Abstract
Background:
Total knee arthroplasty (TKA) is a surgical treatment for patients with knee osteoarthritis (KOA) that no longer experience symptom relief from non-operative or pharmacologic treatments. Non-operative KOA management aims to address patient symptoms and improve function, as well as forestall or mitigate the large costs associated with TKA. The primary objective of this study was to examine the relationship between intra-articular hyaluronic acid (IA-HA) treatment and delaying TKA in patients with KOA compared to patients not receiving IA-HA, as well as to identify differences in KOA-related costs incurred among patients who received or did not receive IA-HA.
Methods:
This was a retrospective analysis of an administrative claims database from October 1st, 2010 through September 30th, 2015. Kaplan–Meier survival analysis was conducted to determine the TKA-free survival of patients who received IA-HA, stratified by the number of injection courses received versus those who did not receive any IA-HA. Median KOA-related costs per year were calculated for 2 comparisons: (1) patients who received IA-HA versus patients who did not receive IA-HA, among patients who eventually had TKA, and (2) patients who received IA-HA versus patients who did not receive IA-HA, among patients who did not have TKA.
Results:
A total of 744 734 patients were included in the analysis. A delay to TKA was observed after IA-HA treatment for patients treated with IA-HA compared to those who did not receive IA-HA. At 1 year, the TKA-free survival was 85.8% (95% CI: 85.6%-86.0%) for patients who received IA-HA and 74.1% (95% CI: 74.0%-74.3%) for those who did not receive IA-HA. At 2 years, the TKA free survival was 70.8% (70.5%-71.1%) and 63.7% (63.5%-63.9%) in the 2 groups, respectively. Patients treated with multiple courses of IA-HA demonstrated an incremental increase in delay to TKA with more courses of IA-HA, suggesting that the risk of TKA over the study time period is reduced with additional IA-HA courses. The hazard ratio for the need of TKA was 0.85 (95% CI 0.84-0.86) for a single course and 0.27 (95% CI 0.25-0.28) for ⩾5 courses, both compared to the no IA-HA group. In patients that eventually had TKA, the median KOA-related costs were lower among those who received IA-HA before their TKA ($860.24, 95% CI: 446.65-1722.20), compared to those who did not receive IA-HA ($2659.49, 95% CI: 891.04-7480.38). For patients who did not have TKA, the median and interquartile range (IQR) KOA-related costs per year were similar for patients who received IA-HA compared with those who did not.
Conclusion:
These results demonstrate that within a large cohort of KOA patients, individuals who received multiple courses of IA-HA had a progressively greater delay to TKA compared to patients who did not receive IA-HA treatment. Also, for patients who progressed to TKA, IA-HA treatment was associated with a large reduction in KOA-related healthcare costs. Based on these results, multiple, repeat courses of IA-HA may be beneficial in substantially delaying TKA in KOA patients, as well as minimizing KOA-related healthcare costs.
Keywords: Knee, osteoarthritis, total knee arthroplasty, hyaluronic acid, observational study, real-world evidence
Introduction
Knee osteoarthritis (KOA) is a progressive and degenerative disease that leads to significant disability for the patient, as well as substantial socioeconomic impacts.1,2 KOA is characterized by the gradual degradation of the cartilage within the knee joint, resulting in pain, inflammation, stiffness, and decreased physical function.2,3 Early stages of the disease are often treated with non-operative approaches, but as the disease progresses, patients typically transition to pharmacologic agents to manage their disease.4,5 In patients who are progressing toward surgical management, it may be advantageous to delay the need for TKA for optimizing other health issues, work related need for delay to surgery, or the need to care for other ill family members, as this procedure has major socioeconomic impacts on patients’ lives.6 From a policy maker perspective, the need for treatments that delay TKA is motivated by the large costs associated with the procedure. Currently, roughly half of all revision surgeries are conducted on patients under the age of 65, with this percentage projected to increase to 62% by the year 2030.7 Revision surgeries for TKA create a $2.7 billion economic burden within the United States annually, with projections estimating this burden to exceed $13 billion by the year 2030.7,8 Payers may also benefit from non-operative treatments that delay surgery and limit the operative osteoarthritis (OA) treatment to a single, lifetime TKA.6,9,10
Intra-articular hyaluronic acid (IA-HA) has demonstrated efficacy in providing significant relief of pain and symptoms for patients with mild to moderate KOA.11 IA-HA has also been shown to be a safe treatment option for both short and long-term use.12 Current guidelines are inconsistent with regard to their recommendations for the use of IA-HA and standardized treatment algorithms are lacking for the management of KOA.13 With inconsistent guidelines for IA-HA use, it is important to investigate possible socioeconomic outcomes that may be impacted by IA-HA use. If treatment with IA-HA were demonstrated to delay the need for TKA, it would be valuable for both patients and policy makers, as delaying the need for this largely disruptive and socioeconomically expensive procedure would aid in reducing costs and burdens on these groups.9,10,14 It is important to consider such costs from a broad perspective inclusive of the costs associated with TKA.
This investigation aims to determine if KOA patients who received IA-HA demonstrate a delay in time to TKA compared to patients who did not receive IA-HA. This investigation also aims to determine if there is a difference in KOA-related costs from a payer perspective between KOA patients who had received IA-HA versus those who did not receive IA-HA among patients who eventually had TKA, as well as those who did not eventually undergo TKA. This will inform the potential benefit of IA-HA in reducing KOA-related healthcare costs for patients with KOA across the spectrum of OA disease progression. A secondary objective of this study is to examine the relationship between the number of IA-HA courses received and the magnitude of the delay until TKA.
Methods
Study design
This study was a retrospective claims analysis of a large commercial database (Health Intelligence Company LLC, Chicago, IL), containing HIPAA compliant de-identified data of more than 100 million patients with continuous coverage from October 1st, 2010 through September 30th, 2015. The database included anonymous claims data for all OA patients seen within the aforementioned timeframe. As a retrospective review of anonymous data, no ethics approval was required for this investigation.
Eligibility criteria
All patients with the diagnosis of knee osteoarthritis in the database were included. Exclusion criteria were as follows: patients under the age of 18, patients without OA, patients with OA other than knee, patients with KOA who had immediate TKA after diagnosis and no treatment, and patients with KOA who had no treatment and no TKA. Eligibility was defined by ICD-9 codes. The ICD-9 codes that were included in our analysis are described in Appendix A.
Comparison groups
The 2 comparison groups within the delay to TKA analysis were: (1) Patients who received IA-HA prior to TKA, and (2) Patients who did not receive IA-HA prior to TKA. The specific HCPCS codes used to identify patients who received IA-HA treatment are described within Appendix B. These codes must have been reported in the same claim as an appropriate ICD-9 diagnosis code from Appendix A. Any other treatment provided at time of an eligible ICD-9 diagnosis code was included within the “No IA-HA” group.
IA-HA treatment courses
Data for IA-HA treatments were analyzed separately based on the number of treatment courses each patient received for the delay to TKA analysis. The number of courses was determined based on the suggested number of injections for each product included (either 1, 3, or 5 injections per course, depending on the product). If the appropriate number of injections was given within a 3-month timeframe for a specific product, it was considered to be 1 treatment course.
Outcome measures
Demographic details were reported for the total population, as well as the IA-HA and those who did not receive IA-HA subgroups. Age, gender, admission status (inpatient or outpatient), comorbidities (diabetes, hypertension, obesity, deep vein thrombosis (DVT), cardiovascular disease, rheumatoid arthritis, chronic obstructive pulmonary disease (COPD), and joint effusion) were summarized for included participants. Admission status was considered as a surrogate aimed to assess patients’ overall health state. Descriptive statistics were reported for both treatment groups. Categorical variables were reported as counts and percentages and continuous variables as mean with standard deviation or median with interquartile range (IQR) when data was not normally distributed.
The outcome of time to TKA was defined as the time from the first record of knee OA within the database to the time of the patient’s TKA. The exact date of treatment was defined as the first date in which an OA treatment code was recorded within the database for that patient.
Delay to TKA analysis
The time to first TKA was compared across treatment groups using Mann–Whitney U test. Kaplan–Meier survival analysis was conducted to analyze and report the TKA-free survival of patients within both treatment groups. TKA-free proportions with the corresponding 95% confidence intervals (CI) were extracted from Kaplan–Meier analysis and reported at the 6-month and yearly time points. The log-rank test was performed for comparisons between groups and P values were reported. A Cox proportional hazards regression analysis was conducted to compare the hazard ratios between patients who did not receive IA-HA and those who did receive IA-HA, adjusting for age, gender, admission status, and comorbidities. Interactions between variables were explored, and incorporated into the model if interaction was present. Additionally, a Cox proportional hazard model was applied comparing patients who did not receive IA-HA and patients who received 1, 2, 3, 4, and 5 or more injection courses of IA-HA adjusting for age, gender and admission status. Hazard ratios (HR) were presented with 95% confidence intervals. All p values were considered statistically significant if α ⩽ 0.05.
Analysis of costs
Median direct KOA-related costs per year were calculated by the authors for 2 distinct comparisons: (1) Patients who received IA-HA versus patients who did not receive IA-HA, among patients who eventually underwent TKA; and (2) Patients who received IA-HA versus patients who did not receive IA-HA, among patients who did not undergo TKA. The direct costs included in this analysis consisted of all KOA-related facility, professional, and pharmaceutical costs incurred within the database prior to the TKA procedure. Differences in KOA-related costs across cost quantiles was assessed through quantile regression adjusted for age, gender and admission status, which provided the median KOA-related costs for each assessed group according to their cost quantiles after adjusting for age, gender, and admission status. Quantile regression provides more robust estimation of skewed data with extreme outliers. The patient demographics were assessed for similarity through descriptive inspection. R software (version 3.6.2, The R Foundation for Statistical Computing) was used for all analyses.
Results
Patient population
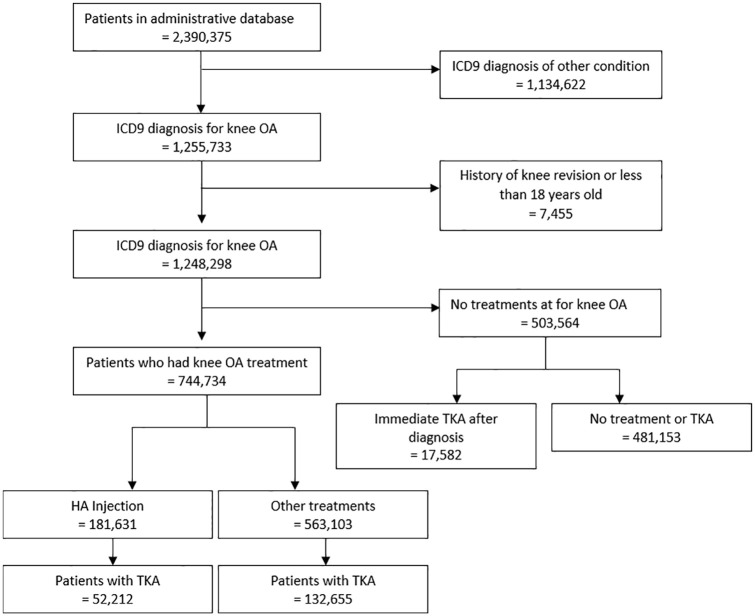
Figure 1 provides a detailed overview of the screening process for identifying eligible patients within the database. Patients were excluded based on each of the aforementioned eligibility criteria: patients with ICD-9 diagnosis for conditions other than KOA (n = 1 134 622); patients with a previous knee revision or patients younger than 18 years old (n = 7455); patients who had immediate TKA within the database timeframe and no other treatment (n = 117 771); and patients who had no treatment after diagnosis (n = 503 564). After these exclusions, 774 734 patients were included in the analysis. Of these patients, 181 631 received IA-HA treatment, while 563 103 did not receive IA-HA treatment. A total of 184 867 patients eventually received a TKA; 52 212 in the IA-HA group and 132 655 in the non-IA-HA group. There was a total of 129 419 patients who received IA-HA but did not progress to TKA within the database timeframe and 430 448 patients who did not receive IA-HA and also did not progress to TKA during the database timeframe.
Figure 1.
Flow diagram shows the selection and inclusion process.
Demographics
The IA-HA treatment group had a mean age of 55.6 ± 8.2 and the group that did not receive IA-HA had a mean age of 56.1 ± 8.5, with similar proportions of females in both groups (60.5% and 57.6%, respectively). Proportions of patient comorbidities were generally similar between the groups, although there were slightly fewer comorbid conditions within the IA-HA group. A summary of baseline demographics and comorbidities of included patients is shown in Table 1.
Table 1.
Baseline demographics by treatment group.
| Variable | IA-HA, N = 181 631 | No IA-HA, N = 563 103 | Total N = 744 734 |
|---|---|---|---|
| Age – Mean (SD) | 55.6 (8.2) | 56.11 (8.5) | 55.98 (8.4) |
| Age category – N (%) | |||
| 18–25 | 523 (0.3) | 2036 (0.4) | 2559 (0.3) |
| 26–35 | 2661 (1.5) | 9804 (1.7) | 12 465 (1.7) |
| 36–45 | 14 751 (8.1) | 44 056 (7.8) | 58 807 (7.9) |
| 46–55 | 57 808 (31.8) | 161 633 (28.7) | 219 441 (29.5) |
| 56–65 | 91 918 (50.6) | 293 610 (52.1) | 385 528 (51.8) |
| >65 | 13 970 (7.7) | 51 964 (9.2) | 65 934 (8.9) |
| Gender – count (%) | |||
| Males | 71 633 (39.4) | 238 420 (42.3) | 310 053 (41.6) |
| Females | 109 886 (60.5) | 324 329 (57.6) | 434 215 (58.3) |
| Unknown | 112 (0.1) | 354 (0.1) | 466 (0.1) |
| Admission at treatment – count (%) | |||
| Inpatient non-acute | 386 (0.2) | 7180 (1.3) | 7566 (1.0) |
| Outpatient | 181 245 (99.8) | 555 923 (98.7) | 737 168 (99.0) |
| TKA | |||
| TKA – count (%) | 52 212 (28.8) | 139 665 (24.8) | 191 877 (25.8) |
| Comorbidities | |||
| Diabetes | 27 231 (15.0) | 95 093 (16.9) | 122 324 (16.4) |
| Hypertension | 75 119 (41.4) | 261 605 (46.5) | 336 724 (45.2) |
| Obesity | 27 065 (14.9) | 98 457 (17.5) | 125 522 (16.9) |
| DVT | 1594 (0.9) | 5586 (1.0) | 7180 (1.0) |
| Cardiovascular disease | 17 861 (9.8) | 64 769 (11.5) | 82 630 (11.1) |
| Rheumatoid arthritis | 6807 (3.7) | 26 642 (4.7) | 33 449 (4.5) |
| COPD | 30 812 (17.0) | 108 832 (19.3) | 139 644 (18.8) |
| Joint effusion | 50 862 (28.0) | 188 447 (33.5) | 239 309 (32.1) |
Time to TKA
The median time to TKA was 1.3 years (IQR 1.57) in the IA-HA group and 0.38 years (IQR 0.95) in the no IA-HA group (P < .0001) (Figure 2). The Cox proportional hazard model found important interaction between the use of IA-HA and the presence of both rheumatoid arthritis and joint effusion. The multivariable hazard ratio for the IA-HA group was 0.69 (95% CI 0.69-0.7) compared to those who did not receive IA-HA, when adjusted for age, gender, admission status, comorbidities, and the aforementioned interactions.
Figure 2.
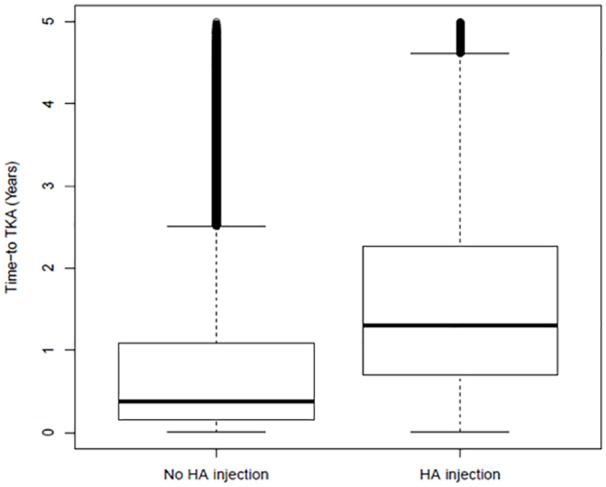
Box and Whisker plot for time to TKA.
Box plot represents median (bold line) and quartiles. Outliers defined as outside 1.5 interquartile range (IQR).
At 1 year, the TKA-free survival was 85.8% (95% CI: 85.6%-86.0%) for patients who received IA-HA and 74.1% (95% CI: 74.0%-74.3%) for those who did not receive IA-HA. At 2 years, the TKA free survival was 70.8% (70.5%-71.1%) and 63.7% (63.5%-63.9%) in the 2 groups, respectively. The overall TKA-free survival was significantly higher in the IA-HA group with a log-rank P value of <.0001 (Figure 3). The proportions of TKA-free survival at each yearly time point are provided in Table 2.
Figure 3.
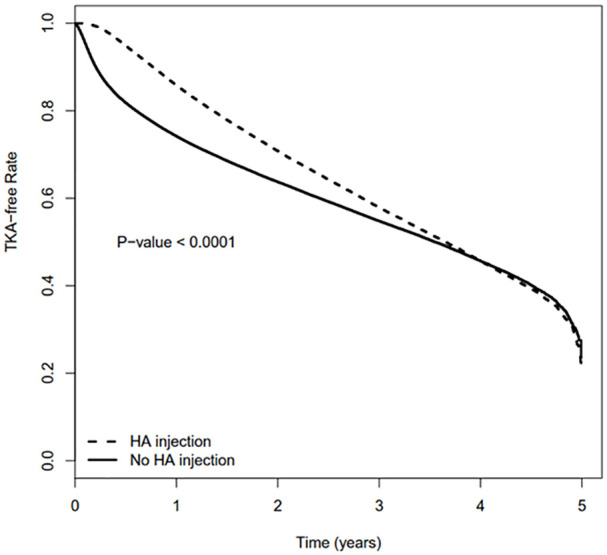
Kaplan–Meier curve comparing IA-HA and No IA-HA groups.
Table 2.
Cumulative proportions of TKA-free survival.
| IA-HA, TKA-free survival (95% CI) | No IA-HA, TKA-free survival (95% CI) | |
|---|---|---|
| 6 Months | 94.9 (94.7–95.0) | 81.8 (81.7–81.9) |
| 1 Year | 85.8 (85.6–86.0) | 74.1 (74.0–74.3) |
| 2 Years | 70.8 (70.5–71.1) | 63.7 (63.5–63.9) |
| 3 Years | 57.9 (57.6–58.2) | 54.8 (54.6–55.0) |
| 4 Years | 45.7 (45.3–46.1) | 45.7 (45.4–46.0) |
Time to TKA was stratified to assess the impact of the number of IA-HA courses received. The Kaplan–Meier curve demonstrated an increasingly prolonged delay to TKA with each subsequent course (Figure 4). A Cox regression analysis adjusting for demographics demonstrated a consistent decrease in hazard ratio with each subsequent course of IA-HA in comparison to the no IA-HA injection group. The hazard ratio was 0.85 (95% CI 0.84-0.86) for a single course and 0.27 (95% CI 0.25-0.28) for ⩾5 courses, both compared to the no IA-HA group. Hazard ratios for all groups are reported in Table 3.
Figure 4.
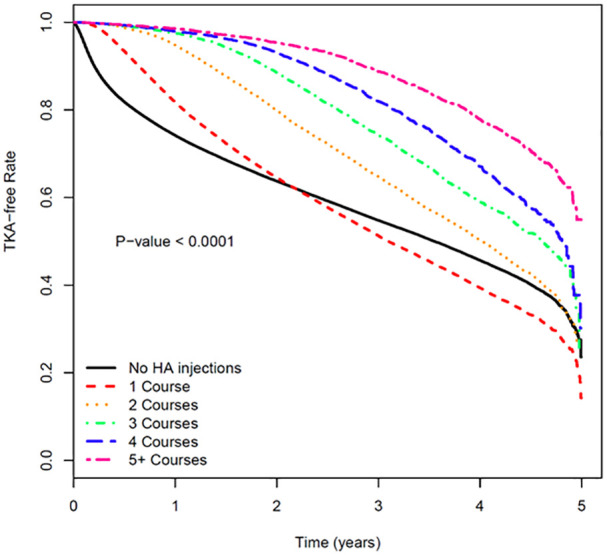
Kaplan–Meier curve by HA courses.
Table 3.
Cox proportional hazards analysis.
| Hazard ratio (95% CI) | P-value | |
|---|---|---|
| No IA-HA* | – | – |
| 1 Course | 0.85 (0.84–0.86) | <.0001 |
| 2 Courses | 0.55 (0.54–0.57) | <.0001 |
| 3 Courses | 0.43 (0.41–0.45) | <.0001 |
| 4 Courses | 0.36 (0.34–0.38) | <.0001 |
| 5+ Courses | 0.27 (0.25–0.28) | <.0001 |
Reference group.
KOA-related costs in TKA patients
In patients who eventually underwent TKA, the median and IQR KOA-related costs per year for those who received IA-HA before their TKA ($860.24, IQR $891.04-$7480.38) were lower than those who did not ($2659.49, IQR $446.65-$1722.20). For patients who were in the highest percentile of KOA-related costs per year, those who received IA-HA had drastically lower KOA-related costs than patients who did not receive IA-HA (Figure 5).
Figure 5.
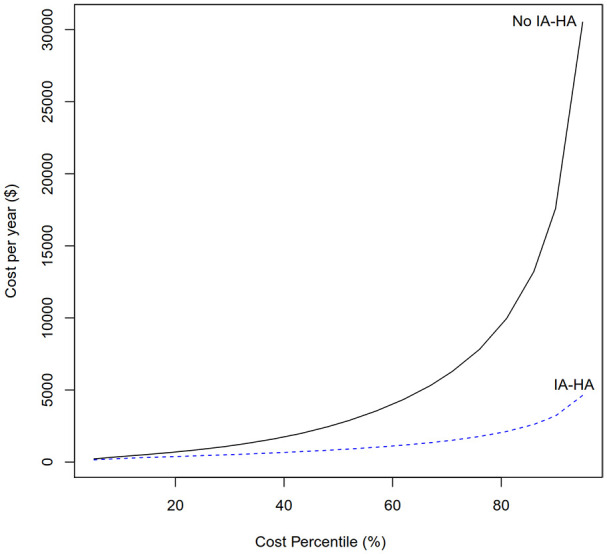
KOA-related costs per year by cost percentile – patients who required TKA.
KOA-related costs in non-TKA patients
The median and IQR for KOA-related costs per year for patients who received IA-HA and did not progress to TKA was $9.66 (IQR $5.01-$30.89), while it was $7.58 (IQR $2.68-$45.17) in patients who did not receive IA-HA and did not require TKA. The median and IQR are largely overlapping, and seem to be similar between treatment groups. The quantile distribution of KOA-related costs for patients who did not have TKA within both the IA-HA treatment group and the group that did not receive IA-HA, is presented in Figure 6.
Figure 6.
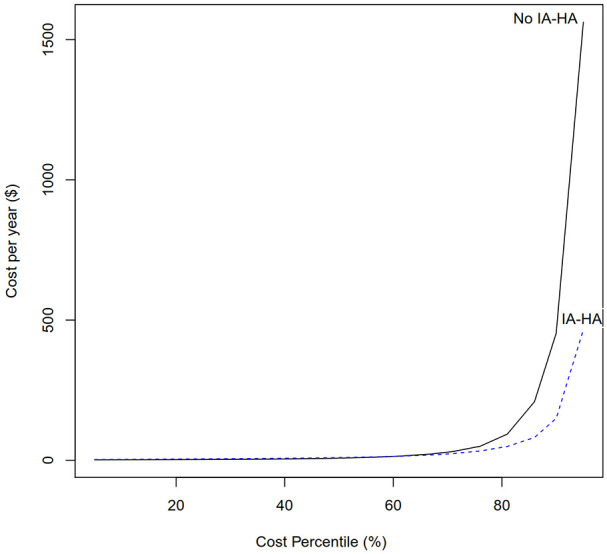
KOA-related costs per year by cost percentile – patients who did not require TKA.
Discussion
The results of this study demonstrate that within a large cohort of KOA patients, individuals who received IA-HA as part of their treatment regimen had a significantly greater delay until their need for TKA in comparison to patients who did not receive IA-HA treatment. The delay to TKA increased with additional IA-HA courses. From the initiation of treatment for KOA until TKA, patients who received IA-HA had a lower median healthcare KOA-related cost per year than those who did not receive IA-HA. For patients who never underwent TKA, median KOA-related costs were similar between all individuals who either received IA-HA or did not receive IA-HA; however, a much larger benefit from receiving IA-HA was demonstrated for those who were in the upper 25% percentile of annual KOA-related costs. These are the direct costs associated with KOA due to the payer perspective of this analysis, but personal and societal costs may be even larger due to the indirect costs accrued. Additionally, patients with rheumatoid arthritis or a joint effusion may have worse outcomes with IA-HA, indicating that these populations are not ideal candidates for IA-HA treatment. The results come from a large database that includes patients from all of the United States, making these results highly generalizable to the US population as a whole.
Previous literature has demonstrated the cost-benefits of IA-HA use in comparison to other treatment options; however, this study utilizes a real-world evidence approach to evaluate all recorded health KOA-related costs rather than employing modeling methods to derive costs.15-21 The direct evaluation of KOA-related costs within a national database provides a more representative assessment of the costs associated with KOA and how those costs may differ when IA-HA is included in the disease management paradigm. From a value-perspective, the potential cost savings seen when IA-HA is utilized should be taken into consideration along with the efficacy and safety evidence for this treatment.11,12,22,23 The results of this study, coupled with the efficacy and safety data, suggest that IA-HA is a safe and effective treatment that may provide substantial TKA delay and cost benefits, particularly among those patients who may eventually progress to TKA. Conversely, the lack of use or availability of IA-HA may actually increase KOA-related costs of care in patients with KOA.
This study is strengthened by the large sample size and analysis of a large administrative database. An additional strength is the conservative approach to the assessment of HA’s delay to TKA. All patients who received HA but never progressed to TKA were excluded from the analysis, which potentially removes the best responders to HA treatment. Patients who were able to forgo the need for TKA completely throughout the study were not considered for the delay to TKA analysis, as all of the included patients eventually required TKA. This was done to ensure patients that were not TKA candidates were removed from the analysis, but in doing so optimal responders to HA would also be omitted. For this reason, the results are strengthened, as the delay to TKA analysis conservatively assesses only those HA patients that eventually received a TKA. For those who did not receive TKA, a separate cost analysis was still conducted to better understand their OA related costs.
There are a number of key limitations of this study. This study is limited by its reliance upon an administrative database. As such, the analysis is limited to the data that had been collected within this database, and the authorship team is unable to consider all relevant variables that were not captured within the database. Specifically, assessments of disease severity and treatment response were unavailable and would have brought significant utility in evaluating the results obtained by the analysis. Analyzing results with admission status was conducted in an attempt to account for the severity patients’ overall health status, although this is not an ideal approach in understanding OA severity. The lack of disease severity within the database presents a confounding variable that is not accounted for within this analysis, but important comorbidities and health status measures have been incorporated into the analysis to help minimize the effect of this limitation. IA-HA patients may have also received other forms of KOA treatment, such as corticosteroids, although patients within the group that did not receive IA-HA group may also have received these other treatment options. Finally, the use of a payer database in the present study restricts the analysis to including only direct, KOA-related costs. While this approach allowed this study to capture any costs that were labeled within the database as being associated with the diagnosis of OA prior to TKA, such as facility, physician, pharmaceutical, and procedure costs, there are some specific costs that were not present within the database. Assessing only direct, KOA-related costs from a payer perspective fails to capture several critical societal costs, including work loss/presenteeism/absenteeism, cost incurred for caregivers of patients with KOA, and patient out-of-pocket costs for nutraceuticals or orthobiologic treatments, among many others.
Despite these limitations, this analysis of a large national database adds important information for policy members and patients alike as to the patterns of TKA timing and KOA-related costs within KOA patients, particularly among individuals who receive IA-HA treatment. It is important for future studies to accurately identify the overall costs of KOA care across the spectrum of disease in assessing of the economic impact of IA-HA. While investigations that utilize modeling are often employed to estimate possible KOA-related costs, evaluations of large administrative databases provide a more direct and thorough assessment of the actual costs incurred during the course of KOA management.
Conclusions
Within a large cohort of KOA patients, individuals who eventually required TKA but received IA-HA as part of their treatment regimen had a significantly greater delay until TKA in comparison to patients who did not receive HA treatment. Among patients who progress to TKA, IA-HA provided a large reduction in healthcare KOA-related costs. In particular, the KOA-related cost of care among the most expensive TKA patients is substantially greater without the use of IA-HA, as opposed to those treated with IA-HA. Based on these results, IA-HA injection may be beneficial in substantially delaying the need for TKA in KOA patients, while reducing KOA-related healthcare costs.
Acknowledgments
Medical writing support was provided by Global Research Solutions Inc. All authors were involved in the conceptualization of the project. All authors provided critical revisions to the manuscript throughout the medical writing process.
Appendix A
Codes Used to Identify Eligible Patients
ICD-9 Diagnosis Codes
711.×6, 712.×6, 715.×6, 716.×6, 717.×, 718.×6, 719.×6, 836.×, 844.×
Appendix B
Codes Used to Identify the IA-HA Treatment Group*
Hyalgan/Supartz: J7321 (eff Jan 1, 2008 to present)
Euflexxa: J7323 (eff Jan 1, 2008 to present)
Orthovisc: J7324 (eff Jan 1, 2008 to present)
Synvisc: J7325 (eff Jan 1, 2010 to present)
Gel-One: J7326 (eff Jan 1, 2012 to present)
Monovisc: J7327 (eff Jan 1, 2015 to present)
*Codes must appear on the same claim as the ICD-9 diagnosis code to limit it to knee injections.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Ferring Pharmaceuticals.
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andrew Concoff: Nothing to declare. Faizan Niazi: Employee of Ferring Pharmaceuticals. Forough Farrokhyar: Nothing to declare. Akram Alyass: Nothing to declare. Jeffrey Rosen: Served on advisory board for Novartis. Mathew Nicholls: Nothing to declare.
References
- 1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(7):1323-1330. [DOI] [PubMed] [Google Scholar]
- 2. Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nicholls MA, Fierlinger A, Niazi F, et al. The disease-modifying effects of Hyaluronan in the osteoarthritic disease state. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117723611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper C, Rannou F, Richette P, et al. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res (Hoboken). 2017;69(9):1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arnold W, Fullerton DS, Holder S, et al. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Losina E, Paltiel AD, Weinstein AM, et al. Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty. Arthritis Care Res (Hoboken). 2015;67(2):203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurtz SM, Lau E, Ong K, et al. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhandari M, Smith J, Miller LE, et al. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ong KL, Anderson AF, Niazi F, et al. Hyaluronic acid injections in medicare knee osteoarthritis patients are associated with longer time to knee arthroplasty. J Arthroplasty. 2016;31(8):1667-1673. [DOI] [PubMed] [Google Scholar]
- 10. Altman R, Lim S, Steen RG, et al. Correction: hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: Evidence from a large U.S. health claims database. PLoS One. 2016;1:e0148591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bannuru RR, Schmid CH, Kent DM, et al. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54. [DOI] [PubMed] [Google Scholar]
- 12. Bannuru RR, Osani M, Vaysbrot EE, et al. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage. 2016;24(12):2022-2041. [DOI] [PubMed] [Google Scholar]
- 13. Altman RD, Schemitsch E, Bedi A. Assessment of clinical practice guideline methodology for the treatment of knee osteoarthritis with intra-articular hyaluronic acid. Semin Arthritis Rheum. 2015;45(2):132-139. [DOI] [PubMed] [Google Scholar]
- 14. Altman R, Fredericson M, Bhattacharyya SK, et al. Association between hyaluronic acid injections and time-to-total knee replacement surgery. J Knee Surg. 2016;29(7):564-570. [DOI] [PubMed] [Google Scholar]
- 15. Hatoum HT, Fierlinger AL, Lin SJ, et al. Cost-effectiveness analysis of intra-articular injections of a high molecular weight bioengineered hyaluronic acid for the treatment of osteoarthritis knee pain. J Med Econ. 2014;17(5):326-337. [DOI] [PubMed] [Google Scholar]
- 16. Rosen J, Sancheti P, Fierlinger A, et al. Cost-effectiveness of different forms of intra-articular injections for the treatment of osteoarthritis of the knee. Adv Ther. 2016;33(6):998-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hermans J, Reijman M, Goossens LMA, et al. Cost-utility analysis of high molecular weight hyaluronic acid for knee osteoarthritis in everyday clinical care in patients at a working age: An economic evaluation of a randomized clinical trial. Arthritis Care Res (Hoboken). 2018;70(1):89-97. [DOI] [PubMed] [Google Scholar]
- 18. Delbarre A, Amor B, Bardoulat I, et al. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis – a Cox model analysis. PLoS One. 2017;12(11):e0187227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ong KL, Niazi F, Lau E, et al. Knee OA cost comparison for hyaluronic acid and knee arthroplasty. J Orthop Surg Res. 2020;15(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shewale AR, Barnes CL, Fischbach LA, et al. Comparison of low-, moderate-, and high-molecular-weight hyaluronic acid injections in delaying time to knee surgery. J Arthropl. 2017;32(10):2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turajane T, Labpiboonpong V, Maungsiri S. Cost analysis of intra-articular sodium hyaluronate treatment in knee osteoarthritis patients who failed conservative treatment. J Med Assoc Thai. 2007;90(9):1839-1844. [PubMed] [Google Scholar]
- 22. Phillips M, Vannabouathong C, Devji T, et al. Differentiating factors of intra-articular injectables have a meaningful impact on knee osteoarthritis outcomes: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2020;28(9):3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hummer CD, Angst F, Ngai W, et al. High molecular weight Intraarticular hyaluronic acid for the treatment of knee osteoarthritis: a network meta-analysis. BMC Musculoskelet Disord. 2020;21(1):702. [DOI] [PMC free article] [PubMed] [Google Scholar]