While the multisystem impact of coronavirus disease 2019 (COVID-19) has been well established [1], electrolyte disorders associated with the disease have only recently been described [2]. Dysnatremias are common in hospitalized patients, and are independent risk factors for mortality, admission to medical intensive care units and prolonged length of hospital stay [3, 4]. Hyponatremia has been reported in the setting of COVID-19 [5, 6], and early reports showed that serum sodium levels varied with disease severity [7, 8]. A subsequent New York study found that hyponatremia in COVID-19 is an independent predictor of in-hospital mortality, and is associated with increased risk of mechanical ventilation and encephalopathy [9]. Data on hypernatremia associated with COVID-19 are limited to case series [10].
In this study of almost 10 000 patients across 13 hospitals in a New York health system, we aimed to (i) describe the prevalence of hyponatremia and hypernatremia and (ii) investigate the association of admission sodium levels with in-hospital death and length of stay. All adult (aged ≥18 years) patients who were hospitalized for COVID-19 from 1 March 2020 to 27 April 2020, and had at least one serum sodium measurement were eligible. Patients with end-stage kidney disease were excluded. For the primary exposure, we used the admission serum sodium and corrected for hyperglycemia using both the Katz and Hillier formulas.
Normonatremia was defined as serum sodium of 136–144 mEq/L and dysnatremias were defined as follows [11]:
moderate/severe hypernatremia (serum sodium ≥150 mEq/L);
mild hypernatremia (serum sodium 145–149 mEq/L);
moderate/severe hyponatremia (serum sodium <130 mEq/L); and
mild hyponatremia (serum sodium 130–135 mEq/L).
We used univariable and multivariable logistic regression to investigate the impact of dysnatremias on in-hospital death (primary outcome) and length of stay (secondary outcome). All analyses were performed using R version 3.6.3. The Northwell Health Institutional Review Board approved the study. The full methods are described in the Supplementary Material.
Among 9946 patients included in the study (Supplementary data, Figure S1), 4808 (48.3%) had normonatremia, 3532 (35.5%) had mild hyponatremia, 904 (9.1%) had moderate/severe hyponatremia, 319 (3.2%) had mild hypernatremia and 383 (3.8%) had moderate/severe hypernatremia (Supplementary data, Figure S2). Supplementary data, Table S1 describes the baseline characteristics of patients (including baseline use of thiazide diuretics and renin–angiotensin–aldosterone system inhibitors) across the different sodium categories. When examined by decile of age, dysnatremia occurred in 46–54% of patients in each group, with hyponatremia the predominant disorder across all age groups (Supplementary data, Figure S3). The prevalence of dysnatremia varied slightly when admission serum sodium was corrected for serum glucose using the Katz and Hillier formulas, with 11.6 and 16.7% of patients being reclassified into different dysnatremia categories (Supplementary data, Table S2 and Figure S4).
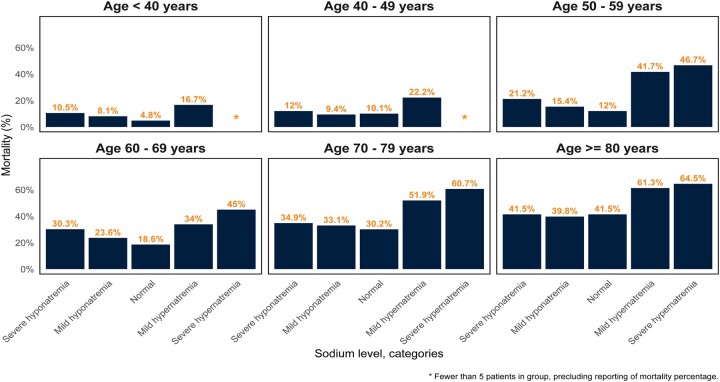
The proportion of patients who experienced in-hospital death was highest for those with moderate/severe hypernatremia [232/383 (60.6%)], followed by mild hypernatremia [163/319 (51.1%)], moderate/severe hyponatremia [261/904 (28.9%)], mild hyponatremia [818/3532 (23.2%)] and normonatremia [1089/4808 (22.6%)], a trend seen across all age groups (Figure 1).
FIGURE 1.
Mortality by admission serum sodium category, stratified by decile of age. The proportion of patients who experienced in-hospital death was highest among those with hypernatremia. Patients with hyponatremia also had a higher proportion of death as compared with normonatremia across age deciles, with the exception of patients ≥80 years old, where hyponatremia and normonatremia had similar proportions of in-hospital death.
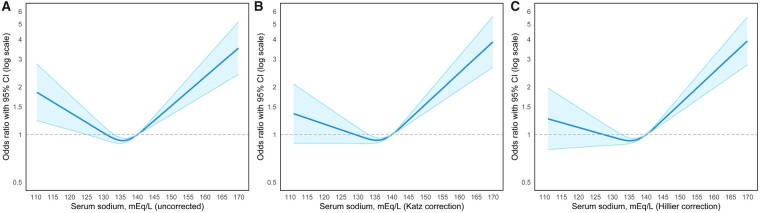
A U-shaped pattern was seen in the relationship between admission serum sodium level and the odds of in-hospital death, with hyponatremia and hypernatremia both significantly associated with mortality, even after full adjustment for demographics, comorbid conditions and illness severity (Figure 2A). Following correction of serum sodium for serum glucose, serum sodium levels in the hypernatremic range remained significantly associated with in-hospital death, but levels in the hyponatremic range were no longer associated with in-hospital death (Figure 2B and C).
FIGURE 2.
The association of serum sodium at hospital presentation and mortality demonstrated a U-shaped pattern. Both hyponatremia and hypernatremia were significantly associated with mortality, which was more pronounced at the extremes of serum sodium, even after adjustment for demographic, comorbid conditions and illness severity (A). Following correction of serum sodium for serum glucose using the Katz (B) and Hillier (C) formulas, serum sodium levels in the hypernatremic range remained significantly associated with in-hospital death, but levels in the hyponatremic range were no longer associated with mortality. A serum sodium value of 140 mEq/L was used as the reference value.
The odds of in-hospital death for patients with mild hyponatremia were not significantly higher than those with normonatremia [odds ratio (OR) = 1.03, 95% confidence interval (CI) 0.93–1.14]. However, the odds of in-hospital death for patients with moderate/severe hyponatremia were higher than those with normonatremia even after adjusting for demographics, comorbid conditions and illness severity [adjusted odds ratio (aOR) = 1.26, 95% CI 1.05–1.51]. Following glucose correction for sodium, this association was no longer present (Supplementary data, Table S3).
When compared with the normonatremic group, those with mild hypernatremia had increased odds of death (OR = 3.57, 95% CI 2.84–4.49), persisting after full adjustment (aOR = 1.91, 95% CI 1.45–2.50). Those with moderate/severe hypernatremia had the highest odds of death after full adjustment (aOR = 2.06, 95% CI 1.57–2.70). The association with death persisted following glucose correction for sodium (Supplementary data, Table S3).
Among survivors, the median and interquartile range length of stay differed by sodium category: normonatremia [5.8 days (3.1–10.0)], mild hyponatremia [6.4 days (3.7–11.2)], moderate/severe hyponatremia [8.8 days (5.7–13.1)], mild hypernatremia [7.1 days (4.4–12.7)] and moderate/severe hypernatremia [8.8 days (5.7–13.1)]. When compared with normonatremia, both hyponatremia and hypernatremia were associated with length of stay ≥7 days, after adjusting for clinical characteristics and illness severity (Supplementary data, Tables S4 and S5).
We found dysnatremias at admission in over half (51.7%) of patients hospitalized with COVID-19, with hyponatremia being most common. Moderate/severe hyponatremia, prior to correction for serum glucose, was associated with increased risk for mortality; however, this association was eliminated after correction for glucose level, similar to non-COVID-19 studies [12]. In this instance, hyperglycemia may be a more important risk factor for death than hyponatremia, although whether this is due to direct glucose effect or ensuing hypertonicity is uncertain.
In contrast, hypernatremia carried a strong association with in-hospital death, in both mild and moderate/severe categories and across all ages, a relationship that persisted even following correction for serum glucose. While hypernatremia has been shown to be a strong predictor of mortality in prior studies, this finding is novel for COVID-19 [13]. Hypernatremia and the related hyperosmolar state can lead to physiologic alterations that may contribute to mortality (negative inotropic effect, increased insulin resistance, impaired hepatic gluconeogenesis and glucose utilization, increased hyperventilation, brain cell shrinkage and vascular rupture) [10, 14].
Both hyponatremia and hypernatremia were also associated with a prolonged hospital length of stay. The magnitude of the OR was substantial, especially for moderate/severe hypo- and hypernatremia, and was not substantively changed after multivariable adjustment. This suggests that at least a portion of the prolonged hospitalization may be directly related to electrolyte disorder management.
This is the first study to describe the prevalence and outcomes of both hyponatremia and hypernatremia in a diverse population of almost 10 000 patients hospitalized with COVID-19. Additionally, we incorporated serum sodium adjustments for hyperglycemia into our analyses. The study is limited by its retrospective and observational design. Thus, the results only reflect the association of dysnatremias with in-hospital death in COVID-19, but do not infer a causal relationship. Although we attempted to adjust for numerous covariates, we limited them to admission data and did not include dynamic changes throughout the hospitalization, and we were unable to determine the chronicity or etiology of dysnatremias. In addition, our hospital sites were all in metropolitan New York during the early part of the pandemic, and may not be representative of later outcomes due to changes in resource capacity and therapeutic refinements.
In conclusion, we found that among patients hospitalized with COVID-19, dysnatremia was commonly present at admission, with hyponatremia being more prevalent than hypernatremia. Both of these disorders were associated with an increased hospital length of stay, and the risk of in-hospital mortality was highest in patients with moderate/severe hypernatremia.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
FUNDING
H.R.-B. is funded by an exploratory/developmental research grant R21DK122023 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The remaining authors declare that they have no relevant financial interests.
AUTHORS’ CONTRIBUTIONS
N.N.U., J.S.H., J.H.N., S.F. and K.D.J. were involved in concept and design; acquisition, analysis and interpretation of data were done by J.S.H., J.H.N., N.N.U., P.S., S.F. and K.D.J.; statistical analysis was performed by J.S.H., J.H.N. and S.F. H.R.-B. helped as an expert consultant on dysnatremia. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
CONFLICT OF INTEREST STATEMENT
K.D.J. is a consultant for Astex Pharmaceuticals and Natera and is a paid contributor for UpToDate.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author, J.H.N. The data are not publicly available due to privacy restrictions.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the Raggio and Hall Families for their support and making this study possible. The authors of Northwell Nephrology COVID-19 Consortium are as follows: Mersema Abate, MD, MPH; Hugo Paz Andrade, MD; Richard L. Barnett, MD; Alessandro Bellucci, MD; Madhu C. Bhaskaran, MD; Antonio G. Corona, MD; Bessy Suyin Flores Chang, MD; Mark Finger, MD; Steven Fishbane, MD; Michael Gitman, MD; Candice Halinski, MBA, MHCDS, MSN, NP-C; Shamir Hasan, MD; Azzour D. Hazzan, MD; Jamie S. Hirsch, MD, MA, MSB; Susana Hong, MD; Kenar D. Jhaveri, MD; Yuriy Khanin, MD; Aireen Kuan, MD; Varun Madireddy, MD; Deepa Malieckal, MD; Abdulrahman Muzib, MD; Gayatri Nair, MD; Vinay V. Nair, DO; Jia Hwei Ng, MD, MSCE; Rushang Parikh, MD; Daniel W. Ross, MD, MPH; Vipulbhai Sakhiya, MBBS; Mala Sachdeva, MD; Richard Schwarz, MD; Hitesh H. Shah, MD; Purva Sharma, MD; Pravin C. Singhal, MD; Nupur N. Uppal, MD; Rimda Wanchoo, MD. Affiliations of the Northwell Nephrology COVID-19 Consortium authors: Division of Kidney Diseases and Hypertension, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Northwell Health, Great Neck, NY 11021, USA.
Contributor Information
the Northwell Nephrology COVID-19 Research Consortium:
Mersema Abate, Hugo Paz Andrade, Richard L Barnett, Alessandro Bellucci, Madhu C Bhaskaran, Antonio G Corona, Bessy Suyin Flores Chang, Mark Finger, Steven Fishbane, Michael Gitman, Candice Halinski, Shamir Hasan, Azzour D Hazzan, Jamie S Hirsch, Susana Hong, Kenar D Jhaveri, Yuriy Khanin, Aireen Kuan, Varun Madireddy, Deepa Malieckal, Abdulrahman Muzib, Gayatri Nair, Vinay V Nair, Jia Hwei Ng, Rushang Parikh, Daniel W Ross, Vipulbhai Sakhiya, Mala Sachdeva, Richard Schwarz, Hitesh H Shah, Purva Sharma, Pravin C Singhal, Nupur N Uppal, and Rimda Wanchoo
REFERENCES
- 1. Gupta S, Hayek SS, Wang W. et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180: 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Malieckal D, Uppal NN, Ng JH. et al. Electrolyte abnormalities in patients hospitalized with COVID-19. Clin Kidney J 2021: sfab060. doi: 10.1093/ckj/sfab060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wald R, Jaber BL, Price LL. et al. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 2010; 170: 294–302 [DOI] [PubMed] [Google Scholar]
- 4. Nair V, Niederman MS, Masani N. et al. Hyponatremia in community-acquired pneumonia. Am J Nephrol 2007; 27: 184–190 [DOI] [PubMed] [Google Scholar]
- 5. De La Flor Merino JC, Mola Reyes L, Linares Gravalos T. et al. Unusual case of severe acute hyponatremia in patient with COVID-19 infection. Nefrología 2020; 40: 356–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Habib MB, Sardar S, Sajid J.. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases 2020; 21: e00859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippi G, South AM, Henry BM.. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann Clin Biochem 2020; 57: 262–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frontera JA, Valdes E, Huang J. et al. Prevalence and impact of hyponatremia in patients with coronavirus disease 2019 in New York City. Crit Care Med 2020; 48: e1211–e1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zimmer MA, Zink AK, Weißer CW. et al. Hypernatremia—a manifestation of COVID-19: a case series. A A Pract 2020; 14: e01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kovesdy CP, Lott EH, Lu JL. et al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012; 125: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waikar SS, Mount DB, Curhan GC.. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 2009; 122: 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Felizardo LI, Dezelée S, Brault D. et al. Prevalence, risk factors and prognosis of hypernatraemia during hospitalisation in internal medicine. Neth J Med 2015; 73: 448–454 [PubMed] [Google Scholar]
- 14. Lindner G, Funk GC, Schwarz C. et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 2007; 50: 952–957 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author, J.H.N. The data are not publicly available due to privacy restrictions.