Abstract
Metastatic triple-negative breast cancer (TNBC) is a heterogeneous disease with a poor prognosis and currently with few treatment options. Treatment of these patients is highly based on systemic chemotherapy. Some targeted drugs were recently approved for these patients: two poly(ADP-ribose) polymerase inhibitors in patients with germline BRCA1/2 mutations (olaparib and talazoparib), immune checkpoint inhibitors in association with chemotherapy if programmed death-ligand 1 positive (atezolizumab plus nabpaclitaxel and pembrolizumab plus chemotherapy [nabpaclitaxel, paclitaxel, and carboplatin plus gemcitabine]), and an antibody-drug conjugate sacituzumab-govitecan in heavily pretreated patients (at least 2 previous lines for the metastatic setting). Combinations using these and other targeted treatment options are under investigation in early and late clinical trials, and we will probably have some practice-changing results in the new future. Other targeted drugs explored in phase II and phase III clinical trials are PI3K/AKT pathway inhibitors and androgen receptor antagonists in patients with alterations in these signaling pathways. The definition of molecular subtypes has been essential for the development of these treatment strategies. Soon, the treatment of metastatic TNBC could be based on personalized medicine using molecular testing for targeted drugs instead of only systemic chemotherapy. The authors present a review of emerging treatment options in metastatic TNBC, focusing on targeted drugs, including the recent data published in 2020.
Keywords: Triple-negative breast cancer, platinum chemotherapy, PARP inhibitors, immune checkpoint inhibitors, androgen receptor antagonists, PI3K/AKT pathway, sacituzumab-govitecan
Introduction
Breast cancer (BC) is associated with approximately 2.1 million new cases in 2018, mostly in women. BC is also the leading cause of cancer mortality in women.1 The incidence and mortality of BC have disparities worldwide.2 Triple-negative breast cancer (TNBC) is defined by the absence of the estrogen receptor, androgen receptor (AR), and human epidermal growth factor receptor 2 (HER2) and accounts for nearly 15% of BC. Patients are usually young (<50 years old), have a shorter time to relapse in the early stages, and are at higher risk for visceral metastasis, including brain metastasis.3 Most TNBC have a high pathological histological grade and high proliferation rates, especially invasive ductal carcinoma, metaplastic, apocrine, and medullary carcinoma. Rare histological subtypes, as salivary gland-type adenoid-cystic and secretory carcinoma, are low-grade neoplasms.4 The prevalence of germline BRCA1/2 mutations is higher in patients with TNBC.5 This tumor subtype is also associated with more tumor-infiltrating lymphocytes (TILs) than other subtypes.6 The survival curves in TNBC, different from other BC subtypes, are characterized by an increase in relapse and a decrease in survival during the first 3 to 5 years after diagnosis. Late recurrences are more frequent in estrogen-positive BC than in TNBC.7,8 Metastatic TNBC (mTNBC) is a heterogeneous and aggressive disease that responds differently to standard chemotherapy and targeted drugs.3
A molecular classification of TNBC described by Lehmann et al identified 6 subtypes using gene expression signatures: basal-like 1, basal-like 2, immunomodulatory (IM), mesenchymal (MES), mesenchymal stem-like, and luminal androgen receptor (LAR). Each subtype is enriched in distinct gene ontologies and gene expression patterns.9 The correlation between molecular subtype, gene expression, and cell pathway is essential to identify targeted agents.10,11 Regarding prognosis, the IM subtype is associated with a better prognosis, and the LAR subtype to a poor prognosis.12 More recently, Burstein et al13 identified 4 TNBC subtypes: LAR, MES, basal-like immunosuppressed, and basal-like immune-activated. Claudin-low tumors are recently discussed. This phenotype involves various intrinsic subtypes associated with high immune and stromal infiltration levels and a low mutation burden, proliferation, and genomic instability.14
Historically, mTNBC used to be treated with cytotoxic chemotherapy.15,16 The growing knowledge of biology, molecular alterations, and genome sequencing has been essential in developing new drugs in recent years. Some targeted drugs, such as poly(ADP-ribose) polymerase (PARP) inhibitors, immune checkpoint inhibitors (ICIs), AR-targeted drugs, drugs targeting the PI3K/AKT pathway, or antibody-drug conjugates (ADCs), have demonstrated clinical benefit in these patients.17 The authors present in this article a review of the most recent drug advances in mTNBC, particularly in the last 5 years, including the new data published in the year 2020.
BRCA1/2 Mutations and Homologous Recombination Deficiency
DNA damage in cells, caused by extracellular agents or endogenous events, is repaired by various DNA repair mechanisms. Those are base excision repair (BER), nucleotide excision repair, mismatch repair, and double-strand break (DSB) repair that includes either homologous recombination during the S and G2 cell cycle phases and nonhomologous end joining.18 BRCA1/2 is directly involved in DNA homologous recombination repair (HRR) and plays an essential role in genome stability. Mutations in BRCA1/2 or other homologous recombination defects result in growth defects and genetic instability.19 Germline BRCA1/2 mutations are present in approximately 10% to 20% of patients with TNBC, especially in those aged <60 years.20-23 The presence of these mutations is associated with hereditary breast and ovarian cancer (HBOC) syndrome.24 Almost all the known BRCA1 mutations have gene expression patterns coincident with basal-like subtype.9 BRCA2 mutation is more associated with lobular histology.25 Testing for germline BRCA1/2 mutations is essential in patients with mTNBC to predict future cancer risk and guide therapeutic strategies.26
There are other multiple genetic alterations beyond BRCA1/2 mutations that are associated with HBOC syndrome. Some of these alterations are also involved in the DNA repair mechanisms, like PALB2, ATM, CHEK2, RAD51, and TP53.27 The discovery of gene mutations that participate in DNA repair pathways supports the possibility for therapeutic targets by the synthetic lethality mechanism as occurs with the BRCA1/2 mutations tumors treated with PARP inhibitors. The evaluation of tumor tissue for loss of heterozygosity, HRR deficiency, or mutational signatures could support therapeutic strategies guided by the patient’s functional status.27 The analysis of somatic mutation genome from cancers permitted identifying more than 20 mutational signatures, including an identified signature 3 associated with the breast, ovarian, and pancreatic cancer and the inactivating BRCA1/2 mutations.28,29 A study performing a comprehensive characterization of signature 3 revealed that it is associated with the biallelic BRCA1/2 inactivation (germline, somatic, or epigenetic silencing). Nevertheless, many samples with signature 3 did not have the BRCA1/2 inactivation, suggesting that other HRR defects can contribute to this signature. The study verified an association with rare truncating variants in other HRR machinery components: germline PALB2 mutations, epigenetic silencing, and somatic mutations in RAD51C. The authors conclude that signature 3 could represent a biomarker for HRR status and could help design the therapeutic trials.30
The Role of Platinum-Based Chemotherapy
In the last few years, chemotherapy was the only treatment strategy approved for mTNBC.15,16,31 The role of platinum drugs, by their mechanism of action—DNA adduct formation with subsequent DSBs, has been questioned in patients with TNBC, as it is associated with a high percentage of BRCA1/2 mutations and other homologous recombination deficiency (HRD).32 In the absence of functional BRCA proteins, tumor cells are more sensitive to platinum drugs due to their mechanism of action.33 A phase II multicentre clinical trial TBCRC009 evaluated the platinum monotherapy (cisplatin 75 mg/m2 or carboplatin area under the curve [AUC] 6) in mTNBC. The results were published in 2015. The study included 86 patients, 69 as a first-line therapy, and 11 with BRCA1/2 mutations. The overall response rate (ORR) was 25.6% (95% confidence interval [CI]: 16.8%-36%) in the overall population, numerically higher with cisplatin (32.6%) versus carboplatin (18.7%) and was 54.5% in patients with BRCA1/2 mutations.34 Three years later, a phase III trial was published, the TNT trial. This study compares carboplatin AUC 6 versus the standard of care, docetaxel 100 mg/m2 in 376 patients with mTNBC. There were no differences in ORR between carboplatin and docetaxel (31.4% vs 34.0%, respectively) in the overall population. There were no differences in median progression-free survival (PFS) and overall survival (OS): 3.1 months (95% CI: 2.4-4.2) and 12.8 months (95% CI: 10.6-15.3) in carboplatin versus 4.4 months (95% CI: 4.1-5.1) and 12.0 months (95% CI: 10.2-13.0) in docetaxel treated group, P = .40 and .96, respectively. There were 43 patients with germline BRCA1/2 mutations included. In the BRCA-mutated patients, there was a significant difference in ORR between carboplatin treated (68%) versus docetaxel (33.3%), P = .03. There is also a significant difference in median PFS in BRCA-mutated: 6.8 months in the carboplatin group versus 4.4 months in the docetaxel group, P = .002. Despite that, there were no differences in the OS evaluation. The safety profile was expected for both drugs. The benefit observed in BRCA-mutated patients was not observed in BRCA1 methylation, BRCA1 mRNA-low tumors, and a high score in Myriad HRD assay.35 In this trial, carboplatin demonstrated similar clinical benefit compared with docetaxel with a favorable toxicity profile and is currently an option for mTNBC.36
Table 1 describes the main characteristics of these two studies. A Cochrane systematic review that included 4418 women evaluated the platinum-containing regimens for metastatic BC. The authors concluded that in women with mTNBC, there is preliminary low-quality evidence of a moderate survival benefit from platinum-based drugs. In contrast, in patients with non-TNBC metastatic disease, there is evidence of no survival benefit and high toxicity from platinum-based regimens.44 Despite these results, platinum agents have been widely used for treating patients with mTNBC. Regarding the presented results, it will be important to develop and understand which biomarkers could predict clinical benefit with platinum chemotherapy in mTNBC beyond the germline BRCA1/2 mutations.
Table 1.
Selected published platinum-based chemotherapy and PARP inhibitors clinical trials in the mTNBC population.
| Author, study | n | Phase | Population | Treatment (experimental vs compared drug) | Target | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|
| Platinum-based chemotherapy | ||||||||
| Isakoff et al,34 (TBCRC009) | 86 | II | First- or second-line in mTNBC | Cisplatin or carboplatin | DNA repair | Cisplatin: 32.6% Carboplatin: 18.7% BRCA1/2: 54.5% |
2.9 | 11 |
| Tutt et al,35 (TNT) | 376 | III | mTNBC (BRCA1/2 in 43 patients) | Carboplatin vs docetaxel | DNA repair BRCA1/2 |
NS (31.4% vs 34%) BRCA1/2: 68% vs 33.3% |
NS (3.1 vs 4.4) BRCA1/2: 6.8 vs 4.4 |
NS (12.8 vs 12.0) |
| PARP inhibitors | ||||||||
| Robson et al,37 (OlympiAD) | 302 | III | Pretreated TNBC and hormone receptor–positive BC with germline BRCA1/2 mutations | Olaparib vs standard chemotherapy | BRCA1/2 | 59.9% vs 28.8% | 7.0 vs 4.2 | NS (19.3 vs 19.6) |
| Litton et al,38 (EMBRACA) | 431 | III | Pretreated TNBC and hormone receptor–positive BC with germline BRCA1/2 mutations | Talazoparib vs standard chemotherapy | BRCA1/2 | 62.6% vs 27.2% | 8.6 vs 5.6 | NS (22.3 vs 19.5) |
| PARP inhibitors plus platinum-based chemotherapy | ||||||||
| Han et al,39 (BROCADE) | 290 | II | Locally recurrent or metastatic breast cancer with germline BRCA1/2 mutations (~40% TNBC) | Carboplatin/paclitaxel plus veliparib vs plus placebo Veliparib plus temozolomide vs placebo plus carboplatin/paclitaxel |
BRCA1/2 DNA repair |
78.0% vs 61.3% 28.6% vs 61.3% |
NS (14.1 vs 12.3) 7.4 vs 12.3 |
NS (28.3 vs 25.9) 19.1 vs 25.9 |
| Diéras et al,40 (BROCADE3) | 509 | III | HER2-negative advanced/metastatic BC with germline BRCA1/2 mutations (48% TNBC) | Carboplatin/paclitaxel plus veliparib vs plus placebo | BRCA1/2 DNA repair |
75.8% vs 74.1% | 14.5 vs 12.6 | NS (33.5 vs 28.2) |
| Sharma et al,41 (SWOG S1416) | 335 | II | Pretreated mTNBC (germline BRCA1/2, BRCA-like with HRD and non-BRCA-like groups) | Cisplatin plus veliparib vs plus placebo | BRCA1/2 HRD |
BRCA-like: NS (45% vs 35%) |
BRCA-like: 5.7 vs 4.3 | BRCA-like: NS (13.7 vs 12.1) |
| PARP inhibitors plus immunotherapy | ||||||||
| Domchek et al,42 (MEDIOLA) | 34 | II | Metastatic BC with germline BRCA1/2 mutations | Olaparib plus durvalumab | BRCA1/2 PD-L1 |
63.3% | 8.2 | 20.5 |
| Vinayak et al,43 (TOPACIO/Keynote-162) | 54 | II | Pretreated mTNBC | Niraparib plus pembrolizumab | DNA repair PD-L1 |
29% (33% in PD-L1 pos vs 15% in PD-L1 neg) | 8.1 (in BRCA-mutated) | – |
Abbreviations: BC, breast cancer; HER2, human epidermal growth factor receptor 2; HRD, homologous recombination deficiency; mTNBC, metastatic triple-negative breast cancer; NS, not significant; ORR, overall response rate; OS, overall survival; PARP, poly(ADP-ribose) polymerase; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TNBC, triple-negative breast cancer.
Key results: Platinum-based chemotherapy showed greater ORR in patients with BRCA1/2 mutations. Poly(ADP-ribose) polymerase inhibitors olaparib and talazoparib showed higher ORR and median PFS than standard chemotherapy, with no difference in OS. Poly(ADP-ribose) polymerase inhibitors combined with platinum-based chemotherapy revealed conflicting results, with higher median PFS in a phase III trial, but without differences in OS. In combination with immunotherapy, PARP inhibitors only have shown few data from phase II studies, mostly in BRCA-mutated patients.
Poly(ADP-Ribose) Polymerase Inhibitors in mTNBC
Poly(ADP-ribose) polymerase 1 is a DNA damage sensor and a signal transducer that binds to DNA breaks. The PARP inhibitors impair the repair of single-strand breaks (SSBs) by the following mechanisms: disruption of the BER pathway and inhibiting the auto-PARylation or PARP release from DNA (PARP1 trapping). The unresolved SSBs lead to DSBs that result in cell death in the HRR-deficient cells.45 Some clinical trials have evaluated multiple PARP inhibitors in patients with BC and BRCA1/2 germline mutations. The first phase III published trial in this setting, demonstrating benefit, was OlympiAD trial in 2017. It is a randomized, open-label study that involves 302 patients with previously treated (⩽2 previous chemotherapy drugs) metastatic HER2-negative BC with a germline BRCA1/2 mutation. The study compares olaparib 300 mg twice daily versus physician’s choice chemotherapy (capecitabine, eribulin, or vinorelbine in the 21-day cycle) in a 2:1 ratio. The median PFS was meaningfully superior in the olaparib group: 7.0 versus 4.2 months, the hazard ratio (HR) 0.58 (95% CI: 0.43-0.80, P < .001). The ORR was almost double in the olaparib group: 59.9% versus 28.8% in standard chemotherapy. The OS was not significantly different in the two groups, numerically nearly 20 months. The safety profile was favorable in the olaparib group: adverse events (AEs) grade ⩾3 Common Terminology Criteria for Adverse Events (CTCAE) were 36.6% in the olaparib group and 50.5% in the standard chemotherapy arm. The most frequent any-grade AEs associated with olaparib were anemia, nausea, vomiting, fatigue, and neutropenia.37 The EMBRACA trial was published in the next year. This study had a similar design: a randomized, open-label trial, including 431 patients with previously treated advanced BC (⩽3 cytotoxic agents) and germline BRCA1/2 mutations. Patients were randomized to talazoparib 1 mg once daily versus physician’s choice chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in a 2:1 ratio. The median PFS is significantly higher in the talazoparib group: 8.6 versus 5.6 months, HR: 0.54 (95% CI: 0.41-0.71, P < .001). The ORR was also almost double in talazoparib-treated patients: 62.6% versus 27.2% in the chemotherapy group, odds ratio 5.0 (95% CI: 2.9-8.8, P < .001). There was no difference in median OS, nearly 20 to 22 months. EMBRACA trial, unlike OlympiAD, permitted crossover to PARP inhibitor after progression, and 18% of patients in the standard group were treated with this drug. The safety profile was worse in talazoparib regarding hematological grade AEs grade ⩾3 CTCAE: 55% versus 38%. The talazoparib group revealed a significant improvement in the estimated overall mean change from the baseline in the global quality-of-life (QoL) on the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ)-C30, compared with a deterioration in the chemotherapy group: 3.0 (95% CI: 1.2-4.8) versus −5.4 (95% CI: −8.8 to −2.0), P < .001.38 These 2 PARP inhibitors are approved and recommended in pretreated metastatic HER2-negative BC with germline BRCA1/2 mutations in the first- to third-line setting.16,36,46
Combining PARP inhibitors and chemotherapy to achieve an enhanced efficacy is also under investigation. There are some recently reported data on this issue. Veliparib, a PARP inhibitor with minimal PARP trapping, alone or with chemotherapy, was studied in early phase trials and demonstrated efficacy and a safety profile.47 A randomized phase II clinical trial (BROCADE) evaluated the combination of veliparib with carboplatin/paclitaxel or with temozolomide in patients with BRCA1/2 mutations in recurrent or metastatic BC with ⩽2 previous therapeutic lines. Patients (n = 290) were randomized in a 1:1:1 ratio to veliparib plus carboplatin/paclitaxel, veliparib plus temozolomide, and placebo plus carboplatin/paclitaxel. The ORR was superior in the veliparib plus carboplatin/paclitaxel group (77.8%) versus placebo plus carboplatin/paclitaxel group (61.3%), P = .027. The median PFS and OS were numerically higher in the group containing veliparib than placebo, but without a statistically significant difference. There was no significant increase in toxicity, comparing the addition of veliparib in the two carboplatin/paclitaxel groups. The veliparib plus temozolomide group was compared with placebo plus carboplatin/paclitaxel. Temozolomide plus veliparib was inferior in terms of ORR, median PFS, and median OS.39 The first results of the phase III trial (BROCADE3) were presented at the European Society for Medical Oncology (ESMO) 2019 Congress and published in 2020. The study included patients with HER2-negative advanced/metastatic BC with ⩽2 previous treatment lines and a germline BRCA1/2 mutation. Patients (n = 509) were randomized in a 2:1 ratio to carboplatin AUC 6 on day 1 and paclitaxel 80 mg/m2 on days 1, 8, and 15, with or without veliparib 120 mg bid on days 2 to 5 in a 21-day cycle. Patients with no progression during the chemotherapy phase maintained veliparib or placebo 300 to 400 mg/day. Prior platinum exposure was allowed. The median PFS was superior in the veliparib group: 14.5 months (95% CI: 12.5-17.7) versus 12.6 months (95% CI: 10.6-14.4), HR: 0.71 (95% CI: 0.57-0.88), P = .0016. The authors verified a durable benefit with a 3-year PFS rate of 25.7% (95% CI: 20.3-31.4) versus 10.7% (95% CI: 5.8-17.3). There were no significant differences in median OS (33.5 vs 28.2 months, HR: 0.95, P = .67), and the ORR was 75.8% and 74.1%, respectively. Emesis, neutropenia, anemia, and thrombocytopenia were the most frequent side effects, occurring similarly between the 2 arms.40 A subgroup analysis of hormone receptor-positive and mTNBC was presented in ESMO Breast Cancer Congress 2020: 243 patients (48%) were TNBC, and in this subgroup, the median PFS was 16.6 months (95% CI: 12.3-22.7) in the veliparib arm versus 14.1 months (95% CI: 11.0-15.8) in placebo, HR: 0.72 (95% CI: 0.52-1.00), P = .051. The benefit was durable, with a PFS rate at 3 years of 35.3% (95% CI: 27.2-43.6) versus 13.0% (95% CI: 5.3-24.2). The median OS was 35.0 and 30.0 months, respectively.48 There were no significant differences in the two subgroups relating QoL evaluation through EORTC QLQ-C30, QLQ-BR23, EQ-5D-5L, and Brief Pain Inventory.49
This year, the results from SWOG S1416 were presented at the American Society of Clinical Oncology 2020 annual meeting. This phase II study evaluated the combination of cisplatin 75 mg/m2 with veliparib or placebo 400 mg bid days 1 to 14 in a 21-day-cycle in pretreated with <1 prior line. Three groups of mTNBC (n = 335) were analyzed: germline BRCA-mutated (n = 37), BRCA-like with HRD evaluated by 4 somatic or germline biomarkers (n = 101), and non-BRCA-like (n = 110). In germline BRCA-mutated, the median PFS difference was not statistically different, although with a better numerically median PFS. In non-BRCA-like patients, there was no benefit when veliparib was associated with cisplatin. In the BRCA-like group, the median PFS was higher in patients treated with veliparib plus cisplatin (5.7 months) in comparison with placebo (4.3 months), HR: 0.58, P = .023. The ORR and median PFS were numerically better but without significant difference. Grade 3 to 4 CTCAE hematological side effects were higher in veliparib groups.41 The last 3 trials evaluated the combination of a platinum agent with a PARP inhibitor. There are no data regarding the comparison of a PARP inhibitor and platinum drugs in mTNBC and specifically in patients with BRCA1/2 mutations. A study in this setting would be extremely relevant to guide the better treatment for this subgroup of patients, including the choice of drug, drug sequence, and to know whether it needs a combination approach. This knowledge is even more critical in countries where patients do not have access to the new targeted drugs like PARP inhibitors, and platinum drugs might have an essential role. So, it is crucial to know the specific role of platinum, PARP inhibitor, or their combination in the future.
Combining PARP inhibitors with ICIs is another combination strategy under investigation with promising results and an acceptable safety profile. The MEDIOLA trial is a phase II basket study that evaluated olaparib plus durvalumab in patients with advanced solid tumors. The metastatic BC with germline BRCA1/2 mutations cohort was presented. Patients (n = 34) were submitted to olaparib 300 mg bid for 4 weeks, and after that, 300 mg bid plus durvalumab 1.5 g every 4 weeks until disease progression. The disease control rate (DCR) was 80% (24 of 30) at 12 weeks and 50% (15 of 30) at 28 weeks. The ORR was 63.3% in the overall population, with better results in patients without previous lines and only 1 prior line. The median PFS was 8.2 months (95% CI: 4.6-11.8), and the median OS was 20.5 months (95% CI: 16.2-23.9). The most frequent grade ⩾3 side effects were anemia, neutropenia, and pancreatitis.42 Another PARP inhibitor, niraparib 200 mg id, was evaluated in association with pembrolizumab 200 mg in a 21-cycle in TOPACIO/Keynote-162 phase II study. Preliminary results involving 54 patients (12 with BRCA1/2 mutation) revealed an ORR of 29% and a DCR of 49%. The ORR was 33% in programmed death-ligand 1 (PD-L1) positive with a combined positive score (CPS) ⩾1% and 15% in PD-L1 negative. Patients had durable responses with 13 patients (6 with BRCA1/2 mutations) with >6 months on treatment.43 The DORA trial (NCT03167619) is a phase II study that evaluates olaparib’s role alone or combined with durvalumab as maintenance therapy in patients with advanced TNBC treated with platinum drugs. The primary endpoint is PFS.50 It warrants more data on these 2-drug combinations after these promising preliminary results.
The combination of PARP inhibitors with other targeted drugs or with radiation therapy is also under investigation. The VIOLETTE study (NCT03330847) is evaluating the efficacy and safety of olaparib in association with AZD1775 (a WEE1 checkpoint inhibitor) or with AZD6738 (an ataxia telangiectasia and Rad3-related protein inhibitor), two DNA damage repair agents, in patients with mTNBC with ⩽2 prior lines.51 The RadioPARP study (NCT03109080) is evaluating the combination of olaparib plus radiation therapy in TNBC.52
Immune Checkpoint Inhibitors
Triple-negative breast cancer is the BC subtype that has demonstrated the highest incidence of TILs.6 The high TILs are also associated with a better prognosis and better treatment response in the early stage in TNBC.53 Tumors may suppress T-cell activity by activating inhibitory checkpoint pathways, limiting the antitumor immune response. The ICIs target the T-cells’ regulatory pathways to enhance antitumor immune responses, as the examples of the cytotoxic T-lymphocyte antigen 4, programmed death-1, or lymphocyte antigen 3.54 The associated immunogenicity related to high TILs led to clinical trials using ICIs in metastatic and early TNBC, with the essential characteristics described in Table 2. The safety and antitumor efficacy of pembrolizumab 10 mg/kg were initially evaluated in a phase Ib trial in patients with heavily treated TNBC with PD-L1 ⩾1%. This trial included 32 patients, and pembrolizumab demonstrated an acceptable toxicity profile and an ORR of 18.5%.55 A phase II clinical trial was then developed in patients with previously pretreated mTNBC (cohort A) and untreated mTNBC (cohort B). It evaluated the efficacy and safety of pembrolizumab 200 mg in a 21-day cycle. In cohort A, 170 patients were treated in second or later lines. The ORR was 5.3% (2.7-9.9) in all patients, and the median duration of response (DOR) was not reached when data were published. Grade 3 to 4 AEs occurred in 12.9%.56 In cohort B, 84 patients with untreated mTNBC without central nervous system metastases and PD-L1 CPS ⩾1% were treated with pembrolizumab. The ORR was 21.4% (95% CI: 13.9-31.4), and the DCR was 23.8% (95% CI: 15.9-34.0). The median DOR was 10.4 months, with 44.4% responses ongoing at the cutoff, and the median PFS was 2.1 months and median OS 18.0 months. Grade 3 AEs occurred in 9.5% of patients without grade 4 AEs, with a manageable safety profile.57
Table 2.
Selected published immune checkpoint inhibitor clinical trials in the mTNBC population.
| Author, study | n | Phase | Population | Treatment (experimental vs compared drug) | Target | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors | ||||||||
| Nanda et al,55 (Keynote-012) | 32 | Ib | Heavily pretreated mTNBC | Pembrolizumab | PD-L1 | 18.5% | 1.9 | 11.2 |
| Adams et al,56 (Keynote-086 cohort A) | 170 | II | Pretreated mTNBC | Pembrolizumab | PD-L1 | 5.3% | 2.0 | 9.0 |
| Adams et al,57 (Keynote-086 cohort B) | 84 | II | Untreated mTNBC | Pembrolizumab | PD-L1 | 21.4% | 2.1 | 18.0 |
| Cortés et al,58 (Keynote-119) | 622 | III | Pretreated mTNBC | Pembrolizumab vs standard chemotherapy | PD-L1 | ITT: 9.6% vs 10.6% CPS ⩾1%: 12.3% vs 9.4% CPS ⩾10%: 17.7% vs 9.2% |
NS (2.1 vs 3.3) | NS (9.9 vs 10.8) |
| Immune checkpoint inhibitors plus chemotherapy | ||||||||
| Schmid et al,59 (IMpassion130) | 902 | III | Untreated advanced TNBC | Nabpaclitaxel plus atezolizumab vs plus placebo | PD-L1 | 56.0% vs 45.9% PD-L1 ⩾1%: 58.9% vs 42.6% |
7.2 vs 5.5 PD-L1 ⩾1%: 7.5 vs 5.0 |
NS ITT: 21.0 vs 18.7 PD-L1 ⩾1%: 25.4 vs 17.9 |
| Cortes et al,60 (Keynote-355) | 847 | III | Untreated mTNBC | Chemotherapy (taxane or carboplatin/gemcitabine) plus pembrolizumab vs plus placebo | PD-L1 | – | CPS ⩾10%: 9.7 vs 5.6 | – |
| Miles et al,61 (Impassion131) | 651 | III | Untreated advanced TNBC | Paclitaxel plus atezolizumab vs plus placebo | PD-L1 | – | NS PD-L1 ⩾1%: 6.0 vs 5.7 ITT: 5.7 vs 5.6 |
NS PD-L1 ⩾1%: 22.1 vs 28.3 ITT: 19.2 vs 22.8 |
| Voorwerk et al,62 (TONIC) | 70 | II | mTNBC | Nivolumab preceded by induction treatments: irradiation to a single lesion, low-dose cyclophosphamide, cisplatin, or doxorubicin, or a 2-week waiting period | PD-L1 Microenvironment |
Overall population: 20% Cisplatin: 23% Doxorubicin: 35% |
1.9 | – |
Abbreviations: CPS, combined positive score; ITT, intention to treat; mTNBC, metastatic triple-negative breast cancer; NS, not significant; ORR, overall response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; TNBC, triple-negative breast cancer.
Key results: Immune checkpoint inhibitors in monotherapy have demonstrated low ORR, without positive PFS and OS results compared with standard chemotherapy. Immune checkpoint inhibitors plus chemotherapy showed some conflicting results: atezolizumab plus nabpaclitaxel revealed higher ORR and PFS in PD-L1 positive. In contrast, atezolizumab plus paclitaxel did not reveal a benefit in survival compared with placebo plus chemotherapy. The PFS was also higher with pembrolizumab plus different backbone chemotherapy in PD-L1 positive, comparing with placebo. These trials did not prolong the OS.
A phase III trial (Keynote-119) compared pembrolizumab 200 mg in a 21-day cycle versus single-agent chemotherapy (capecitabine, eribulin, gemcitabine, or vinorelbine) in previously treated patients with mTNBC (1 or 2 previous lines, anthracycline and/or taxane). The study included 622 patients. The median OS and PFS were not significantly superior when treated with pembrolizumab, in intention to treat (ITT) population, in CPS ⩾1% or ⩾10%. Regarding OS, although not significant, the HR was inferior per PD-L1 percentage (0.78 in CPS ⩾10%, 0.86 in CPS ⩾1%, and 0.97 in the ITT population). An exploratory analysis demonstrated benefit in OS in patients with CPS ⩾20% (14.9 vs 12.5 months, HR: 0.58 [95% CI: 0.38-0.88]). The duration of the response was higher with pembrolizumab. This trial demonstrated a trend toward pembrolizumab to respond accordingly to PD-L1 enrichment. The safety profile was favorable in pembrolizumab, with a grade 3 to 5 CTCAE AEs 14% in pembrolizumab versus 36% in the chemotherapy arm.58 Immune checkpoint inhibitors have shown the role of PD-L1 positive in selecting patients for ICIs as it is associated with better clinical outcomes. Immune checkpoint inhibitors as monotherapy have demonstrated low ORR in previously pretreated patients with better results in first-line setting. Indeed, ICIs as monotherapy in later lines are currently not recommended outside a clinical trial.36
The combination of ICIs with chemotherapy in first-line setting is under evaluation and has already been approved. The first phase III trial in this situation was IMpassion130. Patients (n = 902) with untreated advanced TNBC, that relapsed >12 months after completion of early-stage treatment, were treated with nabpaclitaxel 100 mg/m2 on days 1, 8, and 15 with atezolizumab or placebo 840 mg on days 1 and 15 (28-day cycle) in a 1:1 ratio. The median PFS was superior in the atezolizumab group: 7.2 versus 5.5 months in the placebo group, HR: 0.80 (95% CI: 0.69-0.92), P = .002. In the PD-L1-positive subgroup (the expression on tumor-infiltrating immune cells ⩾1%), the median PFS was also higher in atezolizumab: 7.5 versus 5.0 months, HR: 0.62 (95% CI: 0.49-0.78), P < .001. The ORR was 56.0% in atezolizumab versus 45.9% in placebo, P = .002, and 58.9% in PD-L1 positive treated with atezolizumab and 42.6% when treated with placebo, P = .002. Although not statistically significant, the median OS was numerically higher in the immunotherapy group: 21.3 versus 17.6 months, HR: 0.84 (95% CI: 0.69-1.02), P = .08. In the PD-L1-positive subgroup, formal testing for OS was not performed. The median OS was 25.0 months in atezolizumab, and 15.5 months in placebo, HR: 0.62 (95% CI: 0.45-0.86). The most frequent AEs were similar between the two groups, with a higher hypothyroidism rate in the atezolizumab group.59 The final OS analysis was presented recently with a median follow-up time of 18.8 months, and 73.8% died. In ITT, the median OS was 21.0 months in the atezolizumab arm versus 18.7 months in placebo, HR: 0.87 (95% CI: 0.75-1.02), P = .0770. Although not statically significant, numerically median OS was superior in the PD-L1 population with a 7.5-month median improvement: 25.4 versus 17.9 months, HR: 0.67 (95% CI: 0.53-0.86).63 The nabpaclitaxel plus atezolizumab combination is already approved and recommended for mTNBC with PD-L1-positive patients.16,36 Another phase III trial (Keynote-355) compared pembrolizumab with chemotherapy (nabpaclitaxel, paclitaxel, or gemcitabine/carboplatin) versus placebo plus chemotherapy in the first line. The study included 847 patients with mTNBC, with >6 months from completion of early-staged treatment. This study allowed chemotherapy beyond taxanes, including carboplatin-based treatment, which has an important role in these patients. The PFS analysis was done by a hierarchical model by PD-L1 status using CPS. The median PFS was superior in the pembrolizumab arm in patients with CPS ⩾10%, 9.7 versus 5.6 months, HR: 0.65 (95% CI: 0.49-0.86), P = .0012. The estimated PFS at 12 months was 39.1% in the pembrolizumab group and 23.0% in the placebo group. According to statistics prespecified methods, although with a numerically superior median PFS, PFS was not significantly superior in the CPS ⩾1% subgroup (7.6 and 5.6 months, P = .0014) and was not compared in the ITT population. The benefits of pembrolizumab plus chemotherapy were irrespective of chemotherapy protocol in a subgroup analysis. The OS analysis is ongoing. The rate of grade 3 to 5 AEs was comparable between the 2 arms (68.1% and 66.9%), with no new safety concerns.64,60 In November 2020, based on the Keynote-355 trial, the Food and Drug Administration (FDA) approved pembrolizumab plus chemotherapy for advanced irresectable or metastatic TNBC with CPS ⩾10%.65
The IMpassion130 and Keynote-355 have some concerns that might limit the use of their criteria in clinical practice. They excluded patients with early relapses, and IMpassion130 used nabpaclitaxel to avoid corticoid exposure. Still, this drug is no currently the standard of care, and it would be difficult to have access to this drug in some countries.66 A phase III trial (IMpassion131) evaluates the combination of atezolizumab and paclitaxel in first-line treatment for mTNBC. A phase III trial (IMpassion132) evaluated the combination of atezolizumab and chemotherapy (carboplatin/gemcitabine or capecitabine) in patients with early relapses (within 12 months after completion of the curative intention treatment).67-69 The primary results of the IMpassion131 trial were presented at ESMO 2020 Congress. Patients (n = 651) were randomized in a 2:1 ratio for atezolizumab 840 mg on days 1 and 15 or placebo plus paclitaxel 90 mg/m2 on days 1, 8, and 15 every 28 days. Programmed death-ligand 1 status was evaluated through immune cell expression, positive if ⩾1%, and the primary endpoint is the PFS test hierarchically first in the PD-L1 positive and then in the ITT population. The median PFS was not significantly different between the two arms in the PD-L1 positive or ITT population. In the PD-L1 positive, the median PFS was 5.7 months in the placebo and 6.0 months in atezolizumab, HR: 0.82 (95% CI: 0.60-1.12), P = .20. The median OS was not significantly different between the 2 groups analyzed in PD-L1 and the ITT population. In PD-L1 positive, median OS was 28.3 months in the placebo arm and 22.1 months in the atezolizumab arm, HR: 1.12 (95% CI: 0.76-1.65). Grade ⩾3 side effects were balanced in each arm.61 Contradictory to IMpassion130, combination of atezolizumab with paclitaxel did not improve survival in PD-L1 or in ITT population. The ways why the combination of atezolizumab with nabpaclitaxel improved survival but not with paclitaxel are still unknown. In the future, it will be necessary to understand this question.
The IM agents can also play a role when associated with ICIs. A phase II multicohort trial (TONIC) evaluated some strategies to improve the ICI efficacy. Patients (n = 70) were randomized to 1 of the 5 arms with different induction treatments: irradiation to a single lesion, low-dose cyclophosphamide, cisplatin, or doxorubicin a 2-week waiting period, and after that were submitted to a biopsy and treated with nivolumab. The ORR was 20%, and the median DOR was 9 months (95% CI: 4.7-not reached). The induction cisplatin and doxorubicin arms were associated with a better ORR, 23% and 35%, respectively, which may induce a more favorable tumor microenvironment and increased probability nivolumab responses. The responders had higher levels of TILs and higher levels of CD8 and PD-L1, and low levels of Ca15-3. An increased T-cell infiltration after induction, a higher T-cell receptor clonality, and upregulation of inflammation-related signatures were observed in cisplatin and doxorubicin.62 This trial enhances the importance of microenvironment and other IM-associated agents.
Anti-AR Antagonists
The AR promotes cell proliferation in TNBC and is an emerging biomarker in mTNBC.70 Some trials studied the role of anti-AR antagonists in advanced TNBC (Table 3). Bicalutamide, an oral AR antagonist, was the first drug studied in AR-positive mTNBC. In a phase II study (TBCRC011), AR was positive when immunohistochemistry (IHC) staining was >10%, which corresponds to 12% of all screened patients (n = 51). This trial included 28 patients. The clinical benefit rate (CBR) was the primary endpoint. The CBR at 6 months was 19% (95% CI: 7-39). The median PFS was 12 weeks. The most common AEs were fatigue, hot flashes, limb edema, and aspartate and alkaline aminotransferase elevations.71 Abiraterone is an irreversible and potent inhibitor of CYP17. Abiraterone 1000 mg plus prednisolone 5 mg bid was evaluated in a phase II study (UCBG 12-1) in patients with AR-positive (⩾10% by IHC staining) advanced TNBC. The percentage of AR-positive was 37.6%, but only 34 patients entered the study. The CBR was 20% (95% CI: 7.7-38.6) at 6 months, and the ORR was 6.7% (95% CI: 0.8-22.1). The median PFS was 2.8 months (95% CI: 1.7-5.4). The most frequent AEs were fatigue, hypertension, hypokalemia, and nausea, mostly grades 1 and 2.72 Seviteronel, another inhibitor of CYP17, was studied in a phase I trial with an acceptable safety profile.80 A phase II clinical trial (TBCRC) in advanced TNBC with AR expression evaluated the enzalutamide, a potent AR inhibitor. AR was positive if >0%, which was present in 80% of patients, and negative when 0%. The study involves 118 patients, with 78 of them with evaluable possible. At 16 weeks, the CBR was 33% (23-45) and 22% (19-39) at 24 weeks. The ORR was 8%, the median PFS was 3.3 months (95% CI: 1.9-4.1), and the median OS was 17.6 months (95% CI: 11.6-not reached) in the evaluable group. The most frequent AEs were fatigue, nausea, and decreased appetite.73 The combination of bicalutamide with CDK4/6 palbociclib (NCT02605486) and ribociclib (NCT03090165) is studying in AR-positive metastatic BC in phase II trials.81,82
Table 3.
Selected published clinical trials using targeted drugs in the mTNBC population.
| Author, study | n | Phase | Population | Treatment (experimental vs compared drug) | Target | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|
| AR antagonists | ||||||||
| Gucalp et al,71 (TBCRC011) | 28 | II | Metastatic AR-positive TNBC | Bicalutamide | AR | CBR at 6 months: 19% | 12 weeks | – |
| Bonnefoi et al,72 (UCBG 12-1) | 34 | II | Metastatic AR-positive TNBC | Abiraterone plus prednisolone | AR | CBR at 6 months: 20% ORR: 6.7% |
2.8 | – |
| Traina et al,73 | 78 | II | Metastatic AR-positive TNBC | Enzalutamide | AR | CBR at 16 weeks: 33% | 3.3 | 17.6 |
| AKT inhibitors | ||||||||
| Kim et al,74 (LOTUS) | 166 | II | Metastatic untreated TNBC | Paclitaxel plus ipatasertib vs plus placebo | PI3K/AKT/PTEN | ITT: 40% vs 32% PTEN-low: 48% vs 23% PIK3CA/AKT1/PTEN-altered: 50% vs 44% |
ITT: 6.2 vs 4.9 PTEN-low: NS (6.2 vs 3.7) PIK3CA/AKT1/PTEN-altered: 9.0 vs 4.9 |
– |
| Schmid et al,75 (PAKT) | 140 | II | Metastatic untreated TNBC | Paclitaxel plus capivasertib vs plus placebo | PI3K/AKT/PTEN | ITT: 34.8% vs 28.8% PIK3CA/AKT1/PTEN-altered: 35.3% vs 18.2% |
ITT: NS (5.9 vs 4.2) PIK3CA/AKT1/PTEN-altered: 9.3 vs 3.7 |
ITT: 19.1 vs 12.6 PIK3CA/AKT1/PTEN-altered: NS (NR vs 10.4) |
| Dent et al,76 (IPATunity130) | 255 | III | PIK3CA/AKT1/PTEN-altered advanced irresectable or metastatic TNBC | Paclitaxel plus ipatasertib vs plus placebo | PI3K/AKT/PTEN | 39% vs 35% | NS (7.4 vs 6.1) | – |
| PI3K/AKT pathway plus AR antagonists | ||||||||
| Lehmann et al,77 (TBCRC032) | 17 | Ib/II | Metastatic AR-positive TNBC | Enzalutamide with/without taselisib | AR PI3K/AKT/PTEN |
CBR at 16 weeks: 35.7% | 3.4 | – |
| Antibody-drug conjugate | ||||||||
| Bardia et al,78 (IMMU-132-01) | 108 | I/II | Heavily pretreated mTNBC | Sacituzumab-govitecan | Trop-2 | 33.3% | 5.5 | 13.0 |
| Bardia et al,79 (ASCENT) | 529 | III | Heavily pretreated mTNBC | Sacituzumab-govitecan vs standard chemotherapy | Trop-2 | 35% vs 5% | 5.6 vs 1.7 | 12.1 vs 6.7 |
Abbreviations: AR, androgen receptor; CBR, clinical benefit rate; ITT, intention to treat; mTNBC, metastatic triple-negative breast cancer; NR, not reached; NS, not significant; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PI3K/AKT/PTEN: phosphatidylinositol 3-kinase/AKT/phosphatase and tensin homolog; TNBC, triple-negative breast cancer; Trop-2: trophoblast cell-surface antigen 2.
Key results: Androgen receptor antagonists showed preliminary data in a poor prognostic subpopulation, with a CBR between 20% and 33% between 4 and 6 months. In combination with paclitaxel, AKT inhibitors have shown better ORR and PFS in 2 phase II trials in patients with PIK3CA/AKT1/PTEN alterations. Nevertheless, a phase III trial was negative when ipatasertib was added to paclitaxel comparing with placebo in PIK3CA/AKT1/PTEN-altered advanced irresectable or metastatic TNBC. Sacituzumab-govitecan has demonstrated a higher ORR, PFS, and OS than standard chemotherapy in heavily pretreated patients.
PI3K/AKT Pathway in mTNBC
Genetic alterations in the phosphatidylinositol 3-kinase/AKT/mammalian target of the rapamycin (PI3K/AKT/mTOR) pathway are frequent in all BC subtypes, including TNBC.11 The Cancer Genome Atlas Network reported 7% PIK3CA mutations, 35% PTEN mutations/loss, and 30% INPP4B loss in basal-like BC subtype.83 In patients with TNBC, 70% harbored a PIK3CA, AKT1, or a PTEN aberration, and PI3K mutation is more frequent in the LAR subtype.84 Buparlisib is a pan-class PI3K inhibitor studied as first-line treatment with paclitaxel in HER2-negative advanced BC in phase II/III trial (BELLE-4). The addition of buparlisib did not improve PFS, and the trial was stopped at the end of the phase II part.85
Ipatasertib is an oral AKT inhibitor studied in a randomized phase II study in advanced or metastatic untreated TNBC (LOTUS). Patients (n = 166) were randomized in a 1:1 ratio to paclitaxel 80 mg/m2 on days 1, 8, and 15 plus ipatasertib 400 mg or placebo on days 1 to 21 in a 28-day cycle. The median PFS was superior in the ipatasertib group: 6.2 versus 4.9 months, HR: 0.60 (95% CI: 0.37-0.98), P = .037. In the 48 PTEN-low tumor patients, the median PFS was 6.2 months in ipatasertib and 3.7 months in placebo, HR: 0.59 (95% CI: 0.26-1.32), P = .18. In PIK3CA/AKT1/PTEN-altered tumors, the median PFS was 9.0 months in ipatasertib versus 4.9 months in placebo, HR: 0.44 (95% CI: 0.20-0.99), P = .041. In ITT, the ORR was 40% in ipatasertib and 32% in placebo. The median DOR was comparable in ITT and PTEN-low but was higher in PIK3CA/AKT1/PTEN-altered tumors (11.2 vs 6.1 months). The most frequently documented grade 3 AEs were diarrhea and neutropenia, with a higher proportion of all grade ⩾3 AEs (54% vs 42%). There was no difference in QoL using EORTC QLQ-C30.74 Another AKT inhibitor, capivasertib, was evaluated in combination with paclitaxel as first-line mTNBC therapy in phase II randomized trial (PAKT). Patients (n = 140) were randomized to paclitaxel 90 mg/m2 on days 1, 8, and 15 plus capivasertib or placebo 400 mg bid on days 2 to 5, 9 to 12, and 16 to 19 in a 28-day cycle. The median PFS was 5.9 months in the capivasertib arm and 4.2 months in the placebo arm, HR: 0.74 (95% CI: 0.50-1.08), P = .11. In PIK3CA/AKT1/PTEN-altered subgroup, the median PFS was 9.3 months with capivasertib versus 3.7 months with placebo, HR: 0.30 (95% CI: 0.11-0.79), P = .01. The ORR in the ITT population was 34.8% in capivasertib, and 28.8% in placebo. In PIK3CA/AKT1/PTEN-altered subgroup, the ORR was 35.3% in capivasertib versus 18.2% in placebo. The median OS was significantly higher in the capivasertib arm in the ITT population: 19.1 versus 12.6 months, HR: 0.61 (95% CI: 0.37-0.99), P = .04. The median OS was not reached in capivasertib-treated patients with PIK3CA/AKT1/PTEN-alterations. Diarrhea, neutropenia, infection, rash, and fatigue were the most frequent AEs associated with capivasertib.75
As we can see in these phase II referred trials (Table 3), the addition of an AKT inhibitor to paclitaxel in first line prolongs the PFS, especially in patients with PIK3CA/AKT1/PTEN-altered tumors. The phase III clinical trial (IPATUNITY130) evaluates the combination of ipatasertib and paclitaxel versus placebo and paclitaxel in PIK3CA/AKT1/PTEN-altered advanced irresectable or metastatic untreated TNBC (cohort A) or hormone receptor–positive/HER2-negative BC. The primary endpoint was investigator-accessed PFS.86 The primary results from this trial were published at the end of 2020. In cohort A, 255 patients were enrolled, 51% with PIK3CA/AKT1 mutations and 49% with PTEN alterations. With a median follow-up of 8.3 months and 33% patients remaining on treatment, there was no difference in PFS between the 2 groups: median PFS 7.4 months (95% CI: 5.6-8.5) in the ipatasertib arm and 6.1 months (95% CI: 5.5-9.0) in the placebo arm, HR: 1.02 (95% CI: 0.71-1.45), P = .9237. The proportion of grade ⩾3 AEs was similar in the 2 arms (46% and 44%). The most frequent AEs were diarrhea (80% and 31%), alopecia (46% and 44%), and nausea (36% and 23%).76 These results did not corroborate the previous results from phase II trials. A phase III randomized trial is also investigating the clinical benefit and safety of capivasertib plus paclitaxel combination in first-line treatment in patients with mTNBC (CAPItello290).87
PI3K/AKT pathway inhibitors are also under investigation in combination with other drugs. Luminal androgen receptor subtype is enriched in PIK3CA mutations (40%-50%). Enzalutamide combined with taselisib (PI3K inhibitor) was studied in a phase Ib/II study (TBCRC032) in AR-positive mTNBC. In phase II, 17 pretreated patients were randomized to receive enzalutamide 160 mg with or without taselisib 4 mg. Hyperglycemia and rash were the most frequent AEs. All the patients had disease progression at 16 weeks, and the CBR was 35.7% in the combination arm compared with no one in the enzalutamide arm. The median PFS was 3.4 months. There were no different results when patients were stratified using PIK3CA mutations. The LAR signature corresponded to a better clinical benefit (75.0%) compared with 12.5% in other subtypes.77 Ipatasertib is also under investigation combined with atezolizumab and paclitaxel in phase III randomized trial (IPATUNITY170) in untreated advanced or mTNBC.88
Antibody-Drug Conjugates
The ADC is recently being studied in mTNBC and is showing promising results. There are some ADCs in ongoing trials, as the examples of sacituzumab-govitecan, ladiratuzumab-vedotin, trastuzumab-deruxtecan, AVID100, U3-1402, CAB-ROR2-ADC, Anti-CA6-DM4 immunoconjugate, and glembatumumab-vedotin. Almost all these ADCs are under investigation in early phase trials, except sacituzumab-govitecan and trastuzumab-deruxtecan. There are phase III clinical trials evaluating the last two drugs.89
Sacituzumab-Govitecan
Sacituzumab-govitecan is an ADC that incorporates a humanized monoclonal antibody (hRS7) that targets the target human trophoblast cell-surface antigen 2 (Trop-2) and enables the internalization and delivery of SN-38 (govitecan). This is an active metabolite of irinotecan, a topoisomerase I inhibitor. Trophoblast cell-surface antigen 2 is highly overexpressed in mTNBC and has limited expression in normal tissues.90 Sacituzumab-govitecan delivers more SN-38 than irinotecan, which may overcome RAD51-mediated HRR pathway in Trop-2-expressing tumors. Also, sacituzumab-govitecan delivers SN-38 in its most active nonglucuronidated form, explaining the lower incidence of diarrhea with sacituzumab-govitecan compared with irinotecan.91 A phase I/II basket study (IMMU-132-01) evaluated it in previously treated metastatic cancers. The metastatic BC cohort was published at the end of the year 2019. This trial included 108 heavily treated mTNBC patients with median previous lines of 3 (range: 2-10). They were treated with 10 mg/kg of the studied drug on days 1 and 8 in a 21-day cycle. The most frequent grade ⩾3 side effects were anemia and neutropenia and febrile neutropenia. Nausea and diarrhea were frequent any-grade side effects. The ORR was 33.3% (95% CI: 24.6-43.1), and the median DOR was 7.7 months. Median PFS was 5.5 months (95% CI: 4.1-6.3) and the median OS 13.0 months (95% CI: 11.2-13.7).78 A phase III confirmatory trial (ASCENT) in heavily pretreated mTNBC (⩾2 lines) has compared sacituzumab-govitecan versus physician’s choice chemotherapy (capecitabine, gemcitabine, vinorelbine, and eribulin). This study was stopped earlier this year due to significant potential benefits in these patients. The results of 529 patients enrolled were recently presented. The primary endpoint is PFS in the brain metastasis–negative population. The median PFS was 5.6 months in sacituzumab-govitecan versus 1.7 months in the physician’s choice chemotherapy arm (HR: 0.41; P < .0001). The median OS was also higher in the experimental drug arm: 12.1 versus 6.7 months (HR: 0.48; P < .0001). The most frequent grade ⩾3 side effects were neutropenia (51% vs 31%), diarrhea (10.5% vs <1%), anemia (8% vs 5%), and febrile neutropenia (6% vs 2%). No treatment-related deaths were reported.79 A postbiomarker analysis revealed that the clinical benefit was independent of Trop-2 expression, accessed using IHC.92 The FDA approved the sacituzumab-govitecan for patients who have received at least 2 previous treatment lines in the mTNBC setting.93 Ongoing multiple trials are testing the combination of sacituzumab-govitecan with chemotherapy, talazoparib, and immunotherapy.91
Other Targeted Pathways
Epidermal growth factor repair is highly expressed in basal TNBC subtypes. Epidermal growth factor repair inhibitors were previously evaluated but with disappointing results.94 The role of angiogenesis is also being questioned in TNBC, mostly in neoadjuvant strategies and metastatic disease. Some trials have demonstrated a benefit in PFS, although without benefit in OS.16 Ongoing trials are evaluating the combination of bevacizumab plus chemotherapy or ICIs. Cabozantinib, a multiple tyrosine kinase inhibitor, including MET and vascular endothelial growth factor receptor 2, was evaluated in mTNBC in a phase II study, including 35 patients. The CBR was 31% (95% CI: 17%-49%), and the median PFS was 1.9 months (95% CI: 1.3-3.3).95 More recently, cabozantinib was evaluated in combination with nivolumab, but without achieving the primary endpoint of ORR.96
Mitogen-activated protein kinase activity is 1 of the mechanisms of resistance to taxane-based chemotherapy. This pathway was evaluated in TNBC in a phase II study (COLET). Cobimetinib (MEK inhibitor) was combined with paclitaxel as first-line treatment in advanced TNBC in multiple cohorts. Cohort I compared paclitaxel plus cobimetinib versus paclitaxel plus placebo in 90 patients. The median PFS was 5.5 months for the combination strategy versus 3.8 months, HR: 0.73 (95% CI: 0.43-1.24), P = .2. The ORR was 38.3% versus 20.9%, and the safety profile was expected for both drugs.97 Atezolizumab was combined with cobimetinib and taxane in cohorts II (paclitaxel, n = 63) and III (nabpaclitaxel, n = 62). The ORR was 34% in cohort II (44% in PD-L1 positive and 11% in PD-L1 negative) and 29% in cohort III (33% in PD-L1 positive and 27% in PD-L1 negative). The 6-month PFS was 40.5% in cohort II and 50.1% in cohort III.98
Trilaciclib is a cell cycle selective inhibitor of cyclin-dependent kinases-4/6 that transiently maintain immune cells, hemopoietic stem, and progenitor cells in G1 arrest. So, it is believed to have the potential to optimize antitumor activity and minimize myelotoxicity. The drug was studied in a phase II clinical trial in recurrent or mTNBC with ⩽2 previous lines. Patients (n = 142) were randomized in a 1:1:1 ratio to gemcitabine 100 mg/m2 and carboplatin AUC 2 on days 1 and 8 (group 1), gemcitabine and carboplatin plus trilaciclib 240 mg/m2 on days 1 and 8 (group 2) or on days 1, 2, 8, and 9 on a 21-day cycle. No significant difference was observed in myelosuppression endpoints. Regarding efficacy, median OS was superior in trilaciclib groups: 17.8 months (8.8-not reached) in group 3, 20.1 months (9.4-not reached) in group 2, and 12.6 months in group 1. It was believed that the induced decreased activity of FOXM1, overexpressed in TNBC, induced by trilaciclib, leading to increased sensitivity for chemotherapy, contributed to a better outcome.99
Conclusions
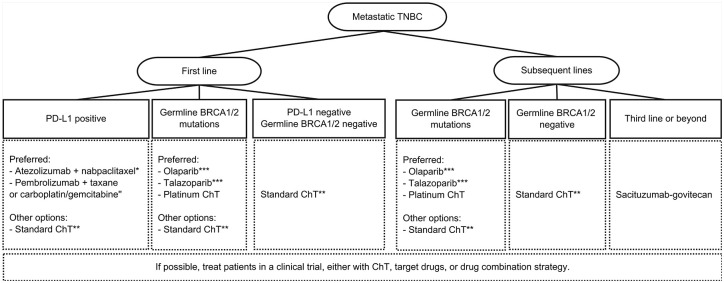
Metastatic TNBC is a heterogeneous disease that used to be treated only with chemotherapy-based strategies. The discovery of genomic alterations like BRCA1/2 mutations, HRD, and the molecular subtypes has led to the development of promising drugs in this disease. Indeed, it is changing the way how we treat mTNBC. Platinum-based chemotherapy is noninferior to other systemic chemotherapy in mTNBC and has better outcomes than a taxane in BRCA1/2-mutated patients regarding ORR and PFS. A platinum drug should be considered in these patients. PARP inhibitors olaparib and talazoparib were both approved for patients with germline BRCA1/2 mutations and metastatic BC. The combinations of PARP inhibitors and chemotherapy or ICIs are under investigation with promising results. Questions remain on what patients should be treated with PARP inhibitors as monotherapy or in combination therapy. Perhaps, PARP inhibitors may have a role in maintenance therapy after platinum chemotherapy leading to chemotherapy-free interval for the patients. Immune checkpoint inhibitors demonstrated clinical benefit associated with chemotherapy in patients with PD-L1 positive. They should be used in the first-line setting, with the two combinations approved: atezolizumab plus nabpaclitaxel or pembrolizumab plus various chemotherapy protocols (nabpaclitaxel, paclitaxel, and carboplatin plus gemcitabine). Also, in the first-line setting, two AKT inhibitors—ipatasertib and capivasertib associated with paclitaxel—have demonstrated promising outcomes in patients with PI3K/AKT pathway alterations in phase II trials. Nevertheless, a phase III trial IPATunity was negative regarding PFS, with immature OS data and other studies ongoing. Androgen receptor antagonists in the LAR subtype showed encouraging results in a poor prognostic subpopulation, with the need for more data in this setting. The ADC sacituzumab-govitecan also showed clinical benefit in heavily pretreated patients and is already approved by the FDA for the third line or beyond in the mTNBC setting. The incorporation into earlier lines is awaited as well as in combination with other drugs. A proposed algorithm that includes approved therapies for treating mTNBC is described in Figure 1. Other targeted drugs and combinations of some referred drugs are under investigation and will bring us results shortly. Knowing the tumor’s biology and targeting drugs in a personalized medicine era brings us new hope for patients with mTNBC.
Figure 1.
Proposed treatment algorithm for the treatment of mTNBC, including the approved drugs.
*If PD-L1 ⩾1% and disease-free survival from (neo)adjuvant ChT >12 months.
"If PD-L1(CPS) ⩾10% and disease-free survival from (neo)adjuvant ChT >6 months.
If both PD-L1 and germline BRCA1/2 are positive, there are no data on which is the best approach.
**Standard chemotherapy includes taxanes, anthracyclines, antimetabolites, and microtubule inhibitors. Carboplatin is an alternative with comparable efficacy and a better toxicity profile than docetaxel. A drug combination or single-agent chemotherapy could be used, depending on the disease’s extension or rapid progression.
***If previous anthracycline and a taxane in the (neo)adjuvant or metastatic setting.
ChT indicates chemotherapy; CPS, combined positive score; mTNBC, metastatic triple-negative breast cancer; PD-L1, programmed death-ligand 1; TNBC, triple-negative breast cancer.
Footnotes
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: ÉC and AM contributed to the conception and design of the work; ÉC was responsible for the acquisition and analysis of data and the draft of the article; AM critically revised the article; and both authors approved the final version of the article.
ORCID iD: Élia Cipriano  https://orcid.org/0000-0003-2838-5777
https://orcid.org/0000-0003-2838-5777
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2. Henry NL, Shah PD, Haider I, Freer PE, Jagsi R, Sabel MS. Cancer of the breast. In: Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE, eds. Abeloff’s Clinical Oncology. 6th ed. Philadelphia, PA: Elsevier; 2020:1560-1603.e12. doi: 10.1016/B978-0-323-47674-4.00088-8. [DOI] [Google Scholar]
- 3. Sharma P. Biology and management of patients with triple-negative breast cancer. Oncologist. 2016;21:1050-1062. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borri F, Granaglia A. Pathology of triple negative breast cancer [published online ahead of print June 13, 2020]. Semin Cancer Biol. doi: 10.1016/j.semcancer.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 5. Petrucelli N, Daly MBPT. BRCA1- | BRCA2-associated hereditary breast and ovarian cancer. GeneReviews. https://www.ncbi.nlm.nih.gov/books/NBK1247/. Updated 2016. Accessed December 31, 2020.
- 6. Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol. 2016;2:1354-1360. doi: 10.1001/jamaoncol.2016.1061. [DOI] [PubMed] [Google Scholar]
- 7. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938-1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 8. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429-4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9. Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750-2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehmann BD, Pietenpol JA. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast. 2015;24:S36-S40. doi: 10.1016/j.breast.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sporikova Z, Koudelakova V, Trojanec R, Hajduch M. Genetic markers in triple-negative breast cancer. Clin Breast Cancer. 2018;18:e841-e850. doi: 10.1016/j.clbc.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 12. Bareche Y, Venet D, Ignatiadis M, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29:895-902. doi: 10.1093/annonc/mdy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burstein MD, Tsimelzon A, Poage GM, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688-1698. doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fougner C, Bergholtz H, Norum JH, Sørlie T. Re-definition of Claudin-low as a breast cancer phenotype. Nat Commun. 2020;11:1-11. doi: 10.1038/s41467-020-15574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29:1634-1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. NCCN guidelines . Breast cancer. NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Updated 2020. Accessed July 11, 2020.
- 17. Lyons TG, Traina TA. Emerging novel therapeutics in triple-negative breast cancer. Adv Exp Med Biol. 2019;1152:377-399. doi: 10.1007/978-3-030-20301-6_20. [DOI] [PubMed] [Google Scholar]
- 18. Fleck O, Nielsen O. DNA repair. J Cell Sci. 2004;117:515-517. doi: 10.1242/jcs.00952. [DOI] [PubMed] [Google Scholar]
- 19. Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12:68-78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez-Angulo AM, Timms KM, Liu S, et al. Incidence and outcome of BRCA mutations in unselected patients with triple receptor-negative breast cancer. Clin Cancer Res. 2011;17:1082-1089. doi: 10.1158/1078-0432.CCR-10-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Armstrong N, Ryder S, Forbes C, Ross J, Quek RG. A systematic review of the international prevalence of BRCA mutation in breast cancer. Clin Epidemiol. 2019;11:543-561. doi: 10.2147/CLEP.S206949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fostira F, Tsitlaidou M, Papadimitriou C, et al. Prevalence of BRCA1 mutations among 403 women with triple-negative breast cancer: implications for genetic screening selection criteria: a Hellenic Cooperative Oncology Group Study. Breast Cancer Res Treat. 2012;134:353-362. doi: 10.1007/s10549-012-2021-9. [DOI] [PubMed] [Google Scholar]
- 23. Rummel S, Varner E, Shriver CD, Ellsworth RE. Evaluation of BRCA1 mutations in an unselected patient population with triple-negative breast cancer. Breast Cancer Res Treat. 2013;137:119-125. doi: 10.1007/s10549-012-2348-2. [DOI] [PubMed] [Google Scholar]
- 24. Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2 -associated hereditary breast and ovarian cancer summary genetic counseling suggestive findings. GeneReviews. Seattle, WA: University of Washington; 1993:1-37. [Google Scholar]
- 25. Hatano Y, Tamada M, Matsuo M, Hara A. Molecular trajectory of BRCA1 and BRCA2 mutations. Front Oncol. 2020;10:361. doi: 10.3389/fonc.2020.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tung NM, Garber JE. BRCA1/2 testing: therapeutic implications for breast cancer management. Br J Cancer. 2018;119:141-152. doi: 10.1038/s41416-018-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielsen FC, van Overeem Hansen T, Sørensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer. 2016;16:599-612. doi: 10.1038/nrc.2016.72. [DOI] [PubMed] [Google Scholar]
- 28. Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415-421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nik-Zainal S, Davies H, Staaf J, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47-54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Polak P, Kim J, Braunstein LZ, et al. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476-1486. doi: 10.1038/ng.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomssen C, Lüftner D, Untch M, et al. International consensus conference for advanced breast cancer, Lisbon 2019: ABC5 consensus—assessment by a German group of experts. Breast Care. 2020;15:82-95. doi: 10.1159/000505957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerratana L, Fanotto V, Pelizzari G, Agostinetto E, Puglisi F. Do platinum salts fit all triple negative breast cancers. Cancer Treat Rev. 2016;48:34-41. doi: 10.1016/j.ctrv.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 33. Torrisi R, Zuradelli M, Agostinetto E, et al. Critical reviews in oncology / hematology platinum salts in the treatment of BRCA -associated breast cancer: a true targeted chemotherapy ? Crit Rev Oncol / Hematol. 2019;135:66-75. doi: 10.1016/j.critrevonc.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 34. Isakoff SJ, Mayer EL, He L, et al. TBCRC009: a multicenter phase II clinical trial of platinum monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33:1902-1909. doi: 10.1200/JCO.2014.57.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT trial. Nat Med. 2018;24:628-637. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 2020;31:1623-1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523-533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 38. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a Germline BRCA mutation. N Engl J Med. 2018;379:753-763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han HS, Diéras V, Robson M, et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann Oncol. 2018;29:154-161. doi: 10.1093/annonc/mdx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diéras VC, Han HS, Kaufman B, et al. LBA9—phase III study of veliparib with carboplatin and paclitaxel in HER2-negative advanced/metastatic gBRCA-associated breast cancer. Ann Oncol. 2019;30:v857-v858. doi: 10.1093/annonc/mdz394.008. [DOI] [Google Scholar]
- 41. Sharma P, Rodler E, Barlow WE, et al. Results of a phase II randomized trial of cisplatin +/- veliparib in metastatic triple-negative breast cancer (TNBC) and/or germline BRCA-associated breast cancer (SWOG S1416). J Clin Oncol. 2020;38:1001. doi: 10.1200/JCO.2020.38.15_suppl.1001. [DOI] [Google Scholar]
- 42. Domchek S, Postel-Vinay S, Im S-A, et al. Phase II study of olaparib (O) and durvalumab (D) (MEDIOLA): updated results in patients (pts) with germline BRCA-mutated (gBRCAm) metastatic breast cancer (MBC). Ann Oncol. 2019;30:v477. doi: 10.1093/annonc/mdz253.017. [DOI] [Google Scholar]
- 43. Vinayak S, Tolaney SM, Schwartzberg LS, et al. TOPACIO/Keynote-162: niraparib + pembrolizumab in patients (pts) with metastatic triple-negative breast cancer (TNBC), a phase 2 trial. J Clin Oncol. 2018;36:1011. doi: 10.1200/JCO.2018.36.15_suppl.1011. [DOI] [Google Scholar]
- 44. Egger SJ, Willson ML, Morgan J, et al. Platinum-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2017;6:CD003374. doi: 10.1002/14651858.CD003374.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mateo J, Lord CJ, Serra V, et al. A decade of clinical development of PARP inhibitors in perspective. Ann Oncol. 2019;30:1437-1447. doi: 10.1093/annonc/mdz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tung NM, Boughey JC, Pierce LJ, et al. Management of hereditary breast cancer: American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Guideline. J Clin Oncol. 2020;38:2080-2106. doi: 10.1200/JCO.20.00299. [DOI] [PubMed] [Google Scholar]
- 47. Somlo G, Frankel PH, Arun BK, et al. Efficacy of the PARP inhibitor veliparib with carboplatin or as a single agent in patients with germline BRCA1- or BRCA2-associated metastatic breast cancer: California Cancer Consortium Trial NCT01149083. Clin Cancer Res. 2017;23:4066-4076. doi: 10.1158/1078-0432.CCR-16-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ayoub J-P, Friedlander ML, Dieras VC, et al. 140O veliparib plus carboplatin-paclitaxel in patients with HER2-negative advanced/metastatic gBRCA-associated breast cancer: results in hormone receptor-positive and triple-negative breast cancer subgroups from the phase III BROCADE3 trial. Ann Oncol. 2020;31:S65. doi: 10.1016/j.annonc.2020.03.241. [DOI] [Google Scholar]
- 49. Diéras V, Han HS, Kaufman B, et al. Veliparib with carboplatin and paclitaxel in (BRCA)-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:1269-1282. doi: 10.1016/S1470-2045(20)30447-2. [DOI] [PubMed] [Google Scholar]
- 50. Sammons S, Tan TJY, Traina TA, et al. Dora: a randomized phase II multicenter maintenance study of olaparib alone or olaparib in combination with durvalumab in platinum responsive advanced triple-negative breast cancer (aTNBC). J Clin Oncol. 2019;37:TPS1113. doi: 10.1200/JCO.2019.37.15_suppl.TPS1113. [DOI] [Google Scholar]
- 51. Tutt A, Stephens C, Frewer P, et al. VIOLETTE: a randomized phase II study to assess DNA damage response inhibitors in combination with olaparib (Ola) vs Ola monotherapy in patients (pts) with metastatic, triple-negative breast cancer (TNBC) stratified by alterations in homologous recombinat. J Clin Oncol. 2018;36:TPS1116. doi: 10.1200/JCO.2018.36.15_suppl.TPS1116. [DOI] [Google Scholar]
- 52. Kirova YM, Loirat D, Berger F, et al. Radioparp: a phase I of olaparib with radiation therapy (RT) in patients with inflammatory, locoregionally advanced or metastatic triple-negative breast cancer (TNBC) or patient with operated TNBC with residual disease—preliminary results. J Clin Oncol. 2020;38:571. doi: 10.1200/JCO.2020.38.15_suppl.571. [DOI] [Google Scholar]
- 53. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19:40-50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 54. Pennock GK, Chow LQM. The evolving role of immune checkpoint inhibitors in cancer treatment. Oncologist. 2015;20:812-822. doi: 10.1634/theoncologist.2014-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nanda R, Chow LQM, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib keynote-012 study. J Clin Oncol. 2016;34:2460-2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:397-404. doi: 10.1093/annonc/mdy517. [DOI] [PubMed] [Google Scholar]
- 57. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30:405-411. doi: 10.1093/annonc/mdy518. [DOI] [PubMed] [Google Scholar]
- 58. Cortés J, Lipatov O, Im S-A, et al. KEYNOTE-119: phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol. 2019;30:v859-v860. doi: 10.1093/annonc/mdz394.010. [DOI] [Google Scholar]
- 59. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379:2108-2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 60. Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817-1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 61. Miles DW, Gligorov J, André F, et al. LBA15 Primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC) ± atezolizumab (atezo) for unresectable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31:S1147-S1148. doi: 10.1016/j.annonc.2020.08.2243. [DOI] [PubMed] [Google Scholar]
- 62. Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920-928. doi: 10.1038/s41591-019-0432-4. [DOI] [PubMed] [Google Scholar]
- 63. Emens LA, Adams S, Barrios CH, et al. LBA16 IMpassion130: final OS analysis from the pivotal phase III study of atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in previously untreated locally advanced or metastatic triple-negative breast cancer. Ann Oncol. 2020;31:S1148. doi: 10.1016/j.annonc.2020.08.2244. [DOI] [Google Scholar]
- 64. Cortes J, Cescon DW, Rugo HS, et al. KEYNOTE-355: randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38:1000. doi: 10.1200/JCO.2020.38.15_suppl.1000. [DOI] [PubMed] [Google Scholar]
- 65. FDA grants accelerated approval to pembrolizumab for locally recurrent unresectable or metastatic triple negative breast cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-pembrolizumab-locally-recurrent-unresectable-or-metastatic-triple. Accessed December 31, 2020.
- 66. Kang C, Syed YY. Atezolizumab (in combination with Nab-Paclitaxel): a review in advanced triple-negative breast cancer. Drugs. 2020;80:601-607. doi: 10.1007/s40265-020-01295-y. [DOI] [PubMed] [Google Scholar]
- 67. Cortüs J, André F, Gonçalves A, et al. IMpassion132 phase III trial: atezolizumab and chemotherapy in early relapsing metastatic triple-negative breast cancer. Future Oncol. 2019;15:1951-1961. doi: 10.2217/fon-2019-0059. [DOI] [PubMed] [Google Scholar]
- 68. Dent R, Andre F, Goncalves A, et al. IMpassion132: a double-blind randomized phase 3 trial evaluating chemotherapy (CT) ± atezolizumab (atezo) for early progressing locally advanced/metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2018;36:TPS1115. doi: 10.1200/JCO.2018.36.15_suppl.TPS1115. [DOI] [Google Scholar]
- 69. Miles D, Andre F, Gligorov J, et al. 317TiP—IMpassion131: phase III study comparing 1L atezolizumab with paclitaxel vs placebo with paclitaxel in treatment-naive patients with inoperable locally advanced or metastatic triple negative breast cancer (mTNBC). Ann Oncol. 2017;28:v105. doi: 10.1093/annonc/mdx365.080. [DOI] [Google Scholar]
- 70. Salvi S, Bonafè M, Bravaccini S. Androgen receptor in breast cancer: a wolf in sheep’s clothing? A lesson from prostate cancer. Semin Cancer Biol. 2020;60:132-137. doi: 10.1016/j.semcancer.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 71. Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19:5505-5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bonnefoi H, Grellety T, Tredan O, et al. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1). Ann Oncol. 2016;27:812-818. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 73. Traina TA, Miller K, Yardley DA, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36:884-890. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim SB, Dent R, Im SA, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017;18:1360-1372. doi: 10.1016/S1470-2045(17)30450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the PAKT trial. J Clin Oncol. 2020;38:423-433. doi: 10.1200/JCO.19.00368. [DOI] [PubMed] [Google Scholar]
- 76. Dent R, Kim S-B, Oliveira M, et al. Double-blind placebo-controlled randomized phase III trial evaluating first-line ipatasertib combined with paclitaxel for PIK3CA/AKT1/PTEN-altered locally advanced unresectable or metastatic triple-negative breast cancer (aTNBC): primary results from IPAT. In: SABCS 2020. https://www.abstractsonline.com/pp8/#!/9223/presentation/672. Updated 2020.
- 77. Lehmann BD, Abramson VG, Sanders ME, et al. TBCRC 032 IB/II multicenter study: molecular insights to AR antagonist and PI3K inhibitor efficacy in patients with AR+ metastatic triple-negative breast cancer. Clin Cancer Res. 2020;26:2111-2123. doi: 10.1158/1078-0432.CCR-19-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741-751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 79. Bardia A, Tolaney SM, Loirat D, et al. LBA17 ASCENT: a randomized phase III study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31:S1149-S1150. doi: 10.1016/j.annonc.2020.08.2245. [DOI] [Google Scholar]
- 80. Bardia A, Gucalp A, DaCosta N, et al. Phase 1 study of seviteronel, a selective CYP17 lyase and androgen receptor inhibitor, in women with estrogen receptor-positive or triple-negative breast cancer. Breast Cancer Res Treat. 2018;171:111-120. doi: 10.1007/s10549-018-4813-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gucalp A, Proverbs-Singh TA, Corben A, et al. Phase I/II trial of palbociclib in combination with bicalutamide for the treatment of androgen receptor (AR)+ metastatic breast cancer (MBC). J Clin Oncol. 2016;34:TPS1103. doi: 10.1200/JCO.2016.34.15_suppl.TPS1103. [DOI] [Google Scholar]
- 82. Kwapisz D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: palbociclib, ribociclib, and abemaciclib. Breast Cancer Res Treat. 2017;166:41-54. doi: 10.1007/s10549-017-4385-3. [DOI] [PubMed] [Google Scholar]
- 83. Koboldt DC, Fulton RS, McLellan MD, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61-70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Millis SZ, Gatalica Z, Winkler J, et al. Predictive biomarker profiling of > 6000 breast cancer patients shows heterogeneity in TNBC, with treatment implications. Clin Breast Cancer. 2015;15:473-481e3. doi: 10.1016/j.clbc.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 85. Martín M, Chan A, Dirix L, et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann Oncol. 2017;28:313-320. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 86. Dent R, Kim S-B, Oliveira M, et al. IPATunity130: a pivotal randomized phase III trial evaluating ipatasertib (IPAT) + paclitaxel (PAC) for PIK3CA/AKT1/PTEN-altered advanced triple-negative (TN) or hormone receptor-positive HER2-negative (HR+/HER2–) breast cancer (BC). J Clin Oncol. 2018;36:TPS1117. doi: 10.1200/JCO.2018.36.15_suppl.TPS1117. [DOI] [Google Scholar]
- 87. Schmid P, Cortes J, Robson ME, et al. A phase III trial of capivasertib and paclitaxel in first-line treatment of patients with metastatic triple-negative breast cancer (CAPItello290). J Clin Oncol. 2020;38:TPS1109. doi: 10.1200/JCO.2020.38.15_suppl.TPS1109. [DOI] [Google Scholar]
- 88. A study Of Ipatasertib in combination with atezolizumab and paclitaxel as a treatment for participants with locally advanced or metastatic triple-negative breast cancer. https://clinicaltrials.gov/ct2/show/NCT04177108. Accessed August 5, 2020.
- 89. Nagayama A, Vidula N, Ellisen L, Bardia A. Novel antibody-drug conjugates for triple negative breast cancer. Ther Adv Med Oncol. 2020;12. doi: 10.1177/1758835920915980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. 2018;9:28989-29006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019-1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hurvitz S, Tolaney S, Punie K, Bardia A. Biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. In: SABCS 2020. https://www.abstractsonline.com/pp8/#!/9223/presentation/674. Updated 2020.
- 93. Wahby S, Fashoyin-Aje L, Osgood CL, et al. FDA approval summary: accelerated approval of Sacituzumab govitecan-hziy for third line treatment of metastatic triple-negative breast cancer (mTNBC). Clin Cancer Res. 2020;27:1-5. doi: 10.1158/1078-0432.CCR-20-3119. [DOI] [PubMed] [Google Scholar]
- 94. Nakai K, Hung MC, Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am J Cancer Res. 2016;6:1609-1623. [PMC free article] [PubMed] [Google Scholar]
- 95. Tolaney SM, Ziehr DR, Guo H, et al. A phase II study of cabozantinib for metastatic triple-negative breast cancer (TNBC). J Clin Oncol. 2015;33:1080. doi: 10.1200/jco.2015.33.15_suppl.1080. [DOI] [Google Scholar]
- 96. Barroso-Sousa R, Trippa L, Li T, et al. Abstract P3-09-10: a phase II study of nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2020;80:P30910. doi: 10.1158/1538-7445.SABCS19-P3-09-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brufsky A, Miles D, Zvirbule Z, et al. Abstract P5-21-01: cobimetinib combined with paclitaxel as first-line treatment for patients with advanced triple-negative breast cancer (COLET study): primary analysis of cohort I. 2018. Cancer Res:;78:P52101. doi: 10.1158/1538-7445.SABCS17-P5-21-01. [DOI] [Google Scholar]
- 98. Brufsky A, Kim S-B, Zvirbule Z, et al. Phase II COLET study: atezolizumab (A) + cobimetinib (C) + paclitaxel (P)/nab-paclitaxel (nP) as first-line (1L) treatment (tx) for patients (pts) with locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2019;37:1013. doi: 10.1200/JCO.2019.37.15_suppl.1013. [DOI] [Google Scholar]
- 99. Tan AR, Wright GS, Thummala AR, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019;20:1587-1601. doi: 10.1016/S1470-2045(19)30616-3. [DOI] [PubMed] [Google Scholar]