Abstract
Objective
Patients with autoimmune diseases were advised to shield to avoid coronavirus disease 2019 (COVID-19), but information on their prognosis is lacking. We characterized 30-day outcomes and mortality after hospitalization with COVID-19 among patients with prevalent autoimmune diseases, and compared outcomes after hospital admissions among similar patients with seasonal influenza.
Methods
A multinational network cohort study was conducted using electronic health records data from Columbia University Irving Medical Center [USA, Optum (USA), Department of Veterans Affairs (USA), Information System for Research in Primary Care-Hospitalization Linked Data (Spain) and claims data from IQVIA Open Claims (USA) and Health Insurance and Review Assessment (South Korea). All patients with prevalent autoimmune diseases, diagnosed and/or hospitalized between January and June 2020 with COVID-19, and similar patients hospitalized with influenza in 2017–18 were included. Outcomes were death and complications within 30 days of hospitalization.
Results
We studied 133 589 patients diagnosed and 48 418 hospitalized with COVID-19 with prevalent autoimmune diseases. Most patients were female, aged ≥50 years with previous comorbidities. The prevalence of hypertension (45.5–93.2%), chronic kidney disease (14.0–52.7%) and heart disease (29.0–83.8%) was higher in hospitalized vs diagnosed patients with COVID-19. Compared with 70 660 hospitalized with influenza, those admitted with COVID-19 had more respiratory complications including pneumonia and acute respiratory distress syndrome, and higher 30-day mortality (2.2–4.3% vs 6.32–24.6%).
Conclusion
Compared with influenza, COVID-19 is a more severe disease, leading to more complications and higher mortality.
Keywords: COVID-19, autoimmune condition, mortality, hospitalization, open science, Observational Health Data Sciences and Informatics (OHDSI), Observational Medical Outcomes Partnership (OMOP)
Rheumatology key messages
Most patients with autoimmune diseases hospitalized for COVID-19 were women, older, and had previous comorbidities.
There is a higher prevalence of hypertension, chronic kidney disease, heart disease in patients with autoimmune diseases hospitalized with COVID-19.
Patients with autoimmune diseases, hospitalized with COVID-19, had worse outcomes and 30-day mortality compared to influenza.
Introduction
Millions of people have died from coronavirus disease 2019 (COVID-19) globally [1]. There is concern that patients with autoimmune diseases are at an increased risk of infection and complications, exacerbated by the nature of their disease and/or the use of immunosuppressive therapies [2]. In addition, systemic inflammation is present in many autoimmune diseases [3], leading to an increased risk of cardiovascular [3–5] and thromboembolic disease [6–8], which have also been recently reported to be associated with COVID-19. In patients infected with COVID-19, worse outcomes such as hospitalization, requiring intensive services and death may be associated with a pro-inflammatory cytokine storm [9–11]. Currently identified general risk factors for COVID-19 hospitalization include systemic autoimmune diseases among other comorbidities [12, 13].
As having autoimmune diseases is a recognized risk factor for COVID-19 related complications [2], public health authorities around the world have advised mitigation strategies for those at risk. In the absence of a vaccine and a scarcity of proven therapeutic options, non-pharmacological measures such as shielding, case isolation, strict hand hygiene and social distancing are key measures to protect this vulnerable group of patients [14, 15]. Thus far, characterization studies about COVID-19 infection in people with autoimmune conditions have been limited in sample size and mostly region-specific [12, 13, 16–19]. As such, COVID-19 outcomes among people with autoimmune conditions remain poorly understood.
With the ongoing threat of COVID-19, clinical understanding of the characteristics and prognosis of patients with autoimmune conditions will facilitate the management of care for this group of patients. Given the paucity of evidence, our study aimed to describe the patients’ socio-demographics, comorbidities and 30-day complications and mortality among patients with prevalent autoimmune conditions hospitalized with COVID-19 across North America, Europe and Asia. In addition, we compared their health outcomes and mortality with those seen in patients with autoimmune diseases hospitalized with seasonal influenza in the previous years.
Methods
Study design and data sources
We conducted a multinational network retrospective cohort study as part of the Characterizing Health Associated Risks, and Your Baseline Disease In Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (CHARYBDIS) protocol [20]. At time of publication, there were 18 databases contributing to CHARYBDIS. All data were standardized to the Observational Medical Outcomes Partnership (OMOP) Common Data Model [21], which allowed a federated network analysis without sharing patient-level data. In this study, we selected databases with more than 140 patients meeting our inclusion criteria to secure sufficient precision with a confidence interval width of ± 5% in the study of the prevalence of a previous condition or 30-day risk of an outcome affecting 10% of the study population.We included six data sources from three countries, namely the USA, Spain and South Korea, including hospital out- and inpatient electronic health records (EHRs) from Columbia University Irving Medical Center (CUIMC) USA, Optum (Optum EHR) (USA), Department of Veterans Affairs (VA-OMOP) (USA), primary care EHR linked to hospital admissions data from the Information System for Research in Primary Care-Hospitalization Linked Data (SIDIAP-H) (Spain) [22], and health claims from IQVIA Open Claims (USA) and Health Insurance and Review Assessment (HIRA) (South Korea) [23]. A flowchart of the databases included and excluded from those available in the network is shown in Supplementary Fig. S1, available at Rheumatology online and a detailed description of the included databases can be found in Supplementary Appendix 1, available at Rheumatology online.
Study participants and follow-up
For the COVID-19 cohort, all patients diagnosed and/or hospitalized between January and June 2020 with a clinical or laboratory-confirmed diagnosis of COVID-19 and with one or more prevalent autoimmune diseases were included. For the influenza cohort, all patients diagnosed and/or hospitalized between September 2017 and April 2018 with a clinical or laboratory-confirmed diagnosis of influenza and with one or more prevalent autoimmune diseases were included. The index date (i.e. start time of the cohort) was the date of diagnosis or of hospital admission, respectively. All participants were required to have at least 365 days of observational data prior to the index date. Prevalent autoimmune condition was defined as patients having any of the following conditions captured in the data source, any time prior to the index date: Type 1 diabetes mellitus, RA, psoriasis, PsA, multiple sclerosis, SLE, Addison’s disease, Graves’ disease, SS, Hashimoto thyroiditis, myasthenia gravis, vasculitis, pernicious anaemia, coeliac disease, scleroderma, sarcoidosis, ulcerative colitis or Crohn’s disease.
Participants were followed up for the identification of study outcomes from the index date until the earliest of death, end of the study (June 2020), 30 days after index or last date of data availability.
Baseline characteristics
Socio-demographics (age and sex) at index date were extracted, together with comorbidities and medicines used as recorded in the 365 days prior to the index date. All features recorded in the analysed databases were extracted, and are fully reported together with study outcomes (see below) in an aggregated form in an interactive web application (https://data.ohdsi.org/Covid19CharacterizationCharybdis/).
Study outcomes
For the diagnosed patients, we identified hospitalization episodes in the 30 days after the index date. For the hospitalized patients, we identified the following outcomes in the 30 days after the index date: acute myocardial infarction, cardiac arrhythmia, heart failure, stroke, venous thromboembolism, sepsis, acute respiratory distress syndrome (ARDS), pneumonia, acute kidney injury and mortality. The outcomes were defined using code sets based on Systematized Nomenclature of Medicine, Current Procedural Terminology, 4th Edition or International Classification of Diseases 9th edition (ICD-9)/10th edition (ICD-10) disease or procedure codes. Outcomes were not reported for the SIDIAP-H database as these were all hospital-based diagnoses and therefore highly incomplete in primary care EHR data. Mortality will only be reported for the following data sources which have good quality and complete data: CUIMC, HIRA, SIDIAP-H and VA-OMOP.
Data characterization and analysis
A common analytical package was developed based on the Observational Health Data Sciences and Informatics (OHDSI) Methods library (available at https://github.com/ohdsi-studies/Covid19CharacterizationCharybdis) and run locally in each database in a distributed network fashion [24, 25]. Results were extracted on 3 October 2020, and are constantly updated with new data in the web application.
We reported patient socio-demographics, comorbidities and commonly used medications in the 365 days before index date. The index date for the diagnosed cohort is the earlier of the date of clinical diagnosis or laboratory confirmed diagnosis using SARS-COV2 test; whereas the index date for the hospitalized cohort is the date of admission. We calculated the absolute standardized mean difference (ASMD) for patient characteristics between the diagnosed and hospitalized with COVID-19 cohorts. The ASMD is calculated as a difference in prevalence between the diagnosed and hospitalized groups, divided by the difference in standard deviation of the prevalence of these two groups. Guidelines indicate that ASMD >0.1 represent that the prevalence in the two groups are different from one another [26]. We calculated the proportion of hospitalization among diagnosed patients and the proportion of hospitalized patients having severe outcomes (acute myocardial infarction, cardiac arrhythmia, heart failure, stroke, venous thromboembolism, sepsis, ARDS, pneumonia, acute kidney injury and mortality) within 30 days post index date. We compared outcomes and mortality to patients with a history of autoimmune diseases hospitalized with influenza in the previous 2017–18 season. This study was descriptive in nature, and no causal inference was intended. Multivariable regression or adjustment for confounding was therefore considered beyond the purpose and scope of our study, and not included in our study protocol. All analyses were performed and visualized using R (version 4.0.2) [27].
Patient and public involvement statement
No patients or public were involved in the design, execution or dissemination of this study.
Ethics approval
All the data partners received Institutional Review Board (IRB) approval or exemption. The use of VA data was reviewed by the Department of Veterans Affairs Central IRB and was determined to meet the criteria for exemption under Exemption Category 4(3) and approved the request for Waiver of Health Insurance Portability and Accountability Act (HIPAA) Authorization. The research was approved by the Columbia University Institutional Review Board as an OHDSI network study. The IRB number for use of HIRA data was AJIB-MED-EXP-20–065. SIDIAP analysis was approved by the Clinical Research Ethics Committee of the IDIAPJGol (project code: 20/070-PCV). Other databases used (IQVIA Open Claims and Optum EHR) are commercially available, syndicated data assets that are licensed by contributing authors for observational research. These assets are de-identified commercially available data products that could be purchased and licensed by any researcher. The collection and de-identification of these data assets is a process that is commercial intellectual property and not privileged to the data licensees and the co-authors on this study. Licensees of these data have signed Data Use Agreements with the data vendors which detail the usage protocols for running retrospective research on these databases. All analyses performed in this study were in accordance with Data Use Agreement terms as specified by the data owners. As these data are deemed commercial assets, there is no IRB applicable to the usage and dissemination of these result sets or required registration of the protocol with additional ethics oversight. Compliance with Data Use Agreement terms, which stipulate how these data can be used and for what purpose, is sufficient for these commercial entities. Further inquiry related to the governance oversight of these assets can be made with the respective commercial entities: IQVIA (iqvia.com) and Optum (optum.com). At no point in the course of this study were the authors of this study exposed to identified patient-level data. All result sets represent aggregate, de-identified data that are represented at a minimum cell size of >5 to reduce potential for re-identification.
Results
We included 133 589 patients (129 221 from USA, 3553 from Spain and 815 from South Korea) with prevalent autoimmune diseases and a clinical diagnosis of COVID-19 or a positive SARS-CoV-2 test (Table 1). The claims databases (HIRA and IQVIA Open Claims) did not have information on laboratory confirmed results. The proportions of laboratory-confirmed COVID-19 cases ranged from 37.1 to 64.0% for the diagnosed cohort and 46.2 to 90.3% for the hospitalized cohort. Patients were mainly female in CUIMC (63.8%), HIRA (63.4%), IQVIA Open Claims (60.5%), Optum EHR (65.9%) and SIDIAP-H (62.0%) but were predominantly male in VA-OMOP (88.3%), as expected given the population based on military veterans. The majority of cases were aged ≥50 years. Among these patients with autoimmune diseases who developed COVID-19, the most prevalent autoimmune conditions were psoriasis (3.5–27.9%), RA (4.0–18.9%) and vasculitis (3.3–17.5%). The most prevalent comorbidities were hypertension (42.0–85.2%), heart disease (29.2–71.1%), Type 2 diabetes (21.7–63.3%) and hyperlipidaemia (22.7–59.2%). Except for HIRA, in which obesity recording rate is low, obesity was a frequently diagnosed comorbidity in all other databases (44.4–63.1%). The most frequently prescribed medications in the year prior to COVID-19 diagnosis across all databases were systemic antibiotics (48.2–84.2%), drugs used for gastro-oesophageal reflux disease (GERD) (39.1–80.6%) and NSAIDs (31.3–77.5%).
Table 1.
Baseline characteristics of study participants diagnosed with COVID-19 and had prevalent autoimmune diseases, stratified by data source
| Covariate | CUIMC (USA) (n = 1363) | HIRA (South Korea) (n = 815) | IQVIA open claims (USA) (n = 104874) | Optum EHR (USA) (n = 12897) | SIDIAP-H (Spain) (n = 3553) | VA-OMOP (USA) (n = 10087) |
|---|---|---|---|---|---|---|
| Female | 63.8 | 63.4 | 60.5 | 65.9 | 62.0 | 11.7 |
| Age group | ||||||
| ≤19 | 0.7 | 0.7 | 0.8 | 1.7 | 1.3 | 0 |
| 20–29 | 3.0 | 8.7 | 2.6 | 6.0 | 4.4 | 0.6 |
| 30–39 | 8.7 | 4.5 | 5.6 | 9.8 | 10.2 | 4.5 |
| 40–49 | 11.9 | 9.9 | 9.9 | 14.8 | 18.0 | 7.0 |
| 50–59 | 16.7 | 26.6 | 17.9 | 21.9 | 17.3 | 15.7 |
| 60–69 | 21.4 | 21.8 | 22.1 | 21.9 | 14.5 | 26.2 |
| 70–79 | 17.9 | 15.5 | 19.9 | 13.8 | 13.1 | 34.2 |
| 80–89 | 14.3 | 9.9 | 21.2 | 10.1 | 14.4 | 8.6 |
| 90–99 | 4.3 | 1.8 | 0.0 | 0.0 | 6.8 | 3.2 |
| Autoimmune disease in the year prior to index datea | ||||||
| Type 1 diabetes mellitus | 3.4 | 1.5 | 5.8 | 6.0 | 5.0 | 4.4 |
| RA | 4.0 | 18.9 | 4.8 | 8.7 | 4.1 | 4.7 |
| Psoriasis | 3.7 | 8.2 | 3.5 | 7.4 | 27.9 | 7.1 |
| PsA | 0.8 | 0.7 | 0.8 | 2.4 | 2.2 | 1.5 |
| Multiple sclerosis | 2.1 | <0.6 | 2.2 | 3.3 | 2.2 | 1.9 |
| SLE | 3.4 | 1.7 | 1.9 | 3.6 | 2.3 | 1.1 |
| Graves’ disease | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 |
| Hashimoto thyroiditis | 0.0 | 0.0 | 0.0 | 2.1 | 0.0 | 0.0 |
| Myasthenia gravis | 0.5 | <0.6 | 0.4 | 0.6 | 1.0 | 0.6 |
| Vasculitis | 4.2 | 14.4 | 4.0 | 5.7 | 17.5 | 3.3 |
| Pernicious anaemia | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 |
| Coeliac disease | 0.9 | <0.6 | 0.5 | 1.6 | 5.1 | 0.7 |
| Scleroderma | 0.6 | <0.6 | 0.2 | 0.4 | 0.8 | 0.2 |
| Sarcoidosis | 2.7 | 0.0 | 1.0 | 2.1 | 1.1 | 2.6 |
| Ulcerative colitis | 1.9 | <0.6 | 1.3 | 2.5 | 4.1 | 2.6 |
| Crohn's disease | 2.3 | <0.6 | 1.2 | 3.0 | 2.9 | 1.6 |
| Comorbidities in the year prior to index date | ||||||
| Hyperlipidaemia | 34.2 | 56.4 | 40.1 | 40.9 | 22.7 | 59.2 |
| Asthma | 27.0 | 28.0 | 23.3 | 22.7 | 8.3 | 15.1 |
| CKD | 33.0 | 14.2 | 35.6 | 27.8 | 15.8 | 38.1 |
| COPD | 18.8 | 3.4 | 24.1 | 16.7 | 27.4 | 40.2 |
| Dementia | 12.6 | 12.3 | 19.0 | 5.6 | 7.3 | 13.4 |
| Heart disease | 66.9 | 29.2 | 71.1 | 48.0 | 32.5 | 70.0 |
| HIV | 3.1 | NA | 2.1 | 0.7 | 0.4 | 2.0 |
| Hypertension | 73.5 | 45.6 | 81.7 | 60.4 | 42.0 | 85.2 |
| Cancer | 32.4 | 8.5 | 24.9 | 25.2 | 15.6 | 32.3 |
| Obesity | 59.4 | NA | 44.4 | 63.1 | 45.5 | 63.0 |
| Type 2 diabetes mellitus | 48.1 | 46.3 | 62.6 | 36.7 | 21.7 | 63.3 |
| Cerebrovascular disease | 6.7 | 7.7 | 8.4 | 4.7 | 3.3 | 6.9 |
| Chronic liver disease | 2.8 | 10.2 | 2.0 | 2.5 | 2.7 | 6.4 |
| Pregnancy | 2.7 | 2.0 | 1.1 | 2.3 | 0.9 | 0.1 |
| Venous thromboembolism | 7.4 | 3.4 | 6.1 | 4.9 | 11.5 | 6.4 |
| Drug utilization in the year prior to index date | ||||||
| Agents acting on the renin–angiotensin system | 29.3 | 29.6 | 30.6 | 31.1 | 31.6 | 47.7 |
| Antibacterials | 48.2 | 84.2 | 54.2 | 49.8 | 51.7 | 54.8 |
| Antidepressants | 23.3 | 21.2 | 23.7 | 31.4 | 30.6 | 45.8 |
| Antineoplastic and immunomodulating agents | 22.9 | 10.2 | 15.6 | 21.8 | 13.3 | 21.3 |
| Antithrombotics | 38.0 | 47.1 | 23.5 | 36.2 | 31.9 | 52.7 |
| Beta blockers | 29.2 | 17.3 | 26.8 | 28.0 | 19.2 | 44.7 |
| Calcium channel blockers | 26.7 | 25.5 | 21.4 | 19.6 | 14.5 | 33.8 |
| Corticosteroids | 36.4 | 72.3 | 38.4 | 45.0 | 39.6 | 43.9 |
| Diuretics | 29.3 | 17.5 | 26.2 | 27.1 | 29.0 | 39.3 |
| Drugs for obstructive airway diseases | 31.0 | 30.2 | 39.1 | 41.2 | 28.8 | 55.1 |
| GERD | 39.1 | 80.6 | 51.4 | 39.2 | 50.7 | 72.8 |
| PPI | 28.9 | 50.2 | 24.4 | 31.8 | 49.9 | 44.6 |
| Lipid modifying agents | 37.0 | 34.5 | 35.2 | 36.1 | 26.8 | 64.4 |
| NSAID | 31.3 | 77.5 | 51.2 | 35.4 | 36.8 | 75.5 |
| Opioids | 24.5 | 82.1 | 24.4 | 29.0 | 21.8 | 30.4 |
Figures are presented in percentages; the figures preceded with < denote less than five people in that category. Age groups are collapsed from 5-year age bands; percentages are not summed if one of the age categories had less than five people.
These are not mutually exclusive, and classification is based on recent (1 year prior) records.
CUIMC: Columbia University Irving Medical Center; HIRA: Health Insurance Review and Assessment Service; SIDIAP-H: Information System for Research in Primary Care—Hospitalization Linked Data; VA-OMOP: Department of Veterans Affairs. CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; GERD: gastro-oesophageal reflux disease; NA: not available; PPI: proton pump inhibitor.
A total of 48 418 patients (46 721 from USA, 884 from Spain and 813 from South Korea) with autoimmune diseases were hospitalized with COVID-19 (Table 2). Patients were mainly female in CUIMC (54.8%), HIRA (63.5%), IQVIA Open Claims (54.8%), Optum EHR (59.5%), about equal proportion in SIDIAP-H (49.0%), but were predominantly male in VA-OMOP (93.2%). Majority of cases were aged ≥50 years. Among these patients with autoimmune diseases who were hospitalized with COVID-19, Type 1 diabetes was the most common autoimmune condition in the US databases (4.8–7.5%) whereas RA was most prevalent in HIRA (18.9%) and psoriasis in SIDIAP-H (26.4%). The most prevalent comorbidities were hypertension (45.5–93.2%), heart disease (29.0–83.8%), Type 2 diabetes (30.8–74.3%) and hyperlipidaemia (31.9–64.5%). The most frequently prescribed medications in the year prior to hospitalization across all databases were systemic antibiotics (52.4–84.0%), drugs used for GERD (47.9–80.6%) and NSAIDs (31.4–81.5%). The list of patient characteristics is presented in Tables 1 and 2. A full list of the conditions that make up the prevalent autoimmune diseases is presented in Supplementary Tables S1 and S2, available at Rheumatology online. A complete list of patient characteristics can be found in the aforementioned interactive web application.
Table 2.
Baseline characteristics of study participants hospitalized with COVID-19 and who had prevalent autoimmune disease, stratified by data source
| Covariate | CUIMC (USA) (n = 557) | HIRA (South Korea) (n = 813) | IQVIA Open Claims (USA) (n = 39 900) | Optum EHR (USA) (n = 3112) | SIDIAP-H (Spain) (n = 884) | VA-OMOP (USA) (n = 3152) |
|---|---|---|---|---|---|---|
| Female | 54.8 | 63.5 | 54.8 | 59.5 | 49.0 | 6.8 |
| Age group | ||||||
| ≤19 | <3.6 | 0.7 | 0.8 | 0.8 | <0.6 | 0.2 |
| 20–29 | 2.0 | 8.8 | 1.2 | 3.0 | 0.6 | 0.2 |
| 30–39 | 2.9 | 4.5 | 2.9 | 6.9 | 2.5 | 1.4 |
| 40–49 | 5.2 | 9.9 | 6.0 | 10.5 | 8.0 | 3.3 |
| 50–59 | 10.7 | 26.7 | 14.4 | 18.1 | 14.3 | 10.9 |
| 60–69 | 20.5 | 21.9 | 23.8 | 25.0 | 17.7 | 25.3 |
| 70–79 | 23.4 | 15.2 | 25.5 | 19.3 | 25.3 | 39.3 |
| 80–89 | 23.9 | 9.9 | 25.4 | 16.2 | 24.4 | 13.4 |
| 90–99 | 8.5 | 1.8 | 0.0 | 0.0 | 6.7 | 6.0 |
| Autoimmune disease in the year prior to index datea | ||||||
| Type 1 diabetes mellitus | 4.8 | 1.5 | 7.5 | 7.5 | 4.4 | 5.3 |
| RA | 4.8 | 18.9 | 4.9 | 8.8 | 5.4 | 4.0 |
| Psoriasis | 1.4 | 8.2 | 2.7 | 5.4 | 26.4 | 4.4 |
| PsA | 0.0 | 0.7 | 0.6 | 1.7 | 2.5 | 0.9 |
| Multiple sclerosis | 1.1 | <0.6 | 2.1 | 3.7 | 2.1 | 1.6 |
| SLE | 3.2 | 1.7 | 1.9 | 4.3 | 2.6 | 0.9 |
| Hashimoto thyroiditis | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 |
| Myasthenia gravis | <0.9 | <0.6 | 0.5 | 0.8 | 1.5 | 0.7 |
| Vasculitis | 3.4 | 14.4 | 4.4 | 7.7 | 20.8 | 4.4 |
| Pernicious anaemia | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 |
| Coeliac disease | <0.9 | <0.6 | 0.3 | 0.9 | 1.2 | 0.4 |
| Scleroderma | <0.9 | <0.6 | 0.2 | 0.4 | 0.9 | <0.2 |
| Sarcoidosis | 3.4 | 0.0 | 1.2 | 1.9 | 1.2 | 2.1 |
| Ulcerative colitis | <0.9 | <0.6 | 1.3 | 2.2 | 2.8 | 1.6 |
| Crohn's disease | 1.1 | <0.6 | 1.0 | 2.4 | 2.4 | 1.2 |
| Comorbidities in the year prior to index date | ||||||
| Hyperlipidaemia | 45.8 | 56.5 | 44.9 | 49.2 | 31.9 | 64.5 |
| Asthma | 29.3 | 28.0 | 22.1 | 20.4 | 7.8 | 12.5 |
| CKD | 50.4 | 14.0 | 49.6 | 42.0 | 25.8 | 52.7 |
| COPD | 28.7 | 3.4 | 30.8 | 26.3 | 40.7 | 51.9 |
| Dementia | 23.2 | 12.2 | 21.5 | 8.9 | 6.8 | 23.1 |
| Heart disease | 83.8 | 29.0 | 81.7 | 62.7 | 47.7 | 81.9 |
| HIV | 2.7 | NA | 2.4 | 1.1 | 0.7 | 2.2 |
| Hypertension | 91.4 | 45.5 | 91.6 | 75.7 | 58.9 | 93.2 |
| Cancer | 38.4 | 8.5 | 29.0 | 29.4 | 22.6 | 37.9 |
| Obesity | 67.7 | NA | 48.0 | 67.0 | 57.5 | 64.0 |
| Type 2 diabetes mellitus | 70.0 | 46.1 | 74.1 | 50.6 | 30.8 | 74.3 |
| Cerebrovascular disease | 10.8 | 7.6 | 11.2 | 6.8 | 4.1 | 9.5 |
| Chronic liver disease | 4.1 | 10.2 | 2.9 | 3.4 | 3.3 | 9.7 |
| Pregnancy | 2.0 | 2.0 | 0.7 | 2.7 | 0.6 | NA |
| Venous thromboembolism | 11.3 | 3.4 | 8.7 | 8.8 | 14.4 | 9.3 |
| Drug utilization in the year prior to index date | ||||||
| Agents acting on the renin–angiotensin system | 37.0 | 29.4 | 36.7 | 38.3 | 43.9 | 51.8 |
| Antibacterials | 52.4 | 84.0 | 55.0 | 57.6 | 59.4 | 64.4 |
| Antidepressants | 23.7 | 21.2 | 25.0 | 33.6 | 31.3 | 47.0 |
| Antineoplastic and immunomodulating agents | 23.0 | 10.0 | 15.4 | 23.3 | 18.3 | 19.3 |
| Antithrombotics | 55.3 | 46.9 | 32.8 | 55.5 | 45.5 | 69.3 |
| Beta blockers | 41.8 | 17.1 | 35.1 | 40.3 | 26.1 | 54.9 |
| Calcium channel blockers | 37.0 | 25.3 | 28.1 | 29.4 | 21.2 | 41.4 |
| Corticosteroids | 37.7 | 72.3 | 39.5 | 48.5 | 48.6 | 46.2 |
| Diuretics | 41.8 | 17.3 | 33.4 | 38.9 | 41.4 | 47.9 |
| Drugs for obstructive airway diseases | 35.7 | 30.1 | 39.8 | 46.9 | 31.9 | 58.1 |
| GERD | 47.9 | 80.6 | 54.1 | 49.3 | 61.2 | 77.8 |
| PPI | 36.6 | 50.3 | 28.0 | 40.5 | 60.3 | 48.4 |
| Lipid modifying agents | 49.6 | 34.6 | 42.5 | 47.4 | 39.5 | 71.4 |
| NSAID | 33.0 | 77.6 | 51.9 | 35.6 | 31.4 | 81.5 |
| Opioids | 30.7 | 82.0 | 28.0 | 41.4 | 28.2 | 37.7 |
Figures are presented in percentages; the figures preceded with < denote less than five people in that category. Age groups are collapsed from 5-year age bands; percentages are not summed if one of the age categories had less than five people.
These are not mutually exclusive, and classification is based on recent (1 year prior) records.
CUIMC: Columbia University Irving Medical Center; HIRA: Health Insurance Review and Assessment Service; SIDIAP-H: Information System for Research in Primary Care—Hospitalization Linked Data; VA-OMOP: Department of Veterans Affairs. CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; GERD: gastro-oesophageal reflux disease; NA: not available; PPI: proton pump inhibitor.
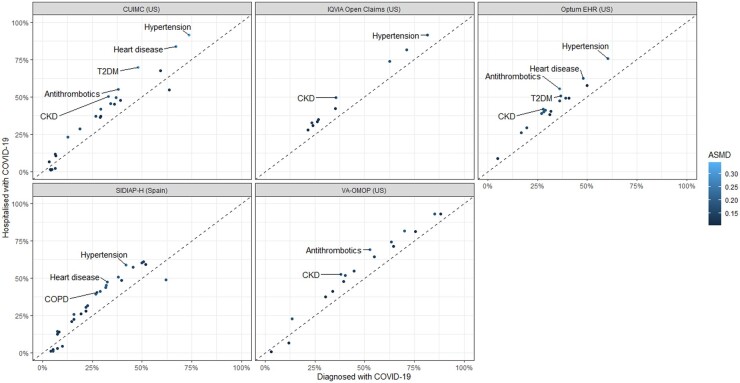
In patients with prevalent autoimmune diseases hospitalised with COVID-19, the prevalence of hypertension (ASMD = 0.18–0.34), chronic kidney disease (ASMD = 0.17–0.25), heart disease (ASMD = 0.18–0.28), Type 2 diabetes (ASMD = 0.15–0.32), chronic obstructive pulmonary disease (COPD) (ASMD = 0.11–0.20) and use of antithrombotics (ASMD = 0.15–0.28) were higher as compared with the larger group of such patients diagnosed with COVID-19 (Fig. 1).
Fig. 1.
Prevalence of patient characteristics in the patients with prevalent autoimmune diseases who were diagnosed with COVID-19 compared with those hospitalized with COVID-19
This scatterplot includes patient characteristics with absolute standardized mean difference (ASMD) ≥0.1. The patient characteristics with ASMD >0.2 are labelled in the scatterplot. HIRA was not included in the scatterplot because of the significant overlap between diagnosed (n = 815) and hospitalized (n = 813) patients. CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; T2DM: Type 2 diabetes mellitus
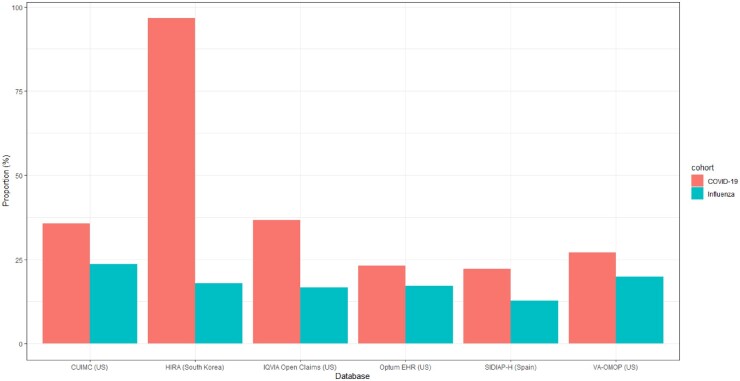
We included 395 784 patients with prevalent autoimmune diseases (392 797 from USA, 2419 from Spain and 568 from South Korea) diagnosed with influenza to compare the proportion of hospitalization episodes. The proportion of hospitalization episodes was higher in the cohort diagnosed with COVID-19 as compared with influenza [35.7% vs 23.6% (CUIMC), 96.6% vs 18.0% (HIRA), 36.7% vs 16.6% (IQVIA Open Claims), 23.1% vs 17.2% (Optum EHR), 22.2% vs 12.8% (SIDIAP-H), 27.1% vs 19.9% (VA-OMOP)] (Fig. 2).
Fig. 2.
Hospitalization in patients with prevalent autoimmune diseases in the 30-day period following a diagnosis of COVID-19 vs influenza
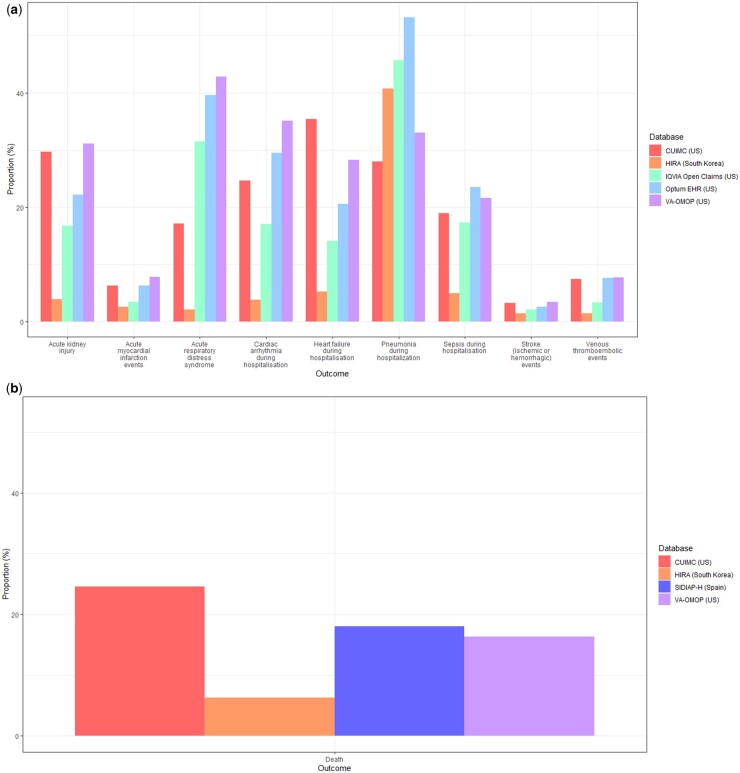
At 30 days post hospitalization, the most frequent severe outcomes were related to the respiratory system, such as ARDS (2.1–42.8%), and pneumonia (12.6–53.2%) (Fig. 3a). Acute kidney injury was the second most common complication, occurring in 9.9–31.1% of patients in US databases, and in 2.8% in HIRA. Cardiac complications were also frequent, including arrhythmia in 3.8–35.1% of patients, heart failure (3.9–24.5%) and acute myocardial infarction (2.4–6.3%). Sepsis occurred during hospitalization in 4.7–23.5% of patients. Ischaemic or haemorrhagic stroke was recorded in 1.4–3.4% of patients, whereas venous thromboembolic events (VTEs) were recorded in 1.4–7.7% of patients across the databases. Mortality as a proportion of those hospitalized was generally higher in the USA and Spain (16.3–24.6%) vs South Korea (6.3%) (Fig. 3b).
Fig. 3.
(a) Severe outcomes in 30 days post hospital admission with COVID-19 in patients with prevalent autoimmune diseases, stratified by database
Hospitalization outcomes data was not available in SIDIAP-H. (b) Mortality in 30 days post hospital admission with COVID-19 in patients with prevalent autoimmune diseases, stratified by database.
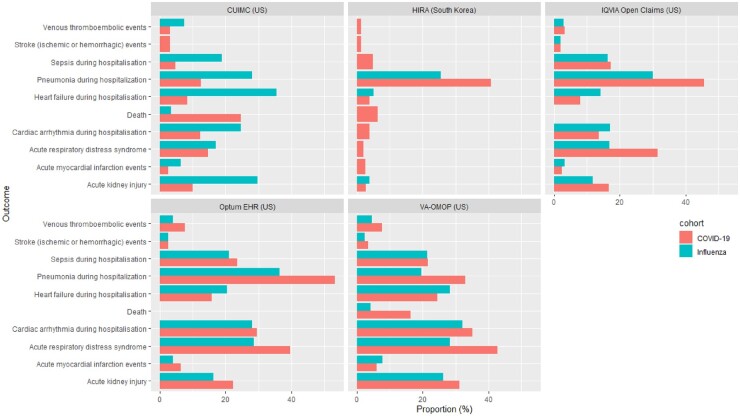
Compared with 70 660 hospitalized individuals (70 184 from USA, 323 from Spain and 153 from South Korea) with influenza in previous years, patients hospitalized with COVID-19 were more likely to have higher respiratory complications such as ARDS (14.7–42.8% vs 16.9–28.7%) and pneumonia (12.6–53.2% vs 19.5–36.3%), and had a higher mortality (6.3–24.6% vs 2.2–4.4%) (Fig. 4) (Supplementary Table S3, available at Rheumatology online).
Fig. 4.
Comparison of outcomes in patients with prevalent autoimmune conditions hospitalized with COVID-19 vs influenza
Outcomes were omitted from the graph if there were less than five people experiencing the event or the data was unavailable in the respective databases. CPRD: Clinical Practice Research Datalink; CUIMC: Columbia University Irving Medical Center; DA Germany: IQVIA disease analyser Germany; DCMC: Daegu Catholic University Medical Center; HIRA: Health Insurance Review and Assessment Service; LPD France: IQVIA Longitudinal Patient Data France; NFHCRD: Nanfang Hospital COVID-19 Research Database; IPCI: Integrated Primary Care Information; SIDIAP: Information System for Research in Primary Care; SIDIAP-H: SIDIAP- Hospitalisation Linked Data; TRDW: Tufts Research Data Warehouse, VA-OMOP: Department of Veterans Affairs.
Discussion
This study represents the hitherto first use of routinely collected health data across the USA, Spain and South Korea to characterize hospitalized COVID-19 patients with prevalent autoimmune diseases. To our knowledge, this is the largest multinational observational study to characterize a cohort of patients with prevalent autoimmune diseases diagnosed/hospitalized with COVID-19 and detail their post-hospitalization outcomes, during the first 6 months of the pandemic. We found that diagnosed autoimmune patients were predominantly female, aged >50 years and had pre-existing comorbidities. Hospitalized autoimmune patients had similar characteristics to those diagnosed but were older and had a higher proportion of pre-existing comorbidities. As compared with patients hospitalized with influenza, more patients infected with COVID-19 died within 30 days of hospitalization. COVID-19 patients also experienced respiratory and cardiac complications during hospitalization.
The patients in our study were predominantly female, except for the VA-OMOP database, of which the majority were male military veterans. This was consistent with the proportion of females across studies of COVID-19 in patients with autoimmune conditions in Spain (59%) [12] and in COVID-19 patients in the Global Rheumatology Alliance (GRA) physician-reported registry (67%) [19]. This is likely due to females having a higher prevalence of most autoimmune diseases, but contrasts with reports of overall COVID-19 patients who were otherwise majority male [16, 28]. A recent meta-analysis has also shown that males had higher in-hospital mortality [29]. The hospitalized patients in our study were mostly aged 65 years and above, with South Korea having more patients in the age group of 50–64 years old. Advanced age has been reported as a poor prognostic factor for COVID-19 [15, 29]. The sociodemographic profile of the patients in VA-OMOP being mostly male and older could be associated with the higher frequency of severe outcomes in that data source. The most prevalent comorbidities in our study were hypertension, heart disease and Type 2 diabetes. This was similarly observed in the GRA registry [19]. These comorbidities were also associated with disease severity and mortality in a meta-analysis involving 12 149 general COVID-19 patients from 15 countries [29].
Our study described post-hospitalization complications in COVID-19 patients with prevalent autoimmune diseases. The most frequent severe outcomes in our study were ARDS, pneumonia and cardiac injury. In the aforementioned meta-analysis [29], the most frequently reported complications associated with COVID-19 were pneumonia, respiratory failure, acute cardiac injury and ARDS; which corroborates our findings regarding the frequency of outcomes. Cardiac injury was also independently associated with in-hospital mortality in a study conducted in Wuhan [30]. The researchers hypothesized that cardiac injury may be precipitated by acute inflammatory response as a result of COVID-infection superimposed on pre-existing cardiovascular disease. In comparison with patients hospitalized with influenza, COVID-19 patients generally had higher proportion of severe outcomes, especially respiratory complications such as ARDS and pneumonia. This phenomenon was also observed in a study conducted in a large tertiary care hospital in the USA, where patients hospitalized with COVID-19 required more mechanical ventilation and had higher mortality than patients with influenza, despite presenting with less pre-existing conditions [31]. Our study showed that up to 8% of hospitalized patients with COVID-19 and prevalent autoimmune diseases suffered VTE and the incidence of VTE is higher in hospitalized COVID-19 patients vs influenza patients in most of the databases. There are extensive evidence demonstrating increased risk of thromboembolism among patients with autoimmune disease, where the mechanism is hypothesized to be a relationship between inflammation and the coagulation pathway [6–8]. The underlying mechanism associated between COVID-19 and intravascular thrombosis has not been fully elucidated. Suggested explanations include the activation of the thrombo-inflammation pathway, endothelial injury and microangiopathy [32]. As COVID-19 and autoimmune disease has been associated with higher risk of VTE, it is plausible that the confluence of both these conditions may heighten this risk. Using a multicentre EHR network, D’Silva et al. [33] found that patients with systemic autoimmune rheumatic diseases diagnosed with COVID-19 had higher risks of VTE as compared with matched patients, and this increased risk of VTE was not mediated by comorbidities.
There was a high prevalence of comorbidities among the patients in our study. According to a meta-analysis [34], the presence of comorbidities such as hypertension, diabetes and obesity among patients with autoimmune diseases was associated with higher rates of hospitalization, ventilation and death due to COVID-19. In a large study using primary care records in England, patients with autoimmune disease had an increased risk of COVID-19 related death after adjustment for various comorbidities such as hypertension, diabetes, cancer, heart disease and respiratory disease [35]. In other patient populations, such as those with obesity, patients with COVID-19 had higher mortality and requirement of intensive services as compared with similar patients with seasonal influenza, despite presenting with fewer comorbidities [36]. In pregnant women, there was a higher frequency of caesarean section and preterm deliveries, as well as poorer outcomes (pneumonia, ARDS, sepsis, acute kidney injury and cardiovascular and thromboembolic events) in those diagnosed with COVID-19 in comparison with seasonal influenza [37]. Like the other databases, CUIMC showed higher mortality in hospitalized patients with COVID-19 than those with influenza, but it showed lower complications in patients hospitalized with COVID-19 than influenza. A possible explanation is that patients hospitalized with influenza had higher incidence of co-morbidities like COPD and Type 2 diabetes, which was also found in a previous study [38], or that data were not well captured during the height of the pandemic.
Study limitations
Although our study found a greater proportion of hospitalization with COVID-19 as compared with influenza, the hospitalization rate may not directly reflect the severity of prognosis in COVID-19. As a novel coronavirus, the higher hospitalization rate could also be contributed by quarantine measures in the hospital after diagnosis or monitoring of patients receiving investigative treatments repurposed for COVID-19. Hence, we have further provided more information regarding severe outcomes within 30 days of hospitalization. COVID-19 cases may be poorly recognized due to shortages in testing capabilities, but this is to some extent mitigated in our study by also including hospitalized patients with a clinical COVID-19 diagnosis. However, even untested hospitalized patients could have been missed if hospitals were understaffed and clinicians did not have time to input proper codes. A known limitation of using routinely collected data is that medical conditions may be misclassified due to erroneous entries or underestimated as they were defined based on the presence of diagnostic or procedural codes, with the absence of records indicative of absence of disease. In particular for healthcare data in the USA, the capturing of codes is largely incentivized by reimbursement from insurance companies. This factor could permit miscoding of Type 2 diabetes as Type 1 and could have enriched the autoimmune disease cohort with Type 2 diabetes patients who might not have autoimmune disease. With the use of claims databases, there may be a discrepancy between the diagnosis recorded and the actual health condition of a patient. However, this is mitigated in more severe conditions and inpatient settings [23]. In the initial stage of the pandemic, the lack of clinical guidance combined with the lack of access to widespread testing means that only more severe patients were seen in healthcare settings. The capture of mortality data is subject to differences by database. For example, data on inpatient deaths are recorded in a hospital EHR but deaths after discharge from hospital will not be captured in such a data source. For data sources linked to primary care, outpatient death events are typically imported into a given database from a national or local death register. It is likely that mortality rates were underestimated in our study. Nevertheless, the consistency of our findings across different healthcare settings in different countries lends credence to our results.
Conclusions
Patients with autoimmune diseases had high rates of respiratory complications and 30-day mortality following a hospitalization with COVID-19. Compared with influenza, COVID-19 is a more severe disease, leading to more complications and higher mortality. Future studies should investigate predictors of poor outcomes in COVID-19 patients with autoimmune diseases.
Supplementary Material
Acknowledgements
A.O., F.N., G.H., K.N., M.S., K.K., D.P.A., P.B.R. and T.D.S. designed the study. K.K., C.R., A.S., S.D., K.L., T.D.S., C.B., J.P. executed the study package on local data and contributed results. E.H.T., S.F.B. analysed the data. E.H.T., A.O., F.N. and D.P.A. interpreted the results. E.H.T. and D.P.A. wrote the original draft of the manuscript. All authors reviewed and edited the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. D.P.A. is the guarantor. The corresponding author has the right to grant on behalf of all authors and does grant on behalf of all authors, a worldwide licence to the Publishers and its licensees in perpetuity, in all forms, formats and media (whether known now or created in the future), to (i) publish, reproduce, distribute, display and store the contribution, (ii) translate the contribution into other languages, create adaptations, reprints, include within collections and create summaries, extracts and/or, abstracts of the contribution, (iii) create any other derivative work(s) based on the contribution, (iv) to exploit all subsidiary rights in the contribution, (v) the inclusion of electronic links from the contribution to third party material where-ever it may be located and (vi) licence any third party to do any or all of the above.
Funding: S.D. reports grants from Anolinx, LLC, grants from Astellas Pharma, Inc, grants from AstraZeneca Pharmaceuticals LP, grants from Boehringer Ingelheim International GmbH, grants from Celgene Corporation, grants from Eli Lilly and Company, grants from Genentech Inc., grants from Genomic Health, Inc., grants from Gilead Sciences Inc., grants from GlaxoSmithKline PLC, grants from Innocrin Pharmaceuticals Inc., grants from Janssen Pharmaceuticals, Inc., grants from Kantar Health, grants from Myriad Genetic Laboratories, Inc., grants from Novartis International AG, grants from Parexel International Corporation through the University of Utah or Western Institute for Veteran Research outside the submitted work; M.E.M. reports funding from VA HSR&D, National Heart, Lung, and Blood Institute, (NHLBI) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDLE), National Institutes of Health (NIH) for grant funding; G.H. reports grants from US National Library of Medicine, during the conduct of the study; grants from Janssen Research, outside the submitted work; K.N. reports grants from NIH, during the conduct of the study; J.L. reports grants from Medical Research Council, grants from Vs Arthritis, outside the submitted work; A.G. is a full-time employee at Regeneron Pharmaceuticals and reports personal fees from Regeneron Pharmaceuticals, outside the submitted work. This work was not conducted at Regeneron Pharmaceuticals; J.J. reports grants from National Health and Medical Research Council, outside the submitted work; V.S. reports grants from National Science Foundation, grants from State of Arizona; Arizona Board of Regents, grants from Agency for Healthcare Research and Quality, grants from National Institutes of Health, outside the submitted work; D.V. reports personal fees from Bayer, during the conduct of the study and outside the submitted work; F.N. reports holding some AstraZeneca shares, outside the submitted work; D.R.M. is supported by a Wellcome Trust Clinical Research Development Fellowship (Grant 214588/Z/18/Z) and reports grants from Chief Scientist Office (CSO), grants from Health Data Research UK (HDR-UK), grants from National Institute of Health Research (NIHR), and Tenovus outside the submitted work; J.P. reports grants from National Library of Medicine, during the conduct of the study; M.S. reports grants from US National Institutes of Health, grants from Department of Veterans Affairs, during the conduct of the study; grants from IQVIA, personal fees from Janssen Research and Development, grants from US Food and Drug Administration, personal fees from Private Health Management, outside the submitted work; D.P.A. reports grants and other from AMGEN; grants, non-financial support and other from UCB Biopharma; grants from Les Laboratoires Servier, outside the submitted work and Janssen, on behalf of Innovative Medicines Initiative (IMI)-funded European Health Data & Evidence Network (EHDEN) and European Medical Information Framework (EMIF) consortiums, and Synapse Management Partners have supported training programs organized by D.P.A.’s department and open for external participants. No other relationships or activities that could appear to have influenced the submitted work. The EHDEN has received funding from the IMI 2 Joint Undertaking (JU) under grant agreement No. 806968. The JU receives support from the European Union’s Horizon 2020 research and innovation programme and European Federation of Pharmaceutical Industries and Associations (EFPIA). The University of Oxford received a grant related to this work from the Bill & Melinda Gates Foundation (Investment ID INV-016201). This research received partial support from the NIHR Oxford Biomedical Research Centre (BRC), US National Institutes of Health, US Department of Veterans Affairs, Janssen Research & Development and IQVIA. This work was also supported by the Bio Industrial Strategic Technology Development Program (20001234) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0992), and the Health Department from the Generalitat de Catalunya with a grant for research projects on SARS-CoV-2 and COVID-19 disease organized by the Direcció General de Recerca i Innovació en Salut. D.P.A. is funded through a NIHR Senior Research Fellowship (Grant number SRF-2018–11-ST2-004). A.P.U. is supported by MRC-DTP [MR/K501256/1, MR/N013468/1] and Fundación Alfonso Martín Escudero (FAME). GH is supported by the US National Library of Medicine (LM006910). The funders did not have any role in the study design, collection, analysis, and interpretation of data, writing of the report, and the decision to submit the article for publication. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Clinician Scientist Award programme, NIHR, Department of Veterans Affairs or the United States Government, NHS or the Department of Health, England. The authors have full access to the results generated and have the ability to work directly with data partners to investigate any discrepancies. Due to privacy laws, data are not moved outside of a site but the use of a common analytics framework and common data model enable the rapid sharing of results.
Disclosure statement: All authors have completed the International Committee of Medical Journal Editors (ICMJE) uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: A.S., C.B., P.R. are employees and shareholders at Janssen Research & Development, a Johnson and Johnson family of companies; C.B. reports personal fees from Janssen R&D, outside the submitted work; C.R. and K.K. report being employees of IQVIA Inc. Lead authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Data availability statement
Analyses were performed locally in compliance with all applicable data privacy laws. Although the underlying data is not readily available to be shared, authors contributing to this paper have direct access to the data sources used in this study. All results (aggregate statistics, not presented at a patient-level with redactions for minimum cell count) are available for public inquiry. These results are inclusive of site-identifiers by contributing data sources to enable interrogation of each contributing site. All analytic code and result sets are made available at: https://github.com/ohdsi-studies/Covid19CharacterizationCharybdis.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University, updated 16 March 2021. https://coronavirus.jhu.edu/map.html (16 March 2021, date last accessed).
- 2. Kastritis E, Kitas GD, Vassilopoulos D. et al. Systemic autoimmune diseases, anti-rheumatic therapies, COVID-19 infection risk and patient outcomes. Rheumatol Int 2020;40:1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furman D, Campisi J, Verdin E. et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hollan I, Meroni PL, Ahearn JM. et al. Cardiovascular disease in autoimmune rheumatic diseases. Autoimmun Rev 2013;12:1004–15. [DOI] [PubMed] [Google Scholar]
- 5. Sitia S, Atzeni F, Sarzi-Puttini P. et al. Cardiovascular involvement in systemic autoimmune diseases. Autoimmun Rev 2009;8:281–6. [DOI] [PubMed] [Google Scholar]
- 6. Yusuf HR, Hooper WC, Beckman MG. et al. Risk of venous thromboembolism among hospitalizations of adults with selected autoimmune diseases. J Thromb Thrombolysis 2014;38:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ.. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zöller B, Li X, Sundquist J, Sundquist K.. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis 2012;2:171–83. [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Wu D, Guo W. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu C, Chen X, Cai Y. et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Int Med 2020;180:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freites Nuñez DD, Leon L, Mucientes A. et al. Risk factors for hospital admissions related to COVID-19 in patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis 2020;79:1393–9. [DOI] [PubMed] [Google Scholar]
- 13. Haberman R, Axelrad J, Chen A. et al. Covid-19 in immune-mediated inflammatory diseases - case series from New York. N Engl J Med 2020;383:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nicola M, O'Neill N, Sohrabi C. et al. Evidence based management guideline for the COVID-19 pandemic - Review article. Int J Surg 2020;77:206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tam L-S, Tanaka Y, Handa R. et al. Care for patients with rheumatic diseases during COVID-19 pandemic: a position statement from APLAR. Int J Rheum Dis 2020;23:717–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y, Chen Z, Wang Y. et al. Clinical characteristics of 17 patients with COVID-19 and systemic autoimmune diseases: a retrospective study. Ann Rheum Dis 2020;79:1163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mathian A, Mahevas M, Rohmer J. et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis 2020;79:837–9. [DOI] [PubMed] [Google Scholar]
- 18. Monti S, Balduzzi S, Delvino P. et al. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis 2020;79:667–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gianfrancesco M, Hyrich KL, Al-Adely S. et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 2020;79:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anthony S, Kristin K. Martijn Schuemie, jdposada. ohdsi-studies/Covid19CharacterizationCharybdis: Charybdis v1.1.1 - Publication Package (Version v1.1.1): Zenodo; 2020. updated 2020, September 16. 10.5281/zenodo.4033034 (16 September 2020, date last accessed).
- 21. Voss EA, Makadia R, Matcho A. et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc 2015;22:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García-Gil Mdel M, Hermosilla E, Prieto-Alhambra D. et al. Construction and validation of a scoring system for the selection of high-quality data in a Spanish population primary care database (SIDIAP). Inform Prim Care 2011;19:135–45. [DOI] [PubMed] [Google Scholar]
- 23. Kim J-A, Yoon S, Kim L-Y, Kim D-S.. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of hira data. J Korean Med Sci 2017;32:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hripcsak G, Duke JD, Shah NH. et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015;216:574–8. [PMC free article] [PubMed] [Google Scholar]
- 25. Hripcsak G, Ryan PB, Duke JD. et al. Characterizing treatment pathways at scale using the OHDSI network. Proc Natl Acad Sci U S A 2016;113:7329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat - Simul Comput 2009;38:1228–34. [Google Scholar]
- 27.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria; 2013.
- 28. Argenziano MG, Bruce SL, Slater CL. et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020;369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jutzeler CR, Bourguignon L, Weis CV. et al. Comorbidities, clinical signs and symptoms, laboratory findings, imaging features, treatment strategies, and outcomes in adult and pediatric patients with COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis 2020;37:101825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi S, Qin M, Shen B. et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donnino MW, Moskowitz A, Thompson GS, Heydrick SJ, Pawar RD, Berg KM. et al. Comparison between influenza and COVID-19 at a Tertiary Care Center. medRxiv. 2020:2020.08.19.20163857. [DOI] [PMC free article] [PubMed]
- 32. Marietta M, Coluccio V, Luppi M.. COVID-19, coagulopathy and venous thromboembolism: more questions than answers. Int Emerg Med 2020;15:1375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. D'Silva KM, Jorge A, Cohen A. et al. COVID-19 Outcomes in Patients with Systemic Autoimmune Rheumatic Diseases (SARDs) compared to the general population: a US Multi-Center Comparative Cohort Study. Arthritis Rheumatol 2020; doi: 10.1002/art.41619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Akiyama S, Hamdeh S, Micic D, Sakuraba A.. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis 2021;80:384–91. [DOI] [PubMed] [Google Scholar]
- 35. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Recalde M, Roel E, Pistillo A, Sena AG, Prats-Uribe A, Ahmed WU-R. et al. Characteristics and outcomes of 627 044 COVID-19 patients with and without obesity in the United States, Spain, and the United Kingdom. medRxiv. 2020:2020.09.02.20185173. [DOI] [PMC free article] [PubMed]
- 37. Lai LYH, Golozar A, Sena A, Margulis AV, Haro N, Casajust P. et al. “Clinical characteristics, symptoms, management and health outcomes in 8,598 pregnant women diagnosed with COVID-19 compared to 27,510 with seasonal influenza in France, Spain and the US: a network cohort analysis”. medRxiv. 2020:2020.10.13.20211821.
- 38. Burn E, You SC, Sena AG. et al. Deep phenotyping of 34,128 adult patients hospitalised with COVID-19 in an international network study. Nat Commun 2020;11:5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Analyses were performed locally in compliance with all applicable data privacy laws. Although the underlying data is not readily available to be shared, authors contributing to this paper have direct access to the data sources used in this study. All results (aggregate statistics, not presented at a patient-level with redactions for minimum cell count) are available for public inquiry. These results are inclusive of site-identifiers by contributing data sources to enable interrogation of each contributing site. All analytic code and result sets are made available at: https://github.com/ohdsi-studies/Covid19CharacterizationCharybdis.