Abstract
Objective
To compare the effects of remifentanil versus fentanyl during light sedation with dexmedetomidine in adults receiving mechanical ventilation (MV) in the intensive care unit.
Methods
In this retrospective cohort study, we compared the use of remifentanil versus fentanyl in adults receiving MV with dexmedetomidine sedation. The primary outcome was the proportion of time under light sedation (Richmond Agitation–Sedation Scale score between −1 and 0) during MV.
Results
We included 94 patients and classified 58 into the remifentanil group and 36 into the fentanyl group. The mean proportion of time under light sedation during MV was 66.6% ± 18.5% in the remifentanil group and 39.9% ± 27.3% in the fentanyl group. In the multivariate analysis with control for confounding factors, patients in the remifentanil group showed a significantly higher proportion of time under light sedation than patients in the fentanyl group (mean difference: 24.3 percentage points; 95% confidence interval: 12.9–35.8).
Conclusions
Remifentanil use might increase the proportion of time under light sedation in patients receiving MV compared with fentanyl administration.
Keywords: Light sedation, remifentanil, fentanyl, dexmedetomidine, mechanical ventilation, opioid
Introduction
According to Pain, Agitation/Sedation, Delirium, Immobility, and Sleep disruption (PADIS) guidelines,1 light sedation is recommended for adults undergoing mechanical ventilation (MV) because it is associated with a shorter time to extubation and a lower incidence of tracheostomy compared with deep sedation. Dexmedetomidine is a highly selective α2-adrenoceptor agonist that induces light sedation, corresponding to a Richmond Agitation–Sedation Scale (RASS) score between −1 and 0.2 However, the SPICE III study3 showed that dexmedetomidine requires additional sedative drugs to maintain light sedation (RASS score between −2 and +1) in patients undergoing MV in the intensive care unit (ICU). The appropriate combination of sedative agents and analgesic drugs was not determined in the SPICE III study and remains controversial.
Remifentanil is an ultra-short-acting opioid. It elicits a deep analgesic state without accumulating in tissues. This opioid is used in anesthetic practice to relieve pain and improve hemodynamic stability during surgery.4 Among surgical patients with short ICU stays, remifentanil is associated with small reductions in MV duration, time to extubation after the cessation of sedation, and length of ICU stay.5 However, limited evidence is available regarding remifentanil, and global standards for its use as an analgesic in the ICU have not been established. In the SPICE III study, the opioid used in combination with dexmedetomidine was mainly fentanyl; remifentanil was prohibited in the protocol. Therefore, the opioid most suitable for light sedation in adults undergoing MV is unknown.
We hypothesized that the combination of dexmedetomidine and remifentanil would allow patients undergoing MV to maintain light sedation more consistently than the combination of dexmedetomidine and fentanyl. We used an ICU database to investigate whether the selection of remifentanil versus fentanyl affected outcomes in patients sedated with dexmedetomidine.
Methods
Study design and approval
We conducted a retrospective cohort study at Hamamatsu University Hospital (Shizuoka, Japan) between January 2010 and July 2019. This study protocol was approved (19-054) by the Ethics Review Board of Hamamatsu University School of Medicine. Because of the retrospective design of the study and absence of follow-up, the Ethics Review Board waived the requirement for written informed consent. This study was conducted according to the STROBE checklist6 and complied with the 1964 Declaration of Helsinki and its later amendments.
Patients
This study included all patients who received MV under sedation with dexmedetomidine combined with remifentanil or fentanyl while in the ICU for >2 consecutive days between January 2010 and July 2019. The exclusion criteria were: (i) continuous infusion of sedative agents other than dexmedetomidine (e.g., propofol and midazolam); (ii) age younger than 18 years; (iii) surgery of the central nervous system (CNS) before or after MV; (iv) post-cardiac arrest syndrome; (v) use of dexmedetomidine, remifentanil, or fentanyl after extubation; (vi) ICU stay of less than 1 day; and (vii) use of both remifentanil and fentanyl as analgesics during ICU stay. Patients were divided into the remifentanil group and fentanyl group.
Data collection
We performed an automatic search of the electronic database of the Data Warehouse (DWH) from Prime GAIA (Nihon Kohden, Tokyo, Japan). The search criteria were the use of a mechanical ventilator and administration of dexmedetomidine with remifentanil or fentanyl. Additional inclusion/exclusion criteria were manually checked by two independent authors.
Demographic data (age, sex, body mass index [BMI], and Acute Physiology and Chronic Health Evaluation [APACHE] II score) were collected. Bolus injection of a sedative drug (e.g., midazolam or propofol for tracheal intubation) and nerve blockade (e.g., epidural or peripheral anesthesia) upon ICU admission were recorded as factors related to the degree of patient sedation and analgesia at the time of inclusion. Outcome-related data, such as the RASS score, hospital mortality, duration of MV, and duration of ICU stay, were also recorded. To maintain data accuracy and address potential bias, the data were collected by a co-author (T.N.) and analyzed by the first author (Y.A.).
Titration of analgesic and sedative agents and extubation criteria
There was no regulation within our facility for the selection of sedative and analgesic agents, and intensivists selected these drugs based on patient information. The intensivists adjusted the dose of the sedative with reference to the RASS score and adjusted the dose of analgesics with reference to the behavioral pain scale (BPS) score. Specific drug dosages were determined by the intensivist; ranges were not used. The decision to extubate was made by intensivists based on the daily evaluation of physical status. Patients who could breathe spontaneously for 30 minutes were extubated by the attending physician.
Outcomes and variables
The primary outcome was the proportion of time under light sedation (RASS score between −1 and 0) during MV. The RASS score was calculated by ICU nurses when patients entered the ICU. The RASS score was recalculated when the level of consciousness changed and then every 4 hours. If the RASS score had not been calculated for >4 hours, the data-collector read the nursing records to assess whether the RASS score had changed. In two types of sensitivity analysis, we excluded patients who died in the hospital and expanded the definition of light sedation from a RASS score between −1 and 0 to a score between −2 and +1. The secondary outcomes were hospital mortality, MV duration, duration of ICU stay, the proportion of patients who required additional analgesics during MV, and the proportion of patients who self-extubated. Adverse events potentially related to the drugs were also investigated. Data up to extubation were collected as the primary outcome. Data up to discharge from the ICU and hospital were the secondary outcomes. We included the APACHE II score, bolus injections of sedative drugs, timing of ICU admission, BMI, sex, nerve block upon ICU admission, and tracheostomy upon ICU admission as clinical covariates.
Statistical analyses
Data are presented as the mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables and number and percentage for categorical variables. To compare the mean values of continuous variables, Student’s t-test was applied. Categorical variables were analyzed with Fisher’s exact test. For the primary outcome, the proportion of time under light sedation was analyzed by using univariate and multivariate linear regression. Sensitivity analysis was also based on univariate and multivariate linear regression. For the secondary outcomes, the continuous variables (duration of MV and ICU stay) were evaluated using the same analytical method as that for the primary outcome. For secondary outcomes that were continuous data, the mean ± SD and 95% confidence interval (CI) of the mean difference (MD) were calculated. Secondary outcomes that were binary variables (hospital mortality and the proportion of patients receiving additional analgesics) were evaluated with univariate and multivariate logistic regression. For secondary outcomes that were binary variables, the odds ratio (OR) and 95% CI were calculated. The proportion of patients who self-extubated was analyzed with Fisher’s exact test. We analyzed continuous variables that did not have a normal distribution (P < 0.05 in Shapiro–Wilk test) after conducting a log transformation. P < 0.05 was considered significant in all analyses. Statistical procedures were conducted with SAS v9.4 (SAS Institute, Cary, NC, USA).
Results
Selection and characteristics of patients
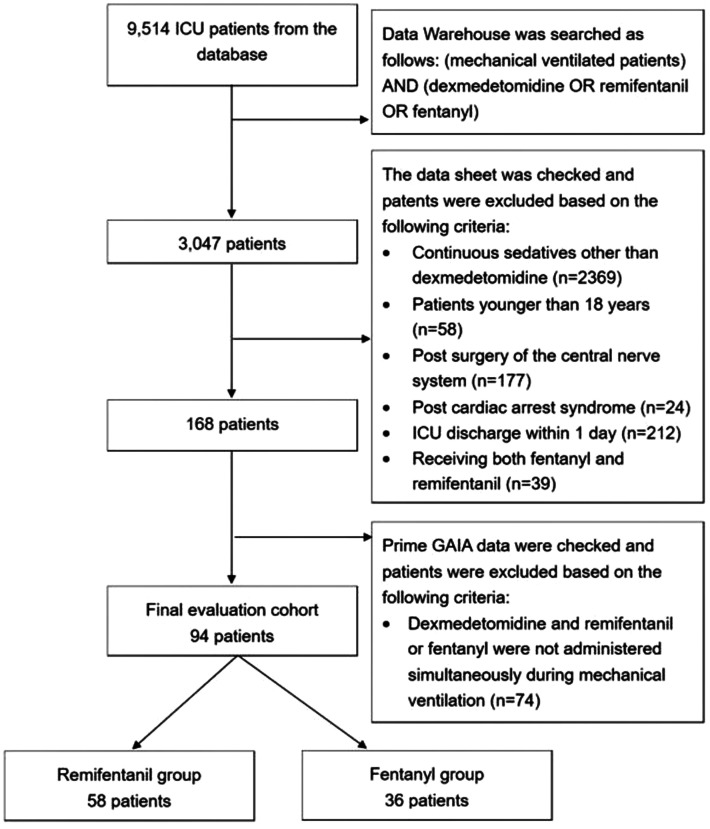
In total, 9,514 patients were admitted to the ICU at Hamamatsu University Hospital during the study period. We searched the DWH data using the preset search criteria and identified 3,047 individuals. Next, we searched the datasheet manually and excluded patients who received continuous administration of sedatives other than dexmedetomidine, were younger than 18 years of age, underwent CNS surgery, had post-cardiac arrest syndrome, left the ICU within 1 day, or received both fentanyl and remifentanil. After these exclusions, 168 patients remained. We also carefully assessed the Prime GAIA data to determine whether dexmedetomidine and remifentanil or fentanyl were administered simultaneously during MV. Finally, 94 patients were included: 58 in the remifentanil group and 36 in the fentanyl group (Figure 1). The characteristics of the enrolled patients are reported in Table 1. The characteristics of the patients at baseline were similar in the two groups, except for the timing of ICU admission, number of postoperative patients, and dose of remifentanil/fentanyl.
Figure 1.
Flowchart of the study.
ICU, intensive care unit.
Table 1.
Characteristics of patients receiving mechanical ventilation with dexmedetomidine sedation
| Remifentanil(n = 58) | Fentanyl(n = 36) | P value | |
|---|---|---|---|
| Age, years | 70.5 (16.2) | 69.2 (16.9) | 0.71 |
| Male sex | 37 (63.8%) | 22 (61.1%) | 0.83 |
| BMI, kg/m2 | 20.1 (3.6) | 20.6 (3.3) | 0.41 |
| APACHE II score | 21.6 (5.9) | 21.7 (7.2) | 0.90 |
| Bolus injection of sedative drug | 27 (46.6%) | 11 (30.6%) | 0.14 |
| Year of ICU admission | 2016.4 (2.1) | 2013.0 (2.1) | <0.0001 |
| Nerve block upon ICU admission | 3 (5.2%) | 1 (2.8%) | 1.00 |
| Tracheostomy upon ICU admission | 3 (5.2%) | 2 (5.6%) | 1.00 |
| Postoperative patients | 23 (39.7%) | 23 (63.9%) | 0.033 |
| Dose of dexmedetomidine, μg/kg/h | 0.31 (0.17) | 0.34 (0.17) | 0.56 |
| Dose of remifentanil/fentanyl, μg/kg/min | 0.038 (0.022) | 0.008 (0.005) | <0.0001 |
Values given are numbers (column %) or mean (standard deviation).
BMI, body mass index; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit.
Primary outcome
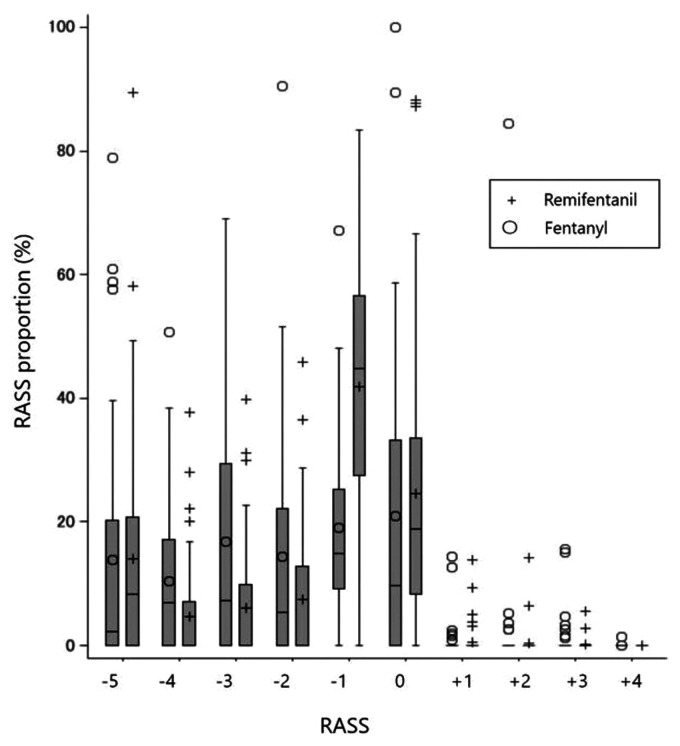
The proportion of time under light sedation was 66.6% ± 18.5% in the remifentanil group and 39.9% ± 27.3% in the fentanyl group (Table 2). After adjusting for potential confounders (APACHE II score, bolus injection of sedative drugs, timing of ICU admission, BMI, sex, nerve block upon ICU admission, and tracheostomy upon ICU admission), the remifentanil group exhibited an increased proportion of time under light sedation during MV compared with the fentanyl group (MD: 24.3 percentage points; 95% CI: 12.9–35.8; P < 0.0001). In the sensitivity analysis excluding patients who died in the hospital, the remifentanil group showed a higher proportion of time under light sedation than the fentanyl group (MD: 20.7 percentage points; 95% CI: 9.8–31.7; P = 0.0002). After additional sensitivity analysis with the expansion of the definition of light sedation from a RASS score between −1 and 0 to a score between −2 and +1, the result was consistent with that of the initial analysis (MD: 18.5 percentage points; 95% CI: 6.3–30.8; P = 0.003). The distribution of RASS scores in all cases is displayed in Figure 2.
Table 2.
Proportion of time under light sedation during mechanical ventilation in patients sedated with dexmedetomidine combined with remifentanil or fentanyl
| Remifentanil | Fentanyl | Univariate analysis | P value | Multivariate regression analysis | P value | |
|---|---|---|---|---|---|---|
| Mean difference, % (95% CI) | Mean difference, % (95% CI) | |||||
| Primary outcome | ||||||
| Proportion of time under light sedation, %, mean (SD) | 66.6 (18.5) | 39.9 (27.3) | 26.7 (17.5 to 35.9) | <0.0001 | 24.3 (12.9 to 35.8) | <0.0001 |
| Sensitivity analysis | ||||||
| With exclusion of patients who died in hospital | 67.8 (16.7) | 44.6 (26.1) | 23.2 (14.3 to 32.1) | <0.0001 | 20.7 (9.8 to 31.7) | 0.0002 |
| With expansion of target RASS score from −1–0 to −2–1 | 75.7 (19.5) | 55.1 (30.5) | 20.6 (10.6 to 30.6) | <0.0001 | 18.5 (6.3 to 30.8) | 0.003 |
SD, standard deviation; CI, confidence interval; RASS, Richmond Agitation–Sedation Scale.
Figure 2.
Distribution of Richmond Agitation–Sedation Scale (RASS) scores in patients admitted to the ICU who received mechanical ventilation under sedation with dexmedetomidine combined with remifentanil or fentanyl. Comparing RASS scores between the remifentanil and fentanyl groups revealed a significant difference for a RASS score of −1 (P < 0.001; Wilcoxon rank-sum test, Bonferroni post hoc test); the differences for the other RASS scores were not significant.
RASS, Richmond Agitation–Sedation Scale; ICU, intensive care unit.
Secondary outcomes
Table 3 shows all secondary outcomes. Hospital mortality and the proportion of patients who self-extubated were not significantly different between the two groups. The proportion of patients receiving additional analgesics was significantly lower in the remifentanil group than in the fentanyl group (OR: 0.09; 95% CI: 0.02–0.46; P = 0.003). The durations of MV and ICU stay were continuous variables without a normal distribution and compared between the two groups after log transformation. The durations of MV and ICU stay did not differ significantly between the two groups.
Table 3.
Secondary outcomes of patients who were mechanically ventilated and had been sedated with dexmedetomidine combined with remifentanil or fentanyl
| A) Binary variables | ||||||
|---|---|---|---|---|---|---|
| Remifentanil | Fentanyl | Univariate analysis | P value | Multivariate analysis | P value | |
| OR (95%CI) | OR (95%CI) | |||||
| Hospital mortality | 2 (3.4%) | 5 (13.9%) | 0.22 (0.04 to 1.21) | 0.08 | 0.22 (1.07 to 1.41) | 0.11 |
| Use of additional analgesics | 2 (3.4%) | 10 (27.8%) | 0.09 (0.02 to 0.45) | 0.003 | 0.09 (0.02 to 0.46) | 0.003 |
| Self-extubation | 0 | 1 (2.8%) | 0.38 | |||
|
| ||||||
|
B) Continuous variables | ||||||
|
Remifentanil |
Fentanyl |
Univariate analysis |
P value |
Multivariate analysis |
P value |
|
|
Difference for log-transformed hour (95% CI) |
Difference for log-transformed hour (95% CI) |
|||||
| Duration of mechanical ventilation, hours | 24.3 (15.5 to 54.3) | 36.8 (14.5 to 87.8) | −0.21 (−0.59 to 0.17) | 0.27 | 0.10 (−0.12 to 0.33) | 0.36 |
| Duration of ICU stay, hours | 80.0 (50.1 to 134.9) | 92.8 (53.4 to 171.8) | −0.11 (−0.44 to 0.21) | 0.49 | 0.23 (−0.06 to 0.53) | 0.12 |
Values are number (column %) or median (interquartile range).
CI, confidence interval; OR, odds ratio; ICU, intensive care unit.
Adverse events
Bradycardia and hypotension occurred sporadically and were addressed with drug reduction or discontinuation. In some of these cases, inotropic and vasopressor drugs were continuously administered. Drug-related cardiac arrest did not occur.
Discussion
We aimed to compare the efficacy of remifentanil and fentanyl in adults undergoing MV with dexmedetomidine sedation. The group receiving remifentanil exhibited an increased proportion of time under light sedation (MD 24.3 percentage points) and a lower percentage of patients receiving additional analgesics. Remifentanil administration did not reduce in-hospital mortality, MV duration, or the risk of self-extubation. Our findings show that remifentanil use resulted in the more consistent maintenance of light sedation than fentanyl use in patients undergoing MV in the ICU.
This is the first study to demonstrate that a combination of dexmedetomidine and remifentanil allows patients to maintain light sedation during MV in the ICU. In other settings, such as ablation,7,8 endoscopic procedures,9 and colonoscopy,10 the usefulness of dexmedetomidine with remifentanil has been reported. In the ICU, dexmedetomidine is an ideal drug for light sedation2 because it can prevent delirium11–13 and improve synchrony with MV.14 However, in the SPICE III study,3 additional sedatives were required to maintain light sedation, and dexmedetomidine was usually combined with fentanyl. Moreover, whereas the SPICE III study allowed dexmedetomidine doses of up to 1.5 µg/kg/hour, Japanese protocols allow a dexmedetomidine dose of no more than 0.7 µg/kg/hour. Nevertheless, MV was possible in this study population, perhaps because of the differences in the type and dosage of opioids. Our results suggest that the combination of dexmedetomidine and remifentanil enables the maintenance of light sedation without the need for other sedatives.
We focused on remifentanil use in the ICU. Remifentanil (a 4-anilidopiperidine derivative of fentanyl) is an ultra-short-acting µ-opioid receptor agonist. Remifentanil is metabolized by nonspecific esterases in blood and tissue and undergoes rapid metabolism independent of the duration of infusion or insufficiency of any organ.15 These unique properties differentiate it from other opioid agents. Although the synthetic opioids fentanyl, alfentanil, and sufentanil have good profiles, they undergo hepatic metabolism, and their continuous infusion leads to accumulation in tissues and prolonged drug effects.16 Although the remifentanil dose in this study was higher than that of fentanyl (Table 1), our intensivists may have administered higher doses because they were not concerned about drug accumulation in tissues. Because bradycardia is the only identified drawback to low-dose remifentanil, remifentanil use in our ICU has increased yearly compared with fentanyl use. Similarly, remifentanil has become a preferred analgesic in the ICU in other countries.17,18 Remifentanil appears to be a good choice for analgesia-based sedation; however, ICU reports are mainly from Europe, and fentanyl remains the mainstream opioid in North America.19 Although remifentanil has a short half-life and is thus more easily titrated than other opioids, the tachyphylaxis and hyperalgesia associated with prolonged infusion limit its use in the ICU to short durations. In the present study, the length of ICU stay and MV were relatively short. Further studies will be needed to demonstrate the superiority of remifentanil in the ICU.
Regarding secondary outcomes, remifentanil was not associated with mortality; this result is consistent with that reported in a systematic review.5 Evidence indicates that the type of opioid analgesic used is not associated with mortality in the ICU. We did not find a significant difference in MV duration between the remifentanil group and the fentanyl group. This result differs from other studies in which remifentanil reduced the MV duration.5,20 This inconsistency suggests that the MV period did not necessarily include the weaning stage in our study. Studies have shown that remifentanil use shortens the duration of ICU stay,5 but we found no significant between-group difference in ICU stay in this study. This finding may be attributed to the fact that patients in this study were not yet at the stage of leaving the ICU. The proportion of patients who received additional analgesics was lower in the remifentanil group than in the fentanyl group; however, this result should be interpreted with caution. We included the use of non-opioid analgesics (e.g., acetaminophen and nonsteroidal anti-inflammatory drugs) because these drugs are recommended in PADIS guidelines.1 However, we could not determine whether the reason for drug administration was pain or fever in our data. The proportion of patients who self-extubated was similar between the groups. Self-extubation appears to be associated with benzodiazepines but not with the type of opioid.21 Because the incidence of self-extubation was low, our result should be interpreted with caution.
Although there were no apparent adverse events in our study, dexmedetomidine has been reported to cause bradycardia more frequently than other sedatives.3,22–24 In addition, asystole was reported with dexmedetomidine use in the SPICE III study.3 Remifentanil is also a bradycardia-inducing drug25,26 and should be used with caution in combination with dexmedetomidine for patients with bradycardia. However, because the doses of both dexmedetomidine and remifentanil were relatively low in this study, side effects were less likely to occur. Our sample size was too small to investigate adverse effects; therefore, the adverse effects of combined dexmedetomidine and remifentanil should be investigated systematically in a larger study.
The definition of light sedation has yet to be confirmed. Earlier reports27 defined light sedation as a RASS score from −2 to +1, but recent reports2 have advocated a narrower range of RASS score (−1 to 0). PADIS guidelines1 state, “Although the prior guideline defined light sedation as a RASS scale score of greater than or equal to –2 and eye opening of at least 10 minutes,28 this level of sedation is probably deeper than required for the management of mechanically ventilated adults in an ICU.” Therefore, in the present study, a RASS score from −1 to 0 was used as the primary endpoint for light sedation, and a range of RASS scores between −2 and +1 was used in the sensitivity analysis. In future studies, it will be necessary to determine the optimal RASS score.
Our cohort study had eight main limitations. First, it was conducted at a single institution, and there was no standardized protocol for sedation or method for reaching the target RASS score. Intensivists at our institution adjusted the dose of dexmedetomidine and remifentanil/fentanyl based on the RASS score and BPS score, but a strict protocol of drug titration was not in place. Although this is a valuable study examining the hypothesis that a combination of dexmedetomidine and remifentanil provides light sedation, our hypothesis must be tested in a prospective study. Second, our observational cohort design could not control for unmeasured or unknown confounding factors that may have influenced the results. For example, we could not collect information regarding rehabilitation, mobilization, postural change, or wound treatment. Third, the accuracy of the evaluated RASS score and data collection may have been suboptimal. However, our ICU nurses are trained thoroughly in calculating the RASS score; moreover, the RASS score is highly reliable among evaluators.29 Because data collection was based on a two-author check, we adequately addressed this issue. Fourth, the tolerance and hyperalgesia observed with remifentanil use were not considered in our study. These side effects have been reported to be dependent on the remifentanil dose, with ≤0.2 µg/kg/minute30 but also 0.1 to 0.15 µg/kg/minute31,32 recommended during general anesthesia. However, in the present study, the mean dose of 0.038 µg/kg/minute was much lower. In addition, dexmedetomidine has both analgesic and sedative effects,33 suggesting the possibility of comfortable conditions with a low dose of remifentanil in patients undergoing MV. Fifth, it was uncertain whether the patients included in our study had a targeted RASS score between −1 and 0. However, light sedation is a standard treatment in the ICU; thus, we often control the administration of sedatives and analgesics to achieve a target RASS score between −1 and 0. If we target deep sedation, we select propofol or midazolam. Sixth, although we selected patients sedated with dexmedetomidine combined with remifentanil or fentanyl during MV, we could not collect information regarding increases and reductions in the dose of each drug or drug discontinuation. Prospective studies will be needed to evaluate the effects of the dose and duration of each drug. Seventh, the RASS score depends on the original level of consciousness. Therefore, we excluded patients who had undergone CNS surgery and those with post-cardiac arrest syndrome, and we performed a sensitivity analysis to exclude cases with a poor prognosis. However, patients might have had diseases that affected their level of consciousness, such as hepatic encephalopathy or septic encephalopathy. Finally, our study included a unique group of patients. Patients under ventilation who are admitted to the ICU and managed with dexmedetomidine and remifentanil or fentanyl are a group that accounts for only ∼1% of all patients admitted to ICUs. In addition, the lack of strict MV weaning and drug adjustment protocols may have significantly affected the results. Therefore, the results of this study are very limited, and we should be cautious about expansive interpretation.
Conclusions
During MV with dexmedetomidine sedation, the use of remifentanil was associated with an approximately 25% greater proportion of time under light sedation than the proportion obtained with fentanyl.
Acknowledgement
We thank Rebecca Tollefson, DVM, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Yoshitaka Aoki https://orcid.org/0000-0002-3750-4160
Yuki Shiko https://orcid.org/0000-0002-3959-9343
References
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018; 46: e825–e873. [DOI] [PubMed] [Google Scholar]
- 2.Romagnoli S, Amigoni A, Blangetti I, et al . Light sedation with dexmedetomidine: a practical approach for the intensivist in different ICU patients. Minerva Anestesiol 2018; 84: 731–746. [DOI] [PubMed] [Google Scholar]
- 3.Shehabi Y, Howe BD, Bellomo R, et al. Early Sedation with Dexmedetomidine in Critically Ill Patients. N Engl J Med 2019; 380: 2506–2517. [DOI] [PubMed] [Google Scholar]
- 4.Rosow CE. An overview of remifentanil. Anesth Analg 1999; 89: S1–S3. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Wang Y, Du B, et al. Could remifentanil reduce duration of mechanical ventilation in comparison with other opioids for mechanically ventilated patients? A systematic review and meta-analysis. Crit Care 2017; 21: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 7.Fu X, Huang F, Chen Y, et al. Application of dexmedetomidine-remifentanil in high-intensity ultrasound ablation of uterine fibroids: a randomised study. BJOG 2017; 124: 23–29. [DOI] [PubMed] [Google Scholar]
- 8.Semenas E, Lönnemark M, Dahlman P, et al. Analgesic effects of dexmedetomidine and remifentanil on periprocedural pain during percutaneous ablation of renal carcinoma. Ups J Med Sci 2020; 125: 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu Z, Li W, Chen H, et al. Efficacy of a Dexmedetomidine-Remifentanil Combination Compared with a Midazolam-Remifentanil Combination for Conscious Sedation During Therapeutic Endoscopic Retrograde Cholangio-Pancreatography: A Prospective, Randomized, Single-Blinded Preliminary Trial. Dig Dis Sci 2018; 63: 1633–1640. [DOI] [PubMed] [Google Scholar]
- 10.Jia L, Xie M, Zhang J, et al. Efficacy of different dose of dexmedetomidine combined with remifentanil in colonoscopy: a randomized controlled trial. BMC Anesthesiol 2020; 20: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reade MC, Eastwood GM, Bellomo R, et al. Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients With Agitated Delirium: A Randomized Clinical Trial. JAMA 2016; 315: 1460–1468. [DOI] [PubMed] [Google Scholar]
- 12.Zaal IJ, Devlin JW, Peelen LM, et al . A systematic review of risk factors for delirium in the ICU. Crit Care Med 2015; 43: 40–47. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Du H, Wei BH, et al. Development and validation of risk-stratification delirium prediction model for critically ill patients: A prospective, observational, single-center study. Medicine (Baltimore) 2017; 96: e7543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conti G, Ranieri VM, Costa R, et al. Effects of dexmedetomidine and propofol on patient-ventilator interaction in difficult-to-wean, mechanically ventilated patients: a prospective, open-label, randomised, multicentre study. Crit Care 2016; 20: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelm W, Kreuer S. The place for short-acting opioids: special emphasis on remifentanil. Crit Care 2008; 12: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Battershill AJ, Keating GM. Remifentanil: a review of its analgesic and sedative use in the intensive care unit. Drugs 2006; 66: 365–385. [DOI] [PubMed] [Google Scholar]
- 17.Talsi O, Kiiski Berggren R, Johansson G, et al. A national survey on routines regarding sedation in Swedish intensive care units. Ups J Med Sci 2019; 124: 199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HH, Choi SC, Ahn JH, et al. Analysis of trends in usage of analgesics and sedatives in intensive care units of South Korea: A retrospective nationwide population-based study. Medicine (Baltimore) 2018; 97: e12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanios M, Nguyen HM, Park H, et al. Analgesia-first sedation in critically ill adults: A U.S. pilot, randomized controlled trial. J Crit Care 2019; 53: 107–113. [DOI] [PubMed] [Google Scholar]
- 20.Greco M, Landoni G, Biondi-Zoccai G, et al. Remifentanil in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth 2012; 26: 110–116. [DOI] [PubMed] [Google Scholar]
- 21.Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth 2001; 13: 24–29. [DOI] [PubMed] [Google Scholar]
- 22.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298: 2644–2653. [DOI] [PubMed] [Google Scholar]
- 23.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301: 489–499. [DOI] [PubMed] [Google Scholar]
- 24.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA 2012; 307: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 25.Reid JE, Mirakhur RK. Bradycardia after administration of remifentanil. Br J Anaesth 2000; 84: 422–423. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi K, Tanaka A. Effect-site concentrations of remifentanil causing bradycardia in hypnotic and non-hypnotic patients. J Clin Monit Comput 2016; 30: 919–924. [DOI] [PubMed] [Google Scholar]
- 27.Brattebø G, Hofoss D, Flaatten H, et al. Effect of a scoring system and protocol for sedation on duration of patients' need for ventilator support in a surgical intensive care unit. BMJ 2002; 324: 1386–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kress JP, Pohlman AS, O'Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000; 342: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 29.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002; 166: 1338–1344. [DOI] [PubMed] [Google Scholar]
- 30.Yu EH, Tran DH, Lam SW, et al. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia 2016; 71: 1347–1362. [DOI] [PubMed] [Google Scholar]
- 31.Delvaux B, Ryckwaert Y, Van Boven M, et al. Remifentanil in the intensive care unit: tolerance and acute withdrawal syndrome after prolonged sedation. Anesthesiology 2005; 102: 1281–1282. [DOI] [PubMed] [Google Scholar]
- 32.Zakhary WZA, Turton EW, Flo Forner A, et al. A comparison of sufentanil vs. remifentanil in fast-track cardiac surgery patients. Anaesthesia 2019; 74: 602–608. doi: 10.1111/anae.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grape S, Kirkham KR, Frauenknecht J, et al. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia 2019; 74: 793–800. [DOI] [PubMed] [Google Scholar]