Abstract
Background:
The risk of primary aristolochic acid (AA)-associated urothelial carcinoma (AA-UC) has been summarized by a 2013-published meta-analysis. Given that additional evidence has been continuously reported by original studies, an updated meta-analysis is needed. Meanwhile, to complete the whole picture, a systematic review of molecular alterations observed in AA-urinary tract cancers (AA-UTC) was also performed.
Methods:
We searched PubMed, Embase and four Chinese databases up to October 2020. Observational studies comparing risk or oncologic outcomes of UTC between patients with and without AA exposure were eligible for systematic review and meta-analysis. Studies investigating molecular alterations in AA-UTC using human tissue samples were eligible for systematic review.
Results:
In total, 38 and 20 studies were included in the systematic review and meta-analysis, respectively. Exposure to AA led to an overall increased risks of primary UTC [UC and renal cell carcinoma (RCC)] (OR 6.085, 95% CI 3.045–12.160) and postoperatively recurrent UC (RR 1.831, 95% CI 1.528–2.194). Subgroup analysis of postoperative primary AA-upper tract UC (AA-UTUC) showed increased risks of bladder recurrence (adjusted RR 1.949, 95% CI 1.462–2.597) and contralateral UTUC recurrence (crude RR 3.760, 95% CI 2.225–6.353), worse overall survival (adjusted HR 2.025, 95% CI 1.432–2.865) and worse disease-specific survival (adjusted HR 3.061, 95% CI 1.190–7.872), but no effect on cancer-specific survival (adjusted HR 0.772, 95% CI 0.269–2.215). High mutation load with AA mutational signature presenting largely in the putative driver genes was observed in AA-UTUC. In contrast, AA mutational signature is rarely found in the mutated RCC driver genes and the mutation load in AA-RCC is low. Therefore, AA has different roles in the genesis of UTUC and RCC.
Conclusions:
Implementing effective strategies to completely protect people from exposure to AA is urgently needed. Additionally, more effort should be made in identifying the precise carcinogenic mechanisms of AA to determine the future treatment strategies.
Plain language summary
Risk, recurrence and survival outcomes after surgery and molecular changes possibly involved in the genesis of aristolochic acid-associated urinary tract cancers
Background: The association between aristolochic acid (AA) and primary urothelial carcinoma (UC) has been summarized by a 2013-published meta-analysis. Given that additional evidence has been reported in the past 7 years, an updated meta-analysis is needed. Meanwhile, to complete the whole picture, a systematic review of molecular changes possibly involved in AA-mediated urinary tract carcinogenesis was also performed. Methods: We searched PubMed, Embase and four Chinese databases for human studies up to October 2020. Studies comparing the risk of urinary tract cancer (UTC) between patients with and without AA exposure and studies investigating the molecular changes in AA-associated UTC (AA-UTC) using human tissue samples were eligible for inclusion. Thirty-eight studies were finally included. Results: The results showed that exposure to AA was associated with a 6-fold increased risk of primary UTC (UC and renal cell carcinoma, RCC) and a 1.8-fold increased risk of postoperatively recurrent UC. After studies reporting primary AA-upper tract UC (AA-UTUC) were analyzed, a 1.9-fold increased risk of bladder recurrence and a 3.8-fold increased risk of contralateral UTUC recurrence was observed. Additionally, exposure to AA worsened the postoperative survival of patients with UTUC by a 2-fold increased risk of overall death and a 3-fold increased risk of death from other diseases and recurrences. However, there was no effect on death due to cancer. Lastly, AA seemed to play different roles in the etiology of UTUC and RCC based on the observations of different mutation loads and different distributions of AA-induced mutations in AA-UTUC and AA-RCC samples. Conclusions: Implementing effective strategies to completely protect people from exposure to AA is urgently needed. Moreover, more effort should be made in identifying the precise carcinogenic mechanisms of AA-UTC to determine the future treatment strategies.
Keywords: aristolochic acid, bladder recurrence, contralateral upper tract urothelial carcinoma recurrence, molecular alterations, oncologic outcomes, updated meta-analysis, updated systematic review, urothelial carcinoma, upper tract urothelial carcinoma
Introduction
Aristolochic acid (AA), a toxic compound existing in plants of genera Aristolochia and Asarum, is mainly composed of a mixture of AAI and AAII.1,2 It seems that the nephrotoxic effect of AA is induced by AAI, while the genotoxic and carcinogenic effects were attributed to both AAI and AAII.2 The amount of AA content varies with genera and species of plants.1 Aristolochic acid nephropathy (AAN) occurring after intake of AA-containing herbal medicines is characterized by chronic tubulointerstitial fibrosis with progression to end-stage renal disease (ESRD) and is accompanied by a high risk of upper tract urothelial carcinoma (UTUC)2,3 and subsequent onset of bladder urothelial carcinoma.4 Balkan endemic nephropathy (BEN), predominantly observed in Balkan countries, is an environmental form of AAN resulting from chronic dietary consumption of wheat flour contaminated by Aristolochia clematitis.2,3,5 The mechanism of carcinogenesis of AA has been extensively studied. After metabolic activation, AA binds covalently to dA and dG residues in DNA to form aristolactam-DNA (AL-DNA) adducts which are concentrated in the renal cortex and causally related to the initiation phase of tumorigenesis.6,7 Both dG and dA adducts block DNA replication and give rise to misincorporation of dA.8,9 The dA-AL adducts are more mutagenic and persistent because, when paired with thymidine (dA-AL:dT), they are repaired by transcription-coupled repair but resistant to global genome nucleotide excision repair (GG-NER).6,9 Such a selective repair results in a mutational pattern of marked nontranscribed strand bias and the persistence of dA-AL adducts in tissues even after stopping exposure to AA for decades.6,9 However, when dAMP is inserted opposite the dA-AL adduct owing to misincorporation,8,9 the resultant dA-AL:dA pair is susceptible to GG-NER by which dA-AL is excised and replaced with dTMP leading to permanent A-to-T transversion.6,9 This distinct single base substitution (SBS) signature, characterized by predominant A:T-to-T:A transversions occurring most commonly in the 5′-Py_A_G-3′ trinucleotide context and enriched on the nontranscribed strand,9 is labeled as SBS22 in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database (v3.1, https://cancer.sanger.ac.uk/cosmic/signatures/). AL-DNA adducts coupled with the AA mutational signature serve as the robust biomarkers of AA-associated UTUC (AA-UTUC).10–12 UTUC tumorigenesis driven by AA-induced mutations was previously considered via affecting the TP53 tumor suppressor gene10–12 but subsequently confirmed also involving many other oncogenic driver genes throughout the genome by whole-genome/exome sequencing (WGS/WES) using next-generation sequencing (NGS) methods.13,14 Due to its potent toxicity, AA was classified as a class I human carcinogen in 2002,15 leading to the official bans of AA-containing herbs and products in many regions/areas.1 However, people still can purchase certain AA-containing herbal products through different methods without prescription (e.g. internet, local markets, or pharmacies).1,2,16 Additionally, AA-containing herbs are still allowed to be used in some areas, such as mainland China,17 Taiwan,18 and Romania.19 As a result, cases of AAN are constantly reported worldwide.1,2,20
Wu and Wang21 conducted a meta-analysis in 2013 to estimate the risk of primary AA-urothelial carcinoma (AA-UC) with the result of pooled odds ratio (OR) 5.97 [95% confidence interval (CI) 2.78–12.84]. However, their literature search was conducted solely using PubMed and they might miss relevant articles collected in mainland China and Taiwan databases. Moreover, the association between AA and another type of urinary tract cancer (UTC), renal cell carcinoma (RCC), has been shown in the past 7 years by the detection of AL-DNA adducts10,19 and genome-wide present A:T-to-T:A transversions22,23 in the RCC patients. Therefore, clinicians need to have the state-of-the-art information to make the most appropriate clinical decision. We aimed to fill the literature gap via performing an updated meta-analysis to summarize the evidence on AA-UTC, and provide the latest estimation. Meanwhile, except for the well-known AA-induced TP53 mutations, other molecular alterations involved in AA-mediated carcinogenesis remain incompletely understood and under investigation. Bara et al.24 performed a systematic review to identify the possible carcinogenic role of various AA-associated cancers (including AA-UTUC and AA-RCC) in 2017. Later, Hassler et al.25 performed another systematic review to investigate the molecular characterization of all causes of UTUC (including AA-UTUC) in 2020. To complete the whole picture of the present systematic review, we updated the information of the two systematic reviews on the molecular alterations observed in AA-UTC, including AA-UTUC, AA-RCC, and AA-bladder cancer (AA-BC), to October 2020.
Materials and methods
Search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for the present systematic review and meta-analysis.26 The electronic database search included PubMed, Embase, and four Chinese databases: Airiti Library, China National Knowledge Infrastructure (CNKI), VIP information/Chinese Scientific Journals database (CSJD-VIP) and Wanfang Data. The search period was between the inception date of every database and 31 October 2020, except for Wanfang Data with the search period up to 31 July 2020. PubMed and Embase were searched with the following search strategy: (Aristolochic acid OR Balkan endemic nephropathy) AND (urothelial carcinoma OR urothelial cancer OR transitional cell carcinoma OR transitional cell cancer OR renal pelvis cancer OR renal pelvic neoplasms OR bladder cancer OR urinary bladder neoplasms OR ureteral cancer OR ureteral neoplasms OR renal cell carcinoma OR renal cell cancer). Except for “Balkan endemic nephropathy,” all of the search key words in Chinese characters were also used in the search of Chinese databases (see Supplemental material Appendix A). The reference lists from relevant studies were surveyed as well to identify additional eligible studies for inclusion.
Selection criteria
We attempted to include original human studies with no restriction on publication dates, and languages. Moreover, the name of AA-containing herbs or herbal products, or the term “aristolochic acid” should be explicitly stated in those studies assessing AA exposure via intake of AA-containing herbs when the information was obtained without objective evidence (i.e. information only obtained from the medical records or patients’ histories). Those studies only used “Chinese herbal medicines” or other similar terms that we could not assess the study participants’ AA exposure were not considered to be included. Studies that compared the risk or oncologic outcomes of UTC between patients with and without a history of AA exposure were evaluated using the following inclusion criteria: (1) the study design was observational; (2) data were reported as OR, relative risks (RR) or hazard ratio (HR) with 95% CI, or number of events with sample sizes; (3) case reports, case series, single-arm descriptive observational studies, and studies without sufficient information for our analysis were excluded. Studies that aimed to identify the molecular alterations possibly involved in AA-mediated urinary tract carcinogenesis were eligible for inclusion if tissue samples from patients with AA-UTC were used. All study designs were acceptable except for case reports. When searching for studies investigating the molecular alterations in AA-UTUC, we focused on those not just identifying AA-induced TP53 A:T-to-T:A transversions.
Data extraction and validity assessment
YCK and MHC independently extracted the data using an extraction form with consensus on all extracted items. Extracted data of studies reporting risk of AA-UTC were publication year and type, study location, design, and period, baseline renal function of patients, study outcome, exposed AA-containing herbs and exposure time, methods of AA exposure assessment, classification and diagnostic criteria of AAN/BEN/AA-UC, follow-up time, outcome data for each included study and adjuvant/neoadjuvant therapy in studies reporting recurrent UC. The methodological quality of the included studies was assessed using the Newcastle–Ottawa scale (NOS)27 with slight modifications adapted for this study (see Supplemental material Appendix B). A study with a total quality score of 5 or less was deemed at high risk of bias and was not included in the meta-analysis. Extracted data of studies investigating the molecular alterations in AA-UTC were publication year and type, country where tissue samples obtained, cancer type of AA-exposed samples, method of AA exposure assessment, type of non-AA-exposed control samples, tissue, analytical method, and molecular alterations identified in the AA-exposed samples.
Statistical analysis
Studies reporting the risk or oncologic outcomes of AA-UTC were analyzed in the meta-analysis. The outcome data of these studies were presented with either number of events with sample sizes or point estimates with 95% CIs. Comprehensive Meta-Analysis version 3 (CMA 3; Biostat Inc., Englewood, NJ, USA) was used to combine the two different formats of data. Because studies reporting primary AA-UTC included cohort and case-control studies, summary ORs were calculated for the meta-analysis. Pooled HRs were calculated for meta-analysis of survival outcomes. Because HR was not available in some studies in studies reporting recurrent AA-UC, RRs were used in these studies and combined with HRs to obtain the summary RRs. All meta-analyses were done by the DerSimonian and Laird (inverse variance) random-effects model.28 A few studies reporting recurrent AA-UC had zero events in control groups and imbalanced sample sizes between AA and control groups; applying inverse variance method with the default constant continuity correction of 0.5 in the CMA may bias the result toward no effect and generate an underestimated summary estimate,29 especially when the proportion of zero-event studies in a meta-analysis is over 50%.30 Under this circumstance, Mantel–Haenszel (M-H) method without zero-cell correction was applied because it provides less biased summary estimate.29 Only M-H pooled OR but not M-H pooled RR has been evaluated in the methodological research of meta-analysis for zero-event studies. However, because the performance of RRs is very similar to their corresponding OR measurements in rare events,31 we used M-H pooled RR without zero-cell correction. The M-H method has two assumptions: (1) studies with little between-study heterogeneity and available data of event numbers and sample sizes are combined; (2) fix-effects model is used.29 When any of the two assumptions was not met, we would still use inverse variance method with 0.5 continuity correction, or did not meta-analyze the data when the proportion of zero-event studies in a meta-analysis was over 50%. For a study presenting different consumption levels of AA, the highest one would be chosen. For a study presenting data of AL-DNA adducts and TP53 A:T-to-T:A mutations separately, the data of AL-DNA adducts would be analyzed in the meta-analysis based on the diagnostic criteria of AAN proposed by Gökmen et al.3 For a study including both patients with and without AAN diagnosis, only the data of cases with AAN diagnosis were analyzed in the meta-analysis. For a study presenting both unadjusted and adjusted estimates, only adjusted estimates were used in the meta-analysis. For a study presenting several adjusted estimates, the one adjusting the largest number of potential confounding factors was used to determine the pooled estimates. Between-study heterogeneity was assessed by the chi-squared (χ2)-based Q-statistic (significance level at p < 0.1) and quantified by I2-measure (25%: low heterogeneity, 50%: moderate heterogeneity, and 75%: high heterogeneity).32 Potential sources of heterogeneity were explored by subgroup analyses. Possible publication bias was assessed by the funnel plot method33 and the Egger’s linear regression test.34 Sensitivity analyses were also conducted to test the robustness of our findings. The meta package in R version 4.0.0 (R-4.0.0, www.r-project.org/) was used to calculate the M-H summary estimates without zero-cell correction and CMA was used to performed all of the other statistical analyses in the present study and generate forest plots.
Results
Comparison of the included studies between the present and previous systematic reviews
The flow diagram for the study selection process is presented in Figure 1. In total, 38 studies, including 22 studies reporting the risk or oncologic outcomes of AA-UTC,35–56 13 studies identifying the molecular alterations in AA-UTC,10,13,14,22,57–65 and three studies investigating both the risk/oncologic outcomes and molecular alterations in AA-UTC,66–68 were included in the present systematic review. Among the eight studies10,12,36,38,39,66,69,70 included in Wu and Wang’s meta-analysis,21 nine studies14,23,49,52,59,61,62,66,67 included in the systematic review of Bara et al.,24 and five studies13,14,58,61,62 included in the systematic review of Hassler et al.,25 five studies reporting the risk36,38,39 or oncologic outcomes49,52 of AA-UC, seven studies reporting the molecular alterations in AA-UTUC10,13,14,58,61,62 or AA-BC,59 and two studies reporting both the risk and molecular alterations in AA-UTUC66 or AA-RCC67 were also included in the present review. Two studies reporting the risk of AA-UC69,70 were excluded because only “Chinese herbs” were reported, and the history of AA exposure could not be confirmed. One study investigating only TP53 mutation in AA-UTUC12 was excluded. One study investigating AA-RCC23 was excluded because the aim of the study was to find the etiology of RCC in BEN regions. Seventeen studies reporting the risk35,37,40–47 or oncologic outcomes44,48,50,51,53–56 of AA-UTC, six studies reporting the molecular alterations in AA-UTUC57,63–65 or AA-RCC,22,60 and one study reporting both the risk and molecular alterations in AA-UTUC68 were newly retrieved in the present systematic review.
Figure 1.
Flow diagram for the selection process of eligible studies.
AA, aristolochic acid; AA-UTC, aristolochic acid-associated urinary tract cancer; BEN, Balkan endemic nephropathy; MNU, morphologically normal human urothelium, NOS, Newcastle–Ottawa scale.
Characteristics of the included studies reporting risk or oncologic outcomes of AA-UTC
Tables 1 and 2 summarize the characteristics of the 25 studies reporting risk or oncologic outcomes of AA-UTC.35–56,66–68 Twenty-four were peer-reviewed articles published from 1991 to 2020,35–42,44–56,66–68 and one was a master’s thesis completed in 2011.43 Among them, 18 were cohort studies37,38,40–44,46,48–55,56,68 and seven were case-control studies.35,36,39,66,45,67,47 Study locations included Taiwan,39,45–46,49,52,67 mainland China,36,38,40–44,48,51,53–56,68 Croatia,35,37 and Serbia.47,50 Baseline renal functions of study participants included chronic kidney disease (CKD) stages 0–5,35,39,45,47–50,52–56,67,68 chronic renal failure (CRF),36,40,43,46 and renal transplant recipients (RTRs).37,38,41,42,44,51,66 The outcome of interest in 15 studies was risk of primary UTC, including UC,35–46,66 RCC,37,41,43,67 and BC.47 In RTRs, post-transplant malignancies were reported. Ten48–56,68 studies reported oncological outcomes of primary AA-UC after surgery; nine48–54,56,68 of them reported recurrence, and five50,54–56,68 of them reported survival outcomes. One study reported both the risk of primary and postoperatively recurrent AA-UC.44 Fourteen studies assessed AA exposure according to the prescription history of AA-containing herbal medicines,39,46,67 medical records,38,41,48,53,55,66 results of the questionnaire survey,45 self-reported data from patients54 or residence in BEN areas.35,47,50 By contrast, in addition to AA exposure history, eight studies assessed AA exposure based on the diagnosis of AAN36,40,42–44,51,56/BEN.37 Three molecular epidemiological studies assessed AA exposure based on the detection of AL-DAN adducts and TP53 gene A:T-to-T:A mutations,49,52 or the genome-wide present AA signature.68 The NOS scores of the 25 included studies were ranged from 4 to 9 (see Supplemental material Appendix B). After excluding four studies reporting primary UTC37,40,41,43 and one study reporting recurrent UC48 with NOS scores of 5 or less, a total of 20 studies35,36,38,39,42,44–47,49–55,66–69 were ultimately identified for inclusion in the meta-analysis.
Table 1.
Characteristics of included studies exploring aristolochic acid exposure and risk of primary urinary tract cancers.
| Author, type | Study location, design, period | Baseline renal function of patients | AA-containing herbs exposed/exposure time (range) | Method of AA exposure assessment | Classification of AAN/BEN diagnosisa (criteriab) | Follow-up time after AA exposure or RT (range) | Study outcome | Primary UTC events/OR, HR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | Non-AA | ||||||||||
| UTC | No UTC | UTC | No UTC | ||||||||
| Sostarić and Vukelić35, PRA | Croatia, retrospective study, 1974–1989 | CKD stages 0–5 | Aristolochia clematitis/permanent exposure | Permanent residence in BEN regions | No diagnosis | NR | UC | 67 | 10027 | 126 | 96180 |
| Li et al.36, PRA | China, HBCC, 2004 | Uremia on dialysis | Guan Mu Tong, Qing Mu Xiang, Xi xin/median: UC: 5 (0.15–40) years, non-UC: 4.5 (0.08–40) years | Medical records; questionnaire | Possible AAN (1, 2, 3, 4) | AA group: median: UC: 10 (3–40) years; non-UC: 7 (2–44) years | UC | 9c | 20c | 2 | 196 |
| Zivcić-cosić et al.37, PRA | Croatia, HBC, 1985–2006 | RTRs | Aristolochia clematitis/NR | Residence in BEN regions | Possible AAN (6) | AA group: median 6.7 (5.2–8.1) years | UC, RCC | 3 | 3 | 3 | 546 |
| Li et al.38, PRA | China, HBC, 1996–2005 | RTRs | NR/⩾2 months | Medical records | No diagnosis | Mean 71.2 (18–132) months | UC | 16 | 279 | 11 | 1123 |
| Lai et al.39, PRA | Taiwan, PBCC, 1997–2002 | CKD stages 0–5 | Mu-Tong, Fangchi, Xi-xin/NR | Prescription records | No diagnosis | 4–6 years | UC | 36 | 577 | 3274 | 121820 |
| AA >500 mg: aOR 2.0 (1.4–2.9)c,f
Mu-Tong >200 g: aOR 2.1 (1.3–3.4)c Fangchi >100 g: aOR 1.3 (0.9–2.0)c Xi-xin >300 g: aOR 1.3 (0.9–2.0)c | |||||||||||
| Wang et al.40, PRA | China, HBC, 2001–2009 | CRF | Qing Mu Xiang, Guan Mu tong/NR | Medical records | Probable AAN (1, 3, 4, 5) | NR | UC | 14 | 58 | 1 | 461 |
| Zhou et al.41, PRA | China, HBC, 2000–2007 | RTRs | Guan Mu tong/median UTC: 2.5 (1–29) years; non-UTC: 1 (0.1–10) years; total: 2.75 (0.33–29) years | Medical records | No diagnosis | NR | UC, RCC | 14 | 9 | 1 | 255 |
| Xiao et al.42, PRA | China, HBC, 2000–2009 | RTRs | NR/⩾6 months | Medical records | Possible AAN (1, 2, 3, 4) | Mean 31 (11–72) months | UC | 8 | 20 | 4 | 614 |
| Gao43, master’s thesis | China, multi-hospital cohort, 1998–2009 | CRF | NR/NR | Medical records | Made diagnosis of AAN (NR) | NR | UC, RCC | 3 | 8 | 4 | 594 |
| Xiao et al.66, PRA | China, HBCC, 1974–2011 | RTRs | NR/NR | Clinical data | No diagnosis | Median 34.5 (2–273) months | UC | 53 | 327 | 47 | 3363 |
| Yang and Liu44, PRA | China, HBC, 2001–2005 | RTRs (528) | NR/NR | Medical records | Possible AAN (1, 4) | AA group: 1–5 years, non-AA group: 5–7 years | UC, RCC | 5 | 34 | 2 | 487 |
| Yang et al.45, PRA | Taiwan, CC, 1985–2001 | Control group excluded uremia patients | Fangchi/NR | Questionnaire | No diagnosis | 4–17 years | UC | AA group: n = 24, control group: n = 140 | |||
| aHR 2.7 (0.6–11.4)d | |||||||||||
| Wang et al.46, PRA | Taiwan, PBC, 1998–2002 | ESRD | Mu-Tong, Fangchi, Xi-xin/NR | Prescription records | No diagnosis | 4–6 years | UC | 9 | 101 | 270 | 32550 |
| AA >300 mg: aHR 5.18 (2.86–9.40)c,f
Mu-Tong >200 g: aHR 3.46 (1.70–7.03)c Fangchi >100 g: aHR 1.89 (0.76–4.68)c Xi-xin >200 g: aHR 0.78 (0.34–1.81)c | |||||||||||
| Hoang et al.67, PRA | Taiwan, PBCC, 1997–2003 | Excluded RTRs | NR/NR | Prescription records | No diagnosis | 3–12 years | ccRCC | 118 | 347 | 3520 | 14281 |
| AA consumption >250 mg: aOR 1.246 (1.004–1.547)e | |||||||||||
| Matic et al.47, PRA | Serbia, HBCC, NR | CKD stages 0–5 | Aristolochia clematitis/NR | Residence in BEN regions | No diagnosis | NR | BC | 67 | 26 | 134 | 96 |
| OR 1.84 (1.08–3.14) | |||||||||||
Classification of AAN diagnosis was based on the criteria proposed by Gökmen et al.3
Diagnostic criteria: (1) history of long-term AA-containing herbs intake before renal impairment, (2) without long-term (⩾3 months) use of antibiotics, antipyretic analgesics or antineoplastic drugs before renal failure, (3) clinical tubulointerstitial nephropathy, (4) ruling out other causes of renal disease, (5) characteristic renal histopathology, (6) WHO criteria for BEN: (Bull World Health Organ 1965; 32: 431–448): medical history, clinical findings, and laboratory results in the familial, geographical, and epidemiological context, ruling out other causes of renal diseases.
Adjusted for age, sex, residence in a township with endemic black foot disease, and history of chronic urinary tract infection.
Adjusted for cigarette smoking.
Adjusted for monthly income, urbanization level, hypertension, diabetes, hyperlipidemia, chronic obstructive pulmonary disease, chronic hepatitis C infection, chronic kidney disease, cystic kidney disease, kidney stones, sickle cell disease, aspirin, non-steroidal anti-inflammatory drugs, and acetaminophen.
Data used in the present meta-analyses.
AA, Aristolochic acid; AAN, Aristolochic acid nephropathy; aHR, adjusted hazard ratio; aOR, adjusted odds ratio; BC, bladder cancer; BEN, Balkan endemic nephropathy; CC, case-control study; ccRCC, clear cell renal cell carcinoma; CKD, chronic kidney disease; CRF, chronic renal failure; ESRD, end-stage renal disease; HBC, hospital-based cohort study; HBCC, hospital-based case-control study; HR, hazard ratio; NR, not reported, OR, odds ratio; PBC, population-based cohort study; PBCC, population-based case-control study; PRA, peer-reviewed article; RCC, renal cell carcinoma; RT, renal transplantation; RTR, renal transplant recipient; UTC, urinary tract cancer; UC, urothelial carcinoma; UTUC, upper tract urothelial carcinoma.
Table 2.
Characteristics of the included studies exploring the oncologic outcomes of aristolochic acid-associated urothelial carcinoma after surgery.
| Author, type | Study location, design, period | Baseline renal function of patients | AA-containing herbs exposed/exposure time (range) | Method of AA exposure assessment | Classification of AAN/BEN/AA-UC diagnosisa (criteriab) | Type of primary UC | (1) Neoadjuvant therapy; (2) Adjuvant therapy | Follow-up time after surgery (range) | Study outcome |
|---|---|---|---|---|---|---|---|---|---|
| Li et al.48, PRA | China, HBC, 2000–2006 | CKD, including RTRs | Guan Mu Tong, Qing Mu Xiang, Guang Fangchi/mean: 1.8 (3–10) years | Medical records; face-to-face or telephone surveys | Only two patients diagnosed with AAN (NR) | UC | (1) NR; (2) NR | NR | Recurrence |
| Yang and Liu44, PRA | China, HBC, 2001–2005 | RTRs | NR/NR | Medical records | Possible AAN (1, 4) | UC | (1) NR; (2) NR | Mean 39 months | Recurrence |
| Chen et al.49, PRA | Taiwan, molecular epidemiological cohort, 1999–2011 | CKD stages 0–5 | NR/NR | AL-DNA adducts and TP53 gene A:T to T:A transversions | AA-UTUC (6, 7) | UTUC | (1) NR; (2) Postoperatively intravesical instillation of chemotherapy or BCG in patients with synchronous bladder tumors | Median 46 (3–144) months | Recurrence |
| Milenkovic-Petronic et al.50, PRA | Serbia, HBC, 1999–2011 | CKD stages 0–5 | Aristolochia clematitis/permanent exposure | Permanent residence in BEN regions | No diagnosis | UTUC | (1) No; (2) Cisplatin-based combination chemotherapy in patients with disease pT3 or pT4 and/or nodal involvement | Median 36 (1–154) months | Recurrence, survival |
| Liu et al.51, PRA | China, HBC, 2006–2013 | RTRs | NR/NR | Medical records | Made diagnosis of AAN (NR) | UTUC | (1) NR; (2) Regularly intravesical instillation of pirarubicin or epirubicin after surgery for one year | Median 38 (12–104) months | Recurrence |
| Chen et al.52, PRA | Taiwan, molecular epidemiological cohort, 1999–2012 | CKD stages 0–5 | NR/NR | AL-DNA adducts | Possible AA-UTUC (6) | UTUC | (1) NR; (2) No | Median 59 (4–208) months | Recurrence |
| Ji et al.53, PRA | China, HBC, 2000–2014 | CKD, including RTRsc | NR/>3 months | Medical records | No diagnosis | UTUC | (1) No; (2) Yes, but treatment details not reported | Mean 70.2 (4–193) months | Recurrence |
| Zhong et al.54, PRA | China, HBC, 1999–2014 | CKD, including RTRsc | NR/>6 years | Self-reported data from patients | No diagnosis | UTUC | (1) No; (2) Yes, but treatment details not reported | Median 60 (IQR 36–100) months | Recurrence, survival |
| Wang et al.55, PRA | China, HBC, 2011–2017 | CKD stages 0–5 | Guan Mu Tong continuous use >15 days or discontinuous use >2 months or other AA-containing herbs >6 months | Clinical data | No diagnosis | UTUC | (1) NR; (2) Yes, 242 patients received postoperative bladder perfusion chemotherapy | Mean 62.5 (18–84) months | Survival |
| Lu et al.68, PRA | China, molecular epidemiological cohort, 2005–2013, 2015–2017 | CKD stages 1–5 | NR/median: patient with recurrence: 60 (0–120) months; patients without recurrence: 12 (0–360) months; total: 12 (0–360) months | Genome-wide present AA signature | AA-UTUC | UTUC | (1) NR; (2) AA group: no; non-AA group: patient with recurrence: 7.7% (1/13) received chemotherapy; patient without recurrence: 12% (6/50) received chemotherapy, 6% (3/50) received radiotherapy, 4% (2/50) received both therapies | Median 31.5 (3–168) months | Recurrence, survival, metastasis |
| Shan et al.56, PRA | China, HBC, 2010–2017 | CKD stages 0–5 | NR/NR | Medical records | Probable AAN (1, 2, 3, 4, 5) | UTUC | (1) Patients with advanced disease received gemcitabine and cisplatin; (2) all patients received postoperative single dose of intravesical mitomycin C and patients with advanced disease received gemcitabine and cisplatin | Mean 43.2 (6–72) months | Recurrence, survival |
| Type of recurrence | Recurrent UC events | HR (95% CI) | |||||||
| AA | Total | Non-AA | Total | Recurrence | Cancer-specific survival | Overall survival | Disease-specific survival | ||
| Li et al.48 | UC | 6 | 18 | 22 | 94 | ||||
| Yang and Liu44 | UC | 3 | 5 | 0 | 2 | ||||
| Chen et al.49 | UC | 23 | 40 | 22 | 52 | ||||
| Contralateral UTUC | 10 | 0 | |||||||
| BC | 13 | 22 | |||||||
| Milenkovic-Petronic et al.50 | BC | NR | 64 | NR | 139 | aHR 2.01 (1.04–4.22)d | aHR 1.28 (0.79–2.06)d | ||
| Liu et al.51 | BC | 7 | 8 | 9 | 29 | aHR 2.179 (1.085–8.093)e | |||
| Chen et al.52 | Metachronous BC | 26 | 79 | 13 | 42 | aHR 0.88 (0.33–2.31)f | |||
| Type of recurrence | Recurrent UC events | HR (95% CI) | |||||||
| AA | Total | Non-AA | Total | Recurrence | Cancer-specific survival | Overall survival | Disease-specific survival | ||
| Ji et al.53 | Contralateral UTUC | 11 | 80 | 44 | 862 | ||||
| Zhong et al.54 | BC | NR | 86 | NR | 856 | aHR 2.117 (1.488–3.013)g | aHR 0.436 (0.214–0.888)g | ||
| Wang et al.55 | BC | NR | 173 | NR | 266 | aHR 1.883 (1.238–2.865)h | |||
| Lu et al.68 | BC | 6 | 27 | 13 | 63 | 1.036 (0.393–2.730) | |||
| Shan et al.56 | UC | 25 | 42 | 84 | 238 | aHR 2.370 (1.428–4.902)i | aHR 3.061 (1.190–7.872)i | ||
| BC | 14 | 47 | |||||||
| Contralateral UTUC | 6 | 12 | |||||||
| Local | 5 | 25 | |||||||
Classification of AAN and AA-UTUC diagnosis was based on the criteria proposed by Gökmen et al.3 and Chen et al.,49 respectively.
Diagnostic criteria: (1) history of long-term AA-containing herbs intake before renal impairment, (2) without long-term (⩾3 months) use of antibiotics, non-steroidal anti-inflammatory drugs, diuretics or Chinese traditional medicines containing minerals or metals, (3) clinical tubulointerstitial nephropathy, (4) ruling out other causes of renal disease, (5) characteristic renal histopathology, (6) AL-DNA adducts, (7) A:T-to-T:A transversions in TP53 gene.
Information available from another study of Zhong et al.: Chin J Urol 2017; 38: 901–904 (in Chinese).
Adjusted for sex, age, concomitant bladder cancer, history of bladder cancer, tumor location, tumor focality, tumor size, tumor grade, tumor stage, lymphovascular invasion, lymph node metastasis, and mode of operation (nephroureterectomy versus conservative).
Adjusted for tumor focality, and distal ureter invasion.
Adjusted for TP 53 mutation other than A > T, diabetes mellitus, and classical prognostic factors.
Adjusted for gender, age, tobacco consumption, tumor side, main tumor location, main tumor size, multifocality, concomitant carcinoma in situ, tumor stage, tumor grade, lymph node status.
Adjusted for smoking, age, sex, number of tumors, history of BC, lymph node metastasis, tumor size, tumor location, tumor stage, tumor grade, operation mode, diabetes mellitus.
Adjusted for age, tumor stage, lymph node status, tumor grade.
AA, Aristolochic acid; AAN, Aristolochic acid nephropathy; AA-UTUC, aristolochic acid-associated upper tract urothelial carcinoma; aHR, adjusted hazard ratio; BC, bladder cancer; BCG, Bacillus Calmette-Guérin; BEN, Balkan endemic nephropathy; CKD, chronic kidney disease; HBC, hospital-based cohort study; HR, hazard ratio; IQR, interquartile range, NR, not reported; pT, primary tumor; PRA, peer-reviewed article; RTR, renal transplant recipient; UC, urothelial carcinoma; UTUC, upper tract urothelial carcinoma.
Partially overlapping study participants were noted in several studies. Two population-based cohort studies reporting primary UC were conducted in Taiwan using the National Health Insurance Research Database (NHIRD).39,46 Lai et al.39 analyzed patients with all stages of CKD from 1997–2002. Wang et al.46 analyzed patients with ESRD from 1998 to 2002. Conducted over almost the same period of time, the patients with ESRD in the studies of Lai et al.39 and Wang et al.46 were overlapped. As a result, the data from the study of Lai et al.39 were selected for the main meta-analysis, and the data from the study of Wang et al.46 was used when subgroup analysis among patients with ESRD was performed. The two molecular epidemiological cohort studies of Chen et al.,49,52 published in 201349 and 2016,52 were conducted in the same hospital with study periods from 1999 to 201149 and from 1999 to 2012,52 respectively. Their study participants were mostly overlapped. Both studies reported bladder and contralateral UTUC recurrences. The data from the study published in 201349 were selected for the main meta-analysis of UC recurrence and the subgroup analysis of contralateral UTUC recurrence because more complete data were presented in the study. The data from the study published in 201652 were selected for the subgroup analysis of bladder recurrence because adjusted estimate was available. Although Ji et al.53 and Zhong et al.54 analyzed on the same 942 UTUC patients, data of both studies were used for meta-analyses due to different reported outcomes (i.e. contralateral UTUC recurrence and bladder recurrence). Ji et al.53 reported AA as an independent risk factor of contralateral UTUC recurrence; however, the adjusted HR was unreasonably less than 1 (HR 0.290, 95%CI 0.097–0.866). Therefore, we calculated the crude risk ratio for the meta-analysis instead. The cohort study of Zhong et al.54 and the molecular epidemiological cohort study of Lu et al.68 were conducted in the same hospital with the same reported outcome of bladder recurrence. Zhong et al.54 analyzed the clinical data of 942 patients from 1999 to 2014 using patients’ self-reported data to assess the AA exposure. Lu et al.68 performed WGS on 47 UTUC patients from 2005 to 2013 and 43 patients from 2015 to 2017 to compare the oncologic outcomes between patients with and without AA mutational signature. Both studies54,68 reported bladder recurrence. Specifically, the multivariate-adjusted estimates calculated by Zhong et al.54 were used in the main meta-analysis of recurrent UC and subgroup analysis of bladder recurrence for studies with multivariate-adjusted data. The AA mutational signature data investigated by Lu et al.68 were used in subgroup analysis of bladder recurrence for studies in which patients with AAN/BEN or AA-UTUC diagnosis were included.
Characteristics of the included studies identifying molecular alterations in AA-UTC
Characteristics and summary findings of the 16 studies reporting the molecular alterations in AA-UTC10,13,14,22,57–68 are presented in Table 3. All were peer-reviewed articles published from 2009 to 2020. Countries where the AA-exposed samples were obtained included Taiwan,10,13,14,59,67 mainland China,60,61,66,68 Belgium,57 Singapore,59 Croatia,58 Bosnia and Herzegovina,58 Serbia,62–65 and Romania.22 Types of AA-exposed tumor samples included UC,10,57,66 UTUC,13,14,58,61–65,68 clear RCC (ccRCC),22,60,67 and BC.59 For comparison, 12 studies10,14,22,58,60,61–66,68 used non-AA-exposed tissue samples; four studies13,67,57,59 compared their findings with the publicly available data. Molecular alterations identified were classified as somatic mutations,10,13,14,22,57–60,66,67 altered microRNA (miRNA) expression61,62 and altered protein expression.63–65 AA exposure assessment among 11 studies identifying somatic mutations10,13,14,22,57–60,66–68 were in one study based on medical records,66 in one study57 based on meeting the definite diagnostic criteria of AAN proposed by Gökmen et al.,3 in one study based on the presence of AL-DNA adducts or TP53 gene A:T-to-T:A transversions,10 in two studies based on the genome-wide present AA signature,60,68 and in six studies,13,14,22,58,59,67 besides the genome-wide present AA signature, two studies also based on patients’ (medical) history,13,59 one study58 also based on meeting the BEN diagnosis criteria proposed by Jelaković et al.,12,71 and the other three studies also based on the presence of AL-DAN adducts14,22,67 or TP53 A:T-to-T:A transversions.14,67 AA exposure assessment among two studies identifying altered miRNA expression61,62 was in one study based on AAN diagnosis61 and in one study based on residence in BEN regions.62 Assessment of AA exposure in three studies identifying altered protein expression was all based on residence in BEN regions.63–65 Although Aydin et al.57 investigated AA-induced TP53 mutation in UTUC, they however focused on the detection of C-to-T mutations and were thus included.
Table 3.
Characteristics and summary of findings of the included studies identifying molecular alterations in aristolochic acid-associated urinary tract cancers.
| Author, type | Country where sample obtained | Cancer type of AA-exposed samples (no. of patients) | Method of AA exposure assessment | Non-AA-exposed control samples (no. of patients) | Tissue | Analytical method | Molecular alterations identified in the AA-exposed samples |
|---|---|---|---|---|---|---|---|
| Somatic mutations | |||||||
| Xiao et al.66, PRA | China | UC (48) | Clinical data | Non-AA-UC from China (42) | FFPE | PCR and Sanger sequencing | Mutated genes: TP53 (4/48, 8.3%), HRAS (3/48, 6.3%) |
| Chen et al.10, PRA | Taiwan | UC (151) | AL-DNA adducts or TP53 gene A:T-to-T:A transversions | RCC from Taiwan (25) | FF | 1. TP53: microarray; 2. FGFR3, HRAS: pyrosequencing |
A:T-to-T:A mutations: TP53 (38/148, 26%), HRAS (7/150, 4.7%), FGFR3 (6/150, 4.0%) |
| Aydin et al.57, PRA | Belgium | UC (5) | Definite diagnosis of AAN | Compared to publicly available data | FF and FFPE | 1. ICH staining; 2. LCM; 3. Nested-PCR and Sanger sequencing |
1. A > T transversion was not only within the TP53 hotspot region and but in p53-negative dysplastic urothelial cells. 2. In addition to A > T, C > T transversions were highly prevalent in TP53 |
| Hoang et al.14, PRA | Taiwan | UTUC (19) | 1. AL-DNA adducts or TP53 gene A:T-to-T:A transversions; 2. Genome-wide present AA signature |
SA-UTUC from Taiwan (7) | FF | WES | Known driver genes identifieda: TP53 (12/18, 66.7%)b, MLL2 (11/18, 61.1%), CREBBP (8/18, 44.4%), STAG2 (5/18, 27.8%), BRCA2 (4/18, 22.2%), NRAS (4/18, 22.2%), KDM6A (3/18, 16.7%), FGFR3 (1/18, 5.6%) |
| Poon et al.13, PRA | Taiwan | UTUC (9) | 1. Medical record and case histories 2. Genome-wide present AA signature |
Compared to publicly available data | NR | WES, WGS | Frequently mutated genes: KDM6A (8/9, 88.9%), LRRK2 (7/9, 77.8%), DCHS2 (7/9, 77.8%), USH2A (7/9, 77.8%), SCN1A (6/9, 66.7%), ADAMTSL1 (5/9, 55.6%), ATRX (5/9, 55.6%), DNAH9 (5/9, 55.6%), MYO5C (5/9, 55.6%), TP53 (5/9, 55.6%), CHD6 (4/9, 44.4%), CDH10 (4/9, 44.4%), CREBBP (4/9, 44.4%), SETX (4/9, 44.4%), ARID1A (3/9, 33.3%) |
| Castells et al.58, PRA | Croatia, Bosnia and Herzegovina | UTUC (10) | 1. Diagnosis of BEN; 2. Genome-wide present AA signature/COSMIC Signature 22 |
Non-AA-UTUC from a metropolitan area of United States (2) | FFPE | LC-WES | 1. Known driver genes carrying nonsynonymous A > T mutations and frequently mutated: XIRP2 (6/10, 60%), ATRX (5/10, 50%), NEB (5/10, 50%), AHNAK (4/10, 40%), SMCHD1 (4/10, 40%), STAG2 (4/10, 40%), TRRAP (4/10, 40%), ARID1B (3/10, 30%), ASH1L (3/10, 30%), CHD5 (3/10, 30%), HDAC9 (3/10, 30%), HUWE1 (3/10, 30%), KDM6A (3/10, 30%), MLL2 (3/10, 30%), NAV3 (3/10, 30%), SYNE1 (3/10, 30%), TRIO (3/10, 30%) 2. APOBEC mutational signature |
| Lu et al.68, PRA | China | UTUC (27) | Genome-wide present AA signature/COSMIC Signature 22 | No-AA signature UTUC from China (63) | FFPE and FF | WGS | Frequently mutated genes: KMT2A,C and D (14/27, 51.9%), MUC16 (13/27, 48.1%), TP53*(13/27, 48.1%), MUC4 (10/27, 37.0%), FRG2C*(7/27, 25.9%), FAT1 (6/27, 22.2%), ARID1A (5/27, 18.5%), COL2A1 (5/27, 18.5%), FBLN2 (5/27, 18.5%), PCM1 (5/27, 18.5%), SPEN (2/27, 7.4%), TERT promoter mutations (C228T and C250T) (6/27, 22.2%) |
| Poon et al.59, PRA | Taiwan, Singapore, China | BC (3) | 1. Know AA exposure history; 2. Genome-wide present AA signature | Compared to and analyzed publicly available data | NR | WES | 1. CpG > TpG signature 2. APOBEC mutational signature |
| Scelo et al.22, PRA | Romania | ccRCC (14) | 1. Genome-wide present AA signature; 2. AL-DNA adductsc |
Non-AA-ccRCC from Czech Republic (38), Russia (38), and the UK (31) | FF | WGS | 1. Know driver genes carrying nonsynonymous A > T mutations: PBRM1 (3/14, 21.4%), TP53 (1/14, 7.1%) 2. Know driver genes carrying other nonsynonymous mutations: PBRM1 (7/14, 50%), VHL (7/14, 50%), KDM5C (4/14, 28.6%), SETD2 (4/14, 28.6%), BAP1 (2/14, 14.3%) |
| Hoang et al.67, PRA | Taiwan | ccRCC (10) | 1. AL-DNA adducts; 2. Genome-wide present AA signature |
Compared to publicly available data | FF | WES | 1. Know driver genes carrying nonsynonymous A > T mutations: PBRM1 (1/10, 10%), SETD2 (1/10, 10%) 2. Know driver genes carrying other nonsynonymous mutation ns: VHL (7/10, 70%), PBRM1 (2/10, 20%), BAP1 (1/10, 10%), EPAS1 (1/10, 10%), GNB2L1 (1/10, 10%), PIK3CA (1/10, 10%) 3. Loss of chromosome 3p (6/10, 60%) |
| Wang et al.60, PRA | China | ccRCC (43) | Genome-wide present AA signature/COSMIC SBS22 | Non-AA-ccRCC from China (109) | FF | WES | Significantly mutated genes: CSMD3* (22.5% AA-exposed samples versus 6.25% non-AA-exposed samples, p = 0.012723) |
| Altered miRNA expression | |||||||
| Tao et al.61, PRA | China | UTUC (5) | AAN diagnosis | Non-AAN-UTUC (5) | FFPE | miRNA microarray | Differentially expressed miRNA: The most upregulated: has-miR-488-3p, has-miR-4434, has-miR-4274, has-miR-224-3p The most downregulated: has-miR-4795-5p, has-miR-4784, has-miR-330-3p, has-miR-181c-5p, has-miR-15a-5p, has-miR-10a-5p, has-miR-200c-3p, has-miR-3916 |
| Popovska-Jankovic et al.62, PRA | Serbia | UTUC (7) | Residence in BEN regions | Non-tumor kidney sample (4) | FFPE | miRNA microarray | Differentially expressed miRNA: Upregulated: hsa-miR-205-5p, hsa-miR-4322, hsa-miR-99b-3p, hsa-miR-3620-3p, hsa-miR-373-5p, hsa-miR-3656, hsa-miR-1290 Downregulated: hsa-miR-30a-5p, hsa-miR-127-3p, hsa-miR-154-5p |
| Altered protein expression | |||||||
| Jankovic-Velickovic et al.63, PRA | Serbia | UTUC (40) | 1. Residence in BEN regions; 2. Seven patients with tubulointerstitial lesions similar to BEN |
Non-BEN-UTUC from non-endemic regions in Serbia (45) | FFPE | IHC staining | E-cadherin: 1. More frequent aberrant expression in BEN UTUC than control (p < 0.01); 2. Decreased expression in BEN UTUC was linked to solid growth pattern |
| Jankovic-Velickovic et al.64, PRA | Serbia | UTUC (44) | Residence in BEN regions | Non-BEN-UTUC from non-endemic regions in Serbia (61) | FFPE | IHC staining | Apoptosis-related biomarkers: 1. Higher expression of antiapoptotic marker Survivin in BEN UTUC with high grade (p < 0.005) and solid growth (p < 0.05) than control; 2. Lower expression of proapoptotic marker Bax in BEN UTUC with high grade (p < 0.05), high stage (p < 0.05), necrosis (p < 0.05), and without metaplastic change (p < 0.05) than control |
| Jankovic-Velickovic et al.65, PRA | Serbia | UTUC (50) | Residence in BEN regions | Non-BEN-UTUC from non-endemic regions in Serbia (60) | FFPE | IHC staining | Angiogenesis-related biomarkers: 1. Lower expression of VEGFR1 in BEN UTUC than control (p < 0.005); 2. Higher expression of MVD CD31 than control was seen in BEN UTUC with papillary growth (p < 0.05) |
Identified from 18 samples after excluding two samples in which the percentages of A:T-to-T:A transversions were not consistent with the AA signature and including a control sample in which AL-DNA adducts and AA signature was found during this study.
Frequency may be skewed because selection of AA-UTUCs was based on A-to-T mutation in TP53.
AL-DNA adducts were later detected in the study of Turesky et al.19
Identified by MutSigCV.
AA, Aristolochic acid; AAN, Aristolochic acid nephropathy; AA-UTUC, AA-associated UTUC; AA-UTC, AA-associated urinary tract cancer; BEN, Balkan endemic nephropathy; ccRCC, clear cell renal cell carcinoma; FF, fresh-frozen; FFPE, formalin-fixed paraffin-embedded; IHC immunohistochemistry staining; LCM, laser capture microdissection; LC-WES, low-coverage whole-exome sequencing; miRNA, microRNA; MVD, microvessel density; NR, not reported; PCR, polymerase chain reaction; PRA, peer-reviewed article; RCC, renal cell carcinoma; SA, smoking-associated; SBS, single base substitution; UC, urothelial carcinoma; UTUC, upper tract urothelial carcinoma; WES, whole-exome sequencing.
Meta-analysis: risk of primary AA-UTC
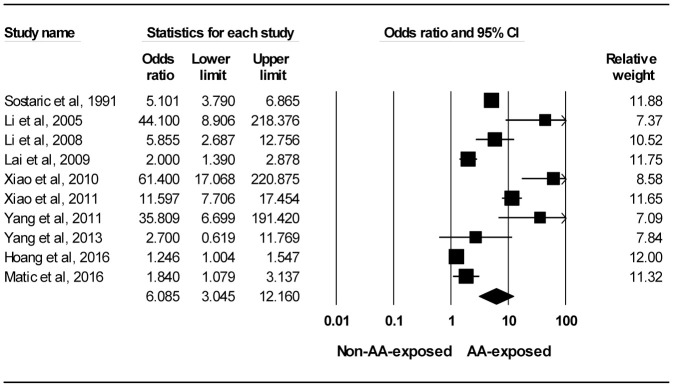
Figure 2 is the forest plot for the 10 studies35,36,38,39,42,44,45,47,66,67 exploring the association between AA exposure and risk of primary AA-UTC. Meta-analysis comprising eight unadjusted35,36,38,39,42,44,47,66 and two adjusted estimates45,67 showed an overall increased risk of UTC with substantial heterogeneity across studies (OR 6.085, 95% CI 3.045–12.160, I2 = 94.632%). Sensitivity analysis conducted by sequentially removing each study led to changes in estimates between 4.827 (95% CI 2.434–9.575) and 7.304 (95% CI 3.773–14.140). Subgroup analyses were undertaken. The pooled OR of UC was 7.304 (95% CI 3.773–14.140, I2 = 90.190%). The pooled OR of RCC was not calculated for two studies reporting RCC44,67 because one44 of them (50%) had zero event in the control group and the between-study heterogeneity was very high (I2 = 75.439%). The pooled ORs was 7.846 (95% CI 3.101–19.850, I2 = 95.197%) when AA exposure was via intake of AA-containing herbal medicines and 3.141 (95% CI 1.158–8.522, I2 = 90.659%) via consumption of AA-contaminated food. The pooled ORs was 2.252 (95% CI 1.169–4.338, I2 = 92.959%) for patients with CKD stages 0–5, 13.218 (95% CI 1.648–106.047, I2 = 83.466%) for patients with CRF and 16.046 (95% CI 6.725–38.290, I2 = 73.142%) for RTRs. After we divided the 10 studies35,36,38,39,42,44,45,47,66,67 into two groups based on the diagnosis of AAN/BEN in the AA-exposed groups, seven studies35,38,39,45,47,66,67 in which patients without diagnosis were responsible for the high heterogeneity (I2 = 95.136%) with a pooled OR of 3.301 (95% CI 1.637–6.657). In contrast, the I2 between the other three studies36,42,44 in which patients were diagnosed with AAN/BEN decreased to 0.000% with a pooled OR of 48.456 (95% CI 20.536–114.339). No asymmetry in the funnel plot was detected by the Egger’s test for assessing publication bias (p = 0.07).
Figure 2.
Forest plot for studies exploring the association between aristolochic acid exposure and risk of primary urinary tract cancer.
AA, aristolochic acid; CI, confidence interval.
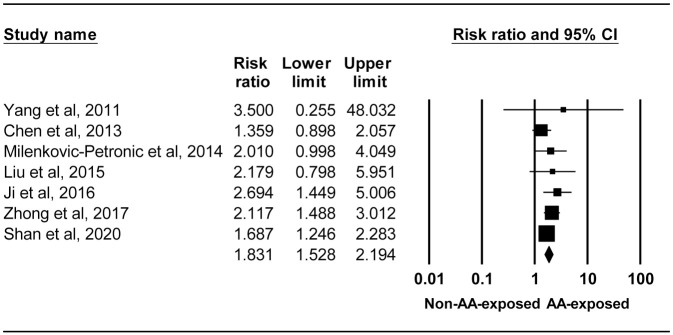
Meta-analysis: oncologic outcomes of primary AA-UC after surgery
Figure 3 is the forest plot showing the pooled RR of risk of postoperative recurrent UC 1.831 (95% CI 1.528–2.194, I2 = 0.000%) from the seven studies reporting recurrent UC following surgical resection for primary AA-UC.44,49,50,51,53,54,56 No funnel plot asymmetry was detected by the Egger’s test (p = 0.327). Although one44 of these seven studies had zero event, the pooled RR was calculated by inverse variance method with 0.5 continuity correction because event numbers and sample sizes were unavailable in two studies50,54 for calculating the pooled M-H RR without zero-cell correction. Sensitivity analysis revealed no change in the statistical significance of the combined RR, ranging from 1.738 (95% CI 1.408–2.146) to 1.964 (95% CI 1.606–2.402). Subgroup analysis by route of exposure to AA showed a pooled RR of 1.819 (95% CI 1.508–2.193, I2 = 0.000%) when exposure was via intake of AA-containing herbal medicines and 2.010 (95% CI 1.040–4.220) via consumption of AA-contaminated food. The pooled RR for patients with and without AAN/BEN diagnosis was 1.684 (95% CI 1.337–2.121, I2 = 21.9%) and 2.206 (95%CI 1.666–2.921, I2 = 0.000%), respectively. Subgroup analyses by sites of postoperative recurrence were conducted for patients with primary AA-UTUC from eight studies.49–54,56,68 The standard surgical treatment, radical nephroureterectomy (RNU) with bladder cuff excision, was performed in seven49,51–54,56,68 of the eight studies.49–54,56,68 Although 86.2% of patients received RNU and 13.8% of selected patients including those with BEN received conservative surgery in the other one study,50 the different modes of operations, however, did not result in significantly different risk of bladder recurrence in the multivariate analysis.50 The pooled RR of bladder recurrence was 1.949 (95% CI 1.462–2.597, I2 = 0.000%) for four studies where RR adjusted for common clinicopathological risk factors were provided,50–52,54 and 1.477 (95%CI 1.015–2.147, I2 = 0.000%) for four studies where patients diagnosed with AAN were included.51,52,56,68 Only unadjusted data were available for studies reporting contralateral UTUC recurrence and local recurrence with a pooled crude RR of 3.760 (95% CI 2.225–6.353, I2 = 0.0%) for three studies49,53,56 and a crude RR of 1.151 (95% CI 0.414–3.198) from one study,56 respectively. Contralateral UTUC recurrence in AAN patients was not further analyzed because one49 of two studies49,56 (50%) had zero event and there was moderate between-study heterogeneity (I2 = 55.470%). For meta-analysis of survival outcomes, after adjustment for common clinicopathological factors in the multivariate analysis, AA exposure in patients with surgically treated primary UTUC showed worse overall survival (HR 2.025, 95% CI 1.432–2.865, I2 = 0.000%) and disease-specific survival (HR 3.061, 95% CI 1.190–7.872), but had no effect on cancer-specific survival (HR 0.772, 95% CI 0.269–2.215, I2 = 83.484%). Table 4 displays the results of meta-analyses and subgroup analyses in the present study.
Figure 3.
Forest plot for studies exploring the association between aristolochic acid exposure and risk of postoperative recurrent urothelial carcinoma.
AA, aristolochic acid; CI, confidence interval.
Table 4.
Results of meta-analyses and subgroups analyses.
| Meta-analyses | Number of studies | Number of patients in analysis | Pooled OR/RR/HR (95% CI) |
p Value | I2 (%) | p for χ2 test |
|---|---|---|---|---|---|---|
| Primary UTC (UC and RCC) | 1035,36,38,39,42,44,45,47,66,67 | 257,480 | OR 6.085 (3.045–12.160) | <0.001 | 94.632 | <0.001 |
| Subgroup analysis by route of AA exposure | ||||||
| AA-containing herbal medicines | 836,38,39,42,44,45,66,67 | 150,757 | OR 7.846 (3.101–19.850) | <0.001 | 95.197 | <0.001 |
| AA-contaminated food | 235,47 | 106,723 | OR 3.141 (1.158–8.522) | 0.025 | 90.659 | 0.001 |
| Subgroup analysis by renal function | ||||||
| CKD stages 0–5 | 535,39,45,47,67 | 250,860 | OR 2.252 (1.169–4.338) | 0.015 | 92.959 | <0.001 |
| CRF (including dialysis) | 236,46 | 33,157 | OR 13.218 (1.648–106.047) | 0.015 | 83.466 | 0.014 |
| RTRs | 438,42,44,66 | 6393 | OR 16.046 (6.725–38.290) | <0.001 | 73.142 | 0.011 |
| Subgroup analysis by AAN diagnosis | ||||||
| No | 735,38,39,45,47,66,67 | 256,079 | OR 3.301 (1.637–6.657) | 0.001 | 95.136 | <0.001 |
| Yes | 336,42,44 | 1401 | OR 48.456 (20.536–114.339) | <0.001 | 0.000 | 0.874 |
| Primary UC | 935,36,38,39,42,44,45,47,66 | 239,214 | OR 7.304 (3.773–14.140) | <0.001 | 90.190 | <0.001 |
| Postoperative recurrent UC | 744,49,50,51,53,54,56 | 2503 | RR 1.831 (1.528–2.194)a | <0.001 | 0.000 | 0.566 |
| Subgroup analysis by route of AA exposure | ||||||
| AA-containing herbal medicines | 644,49,51,53,54,56 | 2300 | RR 1.819 (1.508–2.193) | <0.001 | 0.000 | 0.446 |
| AA-contaminated food | 150 | 203 | RR 2.010 (1.040–4.220) | 0.037 | – | – |
| Subgroup analysis by AAN diagnosis | ||||||
| No | 350,53,54 | 2087 | RR 2.206 (1.666–2.921) | <0.001 | 0.000 | 0.771 |
| Yes | 444,49,51,56 | 416 | RR 1.684 (1.337–2.121)b | <0.0001 | 21.9 | 0.279 |
| Subgroup analysis of postoperative UTUC by sites of recurrence | ||||||
| All studies | ||||||
| Contralateral UTUC recurrence | 349,53,56 | 1314 | RR 3.760 (2.225–6.353)b | <0.0001 | 0.0 | 0.479 |
| Bladder recurrence | 550–52,54,56 | 1583 | RR 1.880 (1.466–2.411) | <0.001 | 0.000 | 0.546 |
| Local recurrence | 156 | 280 | RR 1.151 (0.414–3.198) | 0.787 | – | – |
| Studies with multivariate-adjusted data only | ||||||
| Bladder recurrence | 450–52,54 | 1303 | RR 1.949 (1.462–2.597) | <0.001 | 0.000 | 0.418 |
| Studies included patients with AAN/AA-UTUC diagnosis | ||||||
| Bladder recurrence | 451,52,56,68 | 528 | RR 1.477 (1.015–2.147) | 0.041 | 0.000 | 0.484 |
| Local recurrence | 156 | 280 | RR 1.151 (0.414–3.198) | 0.787 | – | – |
| Survival outcomes of postoperative UTUC | ||||||
| Cancer-specific survival | 250,54 | 1145 | HR 0.772 (0.269–2.215) | 0.631 | 83.484 | 0.014 |
| Overall survival | 255,56 | 719 | HR 2.025 (1.432–2.865) | <0.001 | 0.000 | 0.546 |
| Disease-specific survival | 156 | 280 | HR 3.061 (1.190–7.872) | 0.020 | – | – |
Inverse variance random-effects RR with 0.5 continuity correction.
Mantel–Haenszel fixed-effects RR without zero-cell correction.
AA, aristolochic acid; AAN, aristolochic acid nephropathy; CI, confidence interval; CKD, chronic kidney disease; CRF, chronic renal failure; OR, odds ratio; RR, risk ratio; RCC, renal cell carcinoma; RTRs, renal transplant recipients; UC, urothelial carcinoma; UTC, urinary tract cancer; UTUC, upper tract urothelial carcinoma.
Discussion
The present systematic review and meta-analysis provided updated evidence on the risk, oncologic outcomes and molecular alterations observed in AA-UTC via searching PubMed, Embase and four Chinese databases, Airiti Library, CNKI, CSJD-VIP and Wanfang Data, with time extending up to 31 October 2020, except for Wanfang Data with the search period up to 31 July 2020. In the meta-analysis of the risk of primary AA-UC, the updated OR was 7.304. Moreover, the impact of AA exposure on patients with various degrees of renal dysfunction and diagnosis of AAN on the changes of the risk of primary UTC was investigated. The risk of primary AA-UTC increased to 13.218-fold for patients with CRF and 16.046-fold for RTRs, and further increased to over 48.456-fold if both such patients were diagnosed with AAN. These results were in accordance with a previous consensus statement highlighting the importance and necessity of diagnosis of AAN/BEN among people with history of AA exposure to identify the higher risk ones for close follow-up, especially for CRF patients and RTRs.71 The meta-analysis also showed that AA exposure was associated with an overall 1.831-fold increased risk of UC recurrence following surgical resection for primary AA-UC. In Western countries, bladder tumors account for 90–95% of primary UC while UTUC are account for only 5–10% of UC.72 Postoperative recurrences of UTUCs after RNU also commonly occur at bladder (30%) and locoregional (20%), but only 2–6% at contralateral upper tract.73 Our subgroup analysis showed that AA exposure was associated with 1.949-fold increased risk of intravesical recurrence of UTUC, and 1.477-fold increased risk in patients diagnosed with AAN. It is worth noting that among the three studies reporting contralateral UTUC recurrence,49,53,56 the recurrence rates of 0–5% in the control groups were similar to that of 2–6% in the general population. However, the rates in the AA groups were very high (13.75–25%),49,53,56 which contributed to an overall 3.760-fold increased risk. Two hypotheses (the field cancerization hypothesis and the intraluminal seeding and implantation hypothesis) have been proposed to explain the multifocal nature of urothelial tumors.74 The field cancerization hypothesis describes that the whole uroepithelium is exposed to common carcinogenic insults in each patient and multifocal urothelial tumors arise from independent clones of transformed transitional cells.74 The intraluminal seeding and implantation hypothesis describes that multifocal urothelial tumors are derived from a single progenitor cell and develop by the seeding or implantation of intraluminal dispersed viable cancer cells or by intraepithelial spread.74 The WGS analysis conducted by Lu et al.68 showed that the urothelial tumor that occurred earlier in the renal pelvis of a patient with AA signature and multifocal UTUC shared no genetic alterations with the subsequent renal pelvis tumor or bladder tumor 8 years later. The two subsequently occurring tumors, however, were genetically related. Hence, multifocality and intravesical recurrence of primary AA-UTUC was considered the co-contribution of field cancerization and intraluminal seeding.68 In the study of Ji et al.,53 few patients had vesicoureteral reflux and there were no correlations between ureteroscopy and new contralateral UTUC, the field cancerization thus served as the hypothesis explaining the contralateral recurrence pattern of AA-UTUC. Because the pooled estimate of contralateral UTUC recurrence in the present meta-analysis was calculated from crude RRs,49,53,56 further research designed for adjusting potential confounding factors is needed to corroborate these findings to develop the postoperative monitoring guidelines of primary AA-UTUC. Moreover, due to the lack of sufficient data and limited number of studies, subgroup analyses by sites of recurrence were not further analyzed in patients with advanced CKD. Chen et al.49 reported that all AA-UTUC patients developing metachronous contralateral UTUC recurrence had poor renal function of CKD stage 3 or worse. In the study of Liu et al.51 conducted on RTRs, an increased risk of bladder recurrence was observed in the native AAN group (adjusted HR 2.179, 95%CI 1.085–8.093). However, due to small sample size of the study,51 many potential risk factors for bladder recurrence were unable to be adjusted. More studies are thus required to investigate the recurrence pattern of AA-UTUC in patients with advanced CKD and RTRs. Meta-analyses of survival outcomes showed that patients with surgically treated primary AA-UTUC had worse overall survival and disease-specific survival, but had no effect on cancer-specific survival. In addition to the high rate of postoperative recurrence, the high risk of death from other diseases was considered to be owing to the various cardiovascular and cerebrovascular complications of AAN, especially in patients receiving maintenance dialysis.56
Among the 16 studies identifying the molecular alterations in AA-UTC,10,13,14,22,57–68 eight studies assessed AA exposure by WGS/WES through NGS approaches.13,14,22,58–60,67,68 WGS was performed on AA-UTUC in one study68 and AA-RCC in one study.22 WES or low-coverage WES (LC-WES) was performed on AA-UTUC in one study,58 AA-BC in one study,59 and AA-RCC in three studies.14,60,67 One study performed both WGS and WES on AA-UTUC.13 By sequencing the entire genome/exome, numerous putative driver genes carrying nonsynonymous A-to-T mutations other than TP53 were identified in AA-UTUC (Table 3). The recurrently mutated genes of AA-UTUC varied in different geographic areas. TP53, CREBBP and LRRK2 are mutated mostly in the Taiwanese samples in contrast to AHNAK, ATRX, SMCHD1 and XIRP2 in the BEN samples.58 The possible contributing factors resulting in the difference include modes of AA exposure (short-term high-dose intake of AA-containing herbs in Asia versus long-term low-dose exposure to contaminated food in BEN regions) and disease susceptibility due to different genetic background.58 In addition to identifying the potential mutated driver genes of AA-UTUC from mainland Chinese patients, survival outcomes of the patients analyzed in the study of Lu et al.68 were also based on the result of the WGS analysis. Kaplan–Meier analysis showed that cancer-specific survival and metastasis-free survival were both significantly better in patients with COSMIC Signature 22 than those without COSMIC Signature 22.68 Such a favorable outcome of cancer-specific survival was considered to be related to the lower tumor stage of AA signature-positive UTUC.54,68,75 Thus, the authors68 concluded that AA signature-positive UTUC is a low-risk subtype which can be treated with kidney-sparing surgical management.68 The low tumor stage of AA signature-positive UTUC was similar to the AAN-UTUC in another study from mainland China56 but contrary to the AA signature-positive UTUC from Taiwan.49,52 The reason why AA exposure is associated with the development of the lower-stage UTUC still needs to be investigated.75 In general, the worsening renal function after radical surgery prevents most AA-UTUC patients from receiving chemotherapy. However, previous systematic review25 implied that immune checkpoint inhibitor therapy may have effects on AA-UTUC. Specifically, studies included in the present review indicate that somatic mutations in AA-UTUC are characterized by high mutation load,13,14,58 high TP53 but rare FGFR3 mutation rates10,13,14,58,68 and presence of APOBEC mutational signature58 (Table 3). When considering the aforementioned results together with the findings from Lu et al.68 (i.e. high numbers of predicted neoantigens and tumor-infiltrating lymphocytes), immune checkpoint inhibitor could be an alternative for treating AA-UTUC.25,68 Other molecular alterations observed in AA-UTUC were summarized as follows. The studies of Tao et al.61 and Popovska-Jankovic et al.62 identified two totally different sets of differentially expressed miRNA in AAN-UTUC samples from mainland China and BEN-UTUC samples from Serbia, respectively. These different results were supposed to be due to the small sample size in the two studies and different types of control samples (i.e. non-AA-UTUC samples in the former study61 in contrast to non-tumor kidney samples in the latter study62). Lastly, altered expressions of E-cadherin, apoptosis-related biomarkers, and angiogenesis-related biomarkers have also been observed in BEN-UTUC samples.
However, different mutation loads were observed between AA-UTUC and AA-RCC. In Taiwanese tissue samples, higher mutation load of A:T-to-T:A transversions in patients with AA-UTUC14 (median 188 per exome) than that in patients with AA-RCC67 (median 46 per exome) was found. These observations can be mutually confirmed in two similarly conducted Taiwanese population-based case-control studies of AA-UC39 and AA-RCC,67 where the risk of AA-UC (crude OR 1.9)39 was higher than that of AA-RCC (crude OR 1.4)67 at a similar AA cumulative dosage of more than 250 mg. Such result was presumed that the sensitivity of the renal tissue to the carcinogenic effects of AA is lower than the urothelium, or renal tumorigenesis only occurs in people who are sensitive to the nephrotoxicity of AA.67 Moreover, different distributions of the AA mutational signature were also observed between AA-UTUC and AA-RCC. The AA mutational signature in UTUC is present largely in the putative driver genes, implying that AA is the causative factor of UTUC.9,76 Castells et al.58 meta-analyzed the data of 37 AA signature-positive UTUC samples from their study (n = 10) and the studies of Poon et al.13 (n = 9) and Hoang et al.14 (n = 18). Eighty-three recurrently mutated cancer driver genes carrying nonsynonymous A:T-to-T:A transversions were identified, including many known drivers and chromatin-associated factors such as TP53 (40.5% of samples), MLL2 (40.5%), CREBBP (35.1%), KDM6A (35.1%), ATRX (32.4%), CHD5 (24.3%), ARID1B (18.9%), TRRAP (18.9%), FAT1 (16.2%), SETBP1 (16.2%), CHD8 (10.8%), and CHD2 (8.1%). In contrast, the AA mutational signature is rarely found in the mutated RCC driver genes. We also meta-analyzed the data of both AL-DNA adducts- and AA signature-positive clear cell RCC samples from the studies of Scelo et al.22 (n = 14) and Hoang et al.67 (n = 6). The mutation patterns of the frequently mutated RCC driver genes in the 20 AA-RCC samples were as follows: VHL (0% of samples with A-to-T mutations versus 60% of samples with other mutations), PBRM1 (20% versus 40%), KDM5C (0% versus 20%), SETD2 (5% versus 20%), and BAP1 (0% versus 15%). The different distributions of AA-signature mutations in UTUC and RCC driver genes may indicate the different roles of AA in the etiology of the two types of tumors.9,76 However, it is still possible that these different distributions may just reflect the time of the occurrence of AA exposure (e.g. coincident with or prior to tumor initiation, or at some following time).9,76 Due to the limited number of AA-RCC cases in the published literature, further studies are warranted to clarify the underlying mechanisms of these observations.
In comparison with the relatively low-dose dietary consumption of AA in BEN areas, our results revealed that AA exposure via ingestion of larger dose in the herbal products had greater increased risk of primary UTC (7.846-fold versus 3.141-fold), although the increased risks of UC recurrence were similar (1.819-fold versus 2.010-fold). However, over 20 kinds of AA-containing herbs, including those with high amount of AA, are currently allowed in mainland China.17 Replacing of AA-containing herbs by non-AA-containing herbs has been proposed.77 Unfortunately, some AA-containing herbs are hard to replace because of the unequal efficacy of the alternatives.78 Some studies reported that the amount of AA in raw herbs may be reduced via several detoxification methods, including the pretreatment processes called Paozhi, compatibility of AA-containing herbs with other herbs, or extraction processes.17,78–80 Disappointingly, none of these aforementioned methods can completely avoid the toxicity of AA.17,78,80 Due to different individuals’ susceptibility and detoxification capabilities to AA1,7 and the impact of various degrees of renal impairment on AA excretion,18 it is unclear whether cumulative toxicity would still occur after prolonged use of these products with “attenuated toxicity.” Containing relatively low amount of AA,1,17,81 Xi xin (Herba Asari) is still allowed to be used in many areas in Asia, including Taiwan,82 Hong Kong,83 mainland China,17 Japan,82 and Korea.82 Increased risks of primary UC after intake of herbal products containing Xi xin were not observed in the two studies on a nationwide Taiwanese population.39,46 Nevertheless, these results were obtained only from the prescription records of NHIRD without calculating the risk induced by the herbal products purchased by patients themselves, and were thus unable to fully reflect the real-world condition. The amounts of AA in Xi xin were found to vary between plant parts, species, origins, processing methods and extraction processes.17,84 In general, the underground parts (rhizome and root), especially root, have a lower level of AA than the aboveground parts (leaves, flowers, and stems).79 The medicinal part of Xi xin has thus been stipulated to be switched from the whole plant to the root in Taiwan85 and the root and rhizome in mainland China.17 The extraction process of herbal products containing Xi xin in Taiwan should be done by water decoction to make sure AA is undetectable in the final products.85 However, AA in raw herbs of Xi xin may exceed the standards17,82 and should be used with caution. Researchers found that the level of AA in the underground parts of some species of Xi xin were similar to the whole plants or even higher than the aboveground parts.86,87 Furthermore, it was observed that the level of AAIVa, a less studied AA analog, in Xi xin was higher in the root and stem than in the leaf, and the impact on human health needs to be investigated.17 When herbal products containing Xi xin are generally considered to be less toxic, people more easily ignore its toxicity. Inadvertent use of Xi xin products with considerably high toxicity may put people in dangerous situations.17,79
The evidence shown in the present meta-analysis may raise awareness among healthcare professionals and public concerns regarding the long-term impact of AA on human health. Regulatory authorities across countries may use our findings to implement effective safety strategies to protect people from exposure to AA. In countries where AA-containing herbs have been banned, the government agencies should enforce strict laws and regulations to prevent AA-containing herbs and products from being sold or purchased privately. In countries where AA-containing herbs are not entirely banned, medical personnel should use these herbs with caution. There is also an urgent need to seek out effective alternative medicinal herbs to minimize the use of AA-containing herbs. The public should be educated that taking herbal medicines must be under the instruction of Chinese medicine practitioners or pharmacists, rather than purchasing them privately. Furthermore, prudent reassessment of total prohibition of AA-containing herbs is required. Recently, sporadic forms of BEN were found in patients residing outside of the established endemic regions of Croatia and Bosnia.88 The broader growth of Aristolochia plants in different geographic environments deserves our attention.88 Moreover, due to free AA released from the decayed seeds of Aristolochia clematitis, AAs has been identified as a new contaminant in soil, which will then contaminate food crops through root uptake.89 In the endemic areas of the Balkan countries, developing methods for remediating AA-contaminated farmland is suggested and the residents should be informed of the existence of AAs in their cultivated fields and food.89
Limitations
The present meta-analysis had several limitations. Firstly, using the diagnostic criteria of AAN proposed by Gökmen et al.,3 most AAN patients included in the present meta-analysis were classified as possible36,42,44 or probable56 AAN rather than definite diagnosis. The risk of primary UC in AAN patients might be underestimated. Moreover, because the estimated risk of primary UC in AAN patients was derived from CRF and RTR populations, it may not be generalizable to patients with other degrees of renal impairments. Secondly, through the literature review, we were unable to gauge the level of AA transformation of the cancers (e.g. how the oncogenes are affected by AA in terms of level of mutational signatures), which may also underestimate the true impact of AA-mediated urothelial carcinogenesis in the meta-analysis. Thirdly, only surgically treated patients were included in studies reporting oncologic outcomes of AA-UTUC, and we could not evaluate the impact of AA exposure on the outcomes of patients who did not require surgical treatment. Moreover, neoadjuvant and/or adjuvant therapies were in some studies administered in selected patients without adjustment in the multivariate analysis50,54 and in some studies not clearly reported.44,49,51–55,58 Some studies included RTRs as a subset of the study participants; however, limited information on these patients was provided.53,54 We thus could not analyze the possible effects of neoadjuvant and/or adjuvant therapies and immunosuppression on the postoperative recurrence rate and survival outcomes of AA-UTUC. Fourthly, a few wide CIs were noted when conducting the subgroup analysis of primary UC. These wide CIs were considered as owing to meta-analysis of studies with very rare events in the control groups, severely imbalanced sample sizes between AA and the control groups, and the very large overall effect sizes.31 Fifthly, one44 of the seven studies reporting recurrent UC44,49–51,53,54,56 had zero event in the control group; applying the inverse variance method with 0.5 continuity correction for the meta-analysis might generate an underestimated estimate. Although the low proportion of zero-event study (14.3%) may not lead to serious bias in the overall effect,30 the result should still be interpreted with caution. Lastly, although we have thoroughly searched Chinese databases from Taiwan and mainland China, we did not search other language databases (e.g. Romanian and Korean) and might have missed relevant studies.
Conclusions
AA remains a global public health hazard. Given that new evidence on the impact of AA exposure on humans has been constantly reported, developing and implementing effective safety strategies to completely protect people from both iatrogenic and environmental exposure to AA is urgently needed. Additionally, more effort should be made in identifying the precise carcinogenic mechanisms of AA-UTC to determine the future treatment strategies.
Supplemental Material
Supplemental material, sj-pdf-1-taw-10.1177_2042098621997727 for Aristolochic acid-associated urinary tract cancers: an updated meta-analysis of risk and oncologic outcomes after surgery and systematic review of molecular alterations observed in human studies by Yu-Chan Kang, Ming-Hong Chen, Chung-Ying Lin, Chih-Yun Lin and Yen-Ta Chen in Therapeutic Advances in Drug Safety
Acknowledgments
The authors would like to thank the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work and providing CMA 3 software.
Footnotes
Conflict of interest statement: Yu-Chan Kang, Ming-Hong Chen, Chung-Ying Lin, Chih-Yun Lin and Yen-Ta Chen declare that they have no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financially supported by grants from the Chang Gung Memorial Hospital, Kaohsiung, Taiwan (CMRPG8B1051).
ORCID iD: Yen-Ta Chen  https://orcid.org/0000-0002-3046-6675
https://orcid.org/0000-0002-3046-6675
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yu-Chan Kang, Department of Pharmacy, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung.
Ming-Hong Chen, Department of Pharmacy, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung.
Chung-Ying Lin, Institute of Allied Health Sciences, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan.
Chih-Yun Lin, Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung.
Yen-Ta Chen, Division of Urology, Department of Surgery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University, College of Medicine, Kaohsiung, No.123, Ta-Pei Road, Niao-Sung District, Kaohsiung City 83301.
References
- 1. Han J, Xian Z, Zhang Y, et al. Systematic overview of aristolochic acids: nephrotoxicity, carcinogenicity, and underlying mechanisms. Front Pharmacol 2019; 10: 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jadot I, Declèves AE, Nortier J, et al. An integrated view of aristolochic acid nephropathy: update of the literature. Int J Mol Sci 2017; 18: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gökmen MR, Cosyns JP, Arlt VM, et al. The epidemiology, diagnosis, and management of aristolochic acid nephropathy: a narrative review. Ann Intern Med 2013; 158: 469–477. [DOI] [PubMed] [Google Scholar]
- 4. Lemy A, Wissing KM, Rorive S, et al. Late onset of bladder urothelial carcinoma after kidney transplantation for end-stage aristolochic acid nephropathy: a case series with 15-year follow-up. Am J Kidney Dis 2008; 51: 471–477. [DOI] [PubMed] [Google Scholar]
- 5. Jelaković B, Dika Ž, Arlt VM, et al. Balkan endemic nephropathy and the causative role of aristolochic acid. Semin Nephrol 2019; 39: 284–296. [DOI] [PubMed] [Google Scholar]
- 6. Sidorenko VS, Yeo JE, Bonala RR, et al. Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res 2012; 40: 2494–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stiborova M, Arlt VM, Schmeiser HH. DNA adducts formed by aristolochic acid are unique biomarkers of exposure and explain the initiation phase of upper urothelial cancer. Int J Mol Sci 2017; 18: 2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Attaluri S, Bonala RR, Yang I-Y, et al. DNA adducts of aristolochic acid II: total synthesis and site-specific mutagenesis studies in mammalian cells. Nucleic Acids Res 2010; 38: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenquist TA, Grollman AP. Mutational signature of aristolochic acid: clue to the recognition of a global disease. DNA Repair 2016; 44: 205–211. [DOI] [PubMed] [Google Scholar]
- 10. Chen CH, Dickman KG, Moriya M, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci U S A 2012; 109: 8241–8246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmeiser HH, Kucab JE, Arlt VM, et al. Evidence of exposure to aristolochic acid in patients with urothelial cancer from a Balkan endemic nephropathy region of Romania. Environ Mol Mutagen 2012; 53: 636–641. [DOI] [PubMed] [Google Scholar]
- 12. Jelaković B, Karanović S, Vuković-Lela I, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int 2012; 81: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poon SL, Pang ST, McPherson JR, et al. Genome-wide mutational signatures of aristolochic acid and its application as a screening tool. Sci Transl Med 2013; 5: 197ra101. [DOI] [PubMed] [Google Scholar]
- 14. Hoang ML, Chen CH, Sidorenko VS, et al. Mutational signature of aristolochic acid exposure as revealed by whole-exome sequencing. Sci Transl Med 2013; 5: 197ra102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization and International Agency for Research on Cancer. Some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr Eval Carcinog Risks Hum 2002; 82: 1–556. [PMC free article] [PubMed] [Google Scholar]
- 16. Maggini V, Menniti-Ippolito F, Firenzuoli F. Aristolochia, a nephrotoxic herb, still surfs on the Web, 15 years later. Intern Emerg Med 2018; 13: 811–813. [DOI] [PubMed] [Google Scholar]
- 17. Tian J-Z, Liang A-H, Liu J, et al. Risk control of traditional Chinese medicines containing aristolochis acids (AAs) based on influencing factors of content of AAs [in Chinese]. Zhongguo Zhong Yao Za Zhi 2017; 42: 4679–4686. [DOI] [PubMed] [Google Scholar]
- 18. Yang HY, Chen PC, Wang JD. Chinese herbs containing aristolochic acid associated with renal failure and urothelial carcinoma: a review from epidemiologic observations to causal inference. Biomed Res Int 2014; 2014: 569325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turesky RJ, Yun BH, Brennan P, et al. Aristolochic acid exposure in Romania and implications for renal cell carcinoma. Br J Cancer 2016; 114: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008; 74: 158–169. [DOI] [PubMed] [Google Scholar]
- 21. Wu F, Wang T. Risk assessment of upper tract urothelial carcinoma related to aristolochic acid. Cancer Epidemiol Biomarkers Prev 2013; 22: 812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scelo G, Riazalhosseini Y, Greger L, et al. Variation in genomic landscape of clear cell renal cell carcinoma across Europe. Nat Commun 2014; 5: 5135. [DOI] [PubMed] [Google Scholar]
- 23. Jelakovic B, Castells X, Tomic K, et al. Renal cell carcinomas of chronic kidney disease patients harbor the mutational signature of carcinogenic aristolochic acid. Int J Cancer 2015; 136: 2967–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bara T, Jr, Gurzu S, Sugimura H, et al. A systematic review of the possible carcinogenic role of the aristolochic acid. Rom J Morphol Embryol 2017; 58: 41–44. [PubMed] [Google Scholar]
- 25. Hassler MR, Bray F, Catto JWF, et al. Molecular characterization of upper tract urothelial carcinoma in the era of next-generation sequencing: a systematic review of the current literature. Eur Urol 2020; 78: 209–220. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 29 September 2016).
- 28. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 29. Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health 2018; 21: 72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ren Y, Lin L, Lian Q, et al. Real-world performance of meta-analysis methods for double-zero-event studies with dichotomous outcomes using the Cochrane Database of Systematic Reviews. J Gen Intern Med 2019; 34: 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deeks JJ, Higgins JPT, Altman DG. (eds). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, et al. (eds) Cochrane handbook for systematic reviews of interventions, version 6.0. London: Cochrane, 2019. [Google Scholar]
- 32. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000; 53: 1119–1129. [DOI] [PubMed] [Google Scholar]
- 34. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sostarić B, Vukelić M. Characteristics of urinary tract tumors in the area of Balkan endemic nephropathy in Croatia. IARC Sci Publ 1991: 29–35. [PubMed] [Google Scholar]
- 36. Li W-H, Yang L, Su T, et al. Influence of taking aristolochic acid-containing Chinese drugs on occurrence of urinary transitional cell cancer in uremic patients undergoing dialysis [in Chinese]. Zhonghua Yi Xue Za Zhi 2005; 85: 2487–2491. [PubMed] [Google Scholar]
- 37. Zivcić-Cosić S, Grzetić M, Valencić M, et al. Urothelial cancer in patients with endemic Balkan nephropathy (EN) after renal transplantation. Ren Fail 2007; 29: 861–865. [DOI] [PubMed] [Google Scholar]
- 38. Li XB, Xing NZ, Wang Y, et al. Transitional cell carcinoma in renal transplant recipients: a single center experience. Int J Urol 2008; 15: 53–57. [DOI] [PubMed] [Google Scholar]
- 39. Lai MN, Wang SM, Chen PC, et al. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst 2009; 102: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang SX, Ma HB, Lu Q, et al. Study on the relationship between chronic aristolochic acid nephropathy and malignant tumors [in Chinese]. Shandong Med J 2009; 49: 73–74. [Google Scholar]
- 41. Zhou Y, Huang L, Liang Y, et al. Investigation of risk of developing secondary cancer in patients receiving Chinese herbal medicine containing aristolochic acid after kidney transplant [in Chinese]. ADRJ 2009; 11: 9–12. [Google Scholar]
- 42. Xiao LR, Yang YR, Xia P, et al. Clinical analysis of eight cases of carcinoma of urinary system after renal transplantation for aristolochic acid nephropathy [in Chinese]. Med J Chin PLA 2010; 35: 442–444. [Google Scholar]
- 43. Gao H. Research and analysis on risk factors of chronic renal failure patients in different stages with malignancies. Master’s Thesis [in Chinese], Taishan Medical University, China, 2011. [Google Scholar]
- 44. Yang HW, Liu L. Aristolochic acid nephropathy complicated urinary tumor tract after renal transplantation: a report of 5 cases and literature review [in Chinese]. J Contemp Urol Reprod Oncol 2011; 3: 45. [Google Scholar]
- 45. Yang HY, Wang JD, Lo TC, et al. Occupational exposure to herbs containing aristolochic acids increases the risk of urothelial carcinoma in Chinese herbalists. J Urol 2013; 189: 48–52. [DOI] [PubMed] [Google Scholar]
- 46. Wang SM, Lai MN, Wei A, et al. Increased risk of urinary tract cancer in ESRD patients associated with usage of Chinese herbal products suspected of containing aristolochic acid. PLoS One 2014; 9: e105218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matic M, Dragicevic B, Pekmezovic T, et al. Common polymorphisms in GSTA1, GSTM1 and GSTT1 are associated with susceptibility to urinary bladder cancer in individuals from Balkan endemic nephropathy areas of Serbia. Tohoku J Exp Med 2016; 240: 25–30. [DOI] [PubMed] [Google Scholar]
- 48. Li W, Lun L, Chen Y, et al. Analysis on the status of patients with urinary transitional cell carcinoma after taking aristolochic acid-containing Chinese medicines [in Chinese]. Chinese J Integr Trad Western Nephrol 2007; 8: 212–214. [Google Scholar]
- 49. Chen CH, Dickman KG, Huang CY, et al. Aristolochic acid-induced upper tract urothelial carcinoma in Taiwan: clinical characteristics and outcomes. Int J Cancer 2013; 133: 14–20. [DOI] [PubMed] [Google Scholar]
- 50. Milenkovic-Petronic D, Milojevic B, Djokic M, et al. The impact of tumor size on outcomes in patients with upper urinary tract urothelial carcinoma. Int Urol Nephrol 2014; 46: 563–569. [DOI] [PubMed] [Google Scholar]
- 51. Liu Y-Q, Lu J, Zhao L, et al. Prognostic factors for intravesical recurrence after surgery for upper tract urothelial carcinoma in renal transplant recipients [in Chinese]. Beijing Da Xue Xue Bao Yi Xue Ban 2015; 47: 605–610. [PubMed] [Google Scholar]
- 52. Chen CH, Dickman KG, Huang CY, et al. Recurrence pattern and TP53 mutation in upper urinary tract urothelial carcinoma. Oncotarget 2016; 7: 45225–45236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ji C, Zhong W, Zhang L, et al. Risk factors of new contralateral upper tract urothelial carcinoma after radical nephroureterectomy [in Chinese]. J Clin Urology (China) 2016; 31: 965–973. [Google Scholar]
- 54. Zhong W, Zhang L, Ma J, et al. Impact of aristolochic acid exposure on oncologic outcomes of upper tract urothelial carcinoma after radical nephroureterectomy. Onco Targets Ther 2017; 10: 5775–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Q, Zhang T, Wu J, et al. Prognosis and risk factors of patients with upper urinary tract urothelial carcinoma and postoperative recurrence of bladder cancer in central China. BMC Urol 2019; 19: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shan H, Tian W, Hong Y, et al. Clinicopathologic characteristics and prognosis of upper tract urothelial carcinoma complicated with aristolochic acid nephropathy after radical nephroureterectomy. BMC Complement Med Ther 2020; 20: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Aydin S, Ambroise J, Cosyns JP, et al. TP53 mutations in p53-negative dysplastic urothelial cells from Belgian AAN patients: new evidence for aristolochic acid-induced molecular pathogenesis and carcinogenesis. Mutat Res 2017; 818: 17–26. [DOI] [PubMed] [Google Scholar]
- 58. Castells X, Karanovic S, Ardin M, et al. Low-coverage exome sequencing screen in formalin-fixed paraffin-embedded tumors reveals evidence of exposure to carcinogenic aristolochic acid. Cancer Epidemiol Biomarkers Prev 2015; 24: 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Poon SL, Huang MN, Choo Y, et al. Mutation signatures implicate aristolochic acid in bladder cancer development. Genome Med 2015; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang XM, Lu Y, Song YM, et al. Integrative genomic study of Chinese clear cell renal cell carcinoma reveals features associated with thrombus. Nat Commun 2020; 11: 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tao L, Zeng Y, Wang J, et al. Differential microRNA expression in aristolochic acid-induced upper urothelial tract cancers ex vivo. Mol Med Rep 2015; 12: 6533–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Popovska-Jankovic K, Noveski P, Jankovic-Velickovic L, et al. MicroRNA profiling in patients with upper tract urothelial carcinoma associated with Balkan endemic nephropathy. Biomed Res Int 2016; 2016: 7450461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Velickovic LJ, Hattori T, Visnjic M, et al. E-cadherin expression in upper urothelial carcinoma in Balkan endemic nephropathy and non-endemic regions. Pathol Res Pract 2009; 205: 682–689. [DOI] [PubMed] [Google Scholar]
- 64. Jankovic-Velickovic L, Stojnev S, Ristic-Petrovic A, et al. Pro- and antiapoptotic markers in upper tract urothelial carcinoma associated with Balkan endemic nephropathy. ScientificWorldJournal 2011; 11: 1699–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jankovic-Velickovic L, Ristic-Petrovic A, Stojnev S, et al. Angiogenesis in upper tract urothelial carcinoma associated with Balkan endemic nephropathy. Int J Clin Exp Pathol 2012; 5: 674–683. [PMC free article] [PubMed] [Google Scholar]
- 66. Xiao J, Zhu X, Hao GY, et al. Association between urothelial carcinoma after kidney transplantation and aristolochic acid exposure: the potential role of aristolochic acid in HRas and TP53 gene mutations. Transplant Proc 2011; 43: 3751–3754. [DOI] [PubMed] [Google Scholar]
- 67. Hoang ML, Chen CH, Chen PC, et al. Aristolochic acid in the etiology of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev 2016; 25: 1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu H, Liang Y, Guan B, et al. Aristolochic acid mutational signature defines the low-risk subtype in upper tract urothelial carcinoma. Theranostics 2020; 10: 4323–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu MJ, Lian JD, Yang CR, et al. High cumulative incidence of urinary tract transitional cell carcinoma after kidney transplantation in Taiwan. Am J Kidney Dis 2004; 43: 1091–1097. [DOI] [PubMed] [Google Scholar]
- 70. Chang CH, Yang CM, Yang AH. Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer 2007; 109: 1487–1492. [DOI] [PubMed] [Google Scholar]
- 71. Jelaković B, Nikolić J, Radovanović Z, et al. Consensus statement on screening, diagnosis, classification and treatment of endemic (Balkan) nephropathy. Nephrol Dial Transplant 2014; 29: 2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol 2018; 73: 111–122. [DOI] [PubMed] [Google Scholar]
- 73. Locke JA, Hamidizadeh R, Kassouf W, et al. Surveillance guidelines based on recurrence patterns for upper tract urothelial carcinoma. Can Urol Assoc J 2018; 12: 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Takahashi T, Kakehi Y, Mitsumori K, et al. Distinct microsatellite alterations in upper urinary tract tumors and subsequent bladder tumors. J Urol 2001; 165: 672–677. [DOI] [PubMed] [Google Scholar]
- 75. Boot A, Jiang N, Rozen SG. Toward clinical understanding of aristolochic acid upper-tract urothelial carcinoma. Theranostics 2020; 10: 5578–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rosenquist TA, Chen C, Hoang ML, et al. Mutational signatures as records of carcinogen exposure: assigning roles in cancer etiology. Environ Mol Mutagen 2017; 58: S29. [Google Scholar]
- 77. Liu YY, Li LD, Mao WL, et al. Contrast research of Aristolochia L. plants with substitution materials for medicine [in Chinese]. Chinese J Clin Pharmacol Ther 2006; 11: 841–846. [Google Scholar]
- 78. Li Y, Li J, Yu F. Current situation and research progress on the nephrotoxicity of aristolochic acid compounds [in Chinese]. J Pharm Res 2017; 36: 534–537. [Google Scholar]
- 79. Liu SH, Chuang WC, Lam W, et al. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf 2015; 38: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fan Y, Li Z, Xi J. Recent developments in detoxication techniques for aristolochic acid-containing traditional Chinese medicines. RSC Adv 2020; 10: 1410–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chan W, Hui KM, Poon WT, et al. Differentiation of herbs linked to “Chinese herb nephropathy” from the liquid chromatographic determination of aristolochic acids. Anal Chim Acta 2006; 576: 112–116. [DOI] [PubMed] [Google Scholar]
- 82. Hsieh SC, Lai JN, Chen PC, et al. Is Duhuo Jisheng Tang containing Xixin safe? A four-week safety study. Chin Med 2010; 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Poon WT, Lai CK, Chan AYW. Aristolochic acid nephropathy: the Hong Kong perspective. Hong Kong J Nephrol 2007; 9: 7–14. [Google Scholar]
- 84. Jong TT, Lee MR, Hsiao SS, et al. Analysis of aristolochic acid in nine sources of Xixin, a traditional Chinese medicine, by liquid chromatography/atmospheric pressure chemical ionization/tandem mass spectrometry. J Pharm Biomed Anal 2003; 33: 831–837. [DOI] [PubMed] [Google Scholar]
- 85. Department of Health and Executive Yuan. Regulations for Asarum and its preparations. Announcement No. 0930000756, Taiwan, ROC [in Chinese], https://dep.mohw.gov.tw/DOCMAP/cp-883-5365-108.html (2004).
- 86. Wu Y, Wang Q. Determination of aristolochic acid A in the different parts of Asarum and preparations by HPLC [in Chinese]. Northwest Pharm J 2007; 22: 108–109. [Google Scholar]
- 87. Liu Y, Zhang C, Zhou Y, et al. Determination of effective components and aristolochic acid I in Asarum herb from different habitats [in Chinese]. China Licensed Pharmacist 2010; 7: 29–33. [Google Scholar]
- 88. Samardzić J, Karanović S, Tomic K, et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid out of established endemic nephropathy rural villages. Nephrol Dial Transplant 2017; 32: iii96. [Google Scholar]
- 89. Chan CK, Liu Y, Pavlović NM, et al. Aristolochic acids: newly identified exposure pathways of this class of environmental and food-borne contaminants and its potential link to chronic kidney diseases. Toxics 2019; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taw-10.1177_2042098621997727 for Aristolochic acid-associated urinary tract cancers: an updated meta-analysis of risk and oncologic outcomes after surgery and systematic review of molecular alterations observed in human studies by Yu-Chan Kang, Ming-Hong Chen, Chung-Ying Lin, Chih-Yun Lin and Yen-Ta Chen in Therapeutic Advances in Drug Safety