Abstract
Low levels of the neurotransmitter serotonin have been associated with the onset of depression. While traditional treatments include antidepressants, physical exercise has emerged as an alternative for patients with depressive disorders. Yet there remains the fundamental question of how exercise is sensed by the brain. The existence of a muscle–brain endocrine loop has been proposed: according to this scenario, exercise modulates metabolization of tryptophan into kynurenine within skeletal muscle, which in turn affects the brain, enhancing resistance to depression. But the breakdown of tryptophan into kynurenine during exercise may also alter serotonin synthesis and help limit depression. In this study, we investigated whether peripheral serotonin might play a role in muscle–brain communication permitting adaptation for endurance training. We first quantified tryptophan metabolites in the blood of 4 trained athletes before and after a long-distance trail race and correlated changes in tryptophan metabolism with physical performance. In parallel, to assess exercise capacity and endurance in trained control and peripheral serotonin–deficient mice, we used a treadmill incremental test. Peripheral serotonin–deficient mice exhibited a significant drop in physical performance despite endurance training. Brain levels of tryptophan metabolites were similar in wild-type and peripheral serotonin–deficient animals, and no products of muscle-induced tryptophan metabolism were found in the plasma or brains of peripheral serotonin–deficient mice. But mass spectrometric analyses revealed a significant decrease in levels of 5-hydroxyindoleacetic acid (5-HIAA), the main serotonin metabolite, in both the soleus and plantaris muscles, demonstrating that metabolization of tryptophan into serotonin in muscles is essential for adaptation to endurance training. In light of these findings, the breakdown of tryptophan into peripheral but not brain serotonin appears to be the rate-limiting step for muscle adaptation to endurance training. The data suggest that there is a peripheral mechanism responsible for the positive effects of exercise, and that muscles are secretory organs with autocrine-paracrine roles in which serotonin has a local effect.
Keywords: Tryptophan, Peripheral and Central Serotonin, Kynurenine, Physical performance
Introduction
Through 2 different pathways, the essential amino acid tryptophan (TRP) is the precursor of the well-known neurotransmitter serotonin (5-hydroxytryptamine, or 5-HT) and kynurenine (KYN) metabolites. TRP is hydroxylated by tryptophan hydroxylase (Tph) into 5-hydroxytryptophan (5-HTP), and 5-HTP is then decarboxylated by 5-HTP decarboxylase for the synthesis of 5-HT. The 2 Tph isoforms, Tph1 and Tph2, are expressed respectively in peripheral and in brain tissues.1,2 Hence 5-HT exerts its effects both centrally and in the periphery. While TRP can cross the blood–brain barrier, 5-HT cannot, and each pool of 5-HT has a specific set of functions. TRP is also metabolized through the KYN pathway (see Figure 1A). Kynurenine can cross the blood–brain barrier and be further metabolized, in the periphery as well as the brain. A neuroprotective branch of the pathway, dependent on the enzyme kynurenine aminotransferase (KAT), yields kynurenic acid (KYNA), which cannot pass the blood–brain barrier. There is also a neurotoxic branch, dependent on kynurenine 3-monooxygenase, which shifts the pathway toward the production of potentially neurotoxic metabolites, including 3-hydroxykynurenine (3-HK) and quinolinic acid.3 We and others have shown that a tight control of TRP metabolism is critical for maintaining healthy homeostasis, as changes in TRP concentrations are associated with physiological and behavioral processes.4-7 For instance, low levels of 5-HT and high levels of neurotoxic KYN have been associated with depression. There is much evidence to suggest that physical exercise is an alternative treatment for patients with a depressive disorder. During physical exercise, blood TRP levels have been shown to rise, which leads to higher TRP levels in the central nervous system, and subsequently, more 5-HT in the brain and plasma.8,9 Other studies demonstrate that exercise enhances muscular expression of KAT, which reduces depression-like symptoms by conversion of neurotoxic KYN into neuroprotective KYNA.10 Furthermore, elevated plasma KYNA levels have been observed after endurance exercise in mice and humans.11,12 These findings suggest that muscle-induced peripheral factors enable direct communication between the muscles and the brain that is necessary for adaptation to endurance training.
Figure 1.
Serotonin synthesis via tryptophan metabolism is essential for intense exercise in trained runners. Tryptophan serum metabolites in 4 trained runners (designated A, B, C, and D) were quantified using liquid chromatography–tandem mass spectrometry during 2 different long-distance trail races, as described in Table 1: (A) metabolites that cross the blood–brain barrier are underlined, those having a neuroprotective effect are printed in italic-green, and those that are neurotoxic are printed in red-bold, (B–G) metabolite concentrations in blood samples taken at different points in the race (expressed as percentage of total distance covered on the x-axes) are shown for the 4 runners, using a unique symbol for each (see key to the right of panel (G)).
5-HIAA, 5-hydroxyindoleacetic acid; 5-HTP, 5-hydroxytryptophan; IDO, indoleamine 2,3-dioxygenase; KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase; TDO, tryptophan 2,3-dioxygenase; Tph1, tryptophan hydroxylase 1.
In this study, we investigated whether a peripheral 5-HT pathway might be involved in an exercise-dependent muscle–brain loop. A trained mouse model deficient in peripheral 5-HT and trained athletes were analyzed. Control and 5-HT–deficient mice underwent endurance training, consisting of repeated sessions of endurance exercise, where each session was a treadmill incremental test that ended when the mouse became exhausted. We next measured TRP metabolites and used the 5-HIAA/5-HT ratio as an estimator of serotonergic activity.
Results
TRP conversion into 5-HT essential for intense exercise in trained runners
To examine changes in concentration of TRP metabolites in 4 volunteers (labeled A, B, C, and D), all trained amateur runners, blood samples were collected at several selected time points during their respective races held in 2017 (Table 1). There were 2 races, both long-distance races: the 166-km Diagonale des Fous and 112-km Trail du Bourbon. Serum concentrations of TRP, KYN, 5-HT, and 5-hydroxyindoleacetic acid (5-HIAA) were determined using liquid chromatography–tandem mass spectrometry. In Figure 1B to G, metabolite concentrations are shown for each sampling point, and variation in TRP metabolism is evident. Levels of 5-HT dropped by approximately 55% for both runners of the 166-km race, and by 75% for both runners of the 112-km race (Figure 1B and Table 1). At the end of each race, there was a striking increase in the ratio of 5-HIAA to 5-HT—commonly used as an estimator of serotonergic activity13,14—for all participants: by approximately 210% for the 166-km race 300% for the 112-km race (Figure 1C and Table 1). However, there were no variations in KYN pathway metabolites throughout the race. Except for runner C, KYN levels were constant, and indoleamine 2,3-dioxygenase (IDO) activity, represented by the ratio of KYN to TRP, remained stable for all participants (Figure 1D and E). No significant changes are observed in tryptophan metabolism toward kynurenine or 5-HT production throughout the race (Figure 1F). Moreover, no variation in 5-HIAA concentration was observed throughout the race (Figure 1G). In summary, serum analyses suggested that both TRP conversion into 5-HT and 5-HT turnover are part of adaptation for endurance exercise, as represented by long-distance trail races, in trained runners.
Table 1.
Runners, races, and tryptophan metabolism.
| Jouffroy et al20 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Runners | Long-distance trail race | Metabolite variation (%) before and after race | ||||||||||
| Runner | Sex | Age (years) | Height (cm) | Weight (kg) | Distance (km) | CEG (m) | TRP | KYN | 5-HT | 5-HIAA | IDO activity | 5-HIAA/5-HT |
| A | M | 43 | 180 | 80 | 166 | 9.611 | −8.4 | 25 | −58.9 | 32.1 | 36.5 | 221.4 |
| B | M | 39 | 178 | 77 | 166 | 9.611 | 12.1 | 25.7 | −52.7 | 40.2 | 12.2 | 196.6 |
| C | M | 37 | 172 | 72 | 112 | 6.468 | −13.7 | 16 | −72.6 | −16.8 | 34.5 | 203.3 |
| D | F | 35 | 165 | 56 | 112 | 6.468 | −34.6 | −37.6 | −77.8 | 14.9 | −4.6 | 417.5 |
IDO, indoleamine 2,3-dioxygenase activity is indicated by the kynurenine-to-tryptophan ratio; CEG, cumulative elevation gain; 5-HIAA, 5-hydroxyindolacetic acid; 5-HT, serotonin; F, female; KYN, kynurenine; M, male; TRP, tryptophan.
Tryptophan metabolites in serum from 4 trained runners were quantified by liquid chromatography–tandem mass spectrometry during 2 long-distance trail races.
Reduced Physical Performance in 5-HT–Deficient Mice Undergoing Endurance Training
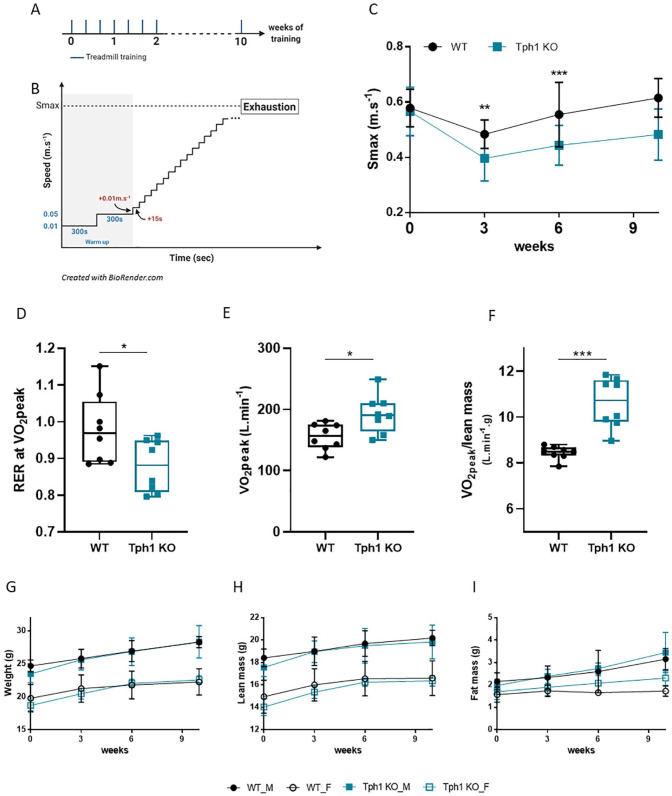
To further understand how 5-HT plays a role in adaptation to endurance training, we used a Tph1 knockout (peripheral 5-HT–deficient mouse model) (Tph1 KO) and wild-type (WT) control mice. All mice had treadmill training 3 times a week (wk) for 10 weeks (Figure 2A). First, we determined body weights and compositions before and after each training session. To measure their performance after endurance training, WT and Tph1 KO mice were subjected to incremental treadmill tests (Figure 2A and B). The maximum speed (Smax), that is, the speed at which the mouse could no longer advance on the treadmill, was recorded. No difference was observed between WT and Tph1 KO mice after the first endurance training (week 0) but after 3 weeks of endurance training, WT mice Smax is higher of 20% (Figure 2C, 3 weeks, P = .0045). Moreover after 6 weeks of endurance training, Smax in Tph1 KO mice was 21% lower than in WT mice (sixth week: WT, 0.56 ± 0.12; Tph1 KO, 0.44 ± 0.07, P = .0191) (Figure 2C). This difference, even if no significant, persisted throughout the period of endurance training (10th week: WT, 0.62 ± 0.07, Tph1 KO: 0.48 ± 0.09, P = .0545). Respiratory exchange ratio (RER) was recorded and analyzed at the peak of O2 consumption (VO2peak) of mice during endurance training. Calorimetric parameters were measured during the last session of 10 weeks of training. RER at VO2peak is decreased in Tph1 KO mice compared to WT (Figure 2D, P = .0499) but their consumption of O2 (VO2peak) is increased compared to WT (Figure 2E, P = .0104). Moreover, the consumption of O2 relative to the lean mass (VO2peak/lean mass) was increased in Tph1 KO mice after 10 weeks of training (Figure 2E, P = .0002). Yet, throughout the training period, body weights and muscle mass were similar for both WT and Tph1 KO mice (Figure 2G–I). Given these findings, we argue that reduced levels of peripheral 5-HT in Tph1 KO mice might be responsible for their lesser response to endurance training.
Figure 2.
Reduced physical performance in 5-HT-deficient mice undergoing endurance training: (A) experimental protocol: mice ran 3 times a week for 10 weeks, (B) incremental treadmill protocol for each session, (C) maximum speed (m s−1) reached by mice at exhaustion, (D) RER at VO2peak reached by mice at the last training session after 10 weeks of training, (E) VO2peak (L min−1) reached by mice at the last training session after 10 weeks of training, (F) VO2peak/lean mass (L min−1 g) reached by mice at the last training session after 10 weeks of training, (G) weight, (H) lean mass, and (I) fat mass of mice at different time points during training. Body composition was measured by nuclear magnetic resonance.
Wild-type male (WT_M), n = 4; wild-type female (WT_F), n = 4; peripheral 5-HT–deficient male (Tph1 KO_M), n = 4; peripheral 5-HT–deficient female (Tph1 KO_F), n = 4. Mean ± SD. Variance homogeneity with Levene test, Mann-Whitney tests were performed as appropriate after interactions were observed with 2-way ANOVA: ***P < .0001.
Inadequate response to endurance training in 5-HT–deficient mice
We also considered TRP metabolism in the plasma of WT and Tph1 KO mice before (steady state) and after endurance training. Our data revealed a significant increase in TRP for Tph1 KO mice after training despite lower levels of circulating TRP in the Tph1 KO mice before training (Figure 3A, P = .0152). However, circulating Kynurenine concentration before and after endurance training did not change (Figure 3B). Endurance training increased plasma 3-HK and KYNA levels in both WT and Tph1 KO mice, whereas no change in anthranilic acid concentration was observed (Figures 1A and 3C: WT P = .0093 and Tph1 KO P = .0411; Figure 3D: WT P = .046 and Tph1 KO P = .0022; Figure 3E). No difference in IDO activity was noted (Figure 3F). In Tph1 KO mice, circulating 5-HT was lower before training (Figure 3G, P = .0043) but increased after endurance training until to have a same plasma 5-HT concentration than WT (Figure 3G, P = .026). In contrast, metabolization of TRP into 5-HT was impaired in Tph1 KO mice. Figure 3I shows that, after endurance training, the ratio of 5-HIAA to 5-HT had increased by 624.7% in WT mice (Figure 3I, P = .0022), yet no variation of this ratio was seen in Tph1 KO mice. This may be explained by the fact that 5-HIAA levels only increased slightly in Tph1 KO mice, relative to WT mice (WT: 282.8 ± 50.9; Tph1 KO: 142 ± 81; P = .0012) (Figure 3H).
Figure 3.
Inadequate response to endurance training in Tph1 KO mice. Metabolite concentrations in plasma from wild-type (WT) and peripheral 5-HT–deficient (Tph1 KO) mice were measured by mass spectrometry before and after training, following the last endurance exercise session: (A) tryptophan, (B) kynurenine, (C) 3-hydroxykynurenine (3-HK), (D) kynurenic acid, (E) anthranilic acid concentrations in plasma, (F) indoleamine 2,3-dioxygenase (IDO) activity (ratio of kynurenine to tryptophan, expressed as percentage), (G) serotonin (5-HT) and (H) 5-hydroxyindoleacetic acid (5-HIAA) concentrations in plasma, and (I) ratio of 5-HIAA to 5-HT, expressed as percentage.
WT, n = 8; Tph1 KO, n = 8. Mean ± SD. Variance homogeneity with Levene test, Mann-Whitney tests were performed appropriate after interactions were observed in repeated measure 2-way ANOVA. The following significance levels were used: *P < .01. **P < .001.
Overall, the data show that, in 5-HT–deficient mice, failure to respond is not associated with decreased TRP levels or insufficient production of KYN metabolites. Rather, the ratio of 5-HIAA to 5-HT suggests that, even though 5-HT is present in the plasma of Tph1 KO mice, it is not metabolized. No decrease in the expression of the Type A or B monoamine oxidase (MAOA or MAOB), which catabolizes monoamine transmitters including 5-HT, further supports this hypothesis (data not shown).
Peripheral but not brain 5-HT necessary for adaptation to endurance exercise session
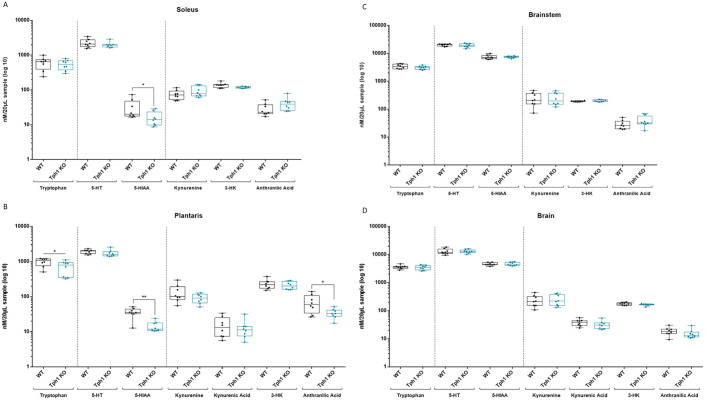
The findings presented above imply that a decrease in both peripheral 5-HT levels and serotonergic activity was responsible for the lesser response to endurance training. We next investigated TRP metabolism in trained mice after 1 endurance exercise session, to mimic the conditions of trained runners after a long-distance trail race. It has been suggested that exercise triggers the release of muscle-derived factors that pass through the blood–brain barrier and influence brain function, constituting a sort of muscle–brain communication. Previous studies have proposed that exercise may have a neuroprotective effect, shifting TRP metabolism away from the production of neurotoxic KYN, which crosses the blood–brain barrier, to the production of nontoxic KYNA. To test this hypothesis, levels of TRP and its metabolites on the 5-HT and KYN pathway branches (Figure 1A) were determined by mass spectrometry. Specifically, 5-HT, 5-HIAA, KYN, 3-HK, KYNA, and anthranilic acid concentrations were measured in the brain, brainstem, and soleus and plantaris muscles. In both muscles, 5-HIAA levels were significantly lower in Tph1 KO mice (5-HIAA in soleus: WT: 31.5 ± 20.9; Tph1 KO: 16 ± 7.6; P = .0207 and 5-HIAA in plantaris: WT: 35.7 ± 11.33; Tph1 KO: 14.5 ± 4.9, P = .0011) while 5-HT levels were identical for Tph1 KO and WT mice (Figure 4A and B). However, anthranilic acid is specifically decreased in plantaris Tph1 KO compared to WT mice (Figure 4B, P = .0499). No variation in TRP metabolism along the KYN or 5-HT branches was observed in any part of the brain or brainstem (Figure 4C and D).
Figure 4.
Peripheral but not brain 5-HT necessary for adaptation to endurance exercise. Metabolite concentrations in the (A) soleus, (B) plantaris muscles, (C) brainstem, and (D) brain of wild-type (WT) and peripheral 5-HT–deficient (Tph1 KO) mice, after 10 weeks of endurance training, as determined by mass spectrometry.
3-HK, 3-hydroxykynurenine; 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine (serotonin).
WT, n = 8; Tph1 KO, n = 8. Mean ± SD. Variance homogeneity with Levene test, Mann-Whitney tests were performed: *P < .01. **P < .001.
No communication between muscle and brain seems necessary for adaptation to endurance exercise session
The previous data prompted us to analyze IDO activity and 5-HT turnover (5-HIAA/5-HT, expressed as percentage), since it has been postulated that exercise prevents depression by increasing expression of skeletal muscle KAT, which in turn shifts KYN metabolism away from the production of neurotoxic KYN and toward that of neuroprotective KYNA (Figure 1A). As shown in Figure 5, there was no observed variation in IDO activity within the brain or muscle, in either WT or Tph1 KO mice, and TRP levels were normal. Moreover, no variation was detected in the ratios of KYNA to KYN and 3-HK to KYN, indicating no variation in KAT activity (Figure 5A and B). While the 5-HIAA–to–5-HT ratio did decrease significantly in the plantaris and soleus muscles of Tph1 KO mice (Soleus: WT: 1.9 ± 0.74; Tph1 KO: 0.9 ± 0.43; P = .0398 and Plantaris: WT 1.3 ± 0.53, Tph1 KO: 0.9 ± 0.43, P = .0095; Figure 5C and D), there was no such variation in the brain or brainstem (Figure 5E and F). These and the previous findings argue against the existence of a muscle–brain endocrine loop; they instead suggest that physical exercise induces a beneficial shift, within the soleus and plantaris muscles, toward the use of peripheral 5-HT for adaptation to endurance training.
Figure 5.
No communication between muscle and brain necessary for adaptation to endurance exercise. Indoleamine 2,3-dioxygenase (IDO), kynurenine aminotransferase (KAT), and kynurenine 3-monooxygenase (KMO) activity and serotonin (5-HT) turnover in different tissues were evaluated after 10 weeks of training: (A) KAT and KMO activity in plantaris muscle, (B) KAT and KMO activity in brain. IDO activity and 5-HT turnover in (C) plantaris muscle, (D) soleus muscle, (E) brainstem, and (F) brain.
Degrees of IDO (KYN/TRP), KAT (KYNA/KYN), and KMO (3-HK/KYN) activity and of 5-HT turnover (5-HIAA/5-HT) are given as percentages. Wild-type (WT), n = 8; peripheral 5-HT–deficient (Tph1 KO), n = 8. Mean ± SD. Variance homogeneity with Levene test, Mann-Whitney tests were performed: *P < .01. **P < .001.
Discussion
Our data showed that no communication between muscle and brain were needed for adaptation to endurance training. Rather, conversion of TRP into peripheral but not brain 5-HT appears to be the rate-limiting step for the adaptation of muscle to endurance training. There is growing evidence that skeletal muscle secretes factors involved in the beneficial effects of exercise on brain health.15,16 It has been hypothesized that TRP metabolites play a role in such a muscle–brain endocrine loop. We confirmed the importance of TRP metabolism in adaptation to endurance training that has been described in the literature. Previous reports have shown that exercise stimulates the expression of KAT in skeletal muscle, which converts neurotoxic KYN in the blood to neuroprotective KYNA, and reduces depression-like symptoms.12 Also, in accordance with published findings, we demonstrated that plasma concentrations of KYN in WT animals significantly increased in response to exercise training. Enhanced peripheral KYN-to-KYNA conversion may prevent accumulation of KYN in the brain of WT mice. However, these changes were not correlated with increased KAT activity in the muscle. Furthermore, despite the change in plasma KYN concentrations, no variations along the KYN metabolic branch were observed in the brain and brainstem of WT and Tph1 KO mice.
Our findings provide evidence that, although exercise is sensed by the brain—suggesting that muscle-derived peripheral factors permit direct muscle–brain communication—peripheral factors alone may account for reduced physical performance. For instance, the trained long-distance runners relied on peripheral 5-HT turnover, rather than IDO activity, during endurance exercise. We also observed lesser adaptation to endurance exercise in trained Tph1 KO mice despite normal levels of 5-HT in the brain. Normal degrees of kynurenine 3-monoxygenase and KAT activity in skeletal muscles of Tph1 KO mice indicate that high levels of KYN metabolites did not impact performance. The KYN-to-TRP ratio, an estimator of IDO activity, was similar in WT and Tph1 KO, implying no difference in IDO breakdown of TRP. Moreover, mass spectrometric analysis revealed no influence of muscle-induced factors, as brain 5-HT consumption and IDO activity were not altered. After 10 weeks of endurance training, the performance of Tph1 KO mice with normal brain levels of 5-HT dropped significantly, due to impaired 5-HT turnover in skeletal muscle. Moreover, the trained long-distance runners relied on peripheral 5-HT turnover, rather than IDO activity, during endurance exercise. Also, the result showing that the RER is decreased in Tph1 KO mice whereas the values for the VO2peak and VO2peak/lean mass were significantly increased may underly a deficit in ATP synthesis. Precisely, the Tph1 KO animals appear to consume more oxygen, still they failed to adapt to endurance training associated with a defect of glucose utilization as energetic substrate. In light of the above, because skeletal muscle is an endocrine organ with autocrine-paracrine functions, we may hypothesize the existence of a muscular microserotonergic system responsive to endurance training. Yet although the results imply a role played by 5-HT in the muscle for adaptation to endurance training, we cannot rule out that the lack of adaptation might be related to the fact that Tph1 KO mice displayed a decrease of cardiac contractility.17 In parallel, data from work by Suidan et al,18 suggested that 5-HT synthesized by Tph1 may have some impact on the nervous system. The authors showed that Tph1 KO animals displayed altered gait dynamics and deficits in rearing behavior as compared to WT. In rodents, rearing behaviors provide a measure of anxiety and the authors proposed that 5-HT deficient mice may have a deficit in motivated locomotion. Accordingly, a potential limitation of our study relates to the use of murine models: unlike trained human athletes, performance is not an important parameter for their well-being. Humans may transcend pain by exercising their will, but it is not known how the same behavior may be elicited in mice. Concerning the gait aspect, interestingly in human, alterations in the fractal properties of gait dynamics have been associated with disease of the central nervous system (eg, Huntington’s disease).19 Overall, whether peripheral 5-HT or other factors are part of a muscle–brain endocrine loop, influence adaptation to physical exercise and can be considered as a treatment for depression will require more studies. Nevertheless, the results presented provide evidence that further attention should be given to peripheral factors potentially having direct or indirect effects on brain function. Such factors could be novel therapeutic targets for neurodegenerative diseases and cognitive enhancers for people of all ages.
Materials and Methods
Study design and participants
TRP metabolite measurements are a secondary study based on Jouffroy et al.20 All human participants gave their full consent as previously described.20 Blood samples were collected at 3 or 4 selected time points during the race: start, checkpoint 1, checkpoint 2, and end. The runners each participated in 1 of 2 long-distance trail races held on Réunion in 2017: La Diagonale des Fous and Trail du Bourbon. Runners A and B participated in La Diagonale des Fous (from Saint-Pierre to Saint-Denis; distance: 166 km; cumulative elevation gain: +9611 m). Checkpoint 1 was at Cilaos Stadium (at 65.3 km); and Checkpoint 2, at Possession Stadium (at 143.4 km). Runners C and D participated in the Trail du Bourbon (from Cilaos to Saint-Denis; distance: 112 km; cumulative elevation gain: +6468 m). There was only 1 checkpoint, at Possession Stadium (at 89 km).
Animals
The study was approved by the Paris Descartes Animal Experimentation Committee for the Performance and Metabolism in Mice (PMM) research facility, as project no. 2017051916196640. The care and treatment of animals followed the guidelines of the French Ministry of Higher Education and Research for the detention, use, and ethical treatment of laboratory animals. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the French Ministry of Agriculture.
In all, 16 mice (C57BL/6) aged 9 weeks were used: 8 Tph1 KO (4 males, 4 females) and 8 WT (4 males, 4 females). They received food and water ad libitum and exposed to a cycle of 12 hours of light and 12 hours of darkness at a temperature of 22°C ± 5°C. All experiments were performed at the PMM research facility.
Weight and body composition
Weight and body composition (including lean mass and fat) were measured before each training session using a nuclear magnetic resonance system (LF50, Bruker, Germany).
Endurance training protocol and evaluation of physical performance
All mice underwent treadmill endurance training 3×/weeks. The treadmill was equipped with an indirect calorimetry system (Phenomaster, TSE, Germany). To evaluate performance, a treadmill incremental test was used: speed was increased by 0.01 m·s−1 every 15 seconds. We recorded the maximum speed (Smax) reached by each mouse before exhaustion, that is, inability to advance any further despite 1.2-mA shocks (duration: 1 second; delay: 1 second) received. At the start of each session, mice warmed up for 5 minutes on the treadmill at 0.01 m·s−1 and an additional 5 minutes at 0.05 m·s−1.21
TRP metabolism measurement
TRP and its metabolites were extracted from tissue with a buffer made of methanol, acetonitrile, and water, kept at −20°C. After addition of extraction buffer (4 µL per mg of tissue), samples were processed with a tissue lyser on ice, with 3 grinding cycles, and centrifuged at 12 000 rpm at 4°C for 10 minutes. Then, 25 µL of lysate were placed in a 300-µL vial and kept at −80°C until analysis.
Internal standard tryptophan-d5 (Cambridge Isotope Laboratories, Inc., MA, USA) was added to samples to reach a final 1 µM concentration. Determination of TRP metabolite concentrations was performed by liquid chromatography–tandem mass spectrometry, in accordance with previously published methods.22,23 A 5-µL sample was injected into an UPLC BEH C18 column (Acquity, Waters, MA, USA; 1.7-µm particle size, 2.1 mm × 100 mm) connected to a UPLC system (Acquity H-Class, Waters) that was interfaced with a triple quadrupole mass spectrometer (Xevo TQ-S, Waters), both controlled by MassLynx software (Waters). Aqueous mobile phase A was water, and organic mobile phase B was acetonitrile, both with 0.1% formic acid. A programmed mobile phase gradient was used during 12-minutes runs: 0 minute, 100% A; 6 minutes, 44% B; 6.5 minutes, 98% B; 9 minutes, 98% B; 9.5 minutes, 100% A; 12 minutes, 100% A. Metabolites were detected and identified in the electrospray positive ion mode, with the multiple reaction monitoring mode activated. The most intense multiple reaction monitoring transitions used for integration and quantification are summarized in Table 2. Quantification was performed using TargetLynx software (Waters).
Table 2.
Multiple reaction monitoring mode.
| Compound name | Cone voltage (V) | Collision energy (eV) | Quantification trace |
|---|---|---|---|
| 3-Hydroxykynurenin (3-HK) | 36 | 16 | 225 > 110 |
| Serotonin (5-HT) | 60 | 20 | 177 > 160 |
| Kynurenine (KYN) | 20 | 20 | 209 > 146 |
| Tryptophan (TRP) | 60 | 16 | 205 > 145 |
| Anthranilic acid | 22 | 15 | 138 > 120 |
| Kynurenic acid (KYNA) | 16 | 28 | 190 > 116 |
| 5-Hydroxyindoleacetic acid (5-HIAA) | 26 | 20 | 192 > 146 |
| Tryptophan-d5 | 28 | 16 | 210 > 150 |
Throughout the manuscript, to make it easier to read, only IDO activity and not TDO is presented. However, the plasma values reflect both IDO and TDO activities (respectively extrahepatic and hepatic enzymes) unlike for the remaining tissues where values only for the IDO enzyme are presented.
Statistical analysis
All statistical analyses were carried out with Prism 6 (GraphPad Software, CA, USA). We assessed variance homogeneity with Levene test, Mann-Whitney tests were performed for tissue measurements as appropriate after interactions were observed with 2-way ANOVA for performance, and body composition, and repeated measure 2-way ANOVA for plasma measurement with Sidák test. The following significance levels were used: *, P < .01; **, P < .001; ***, P < .0001. Data were presented as mean ± SD.
Acknowledgments
The authors thank the LEAT Broussais Animal Facility and technicians for care and breeding of the mice. Performance and metabolic analyses were conducted at the PMM research facility. The authors would also like to thank Jason Miller for editing of the manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was also supported by labex GR-Ex. The labex GR-Ex, reference ANR-11-LABX-0051 is funded by the program “Investissements d’avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MF and PN are responsible for conceptualization and methodology (design of experiments); RJ, MF, ACB, MD, HD, and SS for investigation (execution of experiments); MF, ACB, SS, RB, XC, JFT, OH, PN, and FC for formal analysis of the data; and MF, PN, and FC for writing the paper.
ORCID iDs: Sylvia Sanquer  https://orcid.org/0000-0002-0347-6501
https://orcid.org/0000-0002-0347-6501
Francine Côté  https://orcid.org/0000-0002-6496-7566
https://orcid.org/0000-0002-6496-7566
References
- 1. Walther DJ, Peter J, Bashammakh S. Synthesis of serotonin by a second. Science. 2003;299:76. [DOI] [PubMed] [Google Scholar]
- 2. Côté F, Thévenot E, Fligny C, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525-13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357:369:eaaf9794. [DOI] [PubMed] [Google Scholar]
- 4. Platten M, Wick W, Van Den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435-5440. [DOI] [PubMed] [Google Scholar]
- 5. Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan – kynurenine metabolism. Ann N Y Acad Sci. 2010;1199:1-14. [DOI] [PubMed] [Google Scholar]
- 6. Georgin-Lavialle S, Gaillard R, Moura D, Hermine O. Mastocytosis in adulthood and neuropsychiatric disorders. Transl Res. 2016;174:77-85. [DOI] [PubMed] [Google Scholar]
- 7. Sibon D, Coman T, Rossignol J, Fontenay M, Hermine O, Francine C, et al. Enhanced renewal of erythroid progenitors in myelodysplastic anemia by peripheral serotonin. Cell Rep. 2019;26:3246-3256. [DOI] [PubMed] [Google Scholar]
- 8. Soares J, Naffah-Mazzacoratti MG, Cavalheiro EA. Increased serotonin levels in physically trained men. Braz J Med Biol Res. 1994;27:1635-1638. [PubMed] [Google Scholar]
- 9. Wegner M, Helmich I, Machado S, Nardi A, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta-analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13:1002-1014. [DOI] [PubMed] [Google Scholar]
- 10. Schlittler M, Goiny M, Agudelo LZ, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310:C836-C840. [DOI] [PubMed] [Google Scholar]
- 11. Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agudelo LZ, Femenía T, Orhan F, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33-45. [DOI] [PubMed] [Google Scholar]
- 13. Barton DA, Esler MD, Dawood T, et al. Elevated brain serotonin turnover in patients with depression. Arch Gen Psychiatry. 2008;65:38. [DOI] [PubMed] [Google Scholar]
- 14. Calapai G, Corica F, Corsonello A, et al. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464-472. [DOI] [PubMed] [Google Scholar]
- 16. Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fligny C, Fromes Y, Bonnin P, et al. Maternal serotonin influences cardiac function in adult offspring. FASEB J. 2008;22:2340-2349. [DOI] [PubMed] [Google Scholar]
- 18. Suidan GL, Duerschmied D, Dillon GM, et al. Lack of tryptophan hydroxylase-1 in mice results in gait abnormalities. PLoS One. 2013;8:e59032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hausdorff JM, Mitchell SL, Firtion R, et al. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol. 1997;82:262-269. [DOI] [PubMed] [Google Scholar]
- 20. Jouffroy R, Alves B, Mauvieux B, Mallet L, Beaudeux J-L, Cottart C-H. NSE & S100B protein blood level assessment during a long-distance trail race. Ann Biol Clin (Paris). 2019;77:532-536. [DOI] [PubMed] [Google Scholar]
- 21. Thomasson R, Vignier N, Peccate C, Mougenot N, Noirez P, Muchir A. Alteration of performance in a mouse model of Emery-Dreifuss muscular dystrophy caused by A-type lamins gene mutation. Hum Mol Genet. 2019;28:2237-2244. [DOI] [PubMed] [Google Scholar]
- 22. de Loor H, Poesen R, De Leger W, et al. A liquid chromatography – tandem mass spectrometry method to measure a selected panel of uremic retention solutes derived from endogenous and colonic microbial metabolism. Anal Chim Acta. 2016;936:149-156. [DOI] [PubMed] [Google Scholar]
- 23. Boulet L, Faure P, Flore P, Montérémal J, Ducros V. Simultaneous determination of tryptophan and 8 metabolites in human plasma by liquid chromatography/tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci [Internet]. 2017;1054:36-43. [DOI] [PubMed] [Google Scholar]