Abstract
Odontoblast differentiation is a complex and multistep process regulated by signaling pathways, including the Wnt/β-catenin signaling pathway. Both positive and negative effects of Wnt/β-catenin signaling on dentinogenesis have been reported, but the underlying mechanisms of these conflicting results are still unclear. To gain a better insight into the role of Wnt/β-catenin in dentinogenesis, we used dental pulp cells from a panel of transgenic mice, in which fluorescent protein expression identifies cells at different stages of odontoblast and osteoblast differentiation. Our results showed that exposure of pulp cells to WNT3a at various times and durations did not induce premature differentiation of odontoblasts. These treatments supported the survival of undifferentiated cells in dental pulp and promoted the formation of 2.3GFP+ preodontoblasts and their rapid transition into differentiated odontoblasts expressing DMP1-Cherry and DSPP-Cerulean transgenes. WNT3a also promoted osteogenesis in dental pulp cultures. These findings provide critical information for the development of improved treatments for vital pulp therapy and dentin regeneration.
Keywords: WNT3a, odontoblast, osteoblasts, pulp biology, dentinogenesis, survival
Introduction
The Wnt/β-catenin signaling is an evolutionarily conserved pathway with key and essential roles in embryogenesis and postnatal growth (Clevers and Nusse 2012; Baron and Kneissel 2013; Steinhart and Angers 2018). In the absence of Wnt signaling, cytoplasmic β-catenin is phosphorylated by the serine/threonine kinase GSK-3β, through interactions with the scaffolding proteins Axin and APC, and targeted for degradation by the ubiquitin-proteasome pathway (Clevers and Nusse 2012; Baron and Kneissel 2013; Steinhart and Angers 2018). Activation of Wnt signaling inhibits β-catenin phosphorylation, leading to the stabilization of β-catenin and its accumulation in the nuclei. In the nuclei, β-catenin interacts with T-cell-specific factor/lymphoid enhancer-binding factor 1 (TCF/Lef1) and with transcriptional coactivators that regulate the expression of certain target genes (Clevers and Nusse 2012; Baron and Kneissel 2013; Steinhart and Angers 2018). The Wnt/β-catenin pathway is tightly regulated by a number of antagonists, including Sclerostin (SOST) and Dickkopf (Dkk1 and Dkk2) (Cruciat and Niehrs 2013).
Wnt/β-catenin signaling plays an essential role in the morphogenesis and cellular differentiation of many organs, including tooth (Clevers and Nusse 2012; Baron and Kneissel 2013; Tamura and Nemoto 2016; Steinhart and Angers 2018). During tooth development, this pathway plays multiple essential roles in various stages of tooth morphogenesis, and mutations in various components of Wnt-β-catenin signaling pathway have been identified in syndromic and nonsyndromic tooth agenesis (Tamura and Nemoto 2016).
The roles of Wnt/β-catenin signaling in odontoblast differentiation and dentinogenesis were first suggested by the expression of components of this pathway in odontoblasts. Several Wnts, mediators of the signaling pathway such as Lef-1 and Axin 2 as well as Dkk1, are expressed in developing odontoblasts (Balic and Thesleff 2015; Tamura and Nemoto 2016).
Tissue-specific inhibition of Wnt/β-catenin signaling in young and mature odontoblasts (Bae et al. 2015) caused impaired odontoblast differentiation, suggesting positive roles of this pathway on odontoblast differentiation.
On the other hand, stabilization of β-catenin in young and mature odontoblasts by employing various promotors such as 2.3 kb Col1a1 (Kim et al. 2012), OC (Bae et al. 2013), and DMP1 (Zhao et al. 2019) induced various abnormalities in odontoblasts, including premature differentiation of odontoblasts and formation of immature odontoblasts with lack or reduced levels of Dspp and Nestin (Chen et al. 2009; Kim et al. 2011, 2012; Bae et al. 2013; Zhao et al. 2019). However, all these animals displayed a large amount of thickened and hypomineralized dentin (Chen et al. 2009; Kim et al. 2012; Bae et al. 2013), suggesting roles of this pathway in dentin matrix production and mineralization.
In vitro studies also provided conflicting results regarding the effects of Wnt/β-catenin signaling on odontoblast differentiation and expression of Dspp during the mineralization of pulp cultures. Several studies have shown the positive roles (Yamashiro et al. 2007; Yokose and Naka 2010; Han et al. 2014; Lim et al. 2014; Rahman et al. 2018; Lu et al. 2019), whereas a few studies implicated negative roles (Scheller et al. 2008; Amri et al. 2016) of this pathway on odontoblast differentiation.
Thus, the effects of Wnt/β-catenin signaling on odontoblast differentiation are controversial and still unclear. These discrepancies can be related to many factors, including the possibility of differential responses of various cell populations in dental pulp and in dentinogenic lineage to Wnt/β-catenin signaling.
To gain a better understanding of the progression of progenitor cells in the odontoblast lineage, we have examined and characterized the expression of a series of green fluorescent protein (GFP) reporters during odontoblast differentiation. These studies have shown the stage-specific activation of these transgenes during odontoblast differentiation in vivo and in vitro. pOBCol2.3-GFP (2.3-GFP) transgene is activated at early stages of odontoblast differentiation (preodontoblasts and prior to the expression of Dmp1 and Dspp) (Balic, Aguila, and Mina 2010). DMP1-Cherry and DSPP-Cerulean transgenes are activated and expressed by functional and fully differentiated odontoblasts, respectively (Vijaykumar et al. 2019). More recently, we showed that BSP-GFPtpz identifies cells in the osteogenic lineage and is excluded from cells in the dentinogenic lineage (Vijaykumar et al. 2020). The lineage- and stage-specific activation of these transgenes provides a panel of reporters that can help identify and isolate cells in dentinogenic and osteogenic lineages at various stages of differentiation. Using these reporters, we were able to show that the effects of FGF signaling on cells in odontoblast lineage were stage specific and depended on the stage of cell maturity (Sagomonyants and Mina 2014; Sagomonyants et al. 2015, 2017).
To investigate the role of Wnt/β-catenin signaling in odontoblast differentiation, we took advantage of a well-characterized dental pulp culture from these reporter mice and examined the response of cells in various stages of differentiation to Wnt/β-catenin signaling. Our results show multiple essential roles of Wnt/β-catenin signaling during the in vitro differentiation of odontoblasts from resident progenitors in the dental pulp that are dependent on the stage of maturity/differentiation of cells.
Materials and Methods
Animal Models
All experimental protocols involving animal tissues in the present study were approved by the Institutional Animal Care and use Committee of UConn Health Center. DSPP-Cerulean/DMP1-Cherry, BSP-GFPtpz, pOBCol2.3FP (referred to as 2.3-GFP), TCF/Lef:H2B-EGFP, and nontransgenic mice used in this study have been previously described (Balic, Aguila, Caimano, et al. 2010; Ferrer-Vaquer et al. 2010; Vijaykumar et al. 2019, 2020). All mice were maintained in the CD1 background. In all experiments, pulp cells from nontransgenic littermates served as negative control.
Cell Cultures, Digital Imaging, and Epifluorescence Analysis
Primary pulp cultures were prepared from the coronal pulp of first and second molars from 5- to 7-d-old mice (Balic, Aguila, Caimano, et al. 2010). Cultures were first grown in Dulbecco’s modified Eagle’s medium. At day 7, mineralization was induced by addition of 50 µg/mL fresh ascorbic acid and 4 mM β-glycerophosphate. Medium was changed every other day. Mineralization in live primary cultures was examined by xylenol orange (XO) staining (Vijaykumar et al. 2020). The mean fluorescence intensity of XO staining, DMP1-Cherry, and BSP-GFPtpz was measured using a microplate reader (Safire2; Tecan) (Vijaykumar et al. 2019, 2020).
Activation and Inhibition of Wnt/β-Catenin Signaling
The cultures were grown in the presence of vehicle (VH; 0.1% bovine serum albumin), 50 ng/mL WNT3a (R&D Systems), and 50 ng/mL DKK1 (R&D Systems), with or without simultaneous 50 ng/mL WNT3a between days 3 and 21 (referred to as continuous exposure), days 7 and 21 (referred to as late exposure), and days 3 and 7 (referred to as early exposure).
Immunocytochemistry
Pulp cultures were processed for immunocytochemistry with a 1:1,000 dilution of anti-GFP Alexa Fluor 488–conjugated antibody (Molecular Probes, Invitrogen) (Vijaykumar et al. 2019). Negative controls included primary dental pulp cultures derived from the transgenic littermates without the addition of anti-GFP antibody.
RNA Extraction and Analysis
Total RNA was prepared with TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. After DNase treatment, RNA samples were processed for quantitative polymerase chain reaction (qPCR) analysis with specific primers (Appendix Table 1) purchased from Applied Biosystems (Sagomonyants and Mina 2014).
Flow Cytometric Analysis
Single-cell suspensions from pulp cultures were processed for fluorescence-activated cell sorting (FACS) analysis at different time points using gating strategies shown in Appendix Figure 2. Percentages of GFP+ and GFP− cells were determined with BD FACSDiva 6.2 software (Sagomonyants and Mina 2014).
For fluorescence-activated cell sorting, cultures derived from 2.3-GFP transgenic animals were processed for sorting based on GFP (Balic, Aguila, and Mina 2010; Sagomonyants et al. 2015). Live GFP+ and GFP− cells were replated at the same density as the primary cultures (9.21 × 104 cells/cm2) grown in the presence of VH or WNT3a between days 3 and 14 (continuous exposure) and processed for various extra word gene expression analysis.
Proliferation and Apoptosis Assays
The proliferative cells were detected by incubation of cultures with 10 µM EdU (5-ethynyl-2′-deoxyuridine) for 4 h and Click-iT Plus Flow Cytometry Assay kit (Invitrogen) according to the manufacturer’s instructions.
The apoptotic cells were examined using rabbit cleaved caspase 3 antibody (1:800; Cell Signaling Technology 9661S) for 1 h and goat anti-rabbit Alexa Fluor 594 for 30 min. The number of proliferative and apoptotic cells was calculated by FACS analysis using a BD LSR-II FACS cytometer (BD Biosciences).
Statistical Analysis
Analysis was performed by GraphPad Prism 7 software (GraphPad Software) using an unpaired 2-tailed Student’s t test and 1-way analysis of variance (ANOVA). Values in all experiments represented mean ± SEM of at least 3 independent experiments, and P ≤ 0.05 was considered statistically significant.
Table.
Effects of Wnt/β-Catenin Signaling on the Proliferation and Survival of Cells in Primary Pulp Cultures.
| VH | WNT3a | DKK1 | |
|---|---|---|---|
| % EdU+ cells | |||
| D4 | 16.95 ± 1.61 | 27.1 ± 2.23a | 17.2 ± 0.83b |
| D5 | 12.22 ± 0.83 | 22.25 ± 1.01a | 11.35 ± 0.96b |
| D6 | 18.57 ± 0.37 | 20.62 ± 1.42a | 15.95 ± 0.5b |
| D7 | 10.5 ± 0.89 | 14.35 ± 0.72a | 10.12 ± 0.86b |
| D8 | ND | ND | ND |
| D9 | ND | ND | ND |
| D10 | ND | ND | ND |
| % Col2.3GFP+/EdU+ | |||
| D4 | 7.72 ± 0.79 | 13.85 ± 1.22a | 7 ± 0.41b |
| D5 | 8.27 ± 0.77 | 14.5 ± 0.99a | 7.1 ± 0.73b |
| D6 | 11.17 ± 0.64 | 14.42 ± 0.47a | 9.95 ± 0.41b |
| D7 | 6.54 ± 0.48 | 9.35 ± 0.46 | 6.22 ± 0.78 |
| D8 | ND | ND | ND |
| D9 | ND | ND | ND |
| D10 | ND | ND | ND |
| % Col2.3GFP−/EdU+ | |||
| D4 | 9.22 ± 0.95 | 13.6 ± 1.26a | 10.2 ± 0.45 |
| D5 | 3.95 ± 0.06 | 7.75 ± 0.45a | 4.25 ± 0.32b |
| D6 | 6.4 ± 0.27 | 6.2 ± 0.53 | 6 ± 0.3 |
| D7 | 3.96 ± 0.73 | 4.72 ± 0.48 | 3.7 ± 0.65 |
| D8 | ND | ND | ND |
| D9 | ND | ND | ND |
| D10 | ND | ND | ND |
| % Caspase+ cells in cultures | |||
| D4 | 32.45 ± 2.17 | 29.8 ± 0.57 | 32.36 ± 1.54 |
| D5 | 32.17 ± 5.37 | 31.8 ± 3.1 | 36.8 ± 3.46 |
| D6 | 39.47 ± 1.62 | 25.53 ± 2.53a | 34.3 ± 5.15b |
| D7 | 28.97 ± 2.99 | 22.63 ± 2.03a | 36.16 ± 7.34b |
In these experiments, primary pulp cultures were treated from day 3 onward with vehicle (VH, 0.1% bovine serum albumin), WNT3a (50 ng/mL), or DKK1 (50 ng/mL) and fluorescence-activated cell sorting (FACS) analyzed every 24 h after staining with EdU for proliferation and cleaved caspase 3 for apoptosis as described in the Materials and Methods. The table shows the percentage of EdU+ and EdU− cells in primary pulp cultures from 2.3-GFP mice following various treatments. Note the significant increases in proliferation of 2.3-GFP+ and 2.3-GFP− populations in the WNT3a-treated cultures compared to controls. Note a lack of proliferation during the mineralization phase from days 8 to 10. The table also shows the percentage of cleaved caspase 3+ and cleaved caspase 3− cells in primary pulp cultures following various treatments. Note the significant decreases in apoptosis in the WNT3a-treated cultures compared to controls. DKK1 treatment had no significant effect on apoptosis. Results represent mean ± SEM of at least 3 independent experiments. Analysis was performed using 1-way analysis of variance.
ND, not detected.
P ≤ 0.05 relative to the respective controls at each time point.
P ≤ 0.05 relative to WNT3a at each time point.
Results
Effects of WNT3a on Wnt-Responsive Cells in Pulp Cultures
As the first step, the effects of the activation of Wnt/β-catenin signaling by WNT3a on the status of Wnt-responsive cells in dental pulp were studied by examining the levels of expression of Axin2 (Jho et al. 2002). In addition, we used TCF/Lef:H2B-GFP reporter mice, in which the cells responding to Wnt signaling are identified by the nuclear localization of GFP that can be quantified by FACS analysis (Ferrer-Vaquer et al. 2010).
In these experiments, cultures were treated with 50 ng/mL WNT3a and DKK1 (an antagonist of canonical Wnt/β-catenin) at days 3 and 7. These concentrations were based on their apparent effects on the expression of Axin2 in our preliminary studies (Appendix Fig. 3A, B).
The addition of WNT3a to cultures at both days 3 and 7 resulted in approximately 2.5- to 4-fold increases in the levels of Axin2 (Appendix Fig. 3C, E) and about 1.5-fold increases in the percentage of GFP+ cells (Appendix Fig. 3D, F). DKK1 reduced the WNT3a-induced increases in the levels of Axin2 (Appendix Fig. 3C, E). Together, these results show that primary pulp cultures contain Wnt-responsive cells, and WNT3a induces increases in the expression of Axin2 and percentage of cells with nuclear accumulation of β-catenin.
Effects of WNT3a on Mineralization and Differentiation of Primary Pulp Cultures
Next, we examined the effects of continuous activation of Wnt/β-catenin signaling by the addition of WNT3a on the mineralization and differentiation of pulp cells in vitro. In these experiments, primary pulp cultures were continuously exposed to WNT3a and DKK1 with or without WNT3a between days 3 and 21.
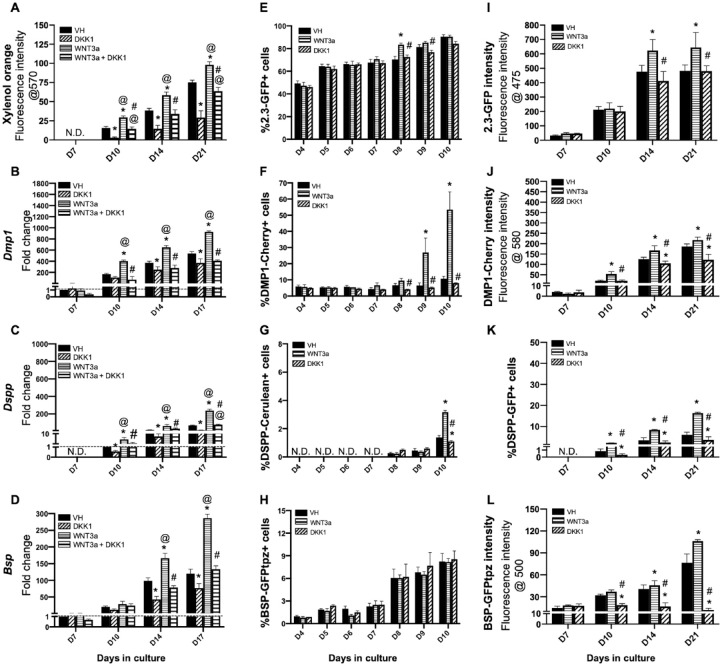
XO staining showed marked increases in the extent of mineralization between days 10 and 21 in the WNT3a-treated cultures compared to VH-treated controls (Fig. 1A and Appendix Fig. 4A, B). DKK1 induced marked decreases in the extent of mineralization and partially reversed the effects of WNT3a on mineralization (Fig. 1A and Appendix Fig. 4A, C).
Figure 1.
Effects of continuous exposure to WNT3a and DKK1 in primary dental pulp cultures. Primary pulp cultures from nontransgenic and transgenic animals were treated continuously from days 3 to 21 with vehicle (VH) (0.1% bovine serum albumin), WNT3a (50 ng/mL), DKK1 (50 ng/mL), or simultaneous WNT3a (50 ng/mL) and DKK1 (50 ng/mL). (A–D) Effects on mineralization and the expression of differentiation markers in primary dental pulp cultures: (A) Bar graphs showing changes in the intensity of xylenol orange (XO) staining (in absolute values) in cultures from nontransgenic animals treated continuously with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a from days 3 to 21. Continuous exposure to WNT3a and DKK1 increased and decreased the intensity of XO at days 10, 14, and 21 as compared to control, respectively. DKK1 treatment also significantly reduced the WNT3a-induced increases in XO intensity. The intensity of XO staining was measured at a 570/610-nm wavelength (excitation/emission) and at a gain of 80. Background fluorescence for XO was measured using cultures without the addition of ascorbic acid and β-glycerophosphate, which lack mineralization potential. The background fluorescence values were subtracted from respective XO measurements. (B–D) Bar graphs showing changes in the expression of Dmp1 and Dspp (odontoblast differentiation markers) and Bsp (osteoblast differentiation marker) in nontransgenic cultures treated continuously with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a from days 3 to 17. Continuous exposure to WNT3a showed significant increases in Dmp1 (B), Dspp (C), and Bsp (D) at days 10 to 17, which were significantly decreased by DKK1 treatment alone. DKK1 treatment significantly reduced the WNT3a-induced increases in Dmp1, Dspp, and Bsp almost to control levels. Expression of Dmp1 and Bsp was normalized to day 7, and Dspp was normalized to day 10 of the VH-treated cultures, which is arbitrarily set to 1 and is indicated by the dashed line. (E–H) Effects on green fluorescent protein (GFP) reporters by fluorescence-activated cell sorting (FACS) analysis: bar graphs showing the changes in the percentage of cells expressing 2.3-GFP (E), DMP1-Cherry (F), DSPP-Cerulean (G), and BSP-GFPtpz (H) transgenes by FACS analysis every 24 h following the start of continuous treatment with VH, WNT3a, and DKK1 at day 3 up to day 10. WNT3a treatment increased the percentage of 2.3-GFP+ cells on day 8, which was significantly decreased by DKK1 at days 8 and 9 compared to WNT3a-treated cultures (E). WNT3a-treated cultures also show significantly increased percentages in DMP1-Cherry+ cells and DSPP-Cerulean+ cells at days 9 and 10. DKK1-treated cultures show decreased percentages of DMP1-Cherry+ and DSPP-Cerulean+ cells that were significant compared to VH-treated controls, as well as WNT3a-treated cultures (F, G). WNT3a and DKK1 treatment showed no effects on the percentage of BSP-GFPtpz+ cells (H). (I–L) Effects on GFP reporters at later stages of mineralization: bar graphs showing the changes in the fluorescence intensity of 2.3-GFP (I), DMP1-Cherry (J), and BSP-GFPtpz (L) transgenes by fluorometric analysis, as well as the percentage of DSPP-Cerulean+ cells (K), detected by immunocytochemistry following continuous treatment with VH, WNT3a, and DKK1 from days 3 to 21. Continuous exposure to WNT3a showed significant increases in the fluorescence intensity of 2.3-GFP (I), DMP1-Cherry (J), BSP-GFPtpz (L), and the percentage of DSPP-Cerulean+ cells (K) at days 10 to 21. DKK1 treatment decreased the intensity of 2.3-GFP, DMP1-Cherry, and BSP-GFPtpz and the percentage of DSPP-Cerulean+ cells at days 10 to 21 compared to VH-treated controls, as well as WNT3a-treated cultures. Results in all graphs represent mean ± SEM of at least 3 independent experiments. Analysis was performed using 1-way analysis of variance. *P ≤ 0.05 relative to VH at each time point; @P ≤ 0.05 relative to DKK1 at each time point; #P ≤ 0.05 relative to WNT3a at each time point. ND, not detected.
The effects of WNT3a on differentiation were studied by examining the relative levels of markers of odontoblast (Dmp1 and Dspp) and osteoblast (Bsp) differentiation. Despite the lack of significant effects on day 7, WNT3a-treated cultures displayed marked increases in the expression of Dmp1 (Fig. 1B), Dspp (Fig. 1C), and Bsp (Fig. 1D) compared to VH-treated controls between days 10 and 17. DKK1 reversed the WNT3a-induced increases in the levels of Dspp, Dmp1, and Bsp to levels similar to those in VH-treated controls (Fig. 1B–D).
Effects of Wnt/β-Catenin Signaling on GFP Reporters
The increases in expression of markers of odontoblast and osteoblast differentiation during the mineralization phase of growth in primary pulp cultures in WNT3a-treated cultures can be related to changes in their levels of transcription and/or changes in the number of cells expressing these markers. To distinguish between these possibilities, the effects of WNT3a on pulp cultures from 2.3-GFP, DSPP-Cerulean/DMP1-Cherry, and BSP-GFPtpz transgenic reporter mice were examined by FACS analysis.
FACS analysis between days 4 and 10 using gating strategies shown in Appendix Figure 2A–C showed no changes in the percentage of cells expressing different transgenes in WNT3a-treated cultures as compared to VH-treated controls (Fig. 1E–H). On the other hand, there were marked increases in the percentages of cells expressing these transgenes in WNT3a-treated cultures as compared to VH-treated controls between days 8 and 10. These changes included increases in the percentage of 2.3-GFP+ cells at day 8 (Fig. 1E) followed by marked increases in the percentage of DMP1-Cherry+ cells at d 9 and 10 (Fig. 1F) and DSPP-Cerulean+ cells at day 10 (Fig. 1G). There were no significant changes in the percentage of BSP-GFPtpz+ (Fig. 1H) cells in the WNT3a-treated cultures as compared to VH-treated controls.
Due to the difficulties in obtaining single cells in mineralized cultures, the changes in the expression of various transgenes at later time points (days 10–21) were analyzed by fluorometric analysis and immunocytochemistry (Fig. 1I–L and Appendix Fig. 4D–F). WNT3a-treated cultures showed continuous increases in the intensity of 2.3-GFP (Fig. 1I), DMP1-Cherry (Fig. 1J), and BSP-GFPtpz (Fig. 1L) as compared to VH-treated controls. Immunocytochemical analysis with the GFP antibody showed marked increases in the number of DSPP-GFP+ cells (Fig. 1K) in WNT3a-treated cultures compared to VH-treated controls.
Stage-Specific Effects of Wnt/β-Catenin Signaling on the Differentiation of Pulp Cells In Vitro
The lack of significant changes in the expression of markers of differentiation and percentage of cells expressing the various transgenes during the first 7 d in the WNT3a-treated suggest that response to activation of Wnt/β-catenin may be dependent on the stage of maturity/differentiation of the cells. This possibility was examined in 2 different sets of experiments.
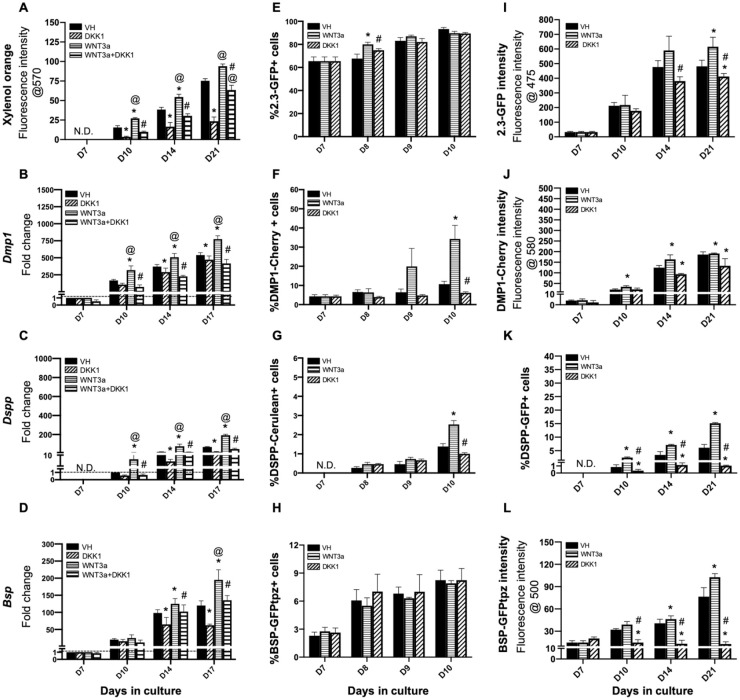
In the first set of experiments, we examined the effects of limited activation of Wnt/β-catenin signaling by WNT3a during the mineralization of primary pulp cultures. In these experiments, the cultures were treated with WNT3a and DKK between days 7 and 21. The changes in the mineralization, the temporal patterns of the expression of various markers of differentiation, and the expression of multiple reporters in cultures treated between days 7 and 21 were similar to the changes observed with continuous treatment (Fig. 2 and Appendix Fig. 5), albeit with a lower magnitude.
Figure 2.
Effects of late exposure of primary dental pulp cultures to WNT3a and DKK1. Primary pulp cultures were treated late from days 7 to 21 with vehicle (VH) (0.1% bovine serum albumin), WNT3a (50 ng/mL), DKK1 (50 ng/mL), or simultaneous WNT3a (50 ng/mL) and DKK1 (50 ng/mL). (A–D) Effects on mineralization and the expression of differentiation markers in primary dental pulp cultures: (A) Bar graphs showing changes in the intensity of xylenol orange (XO) staining (in absolute values) in nontransgenic cultures treated with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a from days 7 to 21. Late exposure to WNT3a and DKK1 increased and decreased the intensity of XO at days 10, 14, and 21 as compared to control, respectively. DKK1 treatment also significantly reduced the WNT3a-induced increases in XO intensity. (B–D) Bar graphs showing changes in the expression of Dmp1, Dspp, and Bsp in nontransgenic cultures treated with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a (50 ng/mL) treatment from days 7 to 17. Late exposure to WNT3a showed significant increases in Dmp1 (B), Dspp (C), and Bsp (D) at days 10 to 17, which were significantly decreased by DKK1 treatment alone. DKK1 treatment also significantly decreased the WNT3a-induced increases in Dmp1, Dspp, and Bsp almost to control levels. Expression of Dmp1 and Bsp was normalized to day 7, and Dspp was normalized to day 10 of the VH-treated cultures, which is arbitrarily set to 1 and is indicated by the dashed line. (E–H) Effects on green fluorescent protein (GFP) reporters by fluorescence-activated cell sorting (FACS) analysis: bar graphs showing the changes in the percentage of cells expressing 2.3-GFP (E), DMP1-Cherry (F), DSPP-Cerulean (G), and BSP-GFPtpz (H) transgenes by FACS analysis every 24 h following the start of late treatment with VH, WNT3a, and DKK1 at day 7 up to day 10. WNT3a treatment significantly increased the percentage of 2.3-GFP+ cells on day 8, which was decreased by DKK1 (E). WNT3a-treated cultures also show significantly increased percentages in DMP1-Cherry+ cells and DSPP-Cerulean+ cells at day 10. DKK1-treated cultures show decreased percentages of DMP1-Cherry+ and DSPP-Cerulean+ cells that were significant compared to WNT3a-treated cultures (F, G). WNT3a and DKK1 treatment showed no effects on the percentage of BSP-GFPtpz+ cells (H). (I–L) Effects on the intensity of GFP and percentages of DSPP-GFP+ at later stages of mineralization: bar graphs showing the changes in the fluorescence intensity of 2.3-GFP (I), DMP1-Cherry (J), and BSP-GFPtpz (L) transgenes, as well as the percentage of DSPP-Cerulean+ cells (K), following late treatment with VH, WNT3a, and DKK1 from days 7 to 21. Late exposure to WNT3a showed significant increases in the fluorescence intensity of 2.3-GFP (I), DMP1-Cherry (J), BSP-GFPtpz (L), and the percentage of DSPP-Cerulean+ cells (K) at days 10 to 21. DKK1 treatment significantly decreased the intensity of 2.3-GFP, DMP1-Cherry, and BSP-GFPtpz and the percentage of DSPP-Cerulean+ cells at days 10 to 21. Results in all graphs represent mean ± SEM of at least 3 independent experiments. Analysis was performed using 1-way analysis of variance. *P ≤ 0.05 relative to VH at each time point; @P ≤ 0.05 relative to DKK1 at each time point; #P ≤ 0.05 relative to WNT3a at each time point. ND, not detected.
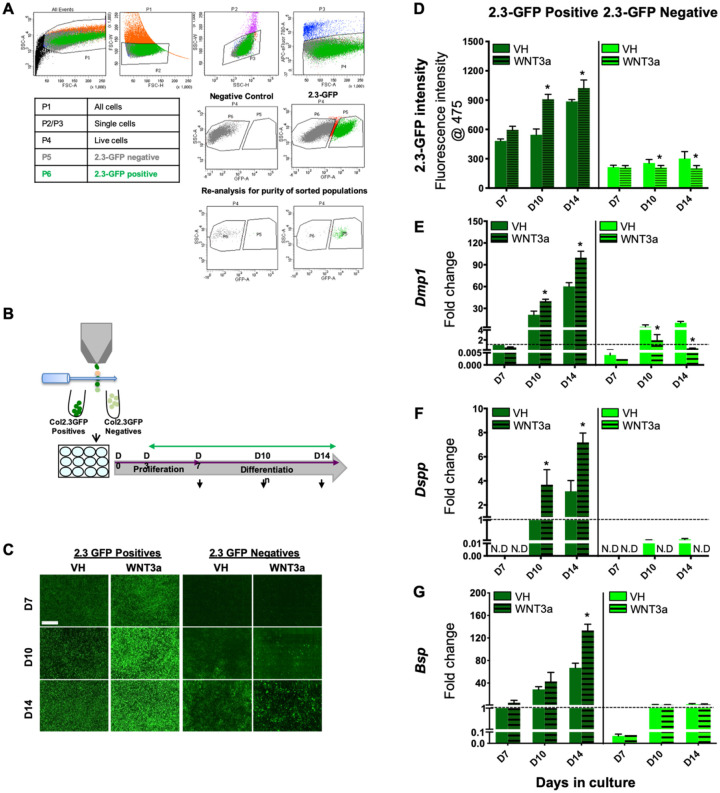
In the second set of experiments, we examined the effects of continuous WNT3a exposure on the differentiation of relatively homogeneous populations of FACS-sorted 2.3-GFP+ and 2.3-GFP− cells (≥98% purity of isolated populations; Fig. 3A, B) that represent populations enriched in preodontoblasts and undifferentiated cells, respectively (Balic, Aguila, Caimano, et al. 2010) (Fig. 3). In cultures established from the 2.3-GFP+ population, WNT3a induced marked increases in the intensity of 2.3-GFP (Fig. 3C, D) and expression of Dmp1 (Fig. 3E), Dspp (Fig. 3F), and Bsp (Fig. 3G) between days 10 and 14. On the other hand, in cultures established from the 2.3-GFP− population, WNT3a induced marked decreases in the intensity of 2.3-GFP (Fig. 3C, D) and expression of Dmp1 (Fig. 3E). In these cultures, WNT3a inhibited the expression of Dspp (Fig. 3F) and had no significant effect on Bsp (Fig. 3G). These observations provide evidence that activation of Wnt/β-catenin signaling promoted the differentiation of preodontoblasts identified by the expression of 2.3-GFP and not the undifferentiated population in the dental pulp.
Figure 3.
Effects of WNT3a on the differentiation of fluorescence-activated cell sorting (FACS)–sorted 2.3-GFP+ and 2.3-GFP− populations. (A) In these experiments, primary pulp cultures from 2.3-GFP transgenic mice were first grown in culture conditions supporting their proliferation and expansion. At day 7, 2.3-GFP+ and 2.3-GFP− cells were separated according to the gating strategy shown. (Top row) Gate P1 includes all cells exclusive of debris. P2 and P3 include single cells identified by forward-scatter and side-scatter profiles, respectively. P4 includes live cells. Dead cells were excluded by staining with fixable viability dye. (Middle row) FACS sorting was performed in 2.3-GFP cultures from molars on day 7. Nontransgenic cultures were used as a negative control. (Bottom row) The isolated populations were reanalyzed for the purity of >98%. The table shows a legend denoting the identity of each gate analyzed by flow cytometry. (B) The sorted 2.3-GFP+ and 2.3-GFP− populations were replated and cultured with continuous exposure to vehicle (VH; 0.1% BSA) or 50 ng/mL WNT3a between days 3 and 14. (C) Representative images of the same areas in cultures from 2.3-GFP+ and 2.3-GFP− populations at various time points analyzed under epifluorescent light using a filter to detect green fluorescent protein (GFP). Scale bar = 1 mm. Note that WNT3a treatment increased and decreased the GFP expression in the 2.3-GFP+ and 2.3-GFP− cultures, respectively. (D) Bar graphs showing significantly increased and decreased fluorescence intensity in the WNT3a-treated 2.3-GFP+ and 2.3-GFP− cultures, respectively, at days 10 and 14. (E–G) Bar graphs showing that continuous exposure of FACS-sorted 2.3-GFP+ cultures to WNT3a showed significant increases in Dmp1 (E), Dspp (F), and Bsp (G) at days 10 to 14 compared to controls. Continuous exposure of FACS-sorted 2.3-GFP− cultures to WNT3a showed significant decreases in Dmp1 expression (E), undetectable Dspp expression (F), and no significant effects in Bsp expression (G) compared to controls. Expression of Dmp1 and Bsp in 2.3-GFP+ and 2.3-GFP− populations was normalized to that in VH-treated 2.3-GFP+ cultures at day 7, which is arbitrarily set to 1 and is indicated by the dashed line. Expression of Dspp in both populations was normalized to that in VH-treated 2.3-GFP+ cultures at day 10, which is arbitrarily set to 1 and is indicated by the dashed line. Results in all graphs represent mean ± SEM of at least 3 independent experiments. Analysis was performed using an unpaired 2-tailed Student’s t test. *P ≤ 0.05 relative to the respective controls at each time point. ND, not detected.
Effects of Wnt/β-Catenin Signaling on Undifferentiated and Early Progenitors in Pulp Cells In Vitro
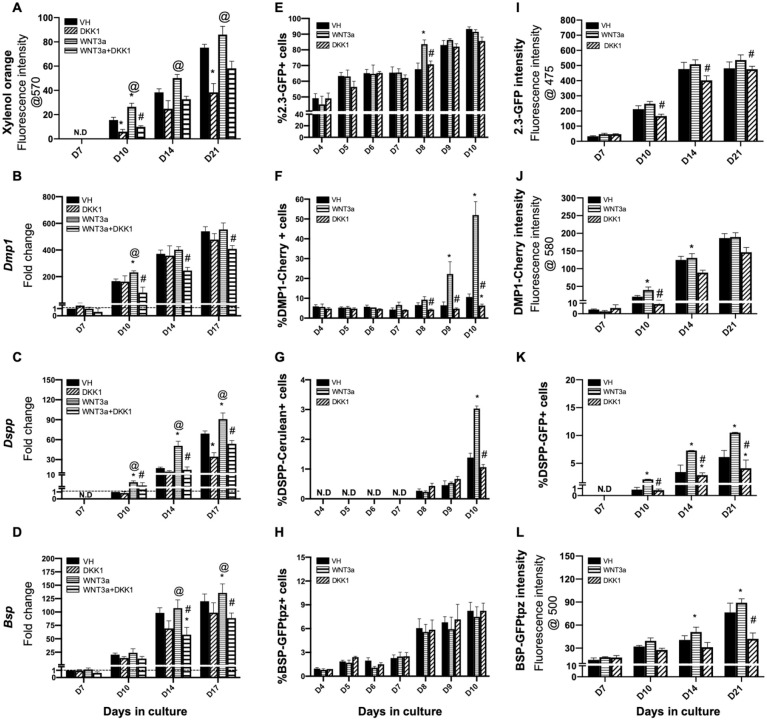
The effects of activation of Wnt/β-catenin signaling by WNT3a on undifferentiated and early progenitors in pulp culture were examined by limited and early exposure of pulp culture to WNT3a. In these experiments, the cultures were treated with WNT3a and DKK1 only between days 3 and 7. Interestingly, early and limited treatment with WNT3a also induced changes similar to those induced by continuous and late treatments but at lower levels (Fig. 4, Appendix Fig. 6). Furthermore, these changes were evident during the mineralization phase of in vitro growth and after the withdrawal of the WNT3a (Fig. 4, Appendix Fig. 6).
Figure 4.
Effects of early exposure of primary dental pulp cultures to WNT3a and DKK1. Primary pulp cultures were treated early from days 3 to 7 with vehicle (VH) (0.1% bovine serum albumin), WNT3a (50 ng/mL), DKK1 (50 ng/mL), or simultaneous WNT3a (50 ng/mL) and DKK1 (50 ng/mL). (A–D) Effects on mineralization and the expression of differentiation markers in primary dental pulp cultures: (A) Bar graphs showing changes in the intensity of xylenol orange (XO) staining (in absolute values) in nontransgenic cultures treated with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a from days 3 to 7. Early exposure to WNT3a and DKK1 increased and decreased the intensity of XO at days 10, 14, and 21 as compared to control, respectively. DKK1 treatment also significantly decreased the WNT3a-induced increases in XO intensity. (B–D) Bar graphs showing changes in the expression of Dmp1, Dspp, and Bsp in nontransgenic cultures treated continuously with VH, WNT3a, and DKK1 alone or with simultaneous WNT3a from days 3 to 7. Early exposure to WNT3a showed significant increases in Dmp1 (B), Dspp (C), and Bsp (D) at days 10 to 17, which were decreased by DKK1 treatment alone. DKK1 treatment also significantly decreased the WNT3a-induced increases in Dmp1, Dspp, and Bsp. Expression of Dmp1 and Bsp was normalized to day 7, and Dspp was normalized to day 10 of the VH-treated cultures, which is arbitrarily set to 1 and is indicated by the dashed line. (E–H) Effects on green fluorescent protein (GFP) reporters by fluorescence-activated cell sorting (FACS) analysis: bar graphs showing the changes in the percentage of cells expressing 2.3-GFP (E), DMP1-Cherry (F), DSPP-Cerulean (G), and BSP-GFPtpz (H) transgenes by FACS analysis every 24 h following the start of early treatment with VH, WNT3a, and DKK1 at day 3 up to day 10. WNT3a treatment significantly increased the percentage of 2.3-GFP+ cells at day 8, which was decreased by DKK1 (E). WNT3a also significantly increased percentages in DMP1-Cherry+ cells and DSPP-Cerulean+ cells at days 9 to 10. DKK1-treated cultures show significantly decreased percentages of DMP1-Cherry+ and DSPP-Cerulean+ cells that were significant compared to VH treated controls, as well as WNT3a-treated cultures (F, G). WNT3a and DKK1 treatment showed no effects on the percentage of BSP-GFPtpz+ cells (H). (I–L) Effects on the intensity of GFP and percentages of DSPP-GFP+ cells at later stages of mineralization: bar graphs showing the changes in the fluorescence intensity of 2.3-GFP (I), DMP1-Cherry (J), and BSP-GFPtpz (L) transgenes, as well as the percentage of DSPP-Cerulean+ cells (K) following late treatment with VH, WNT3a, and DKK1 from days 3 to 7. Early exposure to WNT3a showed significant increases in the fluorescence intensity of DMP1-Cherry (J), BSP-GFPtpz (L), and the percentage of DSPP-Cerulean+ cells (K) at days 10 to 21, with no significant effects on the intensity of Col2.3-GFP (I). DKK1 treatment significantly decreased the intensity of DMP1-Cherry and BSP-GFPtpz and the percentage of DSPP-Cerulean+ cells, at days 10 to 21. Results in all graphs represent mean ± SEM of at least 3 independent experiments. Analysis was performed using 1-way analysis of variance. *P ≤ 0.05 relative to VH at each time point; @P ≤ 0.05 relative to DKK1 at each time point; #P ≤ 0.05 relative to WNT3a at each time point. ND, not detected.
We further examined the effects of Wnt/β-catenin signaling on the survival of primary pulp cultures. FACS analysis using the gating strategy shown in Appendix Figures 2D and E showed marked increases in the percentage of proliferative cells (EdU+) (Table) and decreases in the percentage of apoptotic cells (cleaved caspase 3+) (Table) between days 4 and 7 in WNT3a-treated cultures as compared to VH-treated controls. WNT3a-induced increases in the proliferation in 2.3-GFP+ (Table) and 2.3-GFP− (Table) populations between days 3 and 7. The absence of detectable EdU+ cells (cells in S phase) between days 8 and 10 (after cells reach confluence and initiate mineralization) in unsorted and sorted populations suggests that the increases in 2.3-GFP+ population at day 8 (Fig. 1E) were related to the activation of 2.3-GFP in a new population. These findings indicate that WNT3a promoted the survival of undifferentiated dental pulp cells and enhanced their differentiation into preodontoblasts expressing the 2.3-GFP transgene.
Discussion
Our results also showed essential roles for Wnt/β-catenin signaling in the survival of resident progenitors and are consistent with previous observations in dental pulp (Hunter et al. 2015). Limited and early exposure to WNT3a resulted in increased proliferation and decreased apoptosis in the undifferentiated population.
Our results also showed that exposure of pulp cells to WNT3a at various times and durations did not induce premature differentiation of odontoblasts from resident progenitors. These observations are different from in vivo studies that showed that sustained activation of β-catenin by constitutive stabilization of β-catenin in the dental mesenchyme and odontoblasts led to premature differentiation of immature odontoblasts with lack or reduced levels of Dspp and Nestin (Chen et al. 2009; Kim et al. 2011, 2012; Bae et al. 2013; Zhao et al. 2019). The underlying mechanisms for these differences may be related to differences in the mechanisms of activation of signaling pathways and timing or the duration of activation.
The positive effects of activation of Wnt/β-catenin signaling on odontoblast differentiation in our in vitro study are consistent with the reported effects in other studies (Yamashiro et al. 2007; Yokose and Naka 2010; Lim et al. 2014; Rahman et al. 2018; Lu et al. 2019).
Our results extend these observations by providing evidence that positive effects of Wnt/β-catenin signaling on odontoblast differentiation are mediated through proliferation and the formation of preodontoblasts from residents’ progenitors in the dental pulp. Our observations showed that exposure of pulp cells to WNT3a at various times and durations increased the number of preodontoblasts, odontoblasts, and levels of expression of Dmp1 and Dspp. Since DMP1-Cherry+ and DSPP-Cerulean+ identify functional and fully differentiated odontoblasts (Vijaykumar et al. 2019) that are postmitotic and nonproliferative, the increases in their number in WNT3a-treated cultures in our studies is related to increases in the number of 2.3-GFP+ preodontoblasts and their rapid differentiation into functional and fully differentiated odontoblasts.
This possibility is further supported by our studies on FACS-sorted populations. Exposure of the 2.3-GFP+ population to WNT3a resulted in progressive increases in the intensity of GFP and expression of Dmp1 and Dspp. On the other hand, exposure of the 2.3-GFP− undifferentiated population to WNT3a resulted in marked decreases in the intensity of GFP expression and inhibited the expression of Dmp1 and Dspp.
In our studies, we cannot exclude the possibility that Wnt/β-catenin signaling may also directly affect the transcription of Dspp and Dmp1. Although it has been shown that Lef1 induced Dspp expression via binding to its promoter region in vitro (Yokose and Naka 2010), the precise mechanisms by which Dspp and Dmp1 expression may be induced by Wnt/β-catenin signaling are still unclear and have not been fully elucidated.
Our observations also showed that activation of Wnt/β-catenin signaling also increased the number of BSP-GFP+ osteoblasts from resident progenitors in dental pulp cultures. These observations are consistent with the body of literature that showed one of the mechanisms whereby Wnt/β-catenin signaling increases bone formation by stimulating the proliferation of preosteoblasts and the development of osteoblasts (Kim et al. 2013; Teufel and Hartmann 2019).
Together, these observations showed that in dentinogenic lineage, activation of Wnt/β-catenin signaling by WNT3a promoted the survival of the undifferentiated progenitors, leading to increases in the pool of cells that can form preodontoblasts. Wnt/β-catenin signaling further supports the rapid differentiation of preodontoblasts into functional and fully differentiation odontoblasts.
Author Contributions
A. Vijaykumar, S.H. Root, M. Mina, contributed to conception, design, data acquisition, analysis, or interpretation, drafted and revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520967353 for Wnt/β-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro by A. Vijaykumar, S.H. Root and M. Mina in Journal of Dental Research
Acknowledgments
The authors thank all individuals who provided reagents, valuable input, and technical assistance in various aspects of this study, including Barbara Rodgers and members of the Molecular Core Facility and Flow Cytometry Facility at the University of Connecticut Health Center.
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants R01-DE016689 and R90-DE022526 from the National Institutes of Health (National Institute of Dental and Craniofacial Research).
ORCID iD: A. Vijaykumar  https://orcid.org/0000-0002-9311-8715
https://orcid.org/0000-0002-9311-8715
References
- Amri N, Djole SX, Petit S, Babajko S, Coudert AE, Castaneda B, Simon S, Berdal A. 2016. Distorted patterns of dentinogenesis and eruption in Msx2 null mutants: involvement of sost/sclerostin. Am J Pathol. 186(10):2577–2587. [DOI] [PubMed] [Google Scholar]
- Bae CH, Kim TH, Ko SO, Lee JC, Yang X, Cho ES. 2015. Wntless regulates dentin apposition and root elongation in the mandibular molar. J Dent Res. 94(3):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae CH, Lee JY, Kim TH, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. 2013. Excessive Wnt/β-catenin signaling disturbs tooth-root formation. J Periodontal Res. 48(4):405–410. [DOI] [PubMed] [Google Scholar]
- Balic A, Aguila HL, Caimano MJ, Francone VP, Mina M. 2010. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 46(6):1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Aguila HL, Mina M. 2010. Identification of cells at early and late stages of polarization during odontoblast differentiation using pOBCol3.6GFP and pOBCol2.3GFP transgenic mice. Bone. 47(5):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balic A, Thesleff I. 2015. Tissue interactions regulating tooth development and renewal. Curr Top Dev Biol. 115:157–186. [DOI] [PubMed] [Google Scholar]
- Baron R, Kneissel M. 2013. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 19(2):179–192. [DOI] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. 2009. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 334(1):174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. 2012. Wnt/β-catenin signaling and disease. Cell. 149(6):1192–1205. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Niehrs C. 2013. Secreted and transmembrane wnt inhibitors and activators. Cold Spring Harb Perspect Biol. 5(3):a015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Vaquer A, Piliszek A, Tian G, Aho RJ, Dufort D, Hadjantonakis AK. 2010. A sensitive and bright single-cell resolution live imaging reporter of Wnt/ss-catenin signaling in the mouse. BMC Dev Biol. 10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han N, Zheng Y, Li R, Li X, Zhou M, Niu Y, Zhang Q. 2014. β-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One. 9(2):e88890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Bardet C, Mouraret S, Liu B, Singh G, Sadoine J, Dhamdhere G, Smith A, Tran XV, Joy A, et al. 2015. Wnt acts as a prosurvival signal to enhance dentin regeneration. J Bone Miner Res. 30(7):1150–1159. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. 2002. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 22(4):1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Liu X, Wang J, Chen X, Zhang H, Kim SH, Cui J, Li R, Zhang W, Kong Y, et al. 2013. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther Adv Musculoskelet Dis. 5(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Jang EH, Yoon CY, Bae Y, Ko SO, Taketo MM, Cho ES. 2012. Col1a1-cre mediated activation of β-catenin leads to aberrant dento-alveolar complex formation. Anat Cell Biol. 45(3):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. 2011. Constitutive stabilization of ss-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 412(4):549–555. [DOI] [PubMed] [Google Scholar]
- Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, Williams BO, Sharpe PT, Bardet C, Mah SJ, et al. 2014. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res. 29(4):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chen X, Xing J, Lian M, Huang D, Lu Y, Feng G, Feng X. 2019. miR-140-5p regulates the odontoblastic differentiation of dental pulp stem cells via the Wnt1/β-catenin signaling pathway. Stem Cell Res Ther. 10(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SU, Oh JH, Cho YD, Chung SH, Lee G, Baek JH, Ryoo HM, Woo KM. 2018. Fibrous topography-potentiated canonical Wnt signaling directs the odontoblastic differentiation of dental pulp-derived stem cells. ACS Appl Mater Interfaces. 10(21):17526–17541. [DOI] [PubMed] [Google Scholar]
- Sagomonyants K, Kalajzic I, Maye P, Mina M. 2015. Enhanced dentinogenesis of pulp progenitors by early exposure to FGF2. J Dent Res. 94(11):1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagomonyants K, Kalajzic I, Maye P, Mina M. 2017. FGF signaling prevents the terminal differentiation of odontoblasts. J Dent Res. 96(6):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagomonyants K, Mina M. 2014. Biphasic effects of FGF2 on odontoblast differentiation involve changes in the BMP and Wnt signaling pathways. Connect Tissue Res. 55(suppl 1):53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Chang J, Wang CY. 2008. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 87(2):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart Z, Angers S. 2018. Wnt signaling in development and tissue homeostasis. Development. 145(11):dev146589. [DOI] [PubMed] [Google Scholar]
- Tamura M, Nemoto E. 2016. Role of the Wnt signaling molecules in the tooth. Jpn Dent Sci Rev. 52(4):75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufel S, Hartmann C. 2019. Wnt-signaling in skeletal development. Curr Top Dev Biol. 133:235–279. [DOI] [PubMed] [Google Scholar]
- Vijaykumar A, Dyrkacz P, Vidovic-Zdrilic I, Maye P, Mina M. 2020. Expression of BSP-GFPtpz transgene during osteogenesis and reparative dentinogenesis. J Dent Res. 99(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijaykumar A, Ghassem-Zadeh S, Vidovic-Zdrilic I, Komitas K, Adameyko I, Krivanek J, Fu Y, Maye P, Mina M. 2019. Generation and characterization of DSPP-Cerulean/DMP1-Cherry reporter mice. Genesis. 57(10): e23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, Takano-Yamamoto T, Thesleff I. 2007. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 75(5):452–462. [DOI] [PubMed] [Google Scholar]
- Yokose S, Naka T. 2010. Lymphocyte enhancer-binding factor 1: an essential factor in odontoblastic differentiation of dental pulp cells enzymatically isolated from rat incisors. J Bone Miner Metab. 28(6):650–658. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yuan X, Bellido T, Helms JA. 2019. A correlation between Wnt/beta-catenin signaling and the rate of dentin secretion. J Endod. 45(11):1357–1364.e1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520967353 for Wnt/β-Catenin Signaling Promotes the Formation of Preodontoblasts In Vitro by A. Vijaykumar, S.H. Root and M. Mina in Journal of Dental Research