Abstract
Objective:
To evaluate the comparative efficacy and safety of Janus kinase (JAK) inhibitors and biological disease-modifying antirheumatic drugs (bDMARDs) in patients with rheumatoid arthritis (RA) and an inadequate response to at least one disease-modifying antirheumatic drug (DMARD).
Methods:
PubMed, Embase, Cochrane library and ClinicalTrials.gov were searched for relevant randomized controlled trials (RCTs) from inception to April 2020. The active drugs included three JAK inhibitors and eight bDMARDs while the control drugs included placebo or conventional synthetic disease-modifying antirheumatic drugs (csDMARDs). Outcomes include American College of Rheumatology 20% response (ACR20), Disease Activity Score in 28 joints (DAS28), Health Assessment Questionnaire–Disability Index (HAQ-DI) and discontinuations for adverse events (AEs). We estimated summary odds ratios (ORs) and weighted mean differences (WMDs) using network meta-analysis with random effects.
Results:
Eighty-eight RCTs with 31,566 patients were included. All JAK inhibitors and bDMARDs were more effective than placebo in ACR20 (ORs ranging between 3.05 and 5.61), DAS28 (WMDs ranging between −1.91 and −0.80) and HAQ-DI (WMDs ranging between −0.34 and −0.21). Tocilizumab, certolizumab pegol and upadacitinib showed relatively good efficacy in these three outcomes according to their relative ranking. Notably, tocilizumab was more effective than other active drugs in DAS28 (WMDs ranging between −1.11 and −0.49). Compared with the lower recommended doses, increasing the doses of JAK inhibitors (baricitinib 4 mg versus 2 mg, tofacitinib 10 mg versus 5 mg and upadacitinib 30 mg versus 15 mg) cannot provide significant additional benefits. In terms of discontinuations for AEs, all active drugs showed no significant difference compared with placebo except certolizumab pegol [OR 1.65, 95% credible interval (CrI) 1.06–2.61] and rituximab (3.17, 1.11–10.80).
Conclusions:
Tocilizumab, certolizumab pegol and upadacitinib may have relatively good efficacy in patients with RA after treatment failure with csDMARDs. RA patients taking a JAK inhibitor may have a preference for a lower recommended dose.
Keywords: biological disease-modifying antirheumatic drugs, Janus kinase inhibitors, network meta-analysis, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by damage to cartilage and bone. Early diagnosis and treatment are key to a good prognosis, especially in patients with high disease activity.1
Current guidelines recommend methotrexate (MTX) as the first treatment strategy for RA. For patients who cannot tolerate MTX, other conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) such as leflunomide and sulfasalazine can be considered as part of the treatment strategy.2,3 Janus kinase (JAK) inhibitors and biological disease-modifying antirheumatic drugs (bDMARDs) are currently considered as second-line treatments in RA. For patients with active RA after treatment failure with csDMARDs, the current recommended treatment strategy is to increase the use of a JAK inhibitor or bDMARD.2,3
In recent years, many randomized controlled trials (RCTs) have demonstrated significant efficacy of JAK inhibitors including baricitinib,4–6 tofacitinib7,8 and upadacitinib9,10 in RA. In addition, some studies have shown that baricitinib 4 mg once daily and upadacitinib 15 mg once daily seem to be associated with significant clinical improvement compared with adalimumab in patients with RA after treatment failure with MTX.11,12 These results are exciting but it is not clear whether baricitinib or upadacitinib is superior to other bDMARDs.
There are many types of JAK inhibitors and bDMARDs for the treatment of RA. However, there are still about 20–30% of patients with RA who are refractory to multiple disease-modifying antirheumatic drugs (DMARDs).2,13 The current guidelines do not indicate which active drug is more suitable for patients with RA after treatment failure with csDMARDs,2,3 and clinicians need more evidence to make the best choice for each patient, especially those with RA who have failed multiple DMARDs. Network meta-analysis can combine direct and indirect comparisons to evaluate the relative efficacy of multiple interventions.14 Therefore, we conducted this network meta-analysis to investigate the comparative efficacy and safety of JAK inhibitors and bDMARDs in patients with active RA and an inadequate response (IR) to at least one DMARD.
Methods
This study is registered with PROSPERO (number CRD42020160485) and INPLASY (number INPLASY202030017, doi: 10.37766/inplasy2020.3.0017).
Data sources and searches
We searched PubMed, Embase and Cochrane Central Register of Controlled Trials from inception to April 2020. The full PubMed search strategy is shown in Supplemental Appendix 1. Conference abstracts and ClinicalTrials.gov were also reviewed for possible unpublished trials. In addition, we searched references to the retrieved articles and contacted the drug manufacturers for further relevant publications.
Interventions
The active drugs we studied were three JAK inhibitors including baricitinib, tofacitinib, upadacitinib and eight bDMARDs including adalimumab, etanercept, infliximab, golimumab, certolizumab pegol, tocilizumab, rituximab and abatacept. Control drugs included placebo or csDMARDs. We did not include filgotinib because this latest JAK inhibitor has recently been approved in some countries and is not yet widely used globally.15 We investigated the efficacy and safety of different doses of JAK inhibitors (i.e. baricitinib 2 mg and 4 mg once daily, tofacitinib 5 mg and 10 mg twice daily, upadacitinib 15 mg and 30 mg once daily). For bDMARDs, we chose the current recommended doses (Supplemental Appendix 2). If the recommended dose of an active drug was not mentioned in an article, the dose closest to the recommended dose was selected on the premise of not exceeding the recommended dose.
Study selection
Studies meeting the following criteria were considered for inclusion: (1) Studies must be RCTs comparing the active drugs to the control drugs or another active drug for the treatment of RA. (2) The patients were adults diagnosed with RA16,17 and did not respond adequately to at least one DMARD. (3) The studies reported at least one outcome of interest including American College of Rheumatology 20% response (ACR20), Disease Activity Score in 28 joints (DAS28), Health Assessment Questionnaire–Disability Index (HAQ-DI) and discontinuations for adverse events (AEs). (4) Patients were allowed to use background therapy including at least one csDMARD. (5) Patients did not respond adequately to their background therapy. (6) No language restrictions were applied.
Our exclusion criteria included: (1) Studies investigated the efficacy and safety of active drug monotherapy without background therapy. (2) Studies focused on the efficacy and safety of bDMARDs in maintenance therapy. (3) Studies did not specify which bDMARD [i.e. tumor necrosis factor (TNF) inhibitors or non-TNF biologicals].
According to the current guidelines,2,3 the treatment strategies are different for csDMARD-naive patients and csDMARD-IR patients, and the recommended treatment strategy is a JAK inhibitor or bDMARD in combination with a csDMARD for csDMARD-IR patients. Moreover, some studies have also shown that combination therapy appears to be more effective than monotherapy,18–20 and the inclusion of monotherapy in our analysis may increase the risk of bias. Therefore, we excluded trials that focused on csDMARD-naive patients or active drug monotherapy, which increased the transitivity of our network meta-analysis and reduced the risk of bias. In addition, we excluded studies that focused on maintenance therapy because these patients had already received the first phase of treatment,21 while patients who failed the treatment may have dropped out of the study earlier.
Outcomes
Our efficacy outcomes included ACR20,22 DAS28 and HAQ-DI, while the safety outcome was discontinuations for AEs. ACR20 and discontinuations for AEs were dichotomous outcomes, while DAS28 and HAQ-DI were continuous outcomes. We used ACR20 to assess treatment response because it reflects overall patient improvement and is generally used as the primary outcome measure in studies.11,12 Treatment of RA should aim for sustained remission or low disease activity,2,3 so we investigated the improvement of these active drugs in DAS28. DAS28 is a measure of disease activity based on high-sensitivity C-reactive protein (DAS28-CRP) or erythrocyte sedimentation rate (DAS28-ESR). Total scores range from 0 to 9.4 and higher scores indicated more disease activity. We preferentially selected the data of DAS28-CRP when a study reported both DAS28-CRP and DAS28-ESR. Physical function is another important outcome of RA, so we used HAQ-DI to evaluate the improvement in function. HAQ-DI is a scale ranging from 0 to 3, with higher scores indicating greater disability. The discontinuation rate due to AEs is generally considered to be a reliable outcome for evaluating the safety of long-term treatment,23,24 so we used it to assess the safety of the drugs. To reduce the risk of bias, we selected the time point of follow-up closest to 12 weeks to assess efficacy and safety.
Data extraction and quality assessment
Four authors assessed all trials for eligibility and extracted the data independently. Differences of opinion were settled through discussion. We extracted information including first author, year of publication, interventions, background therapy, subjects, sample size, mean age, outcomes of interest and duration of follow-up. We selected the report with more complete data for duplicate studies. For outcomes that were not reported in detail, we further searched ClinicalTrials.gov for more complete data.
We assessed the quality of the included articles according to the Cochrane Handbook for Systematic Reviews of Interventions. Studies were considered as having a low risk of bias if two or fewer were rated as unclear risk and no high risk. Studies were considered as having a high risk of bias if two or more were rated as high risk. Other conditions were considered as having moderate risk of bias.
Data synthesis and analysis
Our network meta-analysis was based on a Bayesian framework using a random effects model.25 We estimated summary odds ratios (ORs) with 95% credible interval (CrI) for dichotomous outcomes and weighted mean differences (WMDs) with 95% CrI for continuous outcomes using network meta-analysis. We used Markov chain Monte Carlo (MCMC) algorithms to fit the model and used non-informative priors. The primary results were conducted using the gemtc package in R 3.6.1, which recalls JAGS in R for MCMC sampling.26,27 Each result was obtained by 50,000 sample iterations with 20,000 burn-in iterations on four parallel chains. Gelman–Rubin diagnostic and trace plots were used to ensure convergence of the results.26 The computer code for the gemtc package can be found in Supplemental Appendix 3. The surface under the cumulative ranking curve (SUCRA) was used to rank the interventions for each outcome. A treatment is certain to be the best when the SUCRA is 1 and the worst when the SUCRA is 0.28 The design-by-treatment test, loop-specific approach and node-splitting method were used to assess the consistency of the network meta-analysis.29,30 We used I2-statistic and τ² to assess heterogeneity.27,31 Comparison-adjusted funnel plots were used to detect potential publication bias.32 Evaluation of inconsistency and comparison-adjusted funnel plots were done using Stata 14.0.26,27
To explore the efficacy of these active drugs in csDMARD-IR and bDMARD-IR patients, we did a subgroup analysis by subjects. In the subgroup analysis of bDMARD-IR patients, the random effects model was too conservative to detect subtle actual differences in the case of few included studies,33 so we switched to a fixed effects model. To evaluate the robustness of our primary results, we did four sensitivity analyses by excluding trials with follow-up ⩾52 weeks, trials with sample size <50, open-label trials or unpublished trials.
Results
Study selection and characteristics of included studies
We identified 7456 records after duplicates were removed. Finally, 88 trials were included in the analysis (Figure 1). The references to the included trials as well as the PRISMA checklist can be found in the Supplemental Material. Figure 2 shows the evidence network of eligible comparisons for ACR20, and the others are shown in Supplemental Appendix 5.
Figure 1.
Flow diagram according to PRISMA guidelines.
PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis. RCT, randomized controlled trial.
Figure 2.

Network meta-analysis of eligible comparisons for ACR20.
Each circle represents a drug. The connected circles represent the two drugs that have been compared in studies. The width of the lines is proportional to the number of trials. The size of each circle is proportional to the number of randomly assigned patients.
ACR20, American College of Rheumatology 20% response; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs.
In general, 31,566 patients and 16 nodes were included in the analysis (Supplemental Appendix 6). The sample sizes ranged from 24 to 1629, the mean age ranged from 46 to 58 years, and the follow-up time point for evaluation ranged from 12 to 54 weeks. Sixty (68%) of 88 studies used MTX as background therapy, and the remaining 28 (32%) studies also allowed the use of other csDMARDs including leflunomide, sulfasalazine, hydroxychloroquine and so on. The subjects in 71 (81%) studies were csDMARD-IR, in 12 (14%) were bDMARD-IR and in five (6%) were csDMARD-IR or bDMARD-IR patients.
Quality assessment
Most of the studies were double-blind trials, eight (9%) were open-label and one (1%) was single-blind (Supplemental Appendix 7). Overall, 51 (58%) studies were rated as low risk of bias, 28 (32%) as moderate and nine (10%) as high.
Results of network meta-analysis
The forest plots of the four outcomes are shown in Figure 3. In addition, we provide complete results of all comparisons. Comparisons for ACR20 and discontinuations for AEs are shown in Figure 4, while comparisons for DAS28 and HAQ-DI are shown in Figure 5.
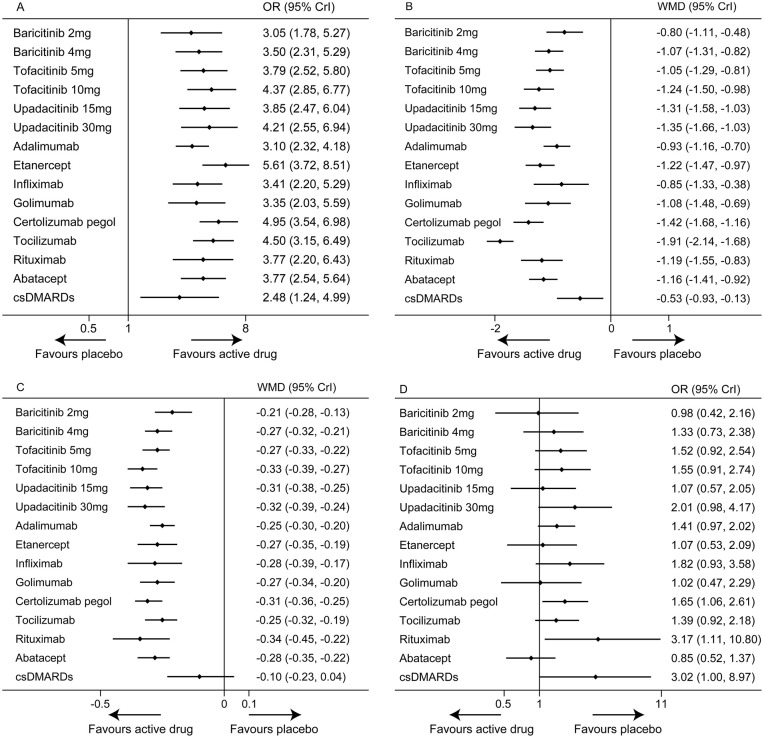
Figure 3.
Forest plots of network meta-analysis of all trials for efficacy and safety. A. ACR20. B. DAS28. C. HAQ-DI. D. Discontinuations for AEs. Drugs compared with placebo, which was the reference compound.
ACR20, American College of Rheumatology 20% response; AEs, adverse events; Crl, credible interval; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, Disease Activity Score in 28 joints; HAQ-DI, Health Assessment Questionnaire–Disability Index; OR, odds ratio; WMD, weighted mean difference.
Figure 4.
Comparisons for ACR20 and discontinuations for AEs in the network meta-analysis.
The numbers below the diagonal represent the ORs and 95% CrI of ACR20. ORs higher than 1 indicate that the drug in that column is better than the drug in that row (i.e. favor the upper treatment). The numbers above the diagonal represent the ORs and 95% CrI of discontinuations for AEs. ORs lower than 1 indicate that the drug in that row is better than the drug in that column (i.e. favor the upper treatment). Significant results are in bold and underscored.
ABT, abatacept; ACR20, American College of Rheumatology 20% response; ADA, adalimumab; AEs, adverse events; Bar, baricitinib; CrI, credible interval; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CZP, certolizumab pegol; ETN, etanercept; GOL, golimumab; IFX, infliximab; ORs, odds ratios; PBO, placebo; RTX, rituximab; TCZ, tocilizumab; Tof, tofacitinib; Upa, upadacitinib.
Figure 5.
Comparisons for DAS28 and HAQ-DI in the network meta-analysis.
The numbers below the diagonal represent the WMDs and 95% CrI of DAS28. WMDs less than 0 indicate that the drug in that column is better than the drug in that row (i.e. favor the upper treatment). The numbers above the diagonal represent the WMDs and 95% CrI of HAQ-DI. WMDs less than 0 indicate that the drug in that row is better than the drug in that column (i.e. favor the upper treatment). Significant results are in bold and underscored.
ABT, abatacept; ADA, adalimumab; Bar, baricitinib; CrI, credible interval; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; CZP, certolizumab pegol; DAS28, Disease Activity Score in 28 joints; ETN, etanercept; GOL, golimumab; HAQ-DI, Health Assessment Questionnaire–Disability Index; IFX, infliximab; PBO, placebo; RTX, rituximab; TCZ, tocilizumab; Tof, tofacitinib; Upa, upadacitinib; WMDs, weighted mean differences.
In terms of ACR20 (82 RCTs, 29,574 patients), all active drugs were significantly superior to placebo, with ORs ranging between 3.05 (95% CrI 1.78–5.27) for baricitinib 2 mg once daily and 5.61 (95% CrI 3.72–8.51) for etanercept. Etanercept was associated with a higher response rate than csDMARDs (OR 2.26, 95% CrI 1.26–4.04), while other active drugs showed no significant difference compared with csDMARDs. In the comparison of different active drugs, the response rate of adalimumab was lower than etanercept (OR 0.55, 95% CrI 0.33–0.92) and certolizumab pegol (OR 0.63, 95% CrI 0.41–0.94).
In terms of DAS28 (63 RCTs, 24,119 patients), all active drugs showed significant improvement over placebo, with WMDs ranging between −1.91 (95% CrI −2.14 to −1.68) for tocilizumab and −0.80 (95% CrI −1.11 to −0.48) for baricitinib 2 mg once daily. Except for baricitinib 2 mg once daily, adalimumab, infliximab and golimumab, all the other active drugs were significantly superior to csDMARDs (WMDs ranging between −1.37 and −0.52). Notably, tocilizumab was observed to be more effective than all of the other active drugs (WMDs ranging between −1.11 and −0.49).
Regarding HAQ-DI (72 RCTs, 25,687 patients), all active drugs showed significant improvement over placebo, with WMDs ranging between −0.34 (95% CrI −0.45 to −0.22) for rituximab and −0.21 (95% CrI −0.28 to −0.13) for baricitinib 2 mg once daily. All active drugs except baricitinib 2 mg once daily were significantly superior to csDMARDs (WMDs ranging between −0.24 and −0.15). In addition, tofacitinib 10 mg twice daily (WMD −0.13, 95% CrI −0.23 to −0.03), upadacitinib 15 mg once daily (WMD −0.11, 95% CrI −0.21 to −0.01) and certolizumab pegol (WMD −0.10, 95% CrI −0.20 to −0.01) were superior to baricitinib 2 mg once daily.
In terms of discontinuations for AEs (83 RCTs, 30,628 patients), all active drugs did not show significant difference compared with placebo except certolizumab pegol (OR 1.65, 95% CrI 1.06–2.61) and rituximab (OR 3.17, 95% CrI 1.11–10.80). Compared with csDMARDs, etanercept (OR 0.35, 95% CrI 0.15–0.82) and abatacept (OR 0.28, 95% CrI 0.09–0.92) were associated with lower discontinuation rates. Furthermore, upadacitinib 30 mg once daily (OR 2.36, 95% CrI 1.01–5.68), certolizumab pegol (OR 1.94, 95% CrI 1.02–3.80) and rituximab (OR 3.76, 95% CrI 1.18–13.85) were associated with higher discontinuation rates than abatacept.
Supplemental Appendix 8 shows the probability plots, cumulative probability plots and SUCRA. The relative ranking of treatments based on SUCRA is summarized in Table 1. We summarize the relative ranking of these treatments because it gives clinicians clearer reference information.
Table 1.
Relative ranking of different drugs assessed by SUCRA.
| Treatment | ACR20 |
DAS28 |
HAQ-DI |
Discontinuations for AEs |
||||
|---|---|---|---|---|---|---|---|---|
| SUCRA | Rank | SUCRA | Rank | SUCRA | Rank | SUCRA | Rank | |
| Baricitinib 2 mg | 0.325 | 13 | 0.195 | 14 | 0.195 | 14 | 0.743 | 3 |
| Baricitinib 4 mg | 0.445 | 10 | 0.446 | 10 | 0.461 | 11 | 0.532 | 7 |
| Tofacitinib 5 mg | 0.531 | 7 | 0.419 | 11 | 0.515 | 8 | 0.421 | 10 |
| Tofacitinib 10 mg | 0.686 | 4 | 0.667 | 5 | 0.855 | 1 | 0.404 | 11 |
| Upadacitinib 15 mg | 0.547 | 6 | 0.737 | 4 | 0.743 | 4 | 0.695 | 6 |
| Upadacitinib 30 mg | 0.640 | 5 | 0.772 | 3 | 0.753 | 3 | 0.242 | 14 |
| Adalimumab | 0.297 | 14 | 0.283 | 12 | 0.327 | 13 | 0.482 | 9 |
| Etanercept | 0.897 | 1 | 0.640 | 6 | 0.505 | 9 | 0.707 | 4 |
| Infliximab | 0.417 | 11 | 0.268 | 13 | 0.551 | 7 | 0.306 | 13 |
| Golimumab | 0.410 | 12 | 0.472 | 9 | 0.492 | 10 | 0.706 | 5 |
| Certolizumab pegol | 0.816 | 2 | 0.844 | 2 | 0.727 | 5 | 0.350 | 12 |
| Tocilizumab | 0.721 | 3 | 0.999 | 1 | 0.398 | 12 | 0.492 | 8 |
| Rituximab | 0.527 | 8 | 0.600 | 7 | 0.812 | 2 | 0.118 | 16 |
| Abatacept | 0.526 | 9 | 0.565 | 8 | 0.588 | 6 | 0.871 | 1 |
| csDMARDs | 0.213 | 15 | 0.094 | 15 | 0.075 | 15 | 0.133 | 15 |
| Placebo | 0.000 | 16 | 0.000 | 16 | 0.005 | 16 | 0.796 | 2 |
ACR20, American College of Rheumatology 20% response; AEs, adverse events; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DAS28, Disease Activity Score in 28 joints; HAQ-DI, Health Assessment Questionnaire–Disability Index; SUCRA, surface under the cumulative ranking curve.
Assessment of inconsistency, heterogeneity and publication bias
The evaluation of inconsistency is shown in Supplemental Appendix 9. The loop-specific approach showed that all loops were consistent for ACR20, DAS28, HAQ-DI, and only one of 15 loops was inconsistent in the analysis of discontinuations for AEs. The node-splitting method showed that no significant inconsistency was seen for all outcomes. The design-by-treatment test also found no significant inconsistency for all outcomes (p = 0.993 for ACR20, p = 0.999 for DAS28, p = 0.818 for HAQ-DI and p = 0.694 for discontinuations for AEs). The global I2 of ACR20, DAS28, HAQ-DI and discontinuations for AEs were 56.4%, 66.9%, 14.5% and 5.3%, respectively. In addition, the τ² of these outcomes were 0.144, 0.080, 0.002 and 0.163, respectively, suggesting low to moderate heterogeneity. The comparison-adjusted funnel plots for all outcomes were approximately symmetric and did not show a significant risk of publication bias (Supplemental Appendix 10).
Subgroup and sensitivity analyses
Our subgroup analysis showed the efficacy of these active drugs in different subjects (Supplemental Appendix 11.1). In csDMARD-IR patients, all active drugs were superior to placebo in ACR20 (ORs ranging between 3.11 and 5.27), DAS28 (WMDs ranging between −1.96 and −0.84), and HAQ-DI (WMDs ranging between −0.35 and −0.21). The addition of other csDMARDs was also superior to placebo in ACR20 (OR 2.22, 95% CrI 1.05–4.86), but had no significant difference in DAS28 (WMD −0.41, 95% CrI −0.85 to 0.03) and HAQ-DI (WMD −0.08, 95% CrI −0.22 to 0.06) compared with placebo. In bDMARD-IR patients, switching to JAK inhibitors or other bDMARDs was still superior to placebo in ACR20 (ORs ranging between 2.22 and 9.05), DAS28 (WMDs ranging between −1.79 and −0.68), and HAQ-DI (WMDs ranging between −0.37 and −0.18). However, most active drugs appeared to be less effective than those in csDMARD-IR patients except tocilizumab and rituximab. Furthermore, our subgroup analysis detailed the comparative efficacy of these active drugs in csDMARD-IR (Supplemental Appendix 11.2) and bDMARD-IR patients (Supplemental Appendix 11.3). In both csDMARD-IR and bDMARD-IR patients, the comparative efficacy of these active drugs was similar to our primary results, and tocilizumab still had an advantage over other active drugs in DAS28. The results of four sensitivity analyses did not change significantly from the primary results, which proved the robustness of our results (Supplemental Appendix 12).
Discussion
In general, our network meta-analysis, which included 88 RCTs and 31,566 patients, investigated the comparative efficacy and safety of JAK inhibitors and bDMARDs in patients with active RA after treatment failure with csDMARDs. Our results showed that all JAK inhibitors and bDMARDs were more effective than placebo in ACR20, DAS28 and HAQ-DI, while tocilizumab, certolizumab pegol and upadacitinib had relatively good efficacy in these three outcomes. Notably, tocilizumab was more effective than JAK inhibitors and other bDMARDs in DAS28. This means that tocilizumab is more likely to achieve remission or low disease activity in RA than other active drugs. Ma et al.18 found that the remission rate of tocilizumab in RA was higher than other treatments, and some studies also showed that monotherapy with interleukin-6 (IL-6) receptor inhibitors was superior to monotherapy with TNF inhibitors.34,35 These findings were consistent with our results. In addition, some studies have shown that tocilizumab has a strong effect of reducing acute reactants including CRP and ESR in patients with RA, which may explain why tocilizumab is superior to other active drugs in DAS28 rather than ACR20 or HAQ-DI.36,37
Concerning the efficacy of different recommended doses of JAK inhibitors, we found that there was no significant difference between baricitinib 2 mg and 4 mg once daily in ACR20, DAS28 and HAQ-DI (Figures 4 and 5), and the same situation was observed with different doses of tofacitinib (5 mg and 10 mg twice daily) and upadacitinib (15 mg and 30 mg once daily). This means that increasing the doses of JAK inhibitors cannot provide significant additional benefits in patients with RA compared with the lower recommended doses. In addition, higher doses of JAK inhibitors may be associated with a greater risk of AEs.38 Considering the safety and high cost of JAK inhibitors, RA patients taking a JAK inhibitor may have a preference for a lower recommended dose.
In terms of discontinuations for AEs, all active drugs showed great safety compared with placebo except certolizumab pegol and rituximab. Furthermore, abatacept was associated with better safety than upadacitinib 30 mg once daily, certolizumab pegol and rituximab. Our results on safety were also similar to those of previous network meta-analyses.23,24
We performed a detailed subgroup analysis to explore differences in the efficacy of these active drugs in different subjects. In csDMARD-IR patients, the results of subgroup analysis were similar to our primary results, and we also found that the addition of other csDMARDs (combination therapy with csDMARDs) was effective in ACR20, but had no significant difference in DAS28 and HAQ-DI compared with placebo. Moreover, the addition of other csDMARDs was associated with a relatively high discontinuation rate for AEs. Therefore, combination therapy with csDMARDs had no advantage over JAK inhibitors or bDMARDs in csDMARD-IR patients. This finding is largely consistent with current guidelines.2,3 A previous abridged Cochrane network meta-analysis found that triple therapy with csDMARDs (MTX plus sulfasalazine plus hydroxychloroquine) was not significantly different from any bDMARD or tofacitinib plus MTX in ACR50 for MTX-naive or MTX-IR patients.39 The reason for this discrepancy may be that more studies we included focused on the combined use of two csDMARDs (MTX plus leflunomide or sulfasalazine or hydroxychloroquine), and only one investigated the efficacy and safety of triple therapy.40 Therefore, the non-inferiority of triple therapy needs to be demonstrated in more RCTs.
With regard to bDMARD-IR patients, the subgroup analysis showed that switching to JAK inhibitors or other bDMARDs was still more effective than placebo in ACR20, DAS28, and HAQ-DI, and these results were consistent with the previous network meta-analyses.33,41 However, most active drugs appeared to be less effective than those in csDMARD-IR patients except tocilizumab and rituximab.
According to current recommendations, another TNF inhibitor can be considered for use in patients with RA if one TNF inhibitor therapy has failed.2,3 Two studies we included showed that golimumab and certolizumab pegol were indeed effective in patients with RA who had failed treatment with other TNF inhibitors.42,43 However, previous studies showed that non-TNF biologicals were more effective than other TNF inhibitors in TNF-IR patients,44,45 which was consistent with our results.
In recent years, some network meta-analyses have investigated the efficacy and safety of tofacitinib and bDMARDs in the treatment of RA,18,23,24,39 but few have found significant differences in the efficacy of these active drugs. Compared with previous network meta-analyses, this study has some strengths. Firstly, we carried out a comprehensive search of the current databases and included several unpublished trials, which enabled us to conduct a more complete analysis of the available evidence. Secondly, we excluded trials that focused on csDMARD-naive patients or active drug monotherapy, which was consistent with the clinical practice recommended by current guidelines.2,3 Finally, we performed several sensitivity analyses to demonstrate the robustness of our results.
Our study had some limitations. Firstly, both csDMARD-IR and bDMARD-IR patients were included in the analysis, which allowed us to include more evidence but may also increase the risk of bias. However, we performed a detailed subgroup analysis to demonstrate that our conclusions were applicable to both csDMARD-IR and bDMARD-IR patients. Secondly, most of the evidence comes from placebo controlled RCTs and head-to-head comparisons are still lacking. Future RCTs should focus more on head-to-head comparisons between active drugs. Thirdly, we did not include other outcomes including ACR50 and ACR70 due to the limitation of article length. Fourthly, we chose the recommended doses for almost all active drugs to reduce the risk of bias, but non-recommended doses may also be common in clinical practice. Finally, we did not take into account the availability of these active drugs. For example, baricitinib and tofacitinib appear earlier and may be more commonly used than upadacitinib. Clinicians also need to consider specific clinical circumstances to make the best choice.
In conclusion, all JAK inhibitors and bDMARDs were more effective than placebo in ACR20, DAS28 and HAQ-DI in patients with RA after treatment failure with csDMARDs, while tocilizumab, certolizumab pegol and upadacitinib had relatively good efficacy in these three outcomes. Notably, tocilizumab was more effective than other active drugs in DAS28. RA patients taking a JAK inhibitor may have a preference for a lower recommended dose. In terms of discontinuations for AEs, all active drugs showed great safety compared with placebo except certolizumab pegol and rituximab. In addition, combination therapy with csDMARDs had no advantage over JAK inhibitors or bDMARDs in csDMARD-IR patients, and switching to JAK inhibitors or other bDMARDs was still effective in bDMARD-IR patients. Clinicians should consider the results of this study and take into account the cost-effectiveness of these drugs to make the best choice for each patient with RA.
Supplemental Material
Supplemental material, sj-pdf-1-tab-10.1177_1759720X21999564 for Comparative efficacy and safety of Janus kinase inhibitors and biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and network meta-analysis by Chenghua Weng, Leixi Xue, Qing Wang, Wentian Lu, Jiajun Xu and Zhichun Liu in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Authors’ contributions: CW and ZL conceived and designed the study. CW, LX, QW and WL searched the articles and extracted data. CW and JX conducted the risk of bias assessment. CW and LX analyzed the data. CW wrote the manuscript. ZL directed the writing and revised the manuscript. All authors read and approved the final version of the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Jiangsu Provincial Key R&D Program (Social Development) (BE2019663), Suzhou Science and Technology Planning Project (sys2018059) and Suzhou Health and Key Talent Project (GSWS2019011).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethical approval: Not applicable.
ORCID iD: Chenghua Weng  https://orcid.org/0000-0001-7253-7745
https://orcid.org/0000-0001-7253-7745
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chenghua Weng, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Leixi Xue, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Qing Wang, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Wentian Lu, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Jiajun Xu, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Suzhou, China.
Zhichun Liu, Department of Rheumatology and Immunology, The Second Affiliated Hospital of Soochow University, Sanxiang Road No.1055, Suzhou, Jiangsu, 215000, P.R. China.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016; 388: 2023–2038. [DOI] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 3. Lau CS, Chia F, Dans L, et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int J Rheum Dis 2019; 22: 357–375. [DOI] [PubMed] [Google Scholar]
- 4. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016; 374: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 5. Keystone EC, Taylor PC, Drescher E, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis 2015; 74: 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dougados M, van der Heijde D, Chen YC, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017; 76: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleischmann R, Mysler E, Hall S, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 2017; 390: 457–468. [DOI] [PubMed] [Google Scholar]
- 8. van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012; 367: 508–519. [DOI] [PubMed] [Google Scholar]
- 9. Burmester GR, Kremer JM, Van den Bosch F, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391: 2503–2512. [DOI] [PubMed] [Google Scholar]
- 10. Genovese MC, Fleischmann R, Combe B, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018; 391: 2513–2524. [DOI] [PubMed] [Google Scholar]
- 11. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017; 376: 652–662. [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019; 71: 1788–1800. [DOI] [PubMed] [Google Scholar]
- 13. Becede M, Alasti F, Gessl I, et al. Risk profiling for a refractory course of rheumatoid arthritis. Semin Arthritis Rheum 2019; 49: 211–217. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet 2015; 386: 628–630. [DOI] [PubMed] [Google Scholar]
- 15. Dhillon S, Keam SJ. Filgotinib: first approval. Drugs 2020; 80: 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 17. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 2010; 69: 1580–1588. [DOI] [PubMed] [Google Scholar]
- 18. Ma KX, Li L, Liu CH, et al. Efficacy and safety of various anti-rheumatic treatments for patients with rheumatoid arthritis: a network meta-analysis. Arch Med Sci 2019; 15: 33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buckley F, Finckh A, Huizinga TW, et al. Comparative efficacy of novel DMARDs as monotherapy and in combination with methotrexate in rheumatoid arthritis patients with inadequate response to conventional DMARDs: a network meta-analysis. J Manag Care Spec Pharm 2015; 21: 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donahue KE, Schulman ER, Gartlehner G, et al. Comparative effectiveness of combining MTX with biologic drug therapy versus either MTX or biologics alone for early rheumatoid arthritis in adults: a systematic review and network meta-analysis. J Gen Intern Med 2019; 34: 2232–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet 2013; 381: 918–929. [DOI] [PubMed] [Google Scholar]
- 22. Felson DT, Anderson JJ, Boers M, et al. American college of rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38: 727–735. [DOI] [PubMed] [Google Scholar]
- 23. Park SK, Lee MY, Jang EJ, et al. A comparison of discontinuation rates of tofacitinib and biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis: a systematic review and Bayesian network meta-analysis. Clin Exp Rheumatol 2017; 35: 689–699. [PubMed] [Google Scholar]
- 24. Desai RJ, Hansen RA, Rao JK, et al. Mixed treatment comparison of the treatment discontinuations of biologic disease-modifying antirheumatic drugs in adults with rheumatoid arthritis. Ann Pharmacother 2012; 46: 1491–1505. [DOI] [PubMed] [Google Scholar]
- 25. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012; 3: 80–97. [DOI] [PubMed] [Google Scholar]
- 26. Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology 2016; 123: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu S, Gao L, Cipriani A, et al. The effects of incretin-based therapies on β-cell function and insulin resistance in type 2 diabetes: a systematic review and network meta-analysis combining 360 trials. Diabetes Obes Metab 2019; 21: 975–983. [DOI] [PubMed] [Google Scholar]
- 28. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64: 163–171. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Jackson D, Barrett JK, et al. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods 2012; 3: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 31. da Costa BR, Reichenbach S, Keller N, et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet 2017; 390: e21–e33. [DOI] [PubMed] [Google Scholar]
- 32. Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8: e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee YH, Bae SC. Comparative efficacy and safety of tocilizumab, rituximab, abatacept and tofacitinib in patients with active rheumatoid arthritis that inadequately responds to tumor necrosis factor inhibitors: a Bayesian network meta-analysis of randomized controlled trials. Int J Rheum Dis 2016; 19: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 34. Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018; 4: 18001. [DOI] [PubMed] [Google Scholar]
- 35. Nam JL, Takase-Minegishi K, Ramiro S, et al. Efficacy of biological disease-modifying antirheumatic drugs: a systematic literature review informing the 2016 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis 2017; 76: 1113–1136. [DOI] [PubMed] [Google Scholar]
- 36. Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: the role of acute-phase reactants. Arthritis Rheum 2011; 63: 43–52. [DOI] [PubMed] [Google Scholar]
- 37. Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008; 112: 3959–3964. [DOI] [PubMed] [Google Scholar]
- 38. Wang F, Sun L, Wang S, et al. Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc 2020; 95: 1404–1419. [DOI] [PubMed] [Google Scholar]
- 39. Hazlewood GS, Barnabe C, Tomlinson G, et al. Methotrexate monotherapy and methotrexate combination therapy with traditional and biologic disease modifying antirheumatic drugs for rheumatoid arthritis: abridged Cochrane systematic review and network meta-analysis. BMJ 2016; 353: i1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Dell JR, Mikuls TR, Taylor TH, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013; 369: 307–318. [DOI] [PubMed] [Google Scholar]
- 41. Singh JA, Hossain A, Tanjong Ghogomu E, et al. Biologics or tofacitinib for people with rheumatoid arthritis unsuccessfully treated with biologics: a systematic review and network meta-analysis. Cochrane Database Syst Rev 2017; 3: CD012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009; 374: 210–221. [DOI] [PubMed] [Google Scholar]
- 43. Schiff MH, von Kempis J, Goldblum R, et al. Rheumatoid arthritis secondary non-responders to TNF can attain an efficacious and safe response by switching to certolizumab pegol: a phase IV, randomised, multicentre, double-blind, 12-week study, followed by a 12-week open-label phase. Ann Rheum Dis 2014; 73: 2174–2177. [DOI] [PubMed] [Google Scholar]
- 44. Gottenberg JE, Brocq O, Perdriger A, et al. Non-TNF-targeted biologic vs a second anti-TNF drug to treat rheumatoid arthritis in patients with insufficient response to a first anti-TNF drug: a randomized clinical trial. JAMA 2016; 316: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 45. Emery P, Gottenberg JE, Rubbert-Roth A, et al. Rituximab versus an alternative TNF inhibitor in patients with rheumatoid arthritis who failed to respond to a single previous TNF inhibitor: SWITCH-RA, a global, observational, comparative effectiveness study. Ann Rheum Dis 2015; 74: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tab-10.1177_1759720X21999564 for Comparative efficacy and safety of Janus kinase inhibitors and biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and network meta-analysis by Chenghua Weng, Leixi Xue, Qing Wang, Wentian Lu, Jiajun Xu and Zhichun Liu in Therapeutic Advances in Musculoskeletal Disease