Abstract
Background:
C9orf72 hexanucleotide repeat expansions are associated with widespread cerebral alterations, including white matter alterations. However, there is lack of information on changes in commissure fibres. Diffusion tensor imaging (DTI) can identify amyotrophic lateral sclerosis (ALS)-associated patterns of regional brain alterations at the group level. The objective of this study was to investigate the structural connectivity of the corpus callosum (CC) in ALS patients with C9orf72 expansions.
Methods:
DTI-based white matter mapping was performed by a hypothesis-guided tractwise analysis of fractional anisotropy (FA) maps for 25 ALS patients with C9orf72 expansion versus 25 matched healthy controls. Furthermore, a comparison with a patient control group of 25 sporadic ALS patients was performed. DTI-based tracts that originate from callosal sub-areas I to V were identified and correlated with clinical data.
Results:
The analysis of white matter integrity demonstrated regional FA reductions for tracts of the callosal areas II and III for ALS patients with C9orf72 expansions while FA reductions in sporadic ALS patients were observed only for tracts of the callosal area III; these reductions were correlated with clinical parameters.
Conclusion:
The tract-of-interest-based analysis showed a microstructural callosal involvement pattern in C9orf72-associated ALS that included the motor segment III together with frontal callosal connections, as an imaging signature of the C9orf72-associated overlap of motor neuron disease and frontotemporal pathology.
Keywords: amyotrophic lateral sclerosis, C9orf72 expansion, diffusion tensor imaging, magnetic resonance imaging, motor neuron disease
Introduction
Amyotrophic lateral sclerosis (ALS) is the most frequent adult onset motor neuron disease, simultaneously affecting upper motor neurons and lower motor neurons in the brain and spinal cord; the neurodegenerative syndrome shares pathobiological features with frontotemporal dementia (FTD) so that up to 15% of patients fulfil criteria of both diseases.1 In 5–10% of ALS patients, a positive family history for ALS can be detected (familial ALS, fALS),2 and there is increasing evidence that the autosomal dominant inheritance of a hexanucleotide expansion in the first intron of the C9orf72 gene is the most common cause of fALS in people of Northern European ancestry and is also a major contributor to frontotemporal pathology in ALS.3–5 The most comprehensive neuroanatomical characterization of the C9orf72 genotype has been provided by detailed postmortem histopathology studies, but the search for in vivo biomarkers of disease onset and progression in C9orf72 repeat expansion carriers has also yielded promising candidates with a focus on neuroimaging.6 By use of diffusion tensor imaging (DTI), the quantification of the diffusion properties, typically described as fractional anisotropy (FA), has been used to evaluate the neuronal fibre integrity, directional coherence and tissue microstructure in the brain. Previous data-driven DTI studies in patients with C9orf72 expansion in cross-sectional and longitudinal design demonstrated alterations in motor tracts6–8; in addition, further white matter areas and several white matter tracts were found to be affected,9 for example, in the frontal white matter.8,10 A recent study demonstrated in vivo histopathological staging specifically in C9orf72-associated ALS by application of a tract of interest (TOI)-based DTI analysis technique.8,11,12
Corpus callosum (CC) degeneration is a recognised feature of ALS and has been consistently highlighted by post mortem studies and demonstrated repeatedly in vivo by computational neuroimaging as part of the widespread white matter changes in ALS.13–19 A recent study demonstrated in vivo histopathological staging specifically in C9orf72-associated ALS by application of a TOI-based DTI analysis technique.8,11,12 It has been demonstrated that genotype-specific changes in C9orf72 associated ALS can be observed in commissure fibres including the anterior CC.20 Pathology of callosal damage in ALS by an ex vivo 7T MRI study showed increased microglial cells in the body of the CC, especially in cases with C9orf72 mutations, and increased reactive astrocytes throughout the CC.21 Obviously, the fibre changes were not distributed equally along the callosal structure but had a distinct pattern along the CC in ALS.22 According to fibre tractography studies,23 the CC can be subdivided into five different structural areas: I–V. How ALS-associated fibre changes of the CC follow these structural areas and whether the CC affectation pattern is distinctly different between genotypes is unclear. Of special interest are those patients carrying C9orf72 mutations as these are expected to be associated with the most severe microstructural alterations and thus the most widespread white matter alterations.24
In the current study, the TOI-based analysis of DTI data was applied to the five different CC areas according to Hofer and Frahm in ALS patients with C9orf72 expansion, in order to investigate in vivo the specific callosal alteration pattern. 23
Materials and methods
Subjects and patient characteristics
A total of 25 ALS patients with confirmed C9orf72 expansion mutations were included. Clinically they met the revised El Escorial diagnostic criteria.25 Four of the C9orf72 expansion carriers fulfilled the criteria for ALS-FTD of the behavioural type (bvFTD) according to Rascovsky criteria.2 Details of demographics, clinical data including the revised ALS functional rating scale (ALS-FRS-R),26 slope of ALS-FRS-R as an indicator for disease progression, upper motor neuron (UMN) burden score,27 site of onset, and disease duration are summarized in Table 1.
Table 1.
Subject characteristics including disease duration, site of onset, ALS-FRS-R, disease progression rate (slope of ALS-FRS-R), UMN burden score,27 and cognitive profile measured by the ECAS.28.
| Gender (m/f) | Age (years) | Disease duration (months median) | Site of onset bulbar/spinal | ALS-FRS-R median | ALS-FRS-R slope median | UMN burden score | ECAS total score | |
|---|---|---|---|---|---|---|---|---|
| C9orf72-associated ALS | 17/8 | 63 ± 11 (range 35–79) | 12, range 3–37 | 10/15 | 41 (range 27–48) | −13/year range (−1 to −31)/year | 7 ± 6 range (2–14) | 30 ± 8 |
| Sporadic ALS | 14/11 | 64 ± 11 (range 31–83) | 11, range 5–28 | 7/18 | 43 (range 30–48) | −8/year range (−1 to −28)/year | 7 ± 4 range (2–14) | Not available |
| Controls | 18/7 | 63 ± 16 (range 26–80) | – | – | – | – | – | – |
| p | Kruskal–Wallis 0.62 | Kruskal–Wallis 0.81 | t test 0.98 | – | t test 0.80 | t test 0.64 | t test 0.16 |
ALS, amyotrophic lateral sclerosis ; ALS-FRS-R, revised ALS functional rating scale; ECAS, Edinburgh cognitive and behavioral ALS screen; f, female; m, male; UMN, upper motor neuron.
ALS patients with C9orf72 expansions were compared first, with a group of 25 age- and gender-matched controls and second, with a group of 25 sporadic ALS patients who had no family history of motor neuron disease (MND) and had been tested negative for the major genes related to ALS (Table 1). Gross brain pathology, including vascular brain alterations, could be excluded by conventional MRI including fluid attenuated inversion recovery sequences. All control individuals lacked a family history of neuromuscular disease and had no history of neurological, psychiatric or other major medical illnesses and were recruited from among spouses of patients and by word of mouth.
All participants provided written informed consent for the study protocol according to institutional guidelines which had been approved by the Ethics Committee of Ulm University, Germany (No. 19/12).
Genetic analysis
DNA was extracted from whole EDTA-containing venous blood samples as previously described. Analysis of the C9orf72 repeat length was performed by fragment length analysis and repeat-primed PCR (RP-PCR) using previously published primers.29 Since PCR-based methods cannot determine the size of larger expanded repeat-alleles, samples with a sawtooth pattern in the RP-PCR were further analysed using Southern blot.3
Magnetic resonance imaging acquisition
Magnetic resonance imaging (MRI) scans were obtained on a 1.5 Tesla Magnetom Symphony scanner (Siemens Medical, Erlangen, Germany). The DTI study protocol consisted of 52 volumes (64 slices, 128 × 128 pixels, slice thickness 2.8 mm, pixel size 2.0 mm × 2.0 mm), representing 48 gradient directions (b = 1000 s/mm2) and four scans with b = 0; TE and TR were 95 ms and 8000 ms, respectively.
Data analysis
DTI data were analysed with the software Tensor Imaging and Fibre Tracking (TIFT).30 The algorithms used in this study have been described previously in detail.11,12,31 In short, stereotaxic normalization to the Montreal Neurological Institute (MNI) space was performed iteratively by combined linear and non-linear registration using study-specific templates.32 From the stereotaxically normalized DTI data sets of all subjects, individual FA maps were calculated quantitatively. A Gaussian filter of 8 mm full width at half maximum was applied for smoothing of the individual FA maps in order to reduce residual individual morphological differences after stereotaxic normalization.33 Finally, data were corrected for the covariate age. Examples for stereotaxic CC matching are presented in Supplemental Figure S1.
From the MNI normalized data of 25 controls, an averaged data set was calculated; this data set was used for fibre tracking. That way, common FT masks could be applied to the individual FA maps. To this end, a conventional streamline tracking was used with a vector product threshold of 0.9 and an FA threshold of 0.2.34,35 Subsequently, the CC could be subdivided into five areas23: area I tracts are callosal fibres comprising bundles projecting into the prefrontal lobe, area II tracts are callosal fibres projecting to frontal areas including premotor and supplementary motor cortices, area III fibres project to the primary motor cortices, area IV fibres project to primary sensory cortices, and area V fibres project to parietal lobe, occipital lobe and temporal lobe. Defined tracts originating in CC areas I–V according to this scheme were identified with the TOI approach.17 Figure 1 shows the separate tracts, calculated from the averaged template (MNI normalized data of 25 controls), with starting seeds for fibre tracking depicted in the median sagittal slice (Supplemental Figure S1). The seed areas were set according to the Hofer and Frahm scheme with respect to an FA threshold of 0.2.23 This seed placement has been performed in callosal areas I-V with FT seed points restricted to the CC (Supplemental Figure S1). Supplemental Figure S2 shows fibre tracking-based end points of tracts originating in the callosal areas I–V. Tract-wise fractional anisotropy statistics (TFAS) were performed by statistically comparing the FA values in each tract between the two groups (Student’s t test),34,36 not considering FA-values below 0.2, since cortical grey matter shows FA values up to 0.2.35
Figure 1.
Tracts of the different segments of the CC with starting seeds for fibre tracking in callosal areas I–V of the median sagittal slice. The fibre tracts were calculated from the averaged data set from 25 controls. Tract volumes are given in mm3, calculated from the number of contributing voxels.
CC, corpus callosum.
A correlation analysis between individual tract-based FA-values and clinical and cognitive scores was performed with Pearson correlation. A threshold of p < 0.05 (two-tailed) was used for statistical interference.
Results
Structural changes in C9orf72 fALS patients
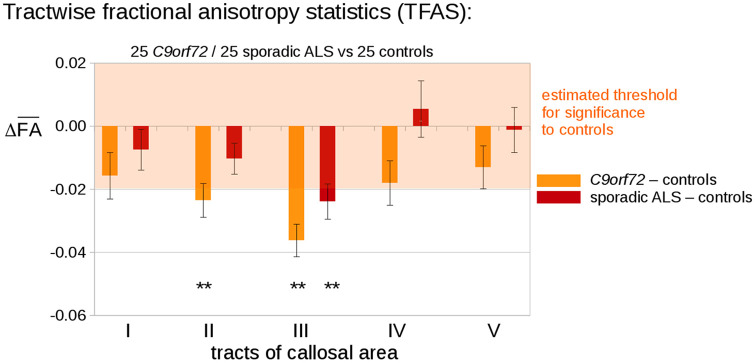
The analysis of the regional FA differences in the CC-associated tracts by use of TFAS showed significant differences in the averaged FA values between the C9orf72 fALS patients and the controls, as demonstrated in Figure 2. The differences were significant in the tracts of callosal areas II and III, while no significant alterations were detected in the tracts of callosal areas I, IV and V (Bonferroni-corrected for multiple comparisons). TFAS demonstrated significant differences in the averaged FA values between the sporadic ALS patients and the controls in the tracts of callosal area III, but not in the tracts of callosal area II (Figure 2). No significant alterations between C9orf72 fALS patients and sporadic ALS patients could be detected. Results for mean FA values and corresponding p values are summarized in Supplemental Table S1.
Figure 2.
TFAS at the group level for C9orf72 fALS patients compared with controls. TFAS demonstrated significant regional FA reductions in tracts of callosal areas II and III in C9orf72 fALS patients compared with matched controls and FA reductions in tracts of callosal area III in sporadic ALS patients. Error bars are SEM.
*p < 0.05, **p < 0.001 (Bonferroni-corrected).
ALS, amyotrophic lateral sclerosis; FA, fractional anisotropy; fALS, familial ALS; SEM, standard error of the mean; TFAS, tractwise fractional anisotropy statistics.
For the C9orf72 group, significant associations with ALS-FRS-R, UMN burden score and disease duration, respectively, were observed for the tracts of callosal area III, while no significant correlations resulted from the analysis of the tracts of the remaining callosal areas including area II. For the sporadic ALS group, significant associations with ALS-FRS-R and UMN burden score were also observed solely for the tracts of callosal area III. The results are summarized in Table 2. No associations were observed for site of onset and disease progression rate.
Table 2.
Association analysis of TFAS-based FA values of tracts of CC areas I–V with the clinical parameters (disease duration, ALS-FRS-R and UMN burden score).
| Clinical parameter | CC area I tracts | CC area II tracts | CC area III tracts | CC area IV tracts | CC area V tracts | |
|---|---|---|---|---|---|---|
| C9orf72-associated ALS | Disease duration | −0.21 | −0.09 | −0.51* | −0.14 | −0.11 |
| ALS-FRS-R | −0.04 | 0.12 | 0.40* | −0.19 | −0.02 | |
| UMN burden score | −0.02 | −0.43* | −0.61* | 0.00 | −0.10 | |
| Sporadic ALS | Disease duration | 0.25 | 0.09 | −0.01 | 0.07 | 0.27 |
| ALS-FRS-R | 0.23 | 0.39 | 0.43* | 0.20 | 0.14 | |
| UMN burden score | −0.36 | −0.56* | −0.52* | −0.38 | −0.31 |
Significant correlation.
ALS, amyotrophic lateral sclerosis ; ALS-FRS-R, revised ALS functional rating scale; CC, corpus callosum; FA, fractional anisotropy; TFAS, tractwise fractional anisotropy statistics; UMN, upper motor neuron.
Level analysis of callosal tracts originating in areas II and III
To provide a more comprehensive profile of the callosal tracts for CC areas II and III it was investigated whether there is higher abnormality at an extra-callosal level or at the level of the CC; to this end, regions were specified along the callosal tracts, separating into CC and extra-CC (Supplemental Figure S3, Supplemental Table S2). TFAS showed significant differences for the C9orf72 patients, related mostly to extra-callosal parts, both for tracts of areas II and III. For the sporadic ALS patients, significant differences from controls could be shown for callosal tracts of area III.
Association of structural and cognitive changes
FA values for the tracts of callosal areas I–V were correlated to the executive function score of the Edinburgh cognitive and behavioral ALS screen (ECAS).28 For the C9orf72 patients, a positive association was observed for tracts of callosal areas I (p = 0.02, R2 = 0.55) and II (p = 0.06, R2 = 0.46) to executive function, whereas tracts of callosal areas III, IV and V showed no association.
Discussion
The search for biomarkers in ALS with C9orf72 repeat expansion has yielded promising candidates in the neuroimaging domain, including thalamic atrophy, global loss of functional connectivity, global volume loss (ventricular atrophy, subcortical atrophy), diffuse cortical thinning and loss of white matter integrity.9,37,38 Recently, a hypothesis-driven DTI study showed a sequential corticoefferent spreading pattern in ALS with C9orf72 expansions according to the principles of the proposed neuropathological staging concept of ALS, which is based on cerebral TDP43 pathology.8 Given that data-driven DTI studies had demonstrated reduced integrity of frontal white matter and association tracts,6 the current study addressed the unresolved question of whether, and to what extent, there is a DTI-based association fibre in the CC signature of C9orf72 repeat expansion.
To this end, a hypothesis-guided TOI-based approach was applied to MRI data of patients with C9orf72 repeat expansion-associated ALS. This technique specifically addressed the callosal white matter fibres that fan out into the white matter of both hemispheres, that is, the radiation of the CC; here, the homotopic commissural connections that interconnect the frontal lobes pass in the anterior half of the CC, while the others pass in the posterior half.39 In order to reflect the DTI approach, the topological classification was performed following the scheme of Hofer and Frahm,23 which was also derived from a DTI-based tract-tracing algorithm.
The results demonstrated, both for the C9orf72 group and the sporadic ALS group that was included as a control sample, a predominant involvement of tracts of callosal area III, which also showed significant correlations with the clinical presentation (ALS-FRS-R, UMN burden score and disease duration, respectively), that is, involvement of the motor cortex; note that, in contrast to patients with sporadic ALS, tracts of callosal area II showed significant FA reductions only in C9orf72 patients. This finding indicates a more marked frontal involvement in familial C9orf72 patients than in sporadic ALS patients. Area II contains fibres projecting to frontal regions (including premotor and supplementary motor cortical areas), while area III comprises fibres projecting into the primary motor cortices. It has already been reported for sporadic ALS that the involvement of the CC is a consistent feature in MRI.22,40,41 More specifically, CC area III, that is, the motor segment,23 has been reported to demonstrate high T2 signal and regional atrophy at individual levels in routine structural MRI in MND,42 and has been discussed as an imaging marker in ALS.14,15 The current data are in agreement with previous studies,15,43 which showed associations of the callosal motor area with UMN burden score. In a recent T1w MRI-based texture analysis, the subtype (sporadic) primary lateral sclerosis (PLS) was associated with focused regional CC atrophy and texture alterations limited to the motor-related callosal area III.44 The tracts of the callosal area III showed significant FA reductions in C9orf72 patients as well as in sporadic ALS patients. In the light of the corticoefferent spread of pathology from the motor cortices in ALS, a separate analysis of the superior cortex-adjacent and the CC-adjacent parts of the tracts demonstrated more marked involvement (lower FA) of the former than the latter areas, indicating indirect support of the corticoefferent model both for C9orf72 patients and sporadic ALS patients. These findings are generally in agreement with previous DTI studies in ALS,45 but remain to be confirmed in a future study with higher subject numbers.
The current DTI study thus supports the segmental involvement of tracts originating from the CC in C9orf72 fALS. The anatomical involvement of the cerebral structures in C9orf72 repeat expansion-associated ALS corresponds to the TDP43 propagation pattern, as reported in the original description.46 Accordingly, it can be mapped by the TOI-based approach in vivo.8 The specific involvement of the fibres interconnecting the motor cortices is another element of the fingerprint of cerebral white matter alterations in fALS patients with C9orf72 expansion. These data are in accordance with the results of previous data-driven DTI studies,6 which showed a widespread reduction of white matter integrity in this patient subgroup. The involvement of area II – which could not be demonstrated in the sporadic ALS group – is of specific interest, indicating that fronto-temporal involvement as a part of the spectrum of C9orf72-associated disorders might be reflected by a reduction in FA in tracts originating from more frontal CC parts, which represent fibres homotopically interconnecting the bilateral frontal cortices.
These findings await confirmation in larger groups of C9orf72-positive patients with clinical presentation of frontal deficits. The concept is further supported by the association of executive dysfunction and changes in frontal callosal areas I and II. Executive dysfunction is the most prominent cognitive change in behavioral variant FTD patients and ALS,47,48 and executive functions are processed in dorsal frontal hemispheres that are interconnected by frontal callosal areas I and II. White matter alterations in fibres of dorsal frontal areas have been shown to be altered in association to executive dysfunction.49 We hereby present evidence that changes in frontal fibres can also be shown on the callosal level. Accordingly, it can be assumed that the pattern of FA changes in frontal parts of the CC in the four FTD patients resembles a correlate of executive dysfunction also in motor type ALS patients. It also underlines previous findings of white matter alterations in frontal areas that are seen in patients with executive dysfunction at early pathological stages of ALS.50
This study was not without limitations. There was no neuropathological confirmation of the neuroimaging results by autopsy. Future studies should include longitudinal data to investigate the possibility of tracking disease propagation, as previously demonstrated for staging schemes of sporadic ALS patients.17,50,51
In summary, the results of this study in C9orf72-fALS demonstrate a segmental CC involvement with predominance of callosal tracts II and III where the correlation to the clinical presentation (ALS-FRS-R, UMN burden score and disease duration) is dominated by callosal tract III alterations, that is, involvement of the motor cortex. This specific characterisation of commissural white matter tracts adds novel insights to an established facet of ALS pathology.19 From the clinical perspective, the involvement of the CC, and especially the callosal tracts of area II, might be utilized as a promising candidate imaging marker for cerebral white matter abnormalities in C9orf72 fALS patients,9 which also addresses the C9orf72-associated overlap of motor neuron disease and frontotemporal pathology. These findings may be of clinical relevance for future clinical trials, for example, persons known to carry the C9orf72 expansion could receive DTI-based analyses at a presymptomatic stage to determine the time point in the disease course when the in vivo detection of the segmental CC alterations is possible.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-4-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-5-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Acknowledgments
Sonja Fuchs is gratefully acknowledged for her help in the acquisition of MRI data. Kornelia Günther is gratefully acknowledged for her help in assembling the clinical data. The authors would like to thank the Ulm University Centre for Translational Imaging MoMAN for its support.
Footnotes
Author contributions: Hans-Peter Müller: Study concept and design, data analysis and interpretation of data, critical revision of manuscript for intellectual content.
Dorothée Lulé: Neuropsychology data analysis and interpretation of data, critical revision of manuscript for intellectual content.
Francesco Roselli: Interpretation of data, critical revision of manuscript for intellectual content.
Anna Behler: Interpretation of data, critical revision of manuscript for intellectual content.
Albert C. Ludolph: Interpretation of data, critical revision of manuscript for intellectual content.
Jan Kassubek: Study concept and design, interpretation of data, study supervision, drafting of manuscript.
Conflict of interest statement: The Associate Editor of Therapeutic Advances in Chronic Disease is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process. The authors declare that there is no conflict of interest.
Ethical approval: All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG Grant Number LU 336/15-1), the German Network for Motor Neuron Diseases (BMBF 01GM1103A) and Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE).
ORCID iD: Jan Kassubek  https://orcid.org/0000-0002-7106-9270
https://orcid.org/0000-0002-7106-9270
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hans-Peter Müller, Department of Neurology, University of Ulm, Ulm, Germany.
Dorothée Lulé, Department of Neurology, University of Ulm, Ulm, Germany.
Francesco Roselli, Department of Neurology, University of Ulm, Ulm, Germany.
Anna Behler, Department of Neurology, University of Ulm, Ulm, Germany.
Albert C. Ludolph, Department of Neurology, University of Ulm, Ulm, Germany
Jan Kassubek, Department of Neurology, University of Ulm, Oberer Eselsberg 45, Ulm, 89081, Germany.
References
- 1. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet 2017; 390: 2084–2098. [DOI] [PubMed] [Google Scholar]
- 2. Chiò A, Calvo A, Moglia C, et al. Phenotypic heterogeneity of amyotrophic lateral sclerosis: a population based study. J Neurol Neurosurg Psychiatry 2011; 82: 740–746. [DOI] [PubMed] [Google Scholar]
- 3. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9orf72 causes chromosome 9p-linked FTD and ALS. Neuron 2011; 72: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Renton AE, Majounie E, Waite A, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011; 72: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci 2014; 17: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Floeter MK, Danielian LE, Braun LE, et al. Longitudinal diffusion imaging across the C9orf72 clinical spectrum. J Neurol Neurosurg Psychiatry 2018; 89: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agosta F, Ferraro PM, Riva N, et al. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging 2017; 57: 206–219. [DOI] [PubMed] [Google Scholar]
- 8. Müller HP, Del Tredici K, Lulé D, et al. In vivo histopathological staging in C9orf72-associated ALS: a tract of interest DTI study. Neuroimage Clin 2020; 27: 102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Floeter MK, Gendron TF. Biomarkers for amyotrophic lateral sclerosis and frontotemporal dementia associated with hexanucleotide expansion mutations in C9orf72. Front Neurol 2018; 9: 1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westeneng HJ, Walhout R, Straathof M, et al. Widespread structural brain involvement in ALS is not limited to the C9orf72 repeat expansion. J Neurol Neurosurg Psychiatry 2016; 87: 1354–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kassubek J, Müller HP. Advanced neuroimaging approaches in amyotrophic lateral sclerosis: refining the clinical diagnosis. Expert Rev Neurother 2020; 20: 237–249. [DOI] [PubMed] [Google Scholar]
- 12. Kassubek J, Müller HP, Del Tredici K, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain 2014; 137: 1733–1740. [DOI] [PubMed] [Google Scholar]
- 13. Agosta F, Pagani E, Petrolini M, et al. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 2010; 31: 1457–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kassubek J, Ludolph AC, Müller HP. Neuroimaging of motor neuron diseases. Ther Adv Neurol Disord 2012; 5: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agosta F, Galantucci S, Riva N, et al. Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp 2014; 35: 1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller HP, Turner MR, Grosskreutz J, et al. A large-scale multicentre cerebral diffusion tensor imaging study in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2016; 87: 570–579. [DOI] [PubMed] [Google Scholar]
- 17. Kassubek J, Müller HP, Del Tredici K, et al. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J Neurol Neurosurg Psychiatry 2018; 89: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tu S, Wang C, Menke RAL, et al. Regional callosal integrity and bilaterality of limb weakness in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2020; 21: 396–402. [DOI] [PubMed] [Google Scholar]
- 19. Bede P, Siah WF, McKenna MC, et al. Consideration of C9orf72-associated ALS-FTD as a neurodevel-opmental disorder: insights from neuroimaging. J Neurol Neurosurg Psychiatry 2020; 91: 1138. [DOI] [PubMed] [Google Scholar]
- 20. Mahoney CJ, Beck J, Rohrer JD, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain 2012; 135: 736–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardenas AM, Sarlls JE, Kwan JY, et al. Pathology of callosal damage in ALS: an ex-vivo, 7 T diffusion tensor MRI study. Neuroimage Clin 2017; 15: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chapman MC, Jelsone-Swain L, Johnson TD, et al. Diffusion tensor MRI of the corpus callosum in amyotrophic lateral sclerosis. J Magn Reson Imaging 2014; 39: 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hofer S, Frahm J. Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006; 32: 989–994. [DOI] [PubMed] [Google Scholar]
- 24. Floeter MK, Bageac D, Danielian LE, et al. Longitudinal imaging in C9orf72 mutation carriers: relationship to phenotype. Neuroimage Clin 2016; 12: 1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ludolph A, Drory V, Hardiman O, et al. A revision of the El Escorial criteria - 2015. Amyotroph Lateral Scler Frontotemporal Degener 2015; 29: 1–2. [DOI] [PubMed] [Google Scholar]
- 26. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci 1999; 169: 13–21. [DOI] [PubMed] [Google Scholar]
- 27. Turner MR, Cagnin A, Turkheimer FE, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 2004; 15: 601–609. [DOI] [PubMed] [Google Scholar]
- 28. Lulé D, Burkhardt C, Abdulla S. et al. The Edinburgh cognitive and behavioural amyotrophic lateral sclerosis screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener 2015; 16: 16–23. [DOI] [PubMed] [Google Scholar]
- 29. Zondler L, Müller K, Khalaji S, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol 2016; 132: 391–411. [DOI] [PubMed] [Google Scholar]
- 30. Müller HP, Unrath A, Ludolph AC, et al. Preservation of diffusion tensor properties during spatial normalisation by use of tensor imaging and fibre tracking on a normal brain database. Phys Med Biol 2007; 52: N99–N109. [DOI] [PubMed] [Google Scholar]
- 31. Müller HP, Kassubek J. MRI-based mapping of cerebral propagation in amyotrophic lateral sclerosis. Front Neurosci 2018; 12: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Müller HP, Unrath A, Riecker A, et al. Intersubject variability in the analysis of diffusion tensor images at the group level: fractional anisotropy mapping and fiber tracking techniques. Magn Reson Imaging 2009; 27: 324–334. [DOI] [PubMed] [Google Scholar]
- 33. Unrath A, Müller HP, Riecker A, et al. Whole brain-based analysis of regional white matter tract alterations in rare motor neuron diseases by diffusion tensor imaging. Hum Brain Mapp 2010; 31: 1727–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Müller HP, Unrath A, Sperfeld AD, et al. Diffusion tensor imaging and tractwise fractional anisotropy statistics: quantitative analysis in white matter pathology. Biomed Eng Online 2007; 6: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kunimatsu A, Aoki S, Masutani Y, et al. The optimal trackability threshold of fractional anisotropy for diffusion tensor tractography of the corticospinal tract. Magn Reson Med Sci 2004; 3: 11–17. [DOI] [PubMed] [Google Scholar]
- 36. Müller HP, Grön G, Sprengelmeyer R, et al. Evaluating multicenter DTI data in Huntington’s disease on site specific effects: an ex post facto approach. Neuroimage Clin 2013; 2: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Floeter MK, Traynor BJ, Farren J, et al. Disease progression in C9orf72 mutation carriers. Neurology 2017; 89: 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chipika RH, Siah WF, McKenna MC, et al. The presymptomatic phase of amyotrophic lateral sclerosis: are we merely scratching the surface? J Neurol. Epub ahead of print 31 October 2020. DOI: 10.1007/s00415-020-10289-5. [DOI] [PubMed] [Google Scholar]
- 39. Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. Heidelberg: Steinkopff-Verlag, 2019. [Google Scholar]
- 40. Filippini N, Douaud G, Mackay CE, et al. Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 2010; 75: 1645–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Müller HP, Unrath A, Huppertz HJ, et al. Neuroanatomical patterns of cerebral white matter involvement in different motor neuron diseases as studied by diffusion tensor imaging analysis. Amyotroph Lateral Scler 2012; 13: 254–264. [DOI] [PubMed] [Google Scholar]
- 42. Osborn AG, Hedlund GL, Salzman KL. Osborn’s brain. Philadelphia, PA: Elsevier - Health Sciences Division, 2017. [Google Scholar]
- 43. Cirillo M, Esposito F, Tedeschi G, et al. Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol 2012; 33: 1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Müller HP, Dreyhaupt J, Roselli F, et al. Focal alterations of the callosal area III in primary lateral sclerosis: an MRI planimetry and texture analysis. Neuroimage Clin 2020; 26: 102223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gabel MC, Broad RJ, Young AL, et al. Evolution of white matter damage in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2020; 7: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013; 74: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011; 134: 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Phukan J, Elamin M, Bede P, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry 2012; 83: 102–108. [DOI] [PubMed] [Google Scholar]
- 49. Pettit LD, Bastin ME, Smith C, et al. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain 2013; 136: 3290–3304. [DOI] [PubMed] [Google Scholar]
- 50. Lulé D, Böhm S, Müller HP, et al. Cognitive phenotypes of sequential staging in amyotrophic lateral sclerosis. Cortex 2018; 101: 163–171. [DOI] [PubMed] [Google Scholar]
- 51. Kalra S, Müller H-P, Ishaque A, et al. A prospective harmonized multicentre DTI study of cerebral white matter degeneration in ALS. Neurology 2020; 95: e943-e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-2-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-3-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-4-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease
Supplemental material, sj-docx-5-taj-10.1177_20406223211002969 for Segmental involvement of the corpus callosum in C9orf72-associated ALS: a tract of interest-based DTI study by Hans-Peter Müller, Dorothée Lulé, Francesco Roselli, Anna Behler, Albert C. Ludolph and Jan Kassubek in Therapeutic Advances in Chronic Disease