Abstract
The clinical application of nanotechnology in medicine is promising for therapeutic, diagnostic, and surgical improvements in the near future. Nanotechnologies in nano-ophthalmology are in the early stages of application in clinical contexts, including ocular drug and gene delivery systems addressing eye disorders, particularly retinopathies. Retinal diseases are challenging to treat as current interventions, such as intravitreal injections, are limited by their invasive nature. This review examines nanotechnological approaches to retinal diseases in a clinical context. Nanotechnology has the potential to transform pharmacological and surgical interventions by overcoming limitations posed by the protective anatomical and physiological barriers that limit access to the retina. Preclinical research in the application of nanoparticles in diagnostics indicates that nanoparticles can enhance existing diagnostic and screening tools to detect diseases earlier and more easily and improve disease progression monitoring precision.
Keywords: nanomaterials, retina, gene therapy, drug delivery
Introduction
Nanotechnology has the potential to be applied within medicine 1–3 overall and in the field of ophthalmology4–6 to improve the safety and efficacy of current clinical practices. Gaining access to the retina is particularly challenging due to its anatomy and physiology.
The retina, the innermost layer of the eye, extends across the posterior two-thirds of the eye and contains protective inner and outer blood-retinal barriers (BRBs).7–9 Nanomaterials can be designed with characteristics and properties that allow them to cross the BRBs when delivered by topical installation or targeted intraocular injections.10
Several nanomaterials can improve the safety and efficacy of current treatments, therapies, and surgical procedures for retinal diseases and improve the detection speed and accuracy of retinal disease diagnoses. Drug delivery systems using nanomaterials in in vitro and in vivo models have been advantageous over other drug delivery systems due to efficacy and safety profile improvements. Nanomaterial-based delivery systems have demonstrated increased bioavailability, prolonged release, and reduced dosing or injection frequency relative to other delivery methods.11–13
Without early intervention that is effective and safe, retinal diseases can have progressive, visually debilitating consequences, including blindness. Two of the leading global causes of moderate to severe vision impairment, age-related macular degeneration (AMD) and diabetic retinopathy (DR), are retinal diseases.14 Retinopathy of prematurity (ROP) and other retinal diseases are among the leading causes of preventable blindness in children.15
Types of retinopathies with limited treatment options that could most benefit from advances in nanotechnology range from retinal degeneration, including retinitis pigmentosa (RP), AMD, and Stargardt disease, to retinal neovascularization, including DR, which can cause tractional retinal detachment.16–19 The standard procedure for treating pathological neovascularization is laser photocoagulation and a series of intravitreal injections of anti-vascular endothelial growth factor (VEGF) antibodies. This treatment effectively slows or prevents neovascularization. However, the laser destroys peripheral retinal tissue, causing discomfort. The repeated intravitreal injections that are often required increase the risk for endophthalmitis and retinal detachment. Slow-release medications or gene therapy using nanoparticles can reduce these side effects.20 Therapeutic interventions involving nanoparticles can target contributors to retinopathy pathogenesis such as retinal inflammation.21 Nanomaterials also show promise for improvements and innovations in diagnostic and screening tests and surgical interventions.
Overall, systemic and local nanotoxicity side effects are limited compared with other treatment strategies. However, nanoparticle concentration14 and size 14,15 significantly contribute to their neuronal and ocular side effect profile. These nanotoxicities affect the continued development of nanomaterial applications for retinal diseases.
Nanomaterials of interest in retinal applications
The structural profile of nanomaterials significantly impacts their function, safety, and efficacy in retinal applications. Nanomaterials such as liposomes and nanomicelles have been used to deliver drugs and genes to the retina using various routes, including topical delivery and injections. Retinal delivery and its challenges introduced in this section are further discussed in the proceeding section.
The specific characteristics of nanomaterials, including material type, shape, size, and concentration, determine their effect on the retina and affect their efficacy and safety. Figure 1 visually organizes the amphiphilic molecules, metal-based nanomaterials, polymer-based nanomaterials, and other nanomaterials discussed herein that have shown promise for retinal clinical applications. This section will discuss the characteristics, potential retinal uses, administration route(s), and associated toxicities for each nanomaterial type studied in the retina.
Figure 1.
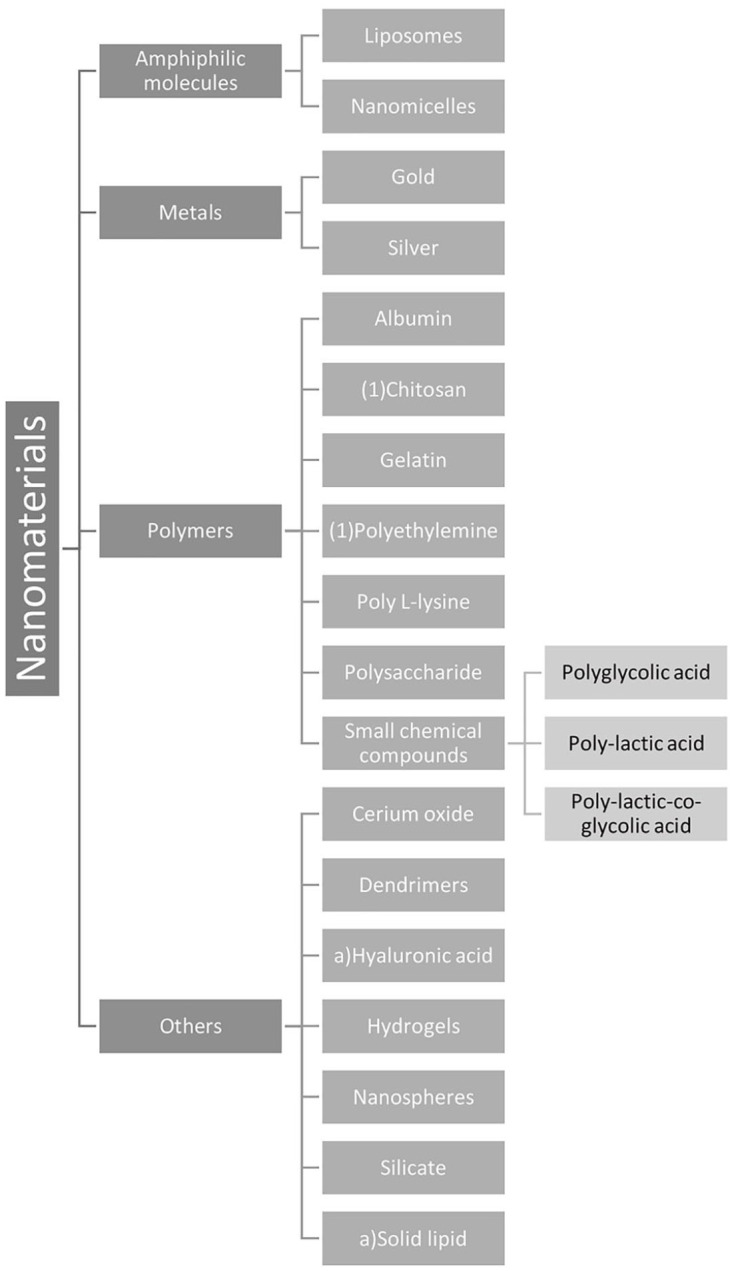
Nanomaterials by category. Most of the nanomaterials that have been studied for retinal disease applications are either made of amphiphilic molecules, metals, or polymers. The small chemical compound polymers with studied applications in drug and gene delivery were further classified.
Amphiphilic molecules
One area of application for nanomaterials in the retina involves nano-based delivery systems of drugs or genes. The specific properties of the nanomaterials are vital to developing a nanomaterial capable of overcoming retinal barriers. Several researchers have studied the amphiphilic nanomaterials of liposomes and nanomicelles due to their ability to overcome retinal barriers and deliver therapeutic materials in a targeted approach.4,22
Liposome-based delivery
Liposomes are comprised of amphiphilic molecules, specifically phospholipids and sterols, with a spherical shape similar to that of a cell membrane. Phospholipids have hydrophilic heads with two nonpolar hydrophobic chains that form membranes. This structure provides the versatility to carry both hydrophilic and hydrophobic drugs and to be modified with carbohydrates or surface polymers to enable targeted delivery.23
The exact physiochemical properties of the liposomal membranes, along with their composition, charge, size, and organization, determine their ability to stably carry therapeutic compounds with limited solubility and absorption capabilities. Liposome-based drug delivery systems are well-characterized as they have been studied since 1960.21 These systems have desirable biocompatibility and biodegradable properties as well as improved retention and permeability. Liposomes with polyethylene glycol (PEG) incorporated within the surface, in addition to other modifications, are being examined as a treatment for choroidal neovascularization via intravitreal administration.10,24
In addition to carrying drugs, liposomes can be modified to deliver genes to the retina. Both horizontal and vertical transfection methods can control the delivery of genes in vitro through magnetic cationic liposomes to the retinal pigment epithelium (RPE).25 In vivo delivery via topical eye drops in rats has been explored for delivering plasmid DNA to the RPE by modifying transferrin on the surface of the liposome.26
Barriers to the successful delivery of liposomes include opsonization, immunogenicity, and limited storage capacity.1,23 There have been attempts to use liposomes to deliver genes to the posterior aspect of the eye. However, liposomal instability remains a hindrance for adequate delivery, and in vivo targeted delivery of RPE protein 65 in mice showed a transfection efficacy of only 50%.27
Nanomicelle-based delivery
Nanomicelles are self-assembled amphoteric and amphiphilic molecules. They consist of a hydrophobic core, which allows hydrophobic drugs to dissolve, along with a hydrophilic shell, which allows delivery via a clear, aqueous formation. Aqueous drops have the potential to be delivered to all parts of the eye, including the retina. Nanomicelles are promising candidates for drug delivery because they can be delivered through aqueous components of the eye with less drug degradation and more drug permeability throughout the ocular epithelial layers. This allows for enhanced safety, bioavailability, lower toxicity, limited irritation, and increased administration convenience, leading to improved patient compliance.28
For instance, nanomicelles composed of polyoxyethylene hydrogenated castor oil 40 and octoxynol 40 have been used to carry anti-viral prodrugs across ocular layers, including to the retina, in vivo following topical administration. They display no cytotoxicity after 24 h in vitro.29 Another nanomicelle formulation involved combining octoxynol 40 with vitamin E tocopherol PEG succinate to make a polymer loading rapamycin to selectively reach the retina and choroid in vivo without significant concentrations in the nearby vitreous humor and with limited signs of cytotoxicity in vitro.30
Conjunctival/scleral injection of nanomicelles appears more promising than intraocular injection as the hydrophilic portion of the nanoparticle limits the ability of the drug to travel through the cornea and the aqueous humor limits transport to the lens. Using the conjunctival/scleral route, nanomicelles made from non-ionic surfactants were able to passively diffuse through the scleral water channels to reach the retina.10,31 Cross-linking the core of the nanomicelles with functional groups or lowering the critical solution temperature of the hydrogel for stabilization can improve the duration and solubility of nanomicellar drug delivery.28
Another delivery method under investigation is intravenous administration. Polyion nanomicelles with encapsulated fluorescein isothiocyanate-labeled poly-L-lysine injected intravenously an in vivo rat model of exudative AMD accumulated around the lesion for 168 h post-administration. However, signs of toxicity indicate the need to further evaluate the specific characteristics of nanomicelles and their administration.32
Metal-based nanomaterials
Gold and silver nanoparticles have the unique property of surface plasmon resonance that differentiates them from other forms of nanoparticles.1 Silver nanoparticles have shown the potential to act as antiangiogenic agents in treating retinal diseases such as AMD. These nanoparticles demonstrated enhanced bioavailability and anti-bacterial, anti-fungal, anti-inflammatory, anti-permeability, and anti-viral film capabilities. The anti-vasopermeability of silver nanoparticles is useful for the vascular endothelial pathological component of some retinal diseases such as ROP.33 These anti-vasopermeability effects can potentially block ICAM-1 expression and reverse the biological effects of advanced glycation end-products implicated in DR and other vascular complications of diabetes.34
Similarly, gold nanoparticles have demonstrated anti-angiogenic and anti-inflammatory properties to treat retinal diseases. In addition, they show diagnostic potential as contrast agents for optical coherence tomography (OCT) imaging of RPE cells.35 Both silver and gold nanoparticles are being assessed for toxic effects within the retina, although the studies are limited. Most notably, metal nanoparticles 20 and 80 nm in diameter were toxic to photoreceptor cells in vitro.36
Polymer-based nanomaterials
A variety of polymer-based nanoparticles have been assessed for their abilities to deliver drugs and genes to the retina. These polymers include albumin, hydrogels, chitosan (also known as hyaluronic acid (HA)), and various small chemical compounds.
The protein albumin can deliver peptides and proteins to the retina. Limited toxicity, biodegradability, and preference for uptake in inflamed tissues and tumors are promising benefits of albumin.37,38
Hydrogels contain biodegradable synthetic or natural polymers with hydrophilic groups that can absorb a large amount of water.39 They have potential as retinal drug delivery agents due to their ability to provide a sustained release of material. The hydrophilic internal structure can hold materials that are challenging to safely secure, such as proteins, antibodies, and peptides.11 In a DR rat model, a chitosan nanoparticle-based hydrogel loaded with insulin had protective effects on the retina’s vascular structure and permeability following subconjunctival delivery.40
HA, also known as chitosan, is a type of hydrogel that can act as an electrostatic coating to deliver drugs to the retina. Chitosan has been used to coat nanoparticles carrying genes and drugs to the retina for intravitreal and suprachoroidal delivery due to its limited toxicity and biodegradability.40–43 In vitro, HA has been coupled with lipoplexes to intravitreally deliver gene therapy to the retina.44 HA used as a nanoparticle core, with liposomes as the shell, has been utilized to target the inflammatory activity of CD44 in the RPE via intravitreal delivery in a rat model of posterior uveitis.45 Since the RPE is implicated in the pathogenesis of inflammation, this may be a useful model in pursuing therapies for other retinopathies.
Small chemical molecules used as nonviral gene carriers to the retina show therapeutic promise in both in vivo and in vitro models when delivered intravenously, intravitreally, or subretinally. Small chemical compounds that have been examined include poly-lactic acid (PLA), poly-lactic-co-glycolic acid (PLGA), and polyglycolic acid (PGA).26,40,42,46,47 One future direction is to test the hypothesis that PLGA nanoparticles in situ can be delivered through injectable light-responsive implants that deliver peptides to the posterior segment of the eye.48 Implants have the potential to improve material delivery for longer periods compared with traditional formations. The current limitations of the procedure’s invasiveness and the need to remove the implant could be reduced using biodegradable implants.11
Other nanomaterials
Several other nanomaterials show potential for use in retinal clinical applications, including nanospheres, dendrimers, hydrogels, solid lipids, and nanomaterials containing cerium oxide and silicate. These nanomaterials range in shape and material type, allowing them to be used for specific purposes, such as drug delivery and gene therapy.
Nanospheres are nanoparticles that are encapsulated in polymer-based delivery systems. These nanoparticles self-assemble to distribute the carried substance uniformly throughout the polymer, limiting the negative effects of tissue damage and irritation. This is advantageous in comparison with the similarly shaped nanocapsules. Nanocapsules are enclosed by a polymer shell to produce materials of various sizes with less uniformity.4 Nanospheres have the potential to be delivered to the retina based on their size. Particles with a diameter of 50 and 200 nm have been successfully delivered to the retina in a rabbit model.23
Dendrimers are tree-like polymers with many branches for functional groups containing both inner and outer shells and a symmetrical core. This structure allows for promising drug and gene delivery applications.49 More specifically, polyamidoamine (PAMAM) dendrimers have been used to deliver DR treatment dexamethasone (DEX) to the retina in vivo via topical and subconjunctival administration in a rat model. However, cytotoxic effects observed in this delivery warrant further study.50 They also have shown promise in targeting retinal microglia/macrophages in vivo through intravenous and intravitreal administration. This could be helpful in various conditions characterized by a prolonged immune response from retinal dysregulation, such as AMD, DR, RP, and retinal vein occlusion.51 The clearance of these dendrimers in this mouse model of ischemia/reperfusion injury was relatively rapid from non-target organs, including the healthy eye, and relatively slow in the target microglia/macrophages compared with the half-life of currently available drugs such as bevacizumab.52
Solid lipid nanoparticles are composed of a solid lipid core combined with surfactants for stabilization. They have been promising candidates for drug and gene delivery to the retina due to their long-term stability, limited toxicity, economic production without solvents at a large scale, sterilization capacity via autoclave, and biodegradability.53,54 Solid lipid nanoparticles delivered nonviral gene vectors intravitreally to the RPE and photoreceptors in a mouse model of X-linked juvenile retinoschisis (XLRS).55 However, some concerns must be addressed, including expulsion of the drug during long-term storage and potential toxicities.54
Cerium oxide nanoparticles, also known as nanoceria, are nanocrystals from the rare earth metal cerium and reduce retinal degeneration in multiple animal models of both AMD and RP through intravitreal delivery with long-term retention of 50% over 1 year after one administration.56 In a murine study, one intravitreal injection of nanoceria indicated sustained nanoceria in the retina for more than 1 year without signs of inflammation or other side effects.57 In a rat model of autosomal dominant RP, nanoceria decreased rod cell apoptosis speed and retinal lipid peroxidation. In these studies, nanoceria demonstrated antioxidant properties that could be beneficial in treating neovascularization.56–58
Like cerium oxide, silicate demonstrates antiangiogenic properties. Specifically, silicate-based nanoparticles were studied in oxygen-induced retinopathy mice that were given an intravitreal injection. This inhibition of neovascularization results from VEGF with no indication of retinal, neural, or endothelial cell toxicity.20
Nanoparticle route of delivery to the retina
The retina is challenging to access, complicating therapeutic and diagnostic nanoparticle delivery in retinal diseases. Current pharmacological treatments of retinal diseases include monoclonal antibodies and fusion proteins for wet AMD and small molecules such as ganciclovir for cytomegalovirus retinitis in immunocompromised individuals. These treatments have to be injected either intravitreally or subretinally due to the physical barriers that limit retinal access.12 However, in a non-clinical setting, drugs can be delivered to the retina via topical installation or intravitreal, subconjunctival, subretinal, and trans-scleral injection. These delivery methods have unique mechanisms with different associated strengths and limitations in the context of nanoparticle delivery.
Subconjunctival injection is an emerging and less-invasive mode of delivery to the retina. The multiple routes to reach the posterior segment of the eye include trans-scleral diffusion, systemic circulation via the choroid, and through the anterior segment, including the tear film, cornea, and both the aqueous and vitreous humor. Although promising, the limitations of these routes involve the multiple barriers to reach the retina and the high drug washout. These limitations could be overcome using nanoparticles to sustain delivery and protect the delivered material from washout and clearance.11
The most convenient way to administer therapeutic materials to the retina clinically is in topical form. Topical delivery is particularly challenging when delivering materials to the retina due to the corneal and conjunctival epithelial barriers in the anterior segment in addition to physiological processes such as lacrimal drainage and tear formation. These factors greatly limit the bioavailability of materials once they reach the retina, reducing the therapeutic potential of this less-invasive delivery mode.12 The challenges can potentially be overcome using sustained drug release to overcome the low bioavailability by focusing on prolonging corneal retention and enhancing drug potency.11
The most used administration route, intravitreal injection, has much higher, targeted, long-lasting bioavailability. Still, it is limited by invasiveness regarding the number of required injections and discomfort during administration.59 Since this route has the advantage of the proximity to the target, unlike other delivery methods, the focus should be on improving the sustainability of delivery using nanoparticles to limit the need for multiple invasive injections while ensuring that the materials do not interfere with vision based on their distribution.11 Overall, the ability of therapeutic materials to overcome the barriers to reaching the retina is predominantly dependent on the formulations and properties of nanoparticle-based delivery systems to overcome mechanistic limitations of the administrative routes of delivery.
Barriers of delivery to the retina
The eye is made up of both an anterior and a posterior segment, which contain barriers to administering therapeutic materials to the retina, including the corneal and conjunctival epithelium, blood-aqueous barriers (BABs), and BRBs. In the anterior portion of the eye, the corneal and conjunctival epithelium and the BAB act as barriers to retinal delivery, especially via topical administration. Mucins present in these layers may play a role in the absorption of nanoparticles, preventing their passage through these layers via adhesion.60 In the posterior portion of the eye, the BRB serves as a hindrance to entry between the nervous and circulatory systems through the two cell types, retinal capillary endothelial cells and RPE cells, which respectfully compose the inner and outer BRBs.61
Characteristics of nanoparticles that enable delivery to the retina
The physical and physiological barriers discussed above can be overcome by considering the properties of nanoparticles, including their size, low molecular weight, small diameter, and negative charge. If the drug is potent enough, topical administration barriers for retinal delivery, especially low bioavailability, could be overcome. For example, rapamycin has been delivered to the RPE in this manner using a nanomicelle-based formulation in male New Zealand white rabbits.30 In a transgenic murine model of retinoblastoma, dendritic nanoparticles sustained the release of carboplatin via subconjunctival delivery without associated retinal toxicity.62
The relatively small size of nanoparticles is beneficial in overcoming retinal delivery barriers. Out of the numerous delivery barriers, the RPE may be considered the rate-limiting factor for delivering hydrophilic or macromolecules via trans-scleral administration, making the nanoparticle size more important than other particle modalities.4,63
The surface charge of nanoparticles considerably impacts both the toxicity and integrity of the BRB.63 Anionic nanoparticles can penetrate all layers of the retina, while cationic nanoparticles get trapped in the vitreous humor without diffusing.26 Similarly, intravitreal delivery of human serum albumin nanoparticles penetrates the RPE and subretinal space with an anionic but not a cationic charge.64
Therapy for retinal disease with nanoparticles
Nanoparticles can be manipulated in various ways to serve therapeutic purposes in the retina, including for the delivery of genes and drugs and for placement of injectable implants (Figure 2). Nanoparticles can deliver genes and drugs to the retina to treat diseases for which safe and effective treatments remain elusive. Table 1 shows the applications of nanoparticles for gene therapy, drug delivery, diagnosis, and surgery. The overlap of nanoparticles used for gene therapy and drug delivery applications is visually illustrated in Figure 3. In addition to novel therapeutics, nanoparticles can also improve existing therapies, such improving laser photocoagulation for inner retina diseases to reduce thermal damage to the RPE and photoreceptor layers.65
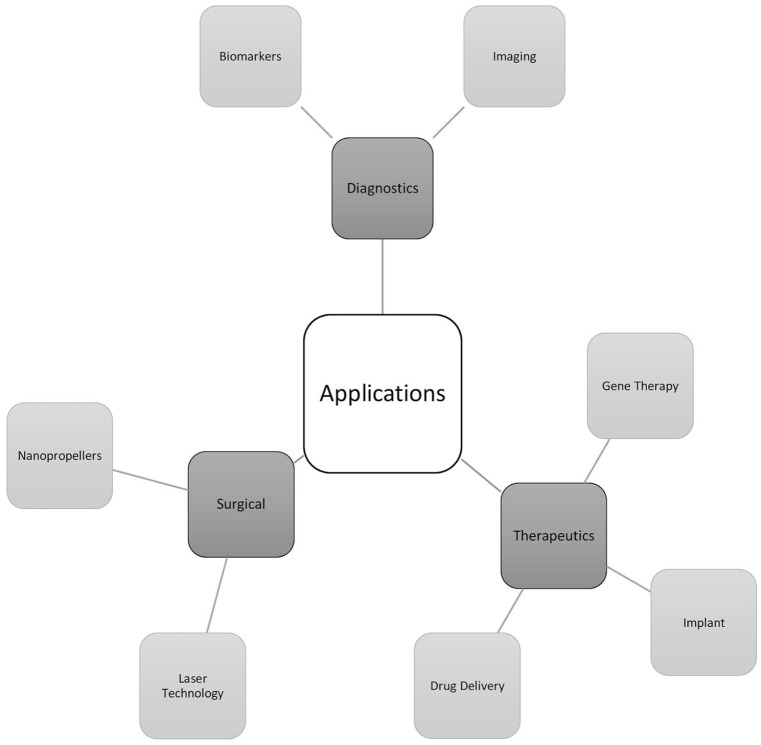
Figure 2.
Nanomaterials by application. Nanomaterials have been assessed for their diagnostic, therapeutic, and surgical use in the retina.
Table 1.
Routes of Delivery, Applications, and Side Effects of Nanoparticles.
| Type of nanoparticle | Route of delivery | Application(s) | Side effects |
|---|---|---|---|
| Albumin | Intravitreal,64 suprachoroidal38 | Drug delivery | No significant toxicities observed |
| Carboplatin | Posterior sub—tenon,66 subconjunctival62 | Drug delivery | No significant toxicities observed |
| Cerium oxide (Nanoceria) | Intravitreal56–58,67,68,69 | Drug delivery | No significant toxicities observed |
| Chitosan (hyaluronic acid) | Intravitreal,41,44,45 subconjunctival,40 suprachoroidal43 | Drug delivery, gene therapy | No significant toxicities observed |
| Dendrimer | Intravenous,52 intravitreal,52 subconjunctival,50,62 topical50 | Drug delivery | Cytotoxic effects from cationic amine group |
| Gold | Intravenous,35,70 intravitreal,35,71 subretinal35,72 | Biomarker, diagnostic imaging, drug delivery, implant, laser technology | Neuronal toxicity from increased apoptosis, oxidative stress, and microglial activation; small diameter of 1.4 nm showing oxidative stress and mitochondrial damage |
| Hydrogel | Subconjunctival40 | Drug delivery | No significant toxicities observed |
| Liposome | Intravitreal,23,24,46,59 topical26 | Drug delivery, gene therapy | No significant toxicities observed |
| Nanomicelle | Intravenous,32 topical30,31 | Drug delivery | No significant toxicities observed |
| Nanosphere | Intravitreal23 | Drug delivery, gene therapy | No significant toxicities observed |
| Poly(beta-amino ester) | Subretinal73 | Gene therapy | Less cytotoxicity than other available non-viral gene delivery options |
| Polyethylene glycol (PEG) | Intravenous,74 subretinal75–79,80,81 | Drug delivery, gene therapy | No significant toxicities observed |
| Poly-lactic acid (PLA) | Intravenous74 | Gene therapy | No significant toxicities observed |
| Poly-lactic-co-glycolic acid (PLGA) | Intravenous,82 intravitreal46,48,83,84 | Drug delivery, gene therapy, implant | Limited toxicity from lactic and glycolic acid production |
| Silicate | Intravitreal20 | Drug delivery, gene therapy | No significant toxicities observed |
| Solid lipid | Intravitreal53,55 | Drug delivery, gene therapy | No significant toxicities observed |
Figure 3.
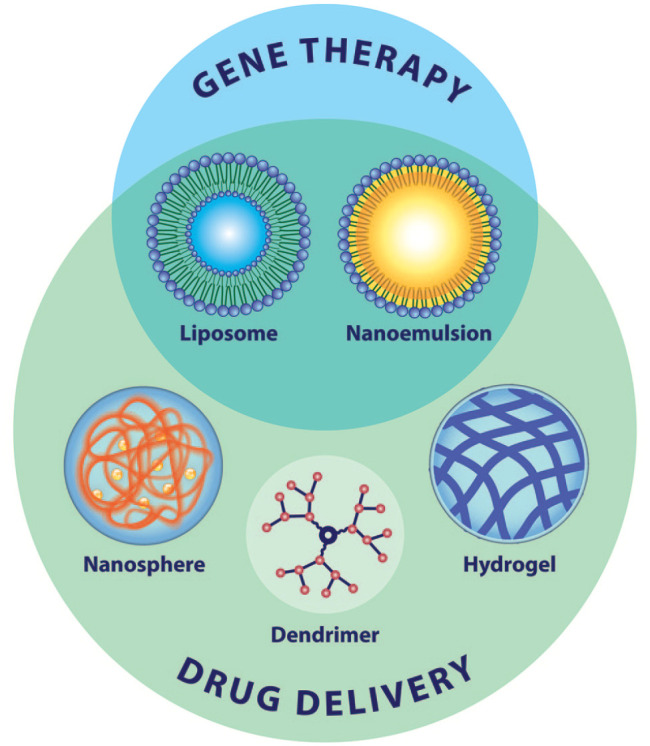
Nanomaterials for use in gene therapy and drug delivery. The three-dimensional structures of nanomaterials examined for use in retinal gene therapy and/or drug delivery are shown. Not shown but discussed in the review are emerging nanoparticles for retinal applications which include nanodiscs and nanorods for diagnostics and nanopropellers for surgical interventions.
A limited number of treatment options exist for retinopathies. Each retinopathy requires a specific approach for clinical prevention, treatment, and specific therapeutic targeting. Some retinopathies, such as AMD, are complex, multifactorial, and most prevalent in the elderly. Others, such as DR, are preventable and occur more commonly in middle-aged people. Some retinopathies are genetically inherited and are without available treatment options, such as Stargardt disease and RP. Below, we discuss potential therapeutic targets for retinopathies with limited therapeutic options.
Angiogenesis-related blindness and neovascularization
Angiogenesis-related blindness is a complication of neovascularization in numerous eye diseases, including many in the retina and is among the highest causes of irreversible blindness worldwide.85 Current treatment options include surgery, cryotherapy, laser photocoagulation, and intravitreal anti-VEGF monoclonal antibody injections, specifically for AMD. Complications of these interventions include damage to healthy retinal tissue, retinal detachment, and endophthalmitis.86
AMD is a progressive disease, which causes geographic atrophy and neovascularization in its late stages to dramatically reduce central vision.87 Only the exudative form can be treated using laser photocoagulation, destroying some healthy retinal tissue. AMD can be diagnosed with angiography. As an alternative to invasive recurrent intravitreal injection treatment, nonviral intravenous gene therapy in a murine and primate AMD model using nanoparticles appears promising. The tripeptide adhesion motif Arg-Gly-Asp of nanoparticles targeted choroidal neovascularization with evidence of vision improvement and no signs of toxicity.82 Although relatively more invasive, intravitreal injection of nanoceria has been studied for its ability to reduce oxidative stress in AMD. For instance, nanoceria has been shown to reduce microglial activation move to the retinal outer nuclear layer 3 weeks after the exposure of 24 h of 1000 lux light in rats.67 This movement through the retinal layers to reach the external retina was demonstrated in rats, resulting in support of photoreceptors and RPE cells through the neuroprotective property of this antioxidant.67,68 Another potential use of nanoparticles for wet AMD involves gold nanoparticles computationally studied using the Monte Carlo simulations to enhance the dose of radiotherapy to macular endothelial cells while limiting the effects to surrounding healthy tissue.88 In addition, materials such as αB-crystallin with anti-apoptotic and anti-inflammatory properties may be beneficial for delivery to the human RPE using protein polymer nanoparticles to enhance survival during oxidative stress in AMD.89
DR is the most common microvascular complication of diabetes mellitus. Secondary to macular edema, DR is the leading cause of blindness and visual impairment in the world. Like AMD, DR can be diagnosed using digital fundus photography and OCT.87 Since nuclear transcription factor-kappa B (NF-kB) is key in angiogenesis promotion for DR, two hyperbranched nanoparticles of cationic polysaccharide derivatives with equal amounts of cationic residues with differing branching structures and molecular weights were found to deliver siRNA to human RPE cells to different degrees while silencing NF-kB.47 Nanoparticle-based methods, including gold nanoparticles, PAMAM dendrimers and chitosan with PGA-PGLA-PLA hydrogel, or PLGA, are promising candidates to deliver drugs or other therapeutic materials, such as interleukin-12 for the treatment of DR, to the retina, based on rat models.40,50,83,70 Gold nanoparticles with resveratrol also showed signs of reducing retinal inflammation by repressing NF-kB.70
For chronic diabetic macular edema, specifically, a prospective, randomized control clinical trial was performed in 22 patients to assess the efficacy of a topically delivered DEX gamma-cyclodextrin nanoparticle eye drop treatment compared with posterior sub-tenon triamcinolone acetonide administration. The nanoparticle-based treatment had a similar therapeutic effect with the noted adverse effect of increased intraocular pressure upon treatment discontinuation compared with the control.90
ROP is prevalent in the population of premature and low birth weight infants, with some treatment options available, including laser therapy and cryotherapy, depending on the severity. The treatment carries a risk of retinal detachment requiring surgery to repair.87
Several different forms of nanoparticles show promising antiangiogenic effects, including anionic peptides that conjugate with Flt1 peptide and hyaluronate,91 nanoceria,58 conjugate with PAMAM and triamcinolone acetonide,92 doxorubicin-PEG-poly(sebacic acid),93 folate-PEG-b-polycaprolactone,94 gold,95 PLGA,84 silicate,20 and silver.96 These effects have the potential to be induced under specific biologic conditions pertinent to laser coagulation treatment in the retina through intravenous74 or intravitreal delivery.95 Liposomes with gold nanoparticles were cytosolically delivered in the biologic conditions of light-induced heat at an acidic pH.95 In addition, nanoparticles have the potential to target retinal capillary endothelial cells to limit damage to surrounding structures, as demonstrated using ligand-modified quantum dots (QDs) in rats delivered intravenously.86
Retinal degeneration
Retinal degeneration is a complication of AMD and DR and a characteristic of inherited conditions, including Stargardt disease, RP, Leber’s congenital amaurosis, and XLRS. The accumulation of reactive oxygen species (ROS) in retinal degeneration is a possible mechanism that leads to cell death and blindness due to stress on photoreceptor cells. This is a target of nanoparticle intervention using the antioxidative properties of nanoceria via intravitreal injection in rat models that have shown reductions in ROS damage across the retinal layers after light damage.56,67–69
For AMD and DR, a rat model of retinal degeneration using bioactive magnetic iron oxide/human serum albumin nanoparticles upon suprachoroidal injection allowed nanoparticles to selectively enter the posterior segment based on tracking with magnetic resonance imaging. The lack of toxicity shown is promising for use in extended drug delivery.38
There are no available treatments for inherited retinal degeneration. Nanoparticles are a promising novel therapeutic tool for gene delivery in these disorders. The most promising application of nanoparticles for treating genetic forms of retinal degeneration is non-viral gene therapy, which has benefits over adeno-associated viruses due to larger vector capacity and localization to the eye instead of additional neural visual pathways.75 Nanoparticles made of conjugated forms of PEG,76–79 chitosan with glycol41 or lipid,44 and poly(beta-amino ester)73 are being assessed in vivo and in vitro for use in retinal gene delivery. For instance, nanoparticles with PEG conjugated to glial cell line-derived neurotrophic factor and a synthetic peptide injected into the murine subretinal space have been shown to reduce apoptosis approximately four to eight folds more than in controls with increased outer nuclear layer thickness.77
Among inherited forms, Stargardt disease is a leading cause of inherited visual impairment and the most common macular dystrophy in children and adults. The condition is autosomal recessive with a heterogeneous phenotype and genotype that can be diagnosed using fundus autofluorescence. Although there are no effective treatment options, clinical trials are in progress for gene replacement therapy, pharmacotherapy, and stem cell therapy.18,87 DNA nanoparticles are promising nonviral delivery options to carry large genes that are too large to be carried with other methods. For instance, in mice, 30-mer cationic poly-lysine nanoparticles conjugated with 10kDa PEG (CK30-PEG-10 K) were able to deliver adenosine triphosphate (ATP)-binding cassette, subfamily A, member 4 (ABCA4) subretinally to a similar extent compared with two recombinant adeno-associated virus delivery systems undergoing clinical trials and effective for ocular delivery, respectively.76
Like Stargardt disease, RP is marked by a heterogeneous genotype but with a specific phenotype of progressive photoreceptor dysfunction. CK30-PEG-10 K nanoparticles carrying opsin promoter and wild-type Rds gene in mice were delivered to the photoreceptor outer segment with signs of structural improvement and cones functioning at control levels without inducing an acute inflammatory response.80,81 Another area of exploration to treat photoreceptor cell degeneration involves nanoparticle-based light-responsive therapeutics. Examples include cadmium sulfide QD-coated lenses to convert ultraviolet to visible light in zebrafish97 and the subretinal implant of gold nanoparticle-decorated titania nanowire in mice.72
XLRS is a leading cause of male macular degeneration involving mutations in the RS1 gene encoding retinoschisin, which causes this protein to be completely absent in the retina in most forms. Solid lipid nanoparticles containing HA were delivered to RS1 h-deficient mice via intravitreal injection to retinal photoreceptor cells at a higher transfection rate in the RPE and inner nuclear layer than amine and dextran, and both experimental groups demonstrated a reduction in both cystic cavity photoreceptor cell loss.55
An area of future exploration involves the use of biodegradable nanoscaffolds to deliver retinal progenitor cells to repair or replace diseased or damaged retinal cells or tissue during the degenerative process. Nanowires in the retina could be used similarly to catheters placing stents to determine if the retinal ganglion cells (RGCs) are viable.98
Retinal tumor formation
The use of nanoparticles to treat tumor formation in retinoblastoma is under investigation. Although treatments for retinoblastoma exist through cryotherapy, photocoagulation, intravenous and intra-arterial chemotherapy, and orbital radiation as well as enucleation in more severe cases, there are no less-invasive drug delivery options available.87,99 Transferrin glycoprotein-coated magnetic nanoparticles were able to raise the temperature at the BRB in an animal model. The nanoparticles were first injected intravenously with sodium fluorescein or Evans blue dye into the left common carotid artery. The nanoparticles were then determined to cross the BRB and could be recovered after the thermal stress. However, this approach’s safety profile is unclear and depends on optimizing the magnetic properties appropriate for the hyperthermia procedure.99
The treatment of advanced intraocular retinoblastoma remains challenging. A more targeted carboplatin chemotherapy would allow for greater tumor control. A small prospective, double-blinded, interventional human clinical case study of six patients examined the safety and delivery efficacy of posterior sub-tenon injection of polymethylmethacrylate nanoparticles containing carboplatin. The patients included were scheduled to undergo enucleation for reasons other than retinoblastoma since the researchers wanted to examine the pharmacokinetics and toxicities with intact BRB, posterior segment, and sclera. The results revealed that carboplatin could more sustainably undergo trans-scleral transport without signs of short-term side effects on histopathology or transmission electron microscopy.66
Potential nanomaterial applications for diagnosis and detection of retinopathies
Some nanomaterials can augment existing diagnostic tests and be used as biomarkers to diagnose and monitor disease progression earlier than the current capacity.4 Research advances in these areas are limited within ophthalmology. Gold nanorods and nanodiscs have been studied in vitro and in vivo in mice for their potential as contrast agents to enhance OCT and improve visualization of ocular structures for earlier diagnoses.35 PEG-molecule-stabilized colloidal gold molecules enhance OCT contrast and photoacoustic microscopy of the retina and surrounding choroidal microvasculature in vivo in a rabbit model. Significant enhancements in both images were obtained following intravenous injection without acute toxicities seven days post-injection. However, the contrast agent accumulated in the liver and spleen.100
Gold nanoparticles have been studied for home screening for DR using a urine-based colorimetric paper sensor system that links with a smartphone to screen DR biomarkers by capturing color with the camera. The studied biomarker, 8-hydroxy-2’-deoxyguanosine, is an oxidative stress DNA breakdown product from hyperglycemia and is a known DR and diabetic nephropathy biomarker. The gold nanoparticles showed 91% sensitivity and 81% specificity. Although there was no correlation with DR severity, the results of this study indicate the potential for nanoparticles in retinal disease screening.101
Nanoparticles may also improve polychromatic angiography to differentiate late stages from earlier disease stages based on the size of the particles that extravasate through the leaky vasculature or BRB. The movement of nanoparticles could indicate the extent of the dysfunction.22
Potential nanomaterial applications for surgical advances
Nanomaterials can be used during surgery in more targeted interventions involving lasers71,102 and nanopropellers.103 A femtosecond laser can specifically target the nanosurgical movement and delivery of particular cells in the retina.71,102 This laser was shown to nanosurgically manipulate retinoblastoma cells’ membrane structure via hemifusion in an in vitro model. This line of study is valuable for understanding the cell membrane and develop targeted cancer-related treatments in retinoblastoma.102 An in vivo murine model using a femtosecond laser delivered conjugated gold nanoparticles containing either siRNAs or isothiocyanate-dextran, both with fluorescent tags, to RGCs without damaging them. These findings demonstrate therapeutic promise in the selective targeting of retinal cells without damage to healthy cells.71
The active propulsion of nanoparticles through magnetism can deliver materials to the retina through the vitreous humor. Slippery micropropellers made of silicon dioxide with nickel have successfully been injected into the vitreous to reach the retina near the optic disk using OCT monitoring in porcine models. This delivery method overcomes the passive diffusional forces that reduce targeted delivery capacity but faces challenges navigating the tight macromolecular matrix of the matrix.103 Drug and gene delivery to the retina using magnetism warrants future study.
Nanotoxicity in the retina
The eye is considered an immune-privileged organ, specifically the anterior chamber, subretinal space, and vitreous chamber. Despite this, nanomaterials still can cause inflammatory damage and other signs of nanotoxicity, including cytotoxicity, genotoxicity, and neuronal toxicity. For instance, dendrimers and liposomes produced allergic and hypersensitivity reactions in animal models.4 Additional in vitro and in vivo studies are needed to characterize the nanotoxic effects of potential therapeutic nanomaterials for use in the retina compared to standard treatment options. Biodegradable byproducts from nanomaterials may also present safety concerns.11
Neuronal toxicity
The safety profile of nanomaterials in the retina, especially its network of neuronal cells, must be thoroughly characterized through studies on both their short-term and long-term impacts, considering the immense implications. One model for the characterization of these evaluated 20 and 80 nm gold and silver nanoparticles in vitro in the post-natal mouse retina. Both metals demonstrated some signs of apoptosis, oxidative stress, and microglial damage at low concentrations.36
ROS production is the most likely mechanism of the neuronal cell and glial cell toxicity of nanomaterials in the retina.104 The prospect of neuronal toxicity of metal nanoparticles is particularly concerning since neuronal systems are vulnerable to metal intoxication during development and into adulthood, leading to the development of neurodegenerative conditions such as Parkinson’s disease.36
Nanotoxicity in the eye
A variety of factors determines nanotoxicity in the eye. The two key factors are the concentration and size of nanomaterials. In some cases, smaller nanomaterials, for example, silver, potentially have a more significant neurotoxic effect due to distribution throughout the retinal layers. Nanomaterials distributed for an extended time, greater concentration, or increased cationic charge are also associated with more nanotoxic effects.104 Other factors that impact nanotoxicity include aggregation, biodistribution, chemical composition, coating, dose, shape, solubility, surface charge, attached chemical groups, and zeta potential.4,104
Despite the future challenges in creating nanomaterials with limited nanotoxicity profiles, nanomaterials have immense potential to reduce toxic effects compared with current treatment options. For example, using nanomaterials to treat AMD can reduce the injection frequency, resulting in reduced infections, irritation, and toxicity compared with the standard treatment of intravitreal anti-VEGF monoclonal antibody injections. Investments in promising nanomaterials that show limited toxicity with maximal efficacy should occur. At the moment, the cytotoxic profiles of nanoparticles are better understood than their effects in animal models. There remains significant unexplored territory in terms of characterizing the nanotoxicity in the retina using animal models.
Conclusion
Nanoparticles are potentially advantageous over current therapeutic, diagnostic, and surgical modalities in the retina. However, nanoparticles’ specific properties and safety profiles require more study via in vitro and in vivo models. Whether or not nanoparticles have desirable safety and therapeutic profiles depends on a range of factors such as biodistribution pattern, concentration, dosage, shape, size, surface charge, and solubility. In areas such as inherited retinopathies without effective treatment options or retinal degeneration that often result in irreversible blindness, nanoparticle research is essential. The diagnostic and surgical potential of nanoparticles requires further study before clinical applications are fully realized, but progress in these realms is clear. Improving current diagnostic tools and reducing the scale of microsurgery applications are directions of promise.
Acknowledgments
The authors would like to thank Dr. Sunu Mathew for help in editing the article.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors recieved fund through NIH/NEI T35EY031282.
ORCID iD: Amir R. Hajrasouliha  https://orcid.org/0000-0002-9326-0391
https://orcid.org/0000-0002-9326-0391
Contributor Information
Melanie Scheive, Indiana University School of Medicine, Indianapolis, IN, USA.
Saeed Yazdani, Indiana University–Purdue University Indianapolis, Indianapolis, IN, USA.
Amir R. Hajrasouliha, Assistant Professor of Ophthalmology, Eugene and Marilyn Glick Eye Institute, Department of Ophthalmology, Indiana University School of Medicine, 1160 W Michigan St., Indianapolis, IN 46202, USA.
References
- 1. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol 2018; 16: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Su H, Wang Y, Gu Y, et al. Potential applications and human biosafety of nanomaterials used in nanomedicine. J Appl Toxicol 2018; 38: 3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rizzo LY, Theek B, Storm G, et al. Recent progress in nanomedicine: therapeutic, diagnostic and theranostic applications. Curr Opin Biotechnol 2013; 24: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamaleddin MA. Nano-ophthalmology: applications and considerations. Nanomedicine 2017; 13: 1459–1472. [DOI] [PubMed] [Google Scholar]
- 5. Zhou H-Y, Hao J-L, Wang S, et al. Nanoparticles in the ocular drug delivery. Int J Ophthalmol 2013; 6: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sultana Y, Maurya DP, Iqbal Z, et al. Nanotechnology in ocular delivery: current and future directions. Drugs Today (Barc) 2011; 47: 441–455. [DOI] [PubMed] [Google Scholar]
- 7. You Q, Sokolov M, Grigartzik L, et al. How nanoparticle physicochemical parameters affect drug delivery to cells in the retina via systemic interactions. Mol Pharm 2019; 16: 5068–5075. [DOI] [PubMed] [Google Scholar]
- 8. Kubo Y, Akanuma SI, Hosoya KI. Recent advances in drug and nutrient transport across the blood-retinal barrier. Expert Opin Drug Metab Toxicol 2018; 14: 513–531. [DOI] [PubMed] [Google Scholar]
- 9. Peynshaert K, Devoldere J, De Smedt SC, et al. In vitro and ex vivo models to study drug delivery barriers in the posterior segment of the eye. Adv Drug Deliv Rev 2018; 126: 44–57. [DOI] [PubMed] [Google Scholar]
- 10. Kaur IP, Kakkar S. Nanotherapy for posterior eye diseases. J Control Release 2014; 193: 100–112. [DOI] [PubMed] [Google Scholar]
- 11. Joseph RR, Venkatraman SS. Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine (Lond) 2017; 12: 683–702. [DOI] [PubMed] [Google Scholar]
- 12. Jiang S, Franco YL, Zhou Y, et al. Nanotechnology in retinal drug delivery. Int J Ophthalmol 2018; 11: 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamada N, Olsen TW. Routes for drug delivery to the retina: topical, transscleral, suprachoroidal and intravitreal gas phase delivery. Dev Ophthalmol 2016; 55: 71–83. [DOI] [PubMed] [Google Scholar]
- 14. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 15. Solebo AL, Teoh L, Rahi J. Epidemiology of blindness in children. Arch Dis Child 2017; 102: 853–857. [DOI] [PubMed] [Google Scholar]
- 16. Moutray T, Chakravarthy U. Age-related macular degeneration: current treatment and future options. Ther Adv Chronic Dis 2011; 2: 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin MK, Tsai Y-T, Tsang SH. Emerging treatments for retinitis pigmentosa: genes and stem cells, as well as new electronic and medical therapies, are gaining ground. Retin Physician 2015; 12: 52–70. [PMC free article] [PubMed] [Google Scholar]
- 18. Tanna P, Strauss RW, Fujinami K, et al. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol 2017; 101: 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu L, Acon D, Wu A, et al. Vascular endothelial growth factor inhibition and proliferative diabetic retinopathy, a changing treatment paradigm. Taiwan J Ophthalmol 2019; 9: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jo DH, Kim JH, Yu YS, et al. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomedicine 2012; 8: 784–791. [DOI] [PubMed] [Google Scholar]
- 21. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomed 2015; 10: 975–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kompella UB, Amrite AC, Pacha Ravi R, et al. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Retin Eye Res 2013; 36: 172–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honda M, Asai T, Oku N, et al. Liposomes and nanotechnology in drug development: focus on ocular targets. Int J Nanomed 2013; 8: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu HA, Liu YL, Ma ZZ, et al. A lipid nanoparticle system improves siRNA efficacy in RPE cells and a laser-induced murine CNV model. Invest Ophthalmol Vis Sci 2011; 52: 4789–4794. [DOI] [PubMed] [Google Scholar]
- 25. Fujii Y, Kachi S, Ito A, et al. Transfer of gene to human retinal pigment epithelial cells using magnetite cationic liposomes. Br J Ophthalmol 2010; 94: 1074–1077. [DOI] [PubMed] [Google Scholar]
- 26. Tsai C-H, Wang P-Y, Lin IC, et al. Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci 2018; 19: 2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zylberberg C, Gaskill K, Pasley S, et al. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther 2017; 24: 441–452. [DOI] [PubMed] [Google Scholar]
- 28. Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine (Lond) 2010; 5: 485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mandal A, Cholkar K, Khurana V, et al. Topical formulation of self-assembled antiviral prodrug nanomicelles for targeted retinal delivery. Mol Pharm 2017; 14: 2056–2069. [DOI] [PubMed] [Google Scholar]
- 30. Cholkar K, Gunda S, Earla R, et al. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS Pharmscitech 2015; 16: 610–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vadlapudi AD, Mitra AK. Nanomicelles: an emerging platform for drug delivery to the eye. Ther Deliv 2013; 4: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ideta R, Yanagi Y, Tamaki Y, et al. Effective accumulation of polyion complex micelle to experimental choroidal neovascularization in rats. FEBS Lett 2004; 557: 21–25. [DOI] [PubMed] [Google Scholar]
- 33. Kalishwaralal K, Barathmanikanth S, Pandian SRK, et al. Silver nano—a trove for retinal therapies. J Control Release 2010; 145: 76–90. [DOI] [PubMed] [Google Scholar]
- 34. Sheikpranbabu S, Kalishwaralal K, Lee KJ, et al. The inhibition of advanced glycation end-products-induced retinal vascular permeability by silver nanoparticles. Biomaterials 2010; 31: 2260–2271. [DOI] [PubMed] [Google Scholar]
- 35. Masse F, Ouellette M, Lamoureux G, et al. Gold nanoparticles in ophthalmology. Med Res Rev 2019; 39: 302–327. [DOI] [PubMed] [Google Scholar]
- 36. Soderstjerna E, Bauer P, Cedervall T, et al. Silver and gold nanoparticles exposure to in vitro cultured retina—studies on nanoparticle internalization, apoptosis, oxidative stress, glial- and microglial activity. PLoS ONE 2014; 9: e105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang D, Chen YS, Rupenthal ID. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery to the retina. Mol Pharm 2017; 14: 533–545. [DOI] [PubMed] [Google Scholar]
- 38. Tzameret A, Ketter-Katz H, Edelshtain V, et al. In vivo MRI assessment of bioactive magnetic iron oxide/human serum albumin nanoparticle delivery into the posterior segment of the eye in a rat model of retinal degeneration. J Nanobiotechnol 2019; 17: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gonçalves C, Pereira P, Gama M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials (Basel) 2010; 3: 1420–1460. [Google Scholar]
- 40. Rong X, Ji Y, Zhu X, et al. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int J Nanomed 2018; 14: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mitra RN, Han Z, Merwin M, et al. Synthesis and characterization of glycol chitosan DNA nanoparticles for retinal gene delivery. Chemmedchem 2014; 9: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pandit J, Sultana Y, Aqil M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif Cells Nanomed Biotechnol 2016; 45: 1397–1407. [DOI] [PubMed] [Google Scholar]
- 43. Patel SR, Berezovsky DE, McCarey BE, et al. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci 2012; 53: 4433–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martens TF, Peynshaert K, Nascimento TL, et al. Effect of hyaluronic acid-binding to lipoplexes on intravitreal drug delivery for retinal gene therapy. Euro J Pharmaceut Sci 2017; 103: 27–35. [DOI] [PubMed] [Google Scholar]
- 45. Gan L, Wang J, Zhao Y, et al. Hyaluronan-modified core-shell liponanoparticles targeting CD44-positive retinal pigment epithelium cells via intravitreal injection. Biomaterials 2013; 34: 5978–5987. [DOI] [PubMed] [Google Scholar]
- 46. Zulliger R, Conley SM, Naash MI. Non-viral therapeutic approaches to ocular diseases: an overview and future directions. J Control Release 2015; 219: 471–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang L, Liu Z, Gong H, et al. Efficient delivery of NF-κ B siRNA to human retinal pigment epithelial cells with hyperbranched cationic polysaccharide derivative-based nanoparticles. Int J Nanomed 2015; 10: 2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bisht R, Jaiswal JK, Rupenthal ID. Nanoparticle-loaded biodegradable light-responsive in situ forming injectable implants for effective peptide delivery to the posterior segment of the eye. Med Hypotheses 2017; 103: 5–9. [DOI] [PubMed] [Google Scholar]
- 49. Abbasi E, Aval SF, Akbarzadeh A, et al. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett 2014; 9: 247–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yavuz B, Pehlivan SB, Vural I, et al. In vitro/in vivo evaluation of dexamethasone—PAMAM dendrimer complexes for retinal drug delivery. J Pharm Sci 2015; 104: 3814–3823. [DOI] [PubMed] [Google Scholar]
- 51. Whitcup SM, Nussenblatt RB, Lightman SL, et al. Inflammation in retinal disease. Int J Inflam 2013; 2013: 724648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kambhampati SP, Clunies-Ross AJM, Bhutto I, et al. Systemic and intravitreal delivery of dendrimers to activated microglia/macrophage in ischemia/reperfusion mouse retina. Invest Ophthalmol Vis Sci 2015; 56: 4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Battaglia L, Serpe L, Foglietta F, et al. Application of lipid nanoparticles to ocular drug delivery. Expert Opin Drug Deliv 2016; 13: 1743–1757. [DOI] [PubMed] [Google Scholar]
- 54. Seyfoddin A, Shaw J, Al-Kassas R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv 2010; 17: 467–489. [DOI] [PubMed] [Google Scholar]
- 55. Apaolaza PS, Del Pozo-Rodriguez A, Solinis MA, et al. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016; 90: 40–49. [DOI] [PubMed] [Google Scholar]
- 56. Maccarone R, Tisi A, Passacantando M, et al. Ophthalmic applications of cerium oxide nanoparticles. J Ocul Pharmacol Ther 2020; 36: 376–383. [DOI] [PubMed] [Google Scholar]
- 57. Cai X, Seal S, McGinnis JF. Non-toxic retention of nanoceria in murine eyes. Mol Vis 2016; 22: 1176–1187. [PMC free article] [PubMed] [Google Scholar]
- 58. Wong LL, Pye QN, Chen L, et al. Defining the catalytic activity of nanoceria in the P23H-1 rat, a photoreceptor degeneration model. PLoS ONE 2015; 10: e0121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thakur SS, Barnett NL, Donaldson MJ, et al. Intravitreal drug delivery in retinal disease: are we out of our depth. Expert Opin Drug Deliv 2014; 11: 1575–1590. [DOI] [PubMed] [Google Scholar]
- 60. Ruponen M, Urtti A. Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 2015; 96: 442–446. [DOI] [PubMed] [Google Scholar]
- 61. Cunha-Vaz JG. The blood-retinal barriers. Doc Ophthalmol 1976; 41: 287–327. [DOI] [PubMed] [Google Scholar]
- 62. Kang SJ, Durairaj C, Kompella UB, et al. Subconjunctival nanoparticle carboplatin in the treatment of murine retinoblastoma. Arch Ophthalmol 2009; 127: 1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lockman PR, Koziara JM, Mumper RJ, et al. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target 2004; 12: 635–641. [DOI] [PubMed] [Google Scholar]
- 64. Kim H, Robinson SB, Csaky KG. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm Res 2009; 26: 329–337. [DOI] [PubMed] [Google Scholar]
- 65. Singh R, Rajaraman S, Balasubramanian M. A novel nanoparticle mediated selective inner retinal photocoagulation for diseases of the inner retina. IEEE Trans Nanobioscience 2017; 16: 542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kalita D, Shome D, Jain VG, et al. In vivo intraocular distribution and safety of periocular nanoparticle carboplatin for treatment of advanced retinoblastoma in humans. Am J Ophthalmol 2014; 157: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 67. Tisi A, Passacantando M, Lozzi L, et al. Cerium oxide nanoparticles reduce the accumulation of autofluorescent deposits in light-induced retinal degeneration: insights for age-related macular degeneration. Exp Eye Res 2020; 199: 108169. [DOI] [PubMed] [Google Scholar]
- 68. Tisi A, Flati V, Delle Monache S, et al. Nanoceria particles are an eligible candidate to prevent age-related macular degeneration by inhibiting retinal pigment epithelium cell death and autophagy alterations. Cells 2020; 9: 1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 2006; 1: 142–150. [DOI] [PubMed] [Google Scholar]
- 70. Dong Y, Wan G, Yan P, et al. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J Photochem Photobiol B 2019; 195: 51–57. [DOI] [PubMed] [Google Scholar]
- 71. Wilson AM, Mazzaferri J, Bergeron É, et al. In vivo laser-mediated retinal ganglion cell optoporation using KV1.1 conjugated gold nanoparticles. Nano Letters 2018; 18: 6981–6988. [DOI] [PubMed] [Google Scholar]
- 72. Tang J, Qin N, Chong Y, et al. Nanowire arrays restore vision in blind mice. Nat Commun 2018; 9: 3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sunshine JC, Sunshine SB, Bhutto I, et al. Poly(β-Amino Ester)-nanoparticle mediated transfection of retinal pigment epithelial cells in vitro and in vivo. PLoS ONE 2012; 7: e37543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Y, Liu C-H, Ji T, et al. Intravenous treatment of choroidal neovascularization by photo-targeted nanoparticles. Nat Commun 2019; 10: 5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Han Z, Conley SM, Makkia R, et al. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS ONE 2012; 7: e52189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Han Z, Conley SM, Makkia RS, et al. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest 2012; 122: 3221–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Read SP, Cashman SM, Kumar-Singh R. POD nanoparticles expressing GDNF provide structural and functional rescue of light-induced retinal degeneration in an adult mouse. Mol Ther 2010; 18: 1917–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Koirala A, Makkia RS, Conley SM, et al. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum Mol Genet 2013; 22: 1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Han Z, Koirala A, Makkia R, et al. Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: expression, repeat delivery and lack of toxicity. Nanomedicine (Lond) 2012; 7: 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cai X, Conley SM, Nash Z, et al. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 2010; 24: 1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cai X, Nash Z, Conley SM, et al. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE 2009; 4: e5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Luo L, Zhang X, Hirano Y, et al. Targeted intraceptor nanoparticle therapy reduces angiogenesis and fibrosis in primate and murine macular degeneration. ACS Nano 2013; 7: 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zeng L, Ma W, Shi L, et al. Poly(lactic-co-glycolic acid) nanoparticle-mediated interleukin-12 delivery for the treatment of diabetic retinopathy. Int J Nanomed 2019; 14: 6357–6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shmueli RB, Ohnaka M, Miki A, et al. Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials 2013; 34: 7544–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol 1998; 43: 245–269. [DOI] [PubMed] [Google Scholar]
- 86. Pollinger K, Hennig R, Ohlmann A, et al. Ligand-functionalized nanoparticles target endothelial cells in retinal capillaries after systemic application. Proc Nat Acad Sci 2013; 110: 6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mathew R, Sivaprasad S, Augsburger JJ, et al. Retina. In: Riordan-Eva P, Augsburger JJ. (eds) Vaughan . . . Asbury’s general ophthalmology, 19e. New York: Mcgraw-Hill Education, 2017: 195–199. [Google Scholar]
- 88. Brivio D, Zygmanski P, Arnoldussen M, et al. Kilovoltage radiosurgery with gold nanoparticles for neovascular age-related macular degeneration (AMD): a Monte Carlo evaluation. Phys Med Biol 2015; 60: 9203–9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang W, Sreekumar PG, Valluripalli V, et al. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J Control Release 2014; 191: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ohira A, Hara K, Johannesson G, et al. Topical dexamethasone gamma-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Acta Ophthalmol 2015; 93: 610–615. [DOI] [PubMed] [Google Scholar]
- 91. Kim H, Choi JS, Kim KS, et al. Flt1 peptide-hyaluronate conjugate micelle-like nanoparticles encapsulating genistein for the treatment of ocular neovascularization. Acta Biomater 2012; 8: 3932–3940. [DOI] [PubMed] [Google Scholar]
- 92. Kambhampati SP, Mishra MK, Mastorakos P, et al. Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells. Eur J Pharm Biopharm 2015; 95: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Iwase T, Fu J, Yoshida T, et al. Sustained delivery of a HIF-1 antagonist for ocular neovascularization. J Control Release 2013; 172: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Suen WL, Chau Y. Specific uptake of folate-decorated triamcinolone-encapsulating nanoparticles by retinal pigment epithelium cells enhances and prolongs antiangiogenic activity. J Control Release 2013; 167: 21–28. [DOI] [PubMed] [Google Scholar]
- 95. Lajunen T, Viitala L, Kontturi LS, et al. Light induced cytosolic drug delivery from liposomes with gold nanoparticles. J Control Release 2015; 203: 85–98. [DOI] [PubMed] [Google Scholar]
- 96. Gurunathan S, Lee KJ, Kalishwaralal K, et al. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009; 30: 6341–6350. [DOI] [PubMed] [Google Scholar]
- 97. Li L, Sahi SK, Peng M, et al. Luminescence- and nanoparticle-mediated increase of light absorption by photoreceptor cells: converting UV light to visible light. Scientific Rep 2016; 6: 20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ellis-Behnke R, Jonas JB. Redefining tissue engineering for nanomedicine in ophthalmology. Acta Ophthalmol 2011; 89: e108–e114. [DOI] [PubMed] [Google Scholar]
- 99. Tabatabaei SN, Tabatabaei MS, Girouard H, et al. Hyperthermia of magnetic nanoparticles allows passage of sodium fluorescein and Evans blue dye across the blood-retinal barrier. Int J Hyperthermia 2016; 32: 657–665. [DOI] [PubMed] [Google Scholar]
- 100. Nguyen VP, Li Y, Qian W, et al. Contrast agent enhanced multimodal photoacoustic microscopy and optical coherence tomography for imaging of rabbit choroidal and retinal vessels in vivo. Scientific Rep 2019; 9: 5945–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hainsworth DP, Gangula A, Ghoshdastidar S, et al. Diabetic retinopathy screening using a gold nanoparticle-based paper strip assay for the at-home detection of the urinary biomarker 8-hydroxy-2’-deoxyguanosine. Am J Ophthalmol 2020; 213: 306–319. [DOI] [PubMed] [Google Scholar]
- 102. Katchinskiy N, Godbout R, Elezzabi AY. Characterization of femtosecond-laser pulse induced cell membrane nanosurgical attachment. Biomed Opt Express 2016; 7: 2749–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wu Z, Troll J, Jeong HH, et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci Adv 2018; 4: eaat4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jo DH, Lee TG, Kim JH. Nanotechnology and nanotoxicology in retinopathy. Int J Mol Sci 2011; 12: 8288–8301. [DOI] [PMC free article] [PubMed] [Google Scholar]