolecules to multi-walled carbon nanotubes is influenced by both pulmonary surfactant lipids and surface chemistry. J Nanobiotechnol. 2010;8:31. doi: 10.1186/1477-3155-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]