Abstract
Existing characterizations of coronavirus disease 2019 (COVID-19) admissions have occurred primarily in urban settings. This report describes demographic and clinical characteristics of the first COVID-19 patients presenting to a 6-hospital integrated health care system in rural/suburban southcentral Pennsylvania. Medical records of adult patients admitted with COVID-19 between March and May of 2020 were retrospectively reviewed for demographics, symptomatology, imaging, and lab values. Results were largely consistent with previous studies, although gastrointestinal manifestations were more prevalent, with diarrhea reported in 25.4% of patients hospitalized due to COVID-19. Nursing home patients represented 10.1% of admissions but accounted for 35.5% of total deaths in our sample. Patients self-identifying as Hispanic were disproportionately affected. Although Hispanic ethnicity was self-reported in only 9% of the community population, Hispanic patients accounted for 34% of admissions. Our data provide a unique focused review of hospitalized COVID-19 patients in a rural/suburban setting.
Keywords: characteristics, COVID-19, demographics, rural, suburban
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease 2019 [COVID-19]) arrived with clinical cases diagnosed in southcentral Pennsylvania in March 2020. Several reports have noted the clinical characteristics and demographics of patients with COVID-19 in urban areas of the United States, Italy, and China, but, to date, little information has been published regarding COVID-19 in rural areas of the United States [1–6]. We report the demographics and clinical characteristics of adult patients hospitalized during the initial local surge from March through May 2020 in the WellSpan Health system, a regional integrated care system covering ~55% of the regional population and serving rural/suburban southcentral Pennsylvania.
METHODS
This study was determined to be exempt with waiver of consent by the WellSpan Health Institutional Review Board. The electronic health record (EHR) system was queried for adult (age ≥18 years) patients admitted to any of 6 WellSpan Health hospitals with COVID-19 detected via nasopharyngeal (NP) sample polymerase chain reaction (PCR) testing. A retrospective review was conducted for all patients hospitalized between March 17, 2020 (the date on which the system’s first COVID-positive patient was confirmed), and May 17, 2020 [7]. Decisions for hospitalization, and subsequent diagnostic testing, were made at the discretion of the physician/provider based on patient hypoxia, respiratory distress/failure, hemodynamic instability, significant comorbidities, or other individual factors.
Data abstracted included demographics, symptoms, laboratory values, chest computed tomography (CT) and radiographs, hospital treatment course, length of hospital and/or intensive care unit (ICU) stay, and discharge disposition. To account for potentially rapid disease progression and deterioration, vital signs, symptoms, and laboratory values were captured at admission or when first recorded. Likewise, only baseline chest CTs and x-rays were reviewed for the presence, type, pattern, and location of infiltrates. Treatment decision-making was per individual physician/provider discretion. To ensure internal consistency, manually abstracted data were cross-referenced with data abstracted via Premier QualityAdvisor (Premier, Inc., Charlotte, NC, USA). Descriptive statistics were reported for all variables. The van Walraven algorithm for the Elixhauser Comorbidity Index was used to summarize the probability of disease burden [8]. Lower comorbidity index scores indicate lower mortality rates, and higher scores indicate higher mortality rates.
RESULTS
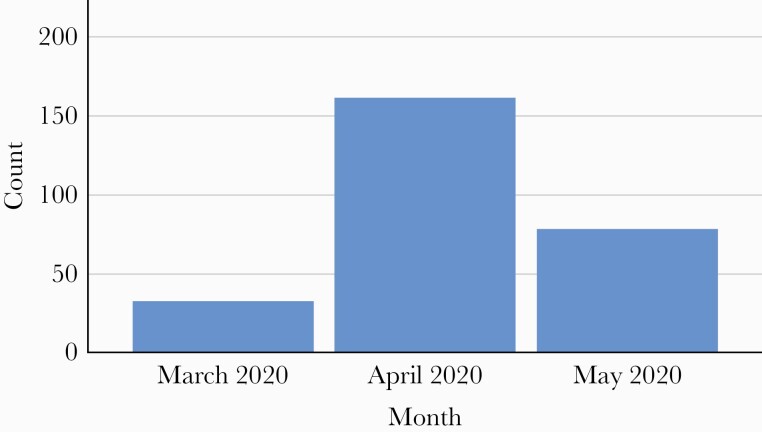
A total of 280 COVID-19 admissions consisting of 272 unique patient admissions between March and May 2020 were reviewed (Figure 1). The mean patient age (range) was 62.5 ± 17.4 (18–97) years. Female patients represented 46.5% of patients, 62.5% were White, and 34% self-reported as Hispanic. The majority, 75.4%, were admitted from home, and 54.3% had government-funded insurance (Medicare/Medicaid) (Table 1).
Figure 1.
COVID admissions by month. Abbreviation: COVID, coronavirus disease 2019.
Table 1.
Demographics and COVID-19-Related Symptoms at Patient Arrival
| Demographics | Count | % | |
|---|---|---|---|
| Gender | Female | 125 | 46.5 |
| Race | Black | 14 | 5.4 |
| White | 163 | 62.5 | |
| Ethnicity-Hispanic | 91 | 34.0 | |
| Preferred language | English | 185 | 69.0 |
| Spanish | 74 | 27.6 | |
| Insurance | Private/self-pay | 114 | 40.7 |
| Medicare/Medicaid (govt) | 152 | 54.3 | |
| Admission source | Home | 211 | 75.4 |
| Nursing home | 27 | 10.1 | |
| Hospital admitted to | Urban/suburban (GSH, YH) | 154 | 55.0 |
| Rural (CH, WH, GH, ECH) | 126 | 45.0 | |
| Symptoms | SOB dyspnea | 212 | 75.7 |
| Cough | 205 | 73.2 | |
| Fever | 199 | 71.1 | |
| Fatigue/malaise/tired | 172 | 61.4 | |
| Chills | 120 | 42.9 | |
| Muscle pain | 76 | 27.1 | |
| Diarrhea | 71 | 25.4 | |
| Headache | 45 | 16.1 | |
| New loss of taste (ageusia) or smell (anosmia) | 24 | 8.6 | |
| Sore throat | 21 | 7.5 |
Abbreviations: CH, Chambersburg Hospital; COVID-19, coronavirus disease 2019; ECH, Ephrata Community Hospital; GH, ;Gettysburg Hospital GSH, Good Samaritan Hospital; SOB, Shortness of breath; WH, Waynesboro Hospital; YH, York Hospital.
Patients presented with various symptoms: dyspnea (75.7%), cough (73.2%), fever (71.1%), fatigue (61.4%), chills (42.9%), muscle pain (27.1%), diarrhea (25.3%), headache (16.1%), loss of taste/smell (8.6%), and sore throat (7.5%) (Table 1). Of these admitted patients, 95.7% had at least 1 symptom, 87.4% had 2 symptoms, 77.6% had 3 symptoms, 62.5% had 4 symptoms, and 41.5% had 5 or more symptoms. The mean Elixhauser Comorbidity Index for patients (range) was 4.7 (–7 to 33).
On hospital admission, patients in the study also presented with a broad range of imaging findings, as detailed in Table 2. Of the 280 patients, 223 (79.6%) underwent a chest x-ray and 135 (48.2%) underwent a CT scan of the chest upon admission. Of the patients who underwent admission chest x-rays, 36.8% had bilateral infiltrates, 17% had peripheral infiltrates, and 16.1% had ground-glass infiltrates. Of the patients who underwent CT scans of the chest on admission, 65.9% had bilateral infiltrates, 49.6% had peripheral infiltrates, and 69.6% had ground-glass infiltrates [7]. Furthermore, 3 patients (1.1%) were diagnosed with new venous thromboembolic disease (1 with deep venous thrombosis and 2 with pulmonary embolism) during their hospital stay.
Table 2.
Findings and Treatment
| Findings | Count (%) | |
|---|---|---|
| Admit/initial chest x-ray completed | 223 (79.6) | |
| CXR infiltrates present? | 169 (75.8) | |
| CXR infiltrate pattern | Bilateral | 82 (36.8) |
| CXR infiltrate location | Peripheral | 38 (17.0) |
| Type | Ground glass | 36 (16.1) |
| Admin/initial CT scan completed? | 135 (48.2) | |
| CT infiltrates present? | 124 (91.9) | |
| CT infiltrate pattern | Bilateral | 89 (65.9) |
| CT infiltrate location | Peripheral | 67 (49.6) |
| Type | Ground glass | 94 (69.6) |
| Treatment | Prone ventilation | 65 (23.2) |
| Endotracheal intubation | 60 (21.4) | |
| Noninvasive low-flow ventilation | 27 (9.6) | |
| High-flow nasal cannula | 70 (25) | |
| Remdesivir | 7 (2.5) | |
| Tocilizumab (Actemra) | 18 (6.4) | |
| Hydroxy-chloroquine | 55 (19.6) | |
| Azithromycin | 85 (30.4) | |
| Convalescent plasma | 60 (21.4) | |
| ICU admission | 69 (24.6) | |
| Floor (non-ICU) | 256 (91.4) | |
| New acute kidney injury | 25 (8.9) | |
| New venous thromboembolic disease (DVT/PE) | 3 (1.1) |
Abbreviations: CT, computed tomography; CXR, Chest x-ray; DVT, deep vein thrombosis; ICU, intensive care unit; PE, pulmonary embolism.
Laboratory findings for patients on admission are summarized in Table 3. A majority of patients had white blood cell counts within normal limits upon admission (median [interquartile range {IQR}], 6.5 [4.8–8.7] K/µL). However, the median lymphocyte count (IQR) was 0.995 (0.7–1.5) K/µL, which is just below the lower limit of the reference range (1.0–4.8 K/µL), indicating a significant prevalence of lymphocytopenia. Median values for creatinine, liver function tests (aspartate aminotransferase and alanine aminotransferase), lactic acid, and B-type Natriuretic Peptide also fell within normal reference ranges, while median values (IQR) for other laboratory findings, including lactate dehydrogenase (270 [198–345] U/L), ferritin (383 [191–727] ng/mL), C-reactive protein (11.4 [5.4–24.1] mg/L), and D-dimer (0.9 [0.5–1.5] mg/dL FEU) were significantly elevated above their respective reference ranges.
Table 3.
Laboratory Findings
| Labs | Reference | Median (IQR) | % Above |
|---|---|---|---|
| WBC | 4.0–11.0 K/µL | 6.5 (4.8–8.7) | 12.2 |
| Lymphocyte | 1.0–4.8 K/µL | 1.0 (0.7–1.5) | 50.0a |
| Creatinine | 0.7–1.3 mg/dL | 0.98 (0.8–1.3) | 25.6 |
| AST | 13–39 IU/L | 30 (22–48) | 35.4 |
| ALT | 7–52 U/L | 24 (15–37) | 12.6 |
| LDH | 107–228 U/L | 270 (198–345) | 62.6 |
| Ferritin | 20–300/19–224 ng/mLb | 383 (191–727) | 71.1 |
| C-reactive protein | <10/<1.0 mg/Lb | 11.4 (5.4–24.1) | 92.4 |
| D-dimer | <0.5 mg/dL FEC | 0.9 (0.5–1.5) | 77.7 |
| BNP | <100 pg/mL | 78.5 (26.0–181.0) | 40.7 |
| Troponin | <0.03 ng/mL | 0.03 (0.02–0.03) | 33.6 |
| Lactic acid | 0.5–2.2 mmol/L | 1.3 (1.0–2.0) | 20.0 |
| Procalcitonin | <0.05 mg/mL | 0.1 (0.1–0.3) | 13.5 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, B-type Natriuretic Peptide; FEC, Fibrinogen Equivalent Unit; IQR, interquartile range; LDH, lactate dehydrogenase; WBC, white blood cell count.
aBelow reference range for this value.
bReference ranges were different due to hospital location, EPIC VS, Meditech.
Patients were managed with a variety of modalities (Table 2). Respiratory support methods included use of a high-flow nasal cannula (25% of cases), prone ventilation (23.2%), endotracheal intubation (21.4%), and noninvasive low-flow ventilation (9.6%). Pharmaceutical treatment consisted of azithromycin for 30.4% of the patients, followed in frequency of use by hydroxychloroquine (19.6%), tocilizumab (6.4%), and remdesivir (2.5%). Convalescent plasma was used in 21.4% of patients. The majority of patients were admitted to a non-ICU hospital unit (91.4%), although at some point during their stay 24.6% of the patients were admitted to the ICU (Table 2), with a mean ICU length of stay (range) of 2.7 (0–34) days. The mean total length of stay in the hospital (range) was 8.6 (1–45) days. Of the 272 patients, 31 (11.4%) patients died. Nursing home patients represented 10.1% of admissions but accounted for 35.5% of total deaths in our sample.
DISCUSSION
There have been multiple case series documenting demographics and clinical characteristics among hospitalized COVID-19 patients in urban areas in the United States and internationally, but few that examine sequentially hospitalized patients with COVID-19 in rural and suburban US populations [9, 10].
This study reviewed data trends for COVID-19 patients in an integrated health system with a clinical area spanning 3500 square miles over 5 predominantly rural counties in southcentral Pennsylvania with a total population of just over 1.3 million people. Within our system, the smaller, rural hospitals experienced the most COVID-19 admissions during the study period, compared with the larger tertiary hospital. Similar to previous studies, patients who self-identify as Hispanic and Spanish-speaking are disproportionately represented in our hospitalized population [11]. Mortality was also disproportionately higher in nursing home COVID-19 patients [9]. Clinical differences from very early reports demonstrate a higher rate of diarrhea as a presenting complaint and a higher percentage of patients with an elevated creatinine/acute kidney injury in our study cohort.
Only 9.32% of patients in our clinical service are identified as Hispanic in the EHR, yet one-third of COVID-19 hospitalizations involved Hispanic patients. Furthermore, 27.6% of hospitalized patients self-reported as primarily Spanish-speaking. Potential explanations for these observations may include clustering of the Hispanic population, multigenerational or crowded households with an inability to socially distance, limited access to preventative care, lack of transportation, inadequate access to testing, preexisting comorbidities, occupations nonconducive to telework, and timely dissemination of public health information secondary to language barriers [11–13]. However, these metrics are beyond the scope of the data collected for the purposes of this study.
Diarrhea was reported in 25.4% of our patients, which is higher than the 12% reported in prior studies [14]. Gastrointestinal manifestations could be due to a predominance of ACE2 receptors on the mucosal surfaces, which in turn facilitate viral tissue invasion [15]. It is unclear why our population had a doubling of previously reported rates of diarrhea, and this area should be considered for future study. Nearly a quarter of our patients had an elevated serum creatinine with a median value of 0.98, which may reflect a higher incidence of AKI, which was not reported in earlier studies but has been seen significantly in more recent studies [16]. One possibility for increased rate of AKI in COVID-19 patients is a higher baseline prevalence of chronic kidney disease in rural populations [17].
Nursing home residents represented 10.1% of our hospitalized COVID-19 patients, but 35.5% of the total COVID-19 deaths during the study period, which is consistent with previous studies. Chidambaram reports that in Pennsylvania 16% of COVID-19-positive cases are in long-term care facilities, but 51% of COVID-19-related deaths are from long-term care facilities [18]. A majority of hospitalized nursing home patients had advanced directives with “Do Not Resuscitate” and/or “Do Not Intubate” orders, which may have contributed to the disproportionate number of deaths among this population.
We recognize several limitations in the present study. Because our population was among the earliest hospitalized, treatment recommendations evolved considerably during and after the study period. At the time of our study, azithromycin and hydroxychloroquine were the recommended therapies. High-dose steroids were not utilized in the early phases of the pandemic, and as such were not part of the treatment course for our patient population. Likewise, remdesivir was administered to only 2.5% of our patients due to limited access and continually emerging information at the time. These circumstances may have contributed to worse outcomes in our population [10, 19]. In future studies, we plan to analyze subsequent cohorts to evaluate the effects of treatment protocols and interventions on mortality and other outcomes.
CONCLUSIONS
Rural areas of the United States have been hard hit by COVID-19, but there are not many studies looking into the clinical characteristics and demographics of this cohort of patients. Our study attempts to define the unique characteristics and presentations. There was a predominance of diarrhea as a presenting complaint in more than 25.4% of our patients, which was an outlier compared with other studies. Hispanic and Spanish-speaking members of our community were disproportionately impacted in our study. We also found that a substantial fraction of our hospitalizations and deaths came from long-term care facilities. We believe that our study will facilitate future research examining the underlying causes of adverse outcomes due to COVID-19 in rural communities.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The design of the work has been approved by the local WellSpan Health Institutional Review Board and conforms to the US Department of Health and Human Services, Office of Human Research Protections regulations regarding waiver of the informed consent process. Informed consent was not sought from patients as this study was conducted retrospectively.
References
- 1. Myers LC, Parodi SM, Escobar GJ, et al. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in Northern California. JAMA 2020; 323:2195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch JS, Narasimhan, M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVD-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lakhani HV, Pillai SS, Zehra M. Systematic review of clinical insights into novel coronavirus (COVID-19) pandemic: persisting challenges in U.S. rural population. Int J Environ Res Public Health 2020; 17:4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paul R, Arif AA, Adeyemi O, et al. Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J Rural Health 2020; 36:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, et al. ; China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennsylvania Department of Health. PA coronavirus (COVID19) update archive March 2020. Available at: https://www.health.pa.gov/topics/disease/coronavirus/Pages/March-Archive.aspx. Accessed 6 January 2021.
- 8. Thompson NR, Fan Y, Dalton JE, et al. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care 2015; 53:374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323:1612–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med 2020; 382:2012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alcendor DJ. Racial-disparities-associated COVID-19 mortality among minority populations in the U.S. J Clin Med 2020; 9:2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tirupathi R, Muradova V, Shekhar R, et al. COVID-19 disparity among racial and ethnic minorities in the US: a cross sectional analysis. Travel Med Infect Dis 2020; 38:101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gil RM, Marcelin JR, Zuniga-Blanco B, et al. COVID-19 pandemic: disparate health impact on the Hispanic/Latinx population in the United States. J Infect Dis 2020; 222:1592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta-analysis. JAMA Netw Open 2020; 3:e2011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du M, Cai G, Chen F, et al. Multiomics evaluation of gastrointestinal and other clinical characteristics of COVID-19. Gastroenterology 2020; 158:2298–301.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch JS, Ng JH, Ross DW, et al. Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States. Available at: http://www.cdc.gov/ckd.linkrel=“stylesheet” type=“text/css” href=https://cdn.ncbi.nlm.nih.gov/pubmed/cc312ade-5231-465b-9a74-aba829269133/core/no-script.css. Accessed 26 April 2020.
- 18. Chidambaram P. Kaiser Family Foundation issue brief: state reporting of cases and deaths due to COVID-19 in long-term care facilities. Available at: https://www.kff.org/medicaid/issue-brief/state-reporting-of-cases-and-deaths-due-to-covid-19-in-long-term-care-facilities/. Accessed 26 April 2020.
- 19. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]