Abstract
The coronavirus disease 2019 (COVID-19) vaccination will be the largest vaccination programme in the history of the NHS. Patients on immunosuppressive therapy will be among the earliest to be vaccinated. Some evidence indicates immunosuppressive therapy inhibits humoral response to the influenza, pneumococcal and hepatitis B vaccines. The degree to which this will translate to impaired COVID-19 vaccine responses is unclear. Other evidence suggests withholding MTX for 2 weeks post-vaccination may improve responses. Rituximab has been shown to impair humoral responses for 6 months or longer post-administration. Decisions on withholding or interrupting immunosuppressive therapy around COVID-19 vaccination will need to be made prior to the availability of data on specific COVID-19 vaccine response in these patients. With this in mind, this article outlines the existing data on the effect of antirheumatic therapy on vaccine responses in patients with inflammatory arthritis and formulates a possible pragmatic management strategy for COVID-19 vaccination.
Keywords: COVID-19, vaccine, biologics, DMARDs, rituximab, methotrexate
Rheumatology key messages
Existing work on vaccine response in DMARDs is an imperfect surrogate for COVID-19 vaccine response.
MTX may impair humoral response; rituximab likely impairs humoral response for 6 months or longer.
Consider risk stratifying rituximab-treated patients and delaying/postponing therapy if appropriate before COVID-19 vaccination.
Introduction
The aim of this viewpoint article is to outline the existing data on the effect of antirheumatic therapy on vaccine responses in patients with inflammatory arthritis, and to formulate a possible pragmatic strategy for the management of therapies in these patients in the context of prospective coronavirus disease 2019 (COVID-19) vaccination. But primarily we aim to facilitate an informed discussion between clinicians and patients in response to issues raised by these data.
COVID-19 needs little introduction, and the three effective vaccines produced by Pfizer (mRNA vector), Moderna (mRNA vector) and AstraZeneca (chimpanzee adenovirus vector ChAdOx-1) have provided a potential exit strategy. The COVID-19 vaccine rollout will be the largest mass vaccination programme in the history of the NHS.
Vaccinations exert their protective effect by stimulating both humoral and cellular immune responses. The relative importance of humoral and cellular immunity in conferring protection from infection varies with each infective organism [1]. B-cell responses are better represented in the literature due to their ease of antibody measurement and the lack of a clear immune correlate of protection for T-cell driven responses. Nevertheless, it is worth noting that emerging evidence suggests a strong role for T-cell mediated immunity in COVID-19 infection [2, 3].
Immunosuppressive therapy such as the DMARDs, used to treat most of our patients, may impair vaccine responses. Existing data on this topic largely focus on influenza, pneumococcal and tetanus vaccines. There is a small amount of data also available on the Zostavax vaccine. Whether these data can be extrapolated to provide guidance for vaccination strategies in COVID-19 remains uncertain. Patients on immunosuppressive therapy are being prioritized for vaccination, so management decisions will need to be made prior to any additional COVID-19 data being available. Caveats when assessing the literature are noted below in Table 1.
Table 1.
Outline of key caveats when assessing the existing literature on vaccine response in the context of DMARD/biologic therapies
|
|
|
Impact of antirheumatic therapy on vaccine response
Table 2 below summarizes a review of the literature on the impact of anti-rheumatic therapy on vaccine response. Further discussion is under the relevant headings.
Table 2.
Summary of the evidence for the effect of common DMARDs/biologic therapies on vaccine response
| Drug | Findings | Interpretation/advice on management |
|---|---|---|
| Corticosteroids |
|
|
| csDMARDs (not MTX) |
|
|
| MTX (alone or in combination) |
|
|
| Anti-TNF |
|
|
| Anti-IL-6 |
|
|
| Abatacept | ||
| JAK inhibitors |
|
|
| Anti-CD20 |
|
csDMARD: conventional synthetic DMARD; JAK: Janus kinase; PCV-7: heptavalent pneumococcal conjugate vaccine; PCV-13: 13 valent pneumococcal conjugate vaccine; PPSV-23: pneumococcal polysaccharide vaccine; RTX: Rituximab; S/C: Subcutaneous injection.
Corticosteroids
Corticosteroids affect vaccine efficacy in a dose-dependent manner. Several studies have assessed the impact of corticosteroid therapy on humoral response to the pneumococcal and influenza vaccines [4–8]. Doses >10 mg prednisolone daily were associated with a degree of impaired humoral immunity in a longitudinal study; however, lower doses had little impact [5]. Steroid doses >10 mg daily prednisolone were associated with poorer outcomes in hospitalized patients with COVID-19[9].
csDMARDs
Other than MTX, there is limited evidence for significant impairment of humoral vaccine responses to other conventional synthetic DMARDs (csDMARDs). Sulfasalazine, hydroxychloroquine, azathioprine and leflunomide may reduce vaccine antibody titres but have not been shown to inhibit a seroprotective response to the pneumococcal or influenza vaccines [10–14].
Much of the trial data on mycophenolate is from organ transplant patients. These trials did not assess responses where mycophenolate was withheld, due to the high risk of graft rejection [14]. Mycophenolate was shown to reduce antibody titres but not below the threshold for seroprotection.
MTX has been shown to impair humoral response to the pneumococcal and influenza vaccines [15–18]. This is unsurprising given its ability (and use) to reduce antibody formation to monoclonal antibodies. Withholding MTX around the time of vaccination has been assessed for 4 weeks before influenza vaccination, 2 weeks either side of vaccination and 4 weeks post-vaccination [16, 18]. Holding MTX for 4 weeks after immunization substantially improved vaccine titres. A subsequent study suggested that the critical period for vaccine-induced humoral immunity was the 2-week period following vaccination [16, 18]. Longer periods of withholding MTX were not shown to confer better vaccine responses but were associated with an increased incidence of disease flare. It appears MTX has the same impact on vaccination when used in combination with other DMARDs. The same risk–benefit assessment is required for a decision on temporary withholding.
TNF inhibition
Several studies have assessed the impact of anti-TNF therapies on pneumococcal and influenza vaccines. There have been no consistent data linking these treatments to significant impairment of the immune response. However, in patients who are taking concurrent MTX, responses have been shown to be impaired. While seroprotective responses are typically maintained, vaccine antibody titres may be lower than for matched controls [4, 8, 10, 19]. TNF inhibition has also been shown to be safe in the context of the live varicella zoster vaccine [20]. In the context of COVID-19, early registry data have shown anti-TNF therapy to be associated with a decreased odds of hospitalization due to COVID-19 [9].
IL-6 inhibitors
Two large Japanese studies have assessed the impact of IL-6 inhibition on influenza and pneumococcal vaccine responses. One showed impaired responses in the IL-6 inhibitor plus MTX combination treatment arm but no impairment with IL-6 inhibition monotherapy [21]. A subsequent study showed no significant impairment in humoral response to the influenza and pneumococcal vaccines at 12 weeks in tocilizumab-treated patients [22].
Abatacept
There is some conflict within the existing data. Abatacept was shown to impair response to the H1N1 influenza vaccine in comparison to age-matched patients [23]. Abatacept was shown to impair heptavalent pneumococcal conjugate vaccine (PCV-7) responses in another small volume study with 17 abatacept-treated patients enrolled [24]. However, subsequent work showed no impairment of response to the trivalent influenza and pneumococcal polysaccharide vaccines in patients treated with subcutaneous abatacept at a dose of 125 mg weekly [25]. Interpretation of data is problematic as the two papers lacked a control group and one study recruited only 17 abatacept-treated patients.
Janus kinase inhibitors
Janus kinase (JAK) inhibition may prove to be problematic in the context of the mRNA COVID-19 vaccines, which induce a strong type 1 interferon-driven immune response. Theoretically, inhibition of this pathway could be associated with a diminished response.
The effect of baricitinib on pneumococcal conjugate and tetanus toxoid vaccine response was assessed and showed that 68% of patients on long-term baricitinib mounted seroprotective responses to the pneumococcal vaccine, although tetanus toxoid responses were less durable [26].
One study assessed the impact of tofacitinib (plus MTX in half of cases) on pneumococcal polysaccharide vaccine (PPSV-23) and influenza vaccine response [27]. Here similar proportions of tofacitinib and control patients achieved a satisfactory response to the influenza vaccine, but pneumococcal responses were impaired, particularly when tofacitinib was combined with MTX. Temporary discontinuation of tofacitinib therapy for 1 week pre-vaccination until 1 week after vaccination was not shown to impact on the humoral response.
Recent data in an abstract from the ACR Convergence 2020 has suggested a satisfactory response to the adjuvant herpes subunit zoster vaccine in JAK-inhibitor-treated patients [28]. However, one-quarter of the JAK-inhibitor-treated patients failed to mount any humoral vaccine response at all. Additionally, the live zoster vaccine Zostavax has been shown to be safe and effective in tofacitinib-treated patients in a study where similar VZV-specific humoral and cell-mediated responses were seen in controls and patients who started tofacitinib 2–3 weeks after live zoster vaccine administration [29].
Anti-CD20
B-cell depleting therapy has been shown to impair humoral responses to the influenza and pneumococcal vaccines in several studies and a subsequent meta-analysis [6, 15, 30, 31]. Biologically, this is consistent with the critical role of B cells in humoral vaccine responses. In 2008, csDMARD-treated patients were compared with csDMARD/rituximab combination therapy patients in the context of the influenza vaccine. Lower antibody titres were identified to all antigens in the combination therapy group and were statistically significant in one case [6]. One study assessed influenza vaccine response in early (4–8 weeks) and late (6–10 months) rituximab-treated patients [31]. Impairment of response was greater in the early rituximab treatment arm. Another study showed general impairment of humoral responses to the influence vaccine after rituximab therapy, but better humoral responses in the late (>5 months post-treatment) rather than early treatment groups [30].
There are some early data suggesting worse outcomes particularly in rituximab treated COVID-19 patients. Case reports have described severe COVID-19 phenotypes in patients treated with rituximab for rheumatological and other B-cell driven disorders [32–34]. Early study data have in some cases suggested poorer outcomes in rituximab-treated patients who become hospitalized with COVID-19 [35, 36]. However, it is likely there is a significant channelling bias as rituximab-treated patients generally have higher rates of interstitial lung disease and other factors associated with poorer outcomes in COVID-19. Nevertheless, such data are concerning and reinforce the need for judicious use of rituximab for only the most clinically necessary cases during a global pandemic.
Risk stratifying and timing vaccinations
Rheumatology departments require guidance on how to manage DMARD/biologic therapies in the context of mass COVID-19 vaccination and this guidance will evolve with time. Existing EULAR guidance is available but may not be sufficient in the context of a global pandemic [37]. In every case the benefits of reducing medication needs to be weighed against the risk of disease flare, which apart from the obvious disadvantage is known to reduce vaccination effectiveness [38]. Key considerations are summarized in Table 3 below.
Table 3.
Outline of key therapeutic considerations when vaccinating against COVID-19 alongside DMARDs/biologic therapies
Where appropriate:
|
A summary of the possible challenges specific to rituximab is depicted in Fig. 1.
Fig. 1.
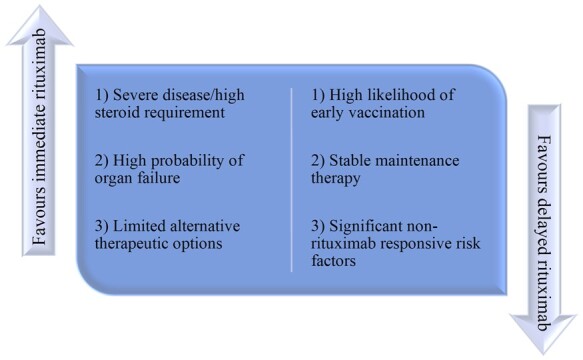
Factors influencing rituximab treatment decisions
For MTX, withholding treatment for 2 weeks following each vaccine dose may help improve humoral response. This is speculative in the context of novel vaccine techniques but could be considered in patients on MTX (and perhaps JAK inhibitors) at low to moderate risk of disease flare. Where a flare occurs, they would require treatment and high doses of prednisolone should be avoided where possible due to its possible effects on vaccine responses and COVID-19 morbidity. However, once again, it is important to stress that the priority is to proceed with vaccination and modification of therapy should not delay this.
The situation with JAK inhibitors is unknown, as unlike the MTX study they have only been withheld for 1 week post-vaccination so far. Some data from work on the influenza and PPSV-23 vaccines and the strong type 1 interferon response generated by the mRNA vaccines suggest that withholding JAK inhibitors might improve COVID-19 vaccine responses, but this is speculative. While for abatacept, the data are conflicting and given its mode of action, which could inhibit T-cell responses, treatment guidance urgently requires further evidence.
In all cases, any decision to delay treatment should be the result of an informed discussion by each patient and physician on a case-by-case basis.
Further work
Additional COVID-19-specific data will be critical in producing more evidence-based recommendations. Quantification of COVID-19 vaccine antibody titres, evidence on T-cell immunity and additional work on the impact of booster vaccinations will all be relevant, and there is ongoing work in Leeds collecting such data with and without medication modification. COVID-19 is likely to be a long-term issue, and the data from such a study should be of value for advice on protection and optimal future vaccination strategy.
Acknowledgements
This report presents independent research supported by the National Institute for Health Research (NIHR) Leeds Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. PE is director of Leeds NIHR BRC.
Funding: No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: J.A. has no conflicts of interest. K.W. has received consulting fees from Pfizer, AbbVie, Union Chimique Belge (UCB), Eli Lilly & Company, Galapagos, GlaxoSmithKline (GSK), Roche, Gilead, BMS, Regeneron, Sanofi, AstraZeneca and Novartis. He has also received research grants paid to his employer from BMS and Pfizer. P.E. has provided expert advice to Pfizer, Abbvie, Amgen, MSD, Roche, Sanofi, BMS, Novartis, Lilly, Gilead, Samsung and Celltrion, and received grants paid to his employer from Abbvie, BMS and Samsung.
Data availability statement
No new data were generated or analysed in support of this review.
References
- 1. Pollard AJ, Bijker EM.. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 2021;21:83–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karlsson AC, Humbert M, Buggert M.. The known unknowns of T cell immunity to COVID-19. Sci Immunol 2020;5:8063. [DOI] [PubMed] [Google Scholar]
- 3. Le Bert N, Tan AT, Kunasegaran K. et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020;584:457–62. [DOI] [PubMed] [Google Scholar]
- 4. Elkayam O, Bashkin A, Mandelboim M. et al. The effect of infliximab and timing of vaccination on the humoral response to influenza vaccination in patients with rheumatoid arthritis and ankylosing spondylitis. Semin Arthritis Rheum 2010;39:442–7. [DOI] [PubMed] [Google Scholar]
- 5. Fischer L, Gerstel PF, Poncet A. et al. Pneumococcal polysaccharide vaccination in adults undergoing immunosuppressive treatment for inflammatory diseases - a longitudinal study. Arthritis Res Ther 2015;17:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oren S, Mandelboim M, Braun-Moscovici Y. et al. Vaccination against influenza in patients with rheumatoid arthritis: the effect of rituximab on the humoral response. Ann Rheum Dis 2008;67:937–41. [DOI] [PubMed] [Google Scholar]
- 7. Coulson E, Saravanan V, Hamilton J. et al. Pneumococcal antibody levels after pneumovax in patients with rheumatoid arthritis on methotrexate. Ann Rheum Dis 2011;70:1289–91. [DOI] [PubMed] [Google Scholar]
- 8.Ben Nessib D, Fazaa A, Miladi S, Sellami M, Ouenniche K, Souabni L et al. Do immunosuppressive agents hamper the vaccination response in patients with rheumatic diseases? A review of the literature. Therapies [Internet]. 2020 [cited 2021 Mar 30]; Available from: https://pubmed.ncbi.nlm.nih.gov/32951867/. [DOI] [PubMed] [Google Scholar]
- 9. Gianfrancesco M, Hyrich KL, Hyrich KL. et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fomin I, Caspi D, Levy V. et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNFα blockers. Ann Rheum Dis 2006;65:191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adler S, Krivine A, Weix J. et al. Protective effect of A/H1N1 vaccination in immune-mediated disease–a prospectively controlled vaccination study. Rheumatology 2012;51:695–700. [DOI] [PubMed] [Google Scholar]
- 12. Franca ILA, Ribeiro ACM, Aikawa NE. et al. TNF blockers show distinct patterns of immune response to the pandemic influenza A H1N1 vaccine in inflammatory arthritis patients. Rheumatology 2012;51:2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gabay C, Bel M, Combescure C. et al. ; H1N1 Study Group. Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum 2011;63:1486–96. [DOI] [PubMed] [Google Scholar]
- 14. Keshtkar-Jahromi M, Argani H, Rahnavardi M. et al. Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: a controlled trial. Am J Nephrol 2008;28:654–60. [DOI] [PubMed] [Google Scholar]
- 15. Hua C, Barnetche T, Combe B, Morel J.. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2014;66:1016–26. [DOI] [PubMed] [Google Scholar]
- 16. Park JK, Choi Y, Winthrop KL, Song YW, Lee EB.. Optimal time between the last methotrexate administration and seasonal influenza vaccination in rheumatoid arthritis: post hoc analysis of a randomised clinical trial. Ann Rheum Dis 2019;78:1283–4. [DOI] [PubMed] [Google Scholar]
- 17. Kapetanovic MC, Saxne T, Sjöholm A. et al. Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis. Rheumatology 2006;45:106–11. [DOI] [PubMed] [Google Scholar]
- 18. Park JK, Lee YJ, Shin K. et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kivitz AJ, Schechtman J, Texter M. et al. Vaccine responses in patients with rheumatoid arthritis treated with certolizumab pegol: results from a single-blind randomized phase IV trial. J Rheumatol 2014;41:648–57. [DOI] [PubMed] [Google Scholar]
- 20. Curtis J, Bridges SL, Cofield SS. et al. Results from a randomized controlled trial of the safety of the live varicella vaccine in TNF-treated patients. Arthritis Rheumatol 2019;71(suppl 10). [Google Scholar]
- 21. Mori S, Ueki Y, Hirakata N. et al. Impact of tocilizumab therapy on antibody response to influenza vaccine in patients with rheumatoid arthritis. Ann Rheum Dis 2012;71:2006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuru T, Terao K, Murakami M. et al. Immune response to influenza vaccine and pneumococcal polysaccharide vaccine under IL-6 signal inhibition therapy with tocilizumab. Mod Rheumatol 2014;24:511–6. [DOI] [PubMed] [Google Scholar]
- 23. Ribeiro AC, Laurindo IM, Guedes LK. et al. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:476–80. [DOI] [PubMed] [Google Scholar]
- 24. Crnkic Kapetanovic M, Saxne T, Jönsson G, Truedsson L, Geborek P.. Rituximab and abatacept but not tocilizumab impair antibody response to pneumococcal conjugate vaccine in patients with rheumatoid arthritis. Arthritis Res Ther 2013;15:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alten R, Bingham CO, Cohen SB. et al. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet Disord 2016;17:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Winthrop KL, Bingham CO, Komocsar WJ. et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther 2019;21:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winthrop KL, Silverfield J, Racewicz A. et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Källmark H, Gullstrand B, Nagel J. et al. Immunogenicity of adjuvanted herpes zoster subunit vaccine in rheumatoid arthritis patients treated with Janus kinase inhibitors and controls: preliminary results. ACR Convergence 2020. Abstract number 1997. https://acrabstracts.org/abstract/immunogenicity-of-adjuvanted-herpes-zoster-subunit-vaccine-in-rheumatoid-arthritis-patients-treated-with-janus-kinase-inhibitors-and-controls-preliminary-results/ (30 March 2021, date last accessed).
- 29. Winthrop KL, Wouters AG, Choy EH. et al. The safety and immunogenicity of live zoster vaccination in patients with rheumatoid arthritis before starting tofacitinib: a randomized phase II Trial. Arthritis Rheumatol 2017;69:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arad U, Tzadok S, Amir S. et al. The cellular immune response to influenza vaccination is preserved in rheumatoid arthritis patients treated with rituximab. Vaccine 2011;29:1643–8. [DOI] [PubMed] [Google Scholar]
- 31. Van Assen S, Holvast A, Benne CA. et al. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum 2010;62:75–81. [DOI] [PubMed] [Google Scholar]
- 32. Tepasse P, Hafezi W, Lutz M. et al. Persisting SARS‐CoV‐2 viraemia after rituximab therapy: two cases with fatal outcome and a review of the literature. Br J Haematol 2020;190:185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guilpain P, Le Bihan C, Foulongne V. et al. Rituximab for granulomatosis with polyangiitis in the pandemic of COVID-19: lessons from a case with severe pneumonia. Ann Rheum Dis 2021;80:e10. [DOI] [PubMed] [Google Scholar]
- 34. Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A.. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis 2020; BMJ Publishing Group; 2020 [cited 2021 Mar 30]. Available from: https://pubmed.ncbi.nlm.nih.gov/32591357/. [DOI] [PubMed] [Google Scholar]
- 35. García-Fernández A, López-Gutiérrez F, Loarce-Martos J. et al. Cohort of rheumatic patients treated with rituximab and COVID-19: does rituximab treatment increases the severity of SARS-COV2 infection? ACR Convergence 2020. Abstract number 0641. https://acrabstracts.org/abstract/cohort-of-rheumatic-patients-treated-with-rituximab-and-covid-19-does-rituximab-treatment-increases-the-severity-of-sars-cov2-infection/ (30 March 2021, date last accessed).
- 36. Nuño L, Novella Navarro M, Bonilla G. et al. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann Rheum Dis 2020;79:1659–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Assen S, Agmon-Levin N, Elkayam O. et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2011;70:414–22. [DOI] [PubMed] [Google Scholar]
- 38. Emery P, Panayi G, Symmons D, Brown G.. Mechanisms of depressed delayed-type hypersensitivity in rheumatoid arthritis: the role of protein energy malnutrition. Ann Rheum Dis 1984;43:430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this review.


