Abstract
The differentiation between influenza and coronavirus disease 2019 (COVID-19) could constitute a diagnostic challenge during the ongoing winter owing to their clinical similitude. Thus, novel biomarkers are required to enable making this distinction. Here, we evaluated whether the surfactant protein D (SP-D), a collectin produced at the alveolar epithelium with known immune properties, was useful to differentiate pandemic influenza A(H1N1) from COVID-19 in critically ill patients. Our results revealed high serum SP-D levels in patients with severe pandemic influenza but not those with COVID-19. This finding was validated in a separate cohort of mechanically ventilated patients with COVID-19 who also showed low plasma SP-D levels. However, plasma SP-D levels did not distinguish seasonal influenza from COVID-19 in mild-to-moderate disease. Finally, we found that high serum SP-D levels were associated with death and renal failure among severe pandemic influenza cases. Thus, our studies have identified SP-D as a unique biomarker expressed during severe pandemic influenza but not COVID-19.
Keywords: SARS-CoV-2, COVID-19, influenza A(H1N1)pdm09, acute respiratory distress syndrome, surfactant protein D
In this study, the authors found that serum surfactant protein D (SP-D) levels are increased in critically ill patients with pandemic influenza A(H1N1) and serve to differentiate them from individuals with severe coronavirus disease 2019 (COVID-19).
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues spreading despite the social distancing measures adopted worldwide. As of 17 January 2021, about 93.2 million new cases of coronavirus disease 2019 (COVID-19) and 2 million deaths have been reported globally [1]. In the absence of sufficient vaccination coverage to control the current pandemic, this could converge with the influenza season in many Northern Hemisphere regions. Besides the burden on hospitals, this overlap will represent a diagnostic dilemma in emergency rooms. The impact could be further heightened by the emergence of the pandemic influenza A(H1N1)pdm09 virus, since this infection, like SARS-CoV-2, also causes severe disease with higher frequency than seasonal influenza viruses [2]. Moreover, the respiratory manifestations of pandemic influenza A(H1N1) and COVID-19 can be similar [3–6]. Hence, novel biomarkers enabling these 2 infections to be distinguished, especially in severely ill patients, are urgently needed.
A variety of cytokines measured in the peripheral circulation have shown some predictive value to differentiate between influenza and COVID-19 [5, 7, 8]. However, their levels could be modified by other inflammatory conditions. Thus, candidate biomarkers for clinical use must have a lung tissue-specific expression pattern and be differentially regulated during influenza and COVID-19. It was previously demonstrated that the surfactant protein D (SP-D), an essential component of the pulmonary surfactant with immune properties, is translocated from the alveoli to the blood of patients with pandemic influenza A(H1N1) and that its serum levels predict mortality risk [9]. Here, we report high SP-D levels in the blood of patients with pandemic influenza A(H1N1) but not those with seasonal influenza or COVID-19. Furthermore, we identified an association between high serum SP-D concentrations and poor clinical outcomes in patients with pandemic influenza. Our results suggest a possible diagnostic usage of SP-D to distinguish pandemic influenza A(H1N1) from COVID-19 in severe disease.
METHODS
Participants
We conducted a cohort study in mechanically ventilated patients with laboratory-confirmed COVID-19 or pandemic influenza A(H1N1), hereinafter referred to as pandemic influenza, admitted to the intensive care unit (ICU) of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ) and the Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas (INER) in Mexico City. Patients with COVID-19 were recruited from March to November 2020, and those with pandemic influenza were enrolled during the 2 immediately preceding flu seasons (2018–2019 and 2019–2020). The infections were detected by means of reverse-transcription polymerase chain reaction (RT-PCR) in swab samples, bronchial aspirates, or bronchoalveolar lavage specimens, as described elsewhere [10]. Study participants were not coinfected with the human immunodeficiency virus. Solid organ transplant recipients and patients with cancer or autoimmune diseases were not eligible.
Clinical and demographic data of all study participants were retrieved by reviewing their medical records. These data included age, sex, anthropometrics, comorbid conditions, symptoms, triage vital signs, admission Sequential Organ Failure Assessment (SOFA) score, and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores, as well as initial laboratory results (the first test results available, typically within 24 hours of admission). Initial laboratory tests included white blood cell counts, liver and kidney function, procalcitonin, blood gas values, and other tissue injury markers. Patients were followed up until hospital discharge or death. The incidence of specific complications, requirement for antibiotics, corticosteroids, antivirals, chloroquine/hydroxychloroquine, azithromycin, and specific intensive care interventions during the follow-up period were recorded.
Samples
Peripheral blood samples were obtained from all participants at their hospital admission by puncturing a superficial vein using yellow top collecting serum tubes without anticoagulant. A second blood sample was taken after 7 days of hospitalization only from patients with pandemic influenza. The serum was collected and aliquoted from whole blood after centrifugation at 400g for 10 minutes and stored at −80 ºC until use. Serum samples from 10 volunteer donors were used as healthy controls. We also obtained serum samples from 20 patients with pulmonary tuberculosis before initiation of antituberculosis drugs and 17 individuals with stable nonexacerbated chronic obstructive pulmonary disease (COPD) that attended INER and were considered disease controls.
A separate group of patients with mild-to-severe COVID-19 (n = 47) and individuals with mild-to-moderate type A(H1N1/H3N2) and B seasonal influenza (n = 41; hereinafter referred to as seasonal influenza) from the EDFLU study were recruited at the emergency room of the Barnes Jewish Hospital in St Louis, Missouri [11], and served as a validation cohort. The levels of SP-D in these patients were analyzed in plasma. Briefly, peripheral blood samples were obtained in the emergency department or within 48 hours after patient admission to the hospital. Blood was obtained using standard phlebotomy techniques and ethylenediaminetetraacetic acid–anticoagulated collection tubes. Blood samples were layered over Ficoll in the laboratory and centrifuged at 400g for 30 minutes at room temperature. The plasma layer was removed, centrifuged for 5 minutes at 350g to remove residual peripheral blood mononuclear cells and then stored at −80 ºC until analysis.
In both the validation cohort and the original Mexican cohort, categories of severity for respiratory disease were defined as follows: patients with mild disease were those with acute respiratory illness that did not require hospitalization; patients with moderate disease, those who were admitted to the hospital but did not require mechanical ventilation; and patients with severe disease, those requiring mechanical ventilation and ICU admission.
SP-D levels
The SP-D levels in serum and plasma were determined by means of enzyme-linked immunosorbent assay using a commercial kit (Human Surfactant Protein D ELISA, BioVendor). Briefly, standards, quality controls, and serum/plasma specimens were brought to room temperature and incubated in microplate wells precoated with polyclonal anti–human SP-D antibodies for 2 hours, followed by a 1-hour incubation with biotin-labeled anti–human SP-D antibodies and 1-hour incubation with a streptavidin–horseradish peroxidase conjugate. The plate was thoroughly washed 5 times with the manufacturer’s washing buffer between each step of the assay. Finally, the plate was incubated with the substrate solution for an additional 15 minutes. The reaction was interrupted by adding a stop acid solution, and the absorbance of each well was determined using a microplate reader set at 450 nm, with the reference wavelength set at 630 nm. Results were calculated by subtracting readings at 630 nm from readings at 450 nm and then interpolating each well’s subtracted absorbances to the standard curve’s absorbances, using a 4-parameter logistic regression model.
Ethical Approval
The current study was reviewed and approved by the institutional review boards of INCMNSZ (approval no. 3349) and INER (approval nos. B28-16 and B09-20) in Mexico City. Clinical samples were managed according to Mexican constitutional law NOM-012-SSA3-2012, which establishes the criteria for executing clinical investigations in humans. The technical procedures used for obtaining biological samples from the validation cohorts were approved by the Institutional Review Board of the Washington University School of Medicine in St Louis (approval nos. 2020-03-085, 2017-10-220, 2018-08-115, and 2019-10-011). All participants or their legal guardians provided written informed consent, following the Declaration of Helsinki for Human Research.
Statistical Analysis
Descriptive statistics were used to characterize the study population clinically. Frequencies and proportions were calculated for categorical data. Medians, interquartile ranges, and 95% confidence intervals were used for continuous variables. Patients were grouped according to their underlying disease, outcome (survival vs death), or specific complications during the follow-up period. Comparisons were made using Fisher exact test, unpaired Mann-Whitney U test, Kruskal-Wallis with post hoc Dunn test, or Wilcoxon signed rank test, as appropriate.
SP-D levels were log-transformed and included in a logistic model with pandemic influenza or COVID-19 as the outcome, using receiver operating characteristic curves, and the Youden index best cutoff was determined and then exponentiated. Afterward, a multiple logistic model was constructed with the SP-D levels categorized as positive or negative, using a cutoff point of 200.11 ng/mL, and other potential confounders to evaluate the magnitude of the association with pandemic influenza or COVID-19 diagnosis. The cutoff value was then evaluated in the validation cohort. Differences in SP-D levels between patients with different clinical outcomes and complications were evaluated in pandemic influenza and COVID-19 groups. The cutoff points and multiple logistic regression models were performed as described above “for each significantly associated outcome”. All analyses were conducted using GraphPad Prism 8 software (GraphPad) and Stata statistical software (release 14 (StataCorp). Differences were considered significant at P ≤ .05 (2 tailed).
RESULTS
Participant Characteristics
We enrolled 93 patients with pandemic influenza and 54 with COVID-19. Their median age was 47 years. Both diseases preferentially affected men (73% of patients with pandemic influenza and 64% of patients with COVID-19). Overall, study participants looked form medical attention after seven days of symptoms onset. All patients required mechanical ventilation and ICU care. Fever, dyspnea, cough, fatigue, myalgia, and arthralgia were the most frequent symptoms presented by both patient groups. The clinical manifestations (Table 1) and laboratory profiles (Table 2) were similar in patients with both diseases. These data indicate that it is complicated to differentiate these diseases based on clinical characteristics. However, some significant differences deserve to be mentioned. For instance, pandemic influenza differed from COVID-19, with a higher prevalence of obesity, increased frequency of fever, myalgia, arthralgia, rhinorrhea, and sputum production, higher levels of aspartate aminotransferase, lactate dehydrogenase, alkaline phosphatase, and procalcitonin, and higher SOFA and APACHE II scores. Conversely, COVID-19 was characterized by dry cough, gastrointestinal symptoms, and higher white blood cell and neutrophil counts, percentage of oxygen saturation, and ratio of the partial pressure of oxygen, arterial, to the fraction of inspired oxygen.
Table 1.
Clinical Characteristics of Study Participants
| Participants, No. (%)a | Participants, No. (%)a | ||||
|---|---|---|---|---|---|
| Characteristics | Influenza (n = 93) |
COVID-19 (n = 54) |
P Valueb | Pulmonary Tuberculosis (n = 20) |
COPD (n = 17) |
| Age, median (range), y | 47 (20–76) | 47 (24–73) | .37 | 52.5 (24–66) | 71 (60–87) |
| Male sex | 68 (73.1) | 35 (64.8) | .35 | 12 (60) | 0 (0) |
| BMI, median (IQR)c | 32.4 (29.6–37.8) | 30.4 (27.4–34.6) | .02 | 23.1 (19.7–26.2) | 25.6 (23.6–28.4) |
| Comorbid conditions | |||||
| Smoking | 39 (41.9) | 11 (20.4) | .01 | 0 (0) | 17 (100) |
| Obesity | 68 (73.1) | 29 (53.7) | .02 | 0 (0) | 2 (11.7) |
| Diabetes | 18 (19.3) | 12 (22.2) | .68 | 11 (55) | 4 (23.5) |
| SAH | 20 (21.5) | 9 (16.6) | .53 | 4 (20) | 6 (35.3) |
| COPD | 2 (2.1) | 1 (1.8) | >.99 | 0 (0) | 17 (100) |
| Symptoms at onset | |||||
| Fever | 85 (91.4) | 42 (77.7) | .03 | … | … |
| Myalgia | 79 (84.9) | 28 (51.8) | <.001 | … | … |
| Arthralgia | 76 (82.6) | 29 (53.7) | <.001 | … | … |
| Headache | 47 (50.5) | 25 (46.2) | .73 | … | … |
| Dyspnea | 85 (91.4) | 48 (88.9) | .77 | … | … |
| Rhinorrhea | 39 (41.9) | 11 (20.4) | .01 | … | … |
| Sore throat | 39 (41.9) | 19 (35.2) | .49 | … | … |
| Thoracic pain | 14 (15) | 6 (11.1) | .62 | … | … |
| Dry cough | 35 (38) | 38 (70.4) | <.001 | … | … |
| Productive cough | 50 (53.7) | 6 (11.1) | <.001 | … | … |
| Fatigue | 64 (68.8) | 35 (64.8) | .72 | … | … |
| Diarrhea | 6 (6.4) | 12 (22.2) | .008 | … | … |
| Nausea | 4 (4.3) | 5 (9.2) | .29 | … | … |
| Vomit | 2 (2.1) | 6 (11.1) | .05 | … | … |
| Duration of symptoms, median (range), d | 7 (0–25) | 7 (1–22) | .97 | … | … |
| Vital signs, median (IQR) | |||||
| Body temperature, oC | 37.5 (36.6–38) | 37 (37–37.7) | .21 | … | … |
| Respirations/min | 25 (20–30) | 27 (24–30) | .22 | … | … |
| Pulse rate, per min | 97 (84–108) | 99 (85–110) | .71 | … | … |
| MAP, mm Hg | 86.6 (75–94) | 88.5 (78–97) | .28 | … | … |
| Sao2 | 78 (66–88) | 87 (69–92) | .04 | … | … |
| Complications | |||||
| Acute kidney injury | 35 (37.6) | 21 (38.9) | >.99 | … | … |
| Secondary infection | 60 (64.5) | 26 (48.1) | .06 | … | … |
| Acute myocardial infarction | 3 (3.2) | 1 (1.8) | >.99 | … | … |
| Deep vein thrombosis | 4 (4.3) | 1 (1.8) | .65 | … | … |
| Stroke | 1 (1.07) | 1 (1.8) | >.99 | … | … |
| Medical treatment | |||||
| Oseltamivir | 93 (100) | 17 (31.5) | <.001 | 0 (0) | 0 (0) |
| Antibiotic therapy | 93 (100) | 54 (100) | >.99 | 20 (100) | 0 (0) |
| No. of antibiotics per patient, median (IQR) | 2 (2–5) | 4 (3–5) | <.001 | … | … |
| Corticosteroids | 12 (12.9) | 28 (51.8) | <.001 | 0 (0) | 3 (17.6) |
| Lopinavir/ritonavir | 0 (0) | 13 (24.1) | <.001 | … | … |
| Chloroquine/hydroxychloroquine | 0 (0) | 32 (59.2) | <.001 | … | … |
| Azithromycin | 0 (0) | 23 (42.6) | <.001 | … | … |
| Intensive support | |||||
| MV | 93 (100) | 54 (100) | >.99 | 0 (0) | 0 (0) |
| Prone position | 48 (51.6) | 26 (48.1) | .73 | 0 (0) | 0 (0) |
| ECMO | 8 (8.6) | 2 (3.7) | .33 | 0 (0) | 0 (0) |
| RRT | 17 (18.2) | 7 (13) | .49 | 0 (0) | 0 (0) |
| Fatality cases | 14 (15) | 23 (42.6) | <.001 | 0 (0) | 0 (0) |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; MV, mechanical ventilation; RRT, renal replacement therapy; SAH, systemic arterial hypertension; Sao2, oxygen saturation.
aData represent no. (%) of participants unless otherwise specified.
bDifferences in continuous variables were estimated using the Mann-Whitney U test, and differences in categorical variables were calculated using the Fisher exact test.
cBMI was calculated as weight in kilograms divided by height in meters squared.
Table 2.
Laboratory Parameters in Patients With Severe Influenza or Coronavirus Disease 2019
| Median Value (IQR)a | |||
|---|---|---|---|
| Parameter | Influenza (n = 93) |
COVID-19 (n = 54) |
P Valueb |
| Blood | |||
| WBC count, ×109/L | 7.0 (5.3–9.8) | 9.4 (7.1–14) | <.001 |
| WBC count, no. (%) | |||
| 4.5 to 11.0 × 109/L | 67 (72) | 35 (64.8) | .36 |
| >12 ×109/L | 17 (18.3) | 17 (31.5) | .07 |
| <4 ×109/L | 9 (9.7) | 2 (3.7) | .33 |
| Neutrophil count, ×109/L | 5.5 (4.1–8.3) | 8.1 (5.6–12.3) | <.001 |
| Lymphocyte count, ×109/L | 0.8 (0.5–1.1) | 0.8 (0.6–1.2) | .36 |
| NLR | 8.5 (5.4–11.8) | 11 (6.9–15) | .05 |
| Hemoglobin, g/dL | 15.3 (13.7–16.8) | 13.6 (12.2–15.1) | <.001 |
| Platelet count, ×109/L | 174 (131–215) | 235 (181–304) | <.001 |
| Metabolic | |||
| Glucose, mg/dL | 144.4 (114.8–218.7) | 132 (99.6–170.5) | .06 |
| Sodium, mmol/L | 137 (133.7–140) | 140 (137–141.8) | <.001 |
| Potassium, mmol/L | 4.1 (3.8–4.5) | 4.1 (3.9–4.6) | .73 |
| Calcium, mg/dL | 7.8 (7.4–8.1) | 8.0 (7.4–8.7) | .03 |
| Renal function | |||
| Creatinine, mg/dL | 1.0 (0.8–1.34) | 0.89 (0.7–1.36) | .09 |
| SUN, mg/dL | 21.0 (14.3–31.6) | 18.7 (12.4–30) | .33 |
| Liver function | |||
| Total bilirubin, mg/dL | 0.65 (0.48–0.9) | 0.56 (0.44–0.75) | .04 |
| AST, U/L | 65.2 (46.1–100.1) | 38.9 (27.7–67.2) | <.001 |
| ALT, U/L | 41.8 (28.1–59) | 35.3 (23.3–62.7) | .23 |
| Tissue injury markers | |||
| LDH, U/L | 640.0 (496.5–901) | 435.8 (318.9–499.3) | <.001 |
| ALP, U/L | 119.9 (95–157.5) | 80.4 (61.3–92.3) | <.001 |
| CPK, U/L | 279.4 (124.5–752.6) | 138.3 (64.5–681.6) | .17 |
| PCT. ng/mL | 0.55 (0.19–1.43) | 0.13 (0.09–0.32) | <.001 |
| Respiratory parameters | |||
| pH | 7.38 (7.31–7.46) | 7.42 (7.36–7.46) | .08 |
| PCo2, mm Hg | 35.0 (29.9–45.9) | 34.1 (27.9–43.2) | .28 |
| Pao2, mmHg | 60.0 (41.8–74.8) | 56.3 (44.7–72.2) | .73 |
| Lactate, mmol/L | 1.3 (0.95–1.65) | 1.2 (1–1.8) | .75 |
| HCO3, mEq/L | 22.2 (20.1–24.6) | 22.6 (19.7–26.1) | .58 |
| Pao2/Fio2, mm Hg | 101.0 (60–155.5) | 129.6 (99.2–192.2) | .002 |
| Severity of illness | |||
| SOFA score | 7 (5–8) | 5 (3–8) | .003 |
| APACHE II score | 10 (7–15) | 8 (5–13) | .057 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; APACHE-II, Acute Physiology and Chronic Health Evaluation II; AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CPK, creatine phosphokinase; Fio2, fraction of inspired oxygen; HCO3, bicarbonate; IQR, interquartile range; LDH, lactate dehydrogenase; NLR, neutrophil-lymphocyte ratio; Pao2, partial pressure of oxygen, arterial; Pco2, partial pressure of carbon dioxide; PCT, procalcitonin; SOFA, Sequential Organ Failure Assessment; SUN, serum urea nitrogen; WBC, white blood cell.
aData represent median value (IQR) unless otherwise specified.
bDifferences in continuous variables were estimated using the Mann-Whitney U test, and differences in categorical variables were calculated using the Fisher exact test.
Importantly, patients with COVID-19 showed a higher mortality rate, despite similar rates of complications (Table 1). All patients with pandemic influenza were treated with oseltamivir, whereas 31.5% of those with COVID-19 received oseltamivir, 24% lopinavir/ritonavir, 59% chloroquine/hydroxychloroquine, and 42% azithromycin. Both patient groups received antibiotics, although individuals with COVID-19 required more antibiotics (Table 1). Notably, patients with COVID-19 received more corticosteroids than those with pandemic influenza (51% vs 13%; P < .001), especially those enrolled after the publication of the RECOVERY trial [12]. Approximately 50% of both patients with pandemic influenza and patients with COVID-19 were ventilated in the prone position. Finally, 8% of patients with pandemic influenza and 4% with COVID-19 were subjected to extracorporeal membrane oxygenation. The clinical characteristics of the comparative and validation cohorts are summarized in Table 1 and Supplementary Table 1, respectively.
SP-D in Patients With Pandemic Influenza and Patients With COVID-19
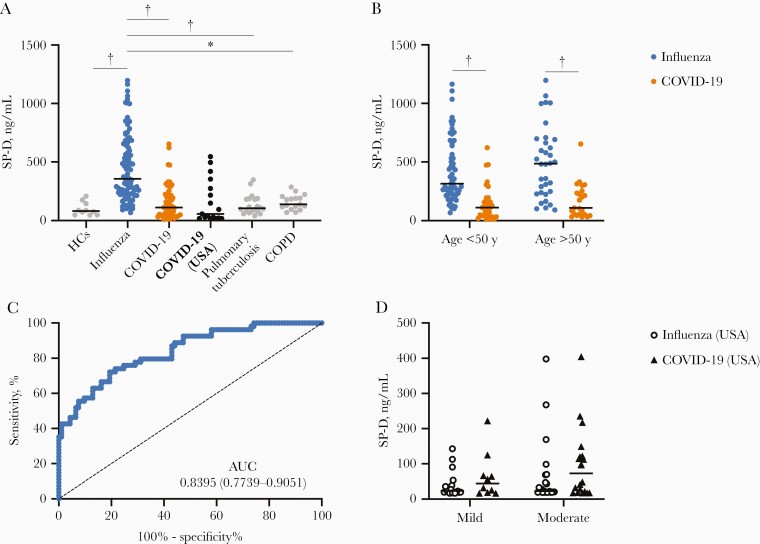
SP-D plays an essential role in innate lung defenses, and its production is dysregulated during inflammatory pulmonary disorders [13]. It was previously observed that SP-D translocates from alveoli to the blood during pandemic influenza A(H1N1) [9]. Hence, this molecule may be a useful readout of lung injury secondary to infections. To address this hypothesis, we measured the serum SP-D levels in pandemic influenza and COVID-19. We observed that serum SP-D levels were elevated in patients with pandemic influenza (Figure 1A) compared with healthy controls, as described elsewhere [9]. Age, sex, BMI, and laboratory parameters in patients with pandemic influenza did not influence their serum SP-D levels (data not shown). Also, differences in serum SP-D levels between patients with severe pandemic influenza and healthy controls remained significant independently of the duration of their symptoms at admission (Supplementary Figure 1).
Figure 1.
Surfactant protein D (SP-D) differentiates pandemic influenza from coronavirus disease 2019 (COVID-19) in patients with severe disease. A, Serum levels of SP-D were determined in healthy controls (HCs; n = 10) and patients with severe pandemic influenza A(H1N1) (n = 93), severe COVID-19 (n = 54), pulmonary tuberculosis (n = 20), or chronic obstructive pulmonary disease (COPD; n = 17). Plasma SP-D levels were also analyzed in a validation cohort of patients with severe COVID-19 from St Louis, Missouri (n = 17). *P < .01; †P < .001 (Kruskal-Wallis test/post hoc Dunn test). B, Serum SP-D levels in patients with severe pandemic influenza or COVID-19 stratified by age. †P < .001 (Mann-Whitney U test). C, Receiver operating characteristic curve of the serum SP-D levels in patients with severe pandemic influenza or COVID-19. Abbreviation: AUC, area under the curve. D, Analysis of plasma SP-D levels in the validation cohort of patients with seasonal influenza or COVID-19 with mild (n = 18 [influenza] vs 10 [COVID-19]) or moderate disease (n = 23 vs 20) (compared using Mann-Whitney U test). Graphs display medians with interquartile ranges or the AUC and 95% confidence intervals.
Strikingly, patients with COVID-19 showed significantly lower serum SP-D levels than patients with pandemic influenza (111.7 vs 355.6 ng/mL; P < .001). This difference was observed both in young and old patients (Figure 1B). Also, mechanically ventilated patients with COVID-19 from the validation cohort had low plasma SP-D levels, similar to the Mexican COVID-19 cohort (Figure 1A). This indicates that SP-D is up-regulated only during pandemic influenza but not COVID-19. Indeed, the serum SP-D levels showed a high performance to differentiate pandemic influenza from COVID-19 in a receiver operating characteristic curve analysis, showing an area under the curve of 0.8395, 80.65% sensitivity, and 72.22% specificity with a cutoff value of 200.11 ng/mL (Figure 1C). The multivariate analysis showed that patients with serum SP-D levels >200.11 ng/mL were 17 times more likely to have pandemic influenza (odds ratio, 17.22; 95% confidence interval [CI], 5.58–53.14; P < .001) after adjustment for age, obesity, smoking status, corticosteroid use, and SOFA score. With adjusment for APACHE-II instead of SOFA, the likelihood decreased to 15.86 (95% CI, 5.37–46.81).
As aforementioned, SP-D can also be induced during chronic pulmonary diseases. Hence, we also tested serum SP-D levels in patients with pulmonary tuberculosis or COPD. Interestingly, the serum SP-D levels of these disease controls were significantly lower than those in patients with severe pandemic influenza (Figure 1A). Together, these results suggest that SP-D can be used as a specific marker to differentiate pandemic influenza from COVID-19 in severe disease and other chronic infectious or inflammatory lung conditions as well.
Despite these findings, the analysis of SP-D in the plasma of non–mechanically ventilated patients with seasonal influenza and COVID-19 showed no differences between groups (Figure 1D). In fact, using the cutoff value determined in the serum of the Mexican cohort, the sensitivity and specificity of plasma SP-D to differentiate seasonal influenza from COVID-19 were 4.9% and 78.7%, respectively, in the whole US cohort. Furthermore, when restricted to hospitalized patients only, this protein showed 8.7% sensitivity and 75.68% specificity. These data indicate that the diagnostic potential of SP-D observed in severe pandemic influenza does not extend to seasonal influenza in patients with mild-to-moderate conditions. However, we could not rule out possible differences in SP-D levels between seasonal influenza and COVID-19 in severe disease.
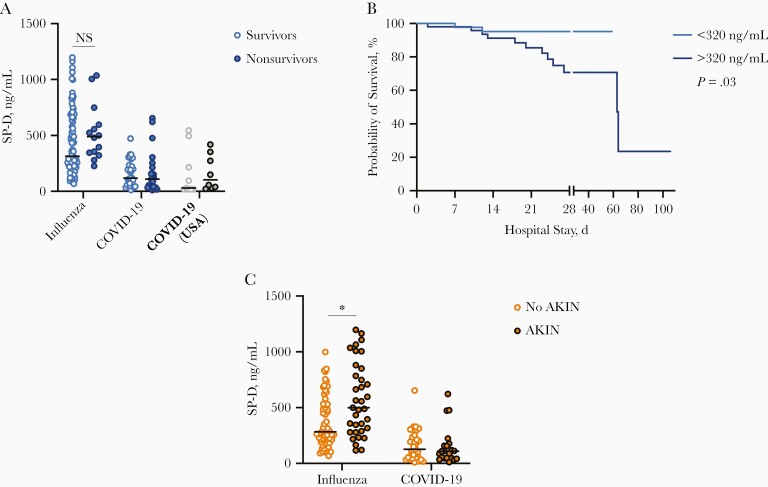
Prognostic Value of Serum SP-D in Severe Pandemic Influenza
As reported elsewhere [9], in the current study we observed that serum SP-D levels tended to be higher among patients who died of pandemic influenza than among survivors (Figure 2A). Indeed, patients with pandemic influenza who had elevated SP-D levels had lower 28-day survival rates than those with lower levels (70.7% vs 95.2%; P = .03) using a cutoff value of 320 ng/mL (Figure 2B). Nonetheless, this association disappeared after covariate adjustment. Interestingly, SP-D levels were increased among patients with pandemic influenza in whom acute kidney injury (AKIN) developed, compared with those who maintained normal renal function during the follow-up period (499.1 vs 282.5 ng/mL, respectively; P = .002) (Figure 2C). A cutoff value of 277.18 ng/mL (area under the curve, 0.70) had a sensitivity of 57.14% and a specificity of 59.34% for AKIN. Multivariate analysis showed that patients with pandemic influenza with serum SP-D levels >277.18 ng/mL were 5 times more likely to develop AKIN (odds ratio, 5.6; 95% CI, 1.85–17.0; P = .002), after adjustment for age and corticosteroid use. Hence, monitoring serum levels of SP-D might help identify individuals with pandemic influenza at risk of renal failure. In contrast, SP-D has no prognostic value among patients with COVID-19 (Figure 2A).
Figure 2.
Prognostic value of serum surfactant protein D (SP-D) levels in severely ill patients with pandemic influenza. A, Serum levels of SP-D were compared between patients with pandemic influenza and those with coronavirus disease 2019 (COVID-19) according to clinical outcome (survival vs death). Abbreviation: NS, not significant (Mann-Whitney U test). B, Survival curves were compared between patients with pandemic influenza according to their serum SP-D levels, using the Kaplan-Meier method and the log rank test. The cutoff value of SP-D for this analysis was estimated using the Younde index. C, Serum SP-D levels were compared between patients with pandemic influenza and patients with COVID-19 in whom acute kidney injury (AKIN) developed, with respect to those who maintained normal renal function during the follow-up period. Graphs display medians and interquartile ranges. *P < .01 (Mann-Whitney U test).
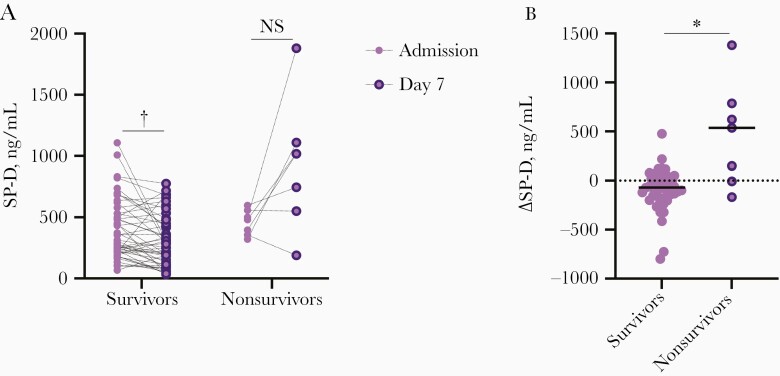
Dynamics of Serum SP-D in Patients With Severe Pandemic Influenza
Because of the diagnostic and prognostic potential of SP-D in severe pandemic influenza, we evaluated longitudinal changes in serum SP-D levels during the course of the disease in 56 patients with pandemic influenza from whom we obtained a second blood sample after 7 days of hospitalization. Interestingly, we found that the dynamics of serum SP-D levels differed between patients who survived (n = 49) and those who died of pandemic influenza (Figure 3A). In fact, 5 of 7 patients who succumbed to the infection showed a longitudinal increase in SP-D levels, whereas most survivors showed stable or decreasing levels during the first week of hospitalization (Figure 3B). This finding suggests that serum SP-D levels could be used to monitor treatment response in patients with severe pandemic influenza, although this hypothesis should be validated in larger cohorts.
Figure 3.
Dynamics of serum surfactant protein D (SP-D) levels in patients with severe pandemic influenza. Serum SP-D levels were determined in serial samples of 56 of 93 patients with pandemic influenza obtained at hospital admission (day 0) and 7 days later. A, Longitudinal dynamics of serum SP-D were analyzed according to their clinical outcome (survival or death). Abbreviation: NS, not significant. †P < .001 (Wilcoxon signed rank test). B, Magnitude of longitudinal change (Δ) in serum SP-D levels were compared between survivor (n = 49) and deceased (n = 7) patients with pandemic influenza. Graph displays medians. *P < .01 (Mann-Whitney U test.).
Discussion
Pandemic influenza A(H1N1) and COVID-19 are the 2 most recent global epidemics associated with the emergence of previously unknown zoonotic viruses. The first appeared in 2009, and ever since, it has acquired a seasonal pattern of transmission in North America. Meanwhile, the outbreak of COVID-19 began in December 2019 and has continued spreading throughout 2020 and 2021, confining the world population into extended quarantine periods. With the lack of control of the current epidemic, it is almost inevitable that COVID-19, seasonal influenza, and pandemic influenza will converge during the current winter in the Northern Hemisphere. This implies that most clinicians in emergency departments will have to distinguish between these infections to provide specific therapeutics for each case. In settings of limited access to RT-PCR tests, solving this diagnostic dilemma will be challenging owing to the clinical similitude between influenza and COVID-19. Indeed, only a few characteristics might differentiate both diseases, as demonstrated here and in previous studies [3–6].
The clinical manifestations of severe pandemic influenza and COVID-19 are related to dysregulated immune responses that cause secondary lung injury. Furthermore, COVID-19 is also characterized by an antiviral immune dysfunction and a skewed reaction with T-helper 1/T-helper 2 components simultaneously elicited by the virus [5, 14]. These different immune responses triggered by both pathogens are translated into distinct lung morphological changes and captured as unique serum cytokine signatures in patients with influenza or COVID-19 [5, 7, 8]. However, it is not entirely understood whether the influenza A(H1N1) pdm09 virus, seasonal influenza viruses, and SARS-CoV-2 differentially regulate local innate lung defenses. In this context, here we evaluated whether the levels of SP-D, a protein component of the lung surfactant with immune properties [13], differed between critically ill patients with pandemic influenza and those with COVID-19.
The molecule SP-D is a type II pneumocyte-derived collectin that participates in the clearance of pathogens at alveolar spaces owing to its opsonizing properties [13]. It also mediates anti-inflammatory activities [13]. Although SP-D production is mainly restricted to the alveolar epithelium, this molecule can be detected in the circulation of patients with different chronic inflammatory disorders of the lung [15, 16], as well as during pulmonary infections [9, 17]. Accordingly, high serum levels of SP-D were observed in our cohort of patients with pandemic influenza, as we also reported before in a prior group of individuals infected with the influenza A(H1N1) pdm09 virus [9]. Changes in the circulating levels of SP-D associated with lung disorders may reflect protein translocation and may serve as a readout of disruption of the alveolar-capillary membrane [15, 16]. As such, we observed slightly higher initial serum SP-D levels and a longitudinal increase of this marker during the first week of hospitalization in patients with pandemic influenza who died but not in survivors, suggesting that this marker could predict poor outcome [9]. Perhaps this observation reflects more severe lung damage in patients who succumb to the infection than in those who recover from pandemic influenza.
Notably, SP-D levels were not elevated in the serum of patients with COVID-19, pulmonary tuberculosis, or COPD, nor in the validation cohorts of patients with seasonal influenza or COVID-19. Based on the translocation hypothesis mentioned above, this finding could indicate that the alveolar-capillary membrane of lungs infected with SARS-CoV-2 conserves its selective permeability for SP-D and other proteins despite the severity of COVID-19. However, previous pathological analyses have shown an extended inflammatory infiltration and diffuse alveolar damage in the lungs of patients who died of COVID-19 [18]. Some possible explanations exist for these discrepancies. First, SARS-CoV-2 likely down-regulates the local expression of SP-D. Assessing the levels of this protein in bronchoalveolar lavage samples from our cohort of COVID-19 would provide additional information in this regard.
Secondly, SP-D might not reach the blood owing to possible obstructions of the lung circulatory system related to the endothelial dysfunction and hypercoagulation observed in patients with COVID-19. Indeed, pathological studies of SARS-CoV-2–infected lungs have revealed damage to pulmonary vasculature and microthrombi [19]. Third, SP-D induction in the lungs could be a very specific defense mechanism against influenza viruses. Indeed, it is known that this collectin inactivates seasonal influenza A viruses by binding to the viral hemagglutinin [20]. Our data only partially support this hypothesis, as we found induction of SP-D during severe pandemic influenza but not in mild-to-moderate seasonal influenza. Independently of the mechanism, our study demonstrates that serum levels of SP-D could be a biomarker with diagnostic applications to distinguish severe pandemic influenza from COVID-19 in the clinical setting.
Finally, our results project a role for SP-D in immunity against the influenza A(H1N1) pdm09 virus. As mentioned above, SP-D can bind to the hemagglutinin and neuraminidase of the influenza A(H3N2) virus and block their receptor and enzyme functions, respectively [20]. Also, mice genetically deficient in SP-D cannot clear the infection with influenza A(H3N2) as wild-type animals do [21]. Although additional confirmatory data from human studies and animal models of influenza A(H1N1) pdm09 virus infection are required, our results might have direct implications in the design and development of novel therapeutics for pandemic influenza based on the administration of protective surfactant components, such as SP-D.
Limitations
A limitation of the study is the small number of participants enrolled. Therefore, our results require validation in larger cohorts. Another caveat is that we did not compare the levels between critically ill patients seasonal influenza and patients with COVID-19. Thus, our observations project a utility of SP-D only to distinguish pandemic influenza from SARS-CoV-2 infection in severe disease. Finally, we did not measure serum SP-D levels in serial samples from patients with COVID-19. Hence, the possibility that serum levels of SP-D were increased late during COVID-19 must be evaluated in future investigations.
In summary, our study brings forward SP-D as a potential biomarker useful to distinguish severe pandemic influenza from COVID-19. This tool could guide initial therapeutic interventions during the wait for definitive RT-PCR results. Future studies must validate whether SP-D discriminates between these diseases better than other clinical factors, explore other covariates associated with the biomarker, and identify settings in which SP-D could provide better diagnostic results before advancing to clinical use.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Data availability. Data are available from the corresponding author on reasonable request.
Author contributions. Collection of clinical data and biological samples: J. A. C. P., L. A. J. A., G. R. M., A. C. L., M. T., L. A. F. L., L. M. H., M. S. V., E. M. H. M., D. L. H. G., J. A. R. N., C. E. R. L., A. D. F., G. Y. Z. L., E. M. G., A. M. M., C. M. M., D. C. R., M. M. T., C. L. R., P. G. O., G. D. C., T. S. R. R., P. A. M., and C. M. H. C. Performance of surfactant protein D enzyme-linked immunosorbent assays: J. A. C. P., L. A. J. A., M. T., and L. A. F. L. Provision of materials: E. A. G. L., P. G. O., F. A. M., G. D. C., T. S. R. R., P. A. M., C. M. H. C., S. A. K., and J. Z. Analysis of data: J. A. C. P., M. C. C, E. M. C. P., S. A. K., and J. Z. Drafting of manuscript: J. A. C. P. and J. Z. All authors read and approved the final version of the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health (NIH). Funders did not play any role in the study design and conduction.
Financial support. This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACyT) grant CVU 737347 to J. A. C. P.; research contracts SECTEI/050/2020, Secretaría de Ciencia, Tecnología e Innovación de la Ciudad de México to J. Z.; CONACYT-Support for scientific research, technological development, and innovation in health during the COVID-19 contingency [projects 313517 and 00311999]) to T. S. R. R. and J. Z.; the Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas, through the interinstitutional collaboration agreement with the Universidad Nacional Autónoma de México (grant 43355-3065-17-XI-15); the Fondo Institucional de Fomento Regional para el Desarrollo Científico y Tecnológico y de Innovación (grant FORDECYT/10SE/2020/05/14-07) to J. Z.; Barnes Jewish Hospital Foundation (P. A. M.); the Washington University Institute of Clinical and Translational Sciences (P. A. M.); Washington University in St Louis (S. A. K.); the NIH (grants R01AI123780, R01AI134236, and COVID grant 3R01AI134236-04W1 to S. A. K.); and the National Center for Advancing Translational Sciences, NIH (grant UL1TR002345 to the Washington University Institute of Clinical and Translational Sciences (P. A. M.)).
Potential conflicts of interests. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. COVID-19 weekly epidemiological Update—19 January 2021.https://www.who.int/publications/m/item/weekly-epidemiological-update---19-january-2021. Accessed 19 January 2021.
- 2. Dunning J, Thwaites RS, Openshaw PJM. Seasonal and pandemic influenza: 100 years of progress, still much to learn. Mucosal Immunol 2020; 13:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang X, Du RH, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest 2020; 158:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li P, Wang Y, Peppelenbosch MP, Ma Z, Pan Q. Systematically comparing COVID-19 with the 2009 influenza pandemic for hospitalized patients. Int J Infect Dis 2021; 102:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choreno-Parra JA, Jimenez-Alvarez LA, Cruz Lagunas A, et al. Clinical and immunological factors that distinguish COVID-19 from pandemic influenza A(H1N1). medRxiv [Preprint: not peer reviewed]. 14 August 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.08.10.20170761v1. [DOI] [PMC free article] [PubMed]
- 6. Jiang C, Yao X, Zhao Y, et al. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect 2020; 22:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Remy KE, Mazer M, Striker DA, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020; 5:e140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mudd PA, Crawford JC, Turner JS, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv 2020; 6:eabe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delgado C, Krötzsch E, Jiménez-Alvarez LA, et al. Serum surfactant protein D (SP-D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung 2015; 193:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castillejos M, Cabello-Gutiérrez C, Alberto Choreño-Parra J, et al. High performance of rapid influenza diagnostic test and variable effectiveness of influenza vaccines in Mexico. Int J Infect Dis 2019; 89:87–95. [DOI] [PubMed] [Google Scholar]
- 11. Turner JS, Lei T, Schmitz AJ, et al. Impaired cellular immune responses during the first week of severe acute influenza infection. J Infect Dis 2020; 222:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Group RC, Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med 2020; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crouch EC. Surfactant protein-D and pulmonary host defense. Respir Res 2000; 1:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucas C, Wong P, Klein J, et al. ; Yale IMPACT Team . Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Winkler C, Atochina-Vasserman EN, Holz O, et al. Comprehensive characterisation of pulmonary and serum surfactant protein D in COPD. Respir Res 2011; 12:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honda Y, Kuroki Y, Matsuura E, et al. Pulmonary surfactant protein D in sera and bronchoalveolar lavage fluids. Am J Respir Crit Care Med 1995; 152:1860–6. [DOI] [PubMed] [Google Scholar]
- 17. Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999; 160:1843–50. [DOI] [PubMed] [Google Scholar]
- 18. Tian S, Xiong Y, Liu H, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol 2020; 33:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polak SB, Van Gool IC, Cohen D, von der Thusen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol 2020; 33:2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reading PC, Morey LS, Crouch EC, Anders EM. Collectin-mediated antiviral host defense of the lung: evidence from influenza virus infection of mice. J Virol 1997; 71:8204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J Immunol 2001; 167:5868–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.