Abstract
Although millions of patients have clinically recovered from COVID-19, little is known about the immune status of lymphocytes in these individuals. In this study, the peripheral blood mononuclear cells of a clinically recovered (CR) cohort were comparatively analyzed with those of an age- and sex-matched healthy donor cohort. We found that CD8+ T cells in the CR cohort had higher numbers of effector T cells and effector memory T cells but lower Tc1 (IFN-γ+), Tc2 (IL-4+), and Tc17 (IL-17A+) cell frequencies. The CD4+ T cells of the CR cohort were decreased in frequency, especially the central memory T cell subset. Moreover, CD4+ T cells in the CR cohort showed lower programmed cell death protein 1 (PD-1) expression and had lower frequencies of Th1 (IFN-γ+), Th2 (IL-4+), Th17 (IL-17A+), and circulating follicular helper T (CXCR5+PD-1+) cells. Accordingly, the proportion of isotype-switched memory B cells (IgM−CD20hi) among B cells in the CR cohort showed a significantly lower proportion, although the level of the activation marker CD71 was elevated. For CD3−HLA-DR− lymphocytes in the CR cohort, in addition to lower levels of IFN-γ, granzyme B and T-bet, the correlation between T-bet and IFN-γ was not observed. Additionally, by taking into account the number of days after discharge, all the phenotypes associated with reduced function did not show a tendency toward recovery within 4‒11 weeks. The remarkable phenotypic alterations in lymphocytes in the CR cohort suggest that severe acute respiratory syndrome coronavirus 2 infection profoundly affects lymphocytes and potentially results in dysfunction even after clinical recovery.
Keywords: COVID-19, recovered individuals, lymphocyte subsets, phenotypic alteration, potential dysfunction
Introduction
The worldwide pandemic of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global threat to humans and society, although the epidemic subsided after control measures were taken in China. As of January 12, 2021, there were >91 million confirmed infections globally, with ∼2 million deaths and >65 million infected individuals who had clinically recovered. Lymphocytes are critical for eliminating infection and for the establishment of long-term immunity (Callaway, 2020; Corey et al., 2020; Leslie, 2020). However, lymphopenia was reported as a typical clinical symptom in COVID-19 patients in addition to specific peripheral ground-glass opacity lung consolidation (GrifoNi et al., 2020; Huang et al., 2020). A growing number of studies have reported a decreased number and impaired function of CD4+ T, CD8+ T, and natural killer (NK) cells in COVID-19 patients, especially in severe cases (Chen et al., 2020; Giamarellos-Bourboulis et al., 2020; MazzoNi et al., 2020; Qin et al., 2020; Zhang et al., 2020). Although lymphocyte counts could gradually increase to the normal range in some patients after viral clearance and clinical recovery (OuYang et al., 2020; Zheng et al., 2020), the activation, differentiation, and function of lymphocytes in individuals who have clinically recovered from COVID-19 remain poorly understood. In a follow-up study of an asymptomatic infected individual without lymphopenia, we found that interferon-γ (IFN-γ)+ CD8+ T cells and interleukin-17A (IL-17A)+ CD4+ T cells were still detected at remarkably reduced percentages even when the SARS-CoV-2 virus had become undetectable for 3 weeks (Yang et al., 2020). These results indicate that SARS-CoV-2 may affect the immune system over a longer period of time than previously thought. Despite the large numbers of recovered individuals, there is a scarcity of information on the composition, phenotype, and functional potential of lymphocytes from these individuals. In this study, we comprehensively investigated the phenotype and potential function of lymphocytes from a COVID-19 clinically recovered (CR) cohort recruited in Wuhan by comparative analysis of peripheral blood nonnuclear cells (PBMCs) from an age- and sex-matched uninfected healthy donor (HD) cohort.
Results
Clinical evaluation of CR individuals
To investigate the immune response post SARS-CoV-2 infection in humans, we performed a cohort study in Wuhan Jinyintan Hospital in April 2020. An HD cohort of 55 healthy donors and a CR cohort of 55 subjects who had recovered from COVID-19 were involved. In the CR cohort, there were only 2 patients (#30 and #32) with a history of severe COVID-19, and the remaining 53 patients had a history of mild to moderate COVID-19. The disease duration from symptom onset to discharge was 13‒60 days, and the median and average durations were 30 and 32 days, respectively. On the day of blood collection, which was performed on average 45.22 days post discharge, all CR subjects showed a normal oxygen saturation of at least 95% by clinical examination. The majority of CR individuals had not presented symptoms in the past 2 weeks, except for four individuals who had experienced shortness of breath, six who had experienced cough, three who had experienced expectoration, and one individual who had required supplemental oxygen (Supplementary Table S1).
The frequencies of CD8+ T cells, B cells, and CD3−HLA-DR− cells but not CD4+ T cells showed normal levels in CR individuals
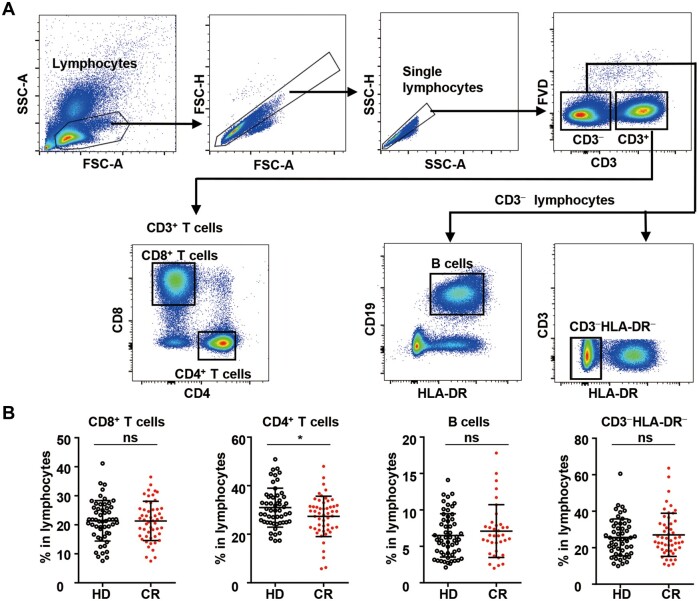
All 55 CR individuals had normal total lymphocyte counts (normal range: 1.0 × 106–3.2 × 106/ml), except for one individual who showed remarkable lymphopenia (0.64 × 106/ml) (Supplementary Table S1). Lymphocyte populations, including CD8+ T cells, CD4+ T cells, CD3−CD19+ B cells, and the non-T non-B CD3−HLA-DR− subset, were analyzed (Figure 1A). To our surprise, the percentage of CD4+ T cells in the lymphocytes of the CR cohort was significantly lower than that in the lymphocytes of the HD cohort, although the percentage of CD8+ T cells, B cells, or CD3−HLA-DR− cells in the lymphocytes of the CR cohort was comparable to that of the HD cohort (Figure 1B). We then assayed and analyzed the differentiation, activation, proliferation, and functional potential of the four lymphocyte subsets individually.
Figure 1.
CD8+ T cells, CD4+ T cells, B cells, and CD3−HLA-DR− lymphocytes in peripheral blood from the HDCR cohorts. (A) Gating strategies used for CD8+ T cells (CD3+CD4−CD8+), CD4+ T cells (CD3+CD8−CD4+), B cells (CD3−CD19−HLA-DR+), and CD3−HLA-DR− lymphocytes (CD3−HLA-DR−) in PBMCs. (B) Frequencies of CD8+ T cells, CD4+ T cells, B cells, and CD3−HLA-DR− lymphocytes among total lymphocytes in the HD and CR cohorts. Data on the CD8+ T cells, CD4+ T cells, and CD3−HLA-DR− lymphocytes were obtained from 55 healthy donors and 55 COVID-19 recovered individuals. Data on the B cells were obtained from 55 healthy donors and 36 COVID-19 recovered individuals. ns, nonsignificant; *P < 0.05.
Remarkable reduction in the Tc1, Tc2, and Tc17 subsets of CD8+ T cells in the CR cohort
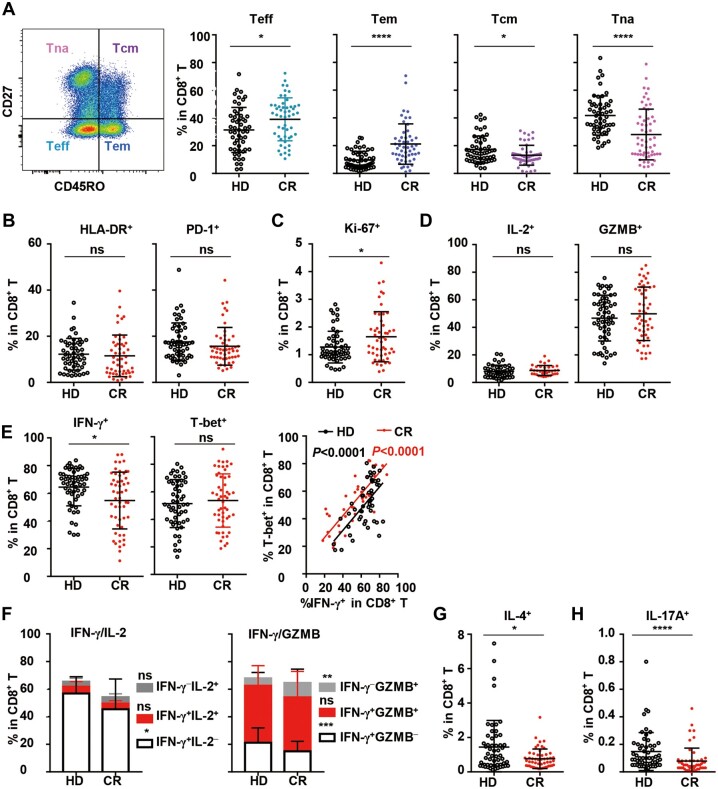
In the context of CD8+ T cells, the frequencies of both effector T cells (CD45RO−CD27−, Teff) and effector memory T cells (CD45RO+CD27−, Tem) in the CR cohort were significantly higher than those in the HD cohort, while the frequencies of naïve T cells (CD45RO−CD27+, Tna) and central memory T cells (CD45RO+CD27+, Tcm) in the CR cohort were lower than those in the HD cohort (Figure 2A). The results above indicated that significant differentiation occurred for CD8+ T cells post SARS-CoV-2 infection. In terms of activation, the expression of human leukocyte antigen receptor (HLA-DR) and programmed cell death protein 1 (PD-1) on CD8+ T cells in the CR cohort was similar to that on CD8+ T cells in the HD cohort (Figure 2B;Supplementary Figure S1A and B), although slightly higher proliferation activity was indicated by the presence of Ki-67+ cells in the Tcm subsets (Figure 2C;Supplementary Figure S1C). Then, the functions of CD8+ T cells were analyzed under polyclonal stimulation. Intriguingly, although the frequencies of IL-2+ and granzyme B (GZMB)+ CD8+ T cells showed no difference between the CR and HD cohorts (Figure 2D), the frequency of IFN-γ+ CD8+ T cells (Tc1) in the CR cohort was notably reduced compared to that in the HD cohort. However, T-bet expression on CD8+ T cells in the CR cohort was not reduced, and the correlation between T-bet+ and IFN-γ+ cells was still observed (Figure 2E). Further analysis showed that the proportions of IFN-γ+IL-2− cells and IFN-γ+GZMB− cells in CD8+ T cells in the CR cohort significantly reduced, while the percentage of IFN-γ−GZMB+ CD8+ T cells remarkably increased compared to that in the HD cohort (Figure 2F). In addition, both the frequencies of IL-4+ CD8+ T cells (Tc2) and IL-17A+ CD8+ T cells (Tc17) in the CR cohort were significantly reduced (Figure 2G and H). Moreover, the frequencies of Tc1, Tc2, and Tc17 cells in the CR cohort were consistently different from those in the HD cohort and showed no tendency to recover with the increase in the number of days post clinical discharge from 4 to 11 weeks of clinical recovery (Supplementary Figure S6A‒C). The data suggested the remarkable functional repression of CD8+ T cells in the CR cohort, although their activation, proliferation, and differentiation might be normal.
Figure 2.
Differentiation, activation, proliferation, and function of CD8+ T cells in peripheral blood from the HD and CR cohorts. (A) Gating strategy and the frequencies of Teff (CD45RO−CD27−), Tem (CD45RO+CD27−), Tcm (CD45RO+CD27+), and Tna (CD45RO−CD27+) subsets among CD8+ T cells in PBMCs. (B and C) Frequencies of HLA-DR+, PD-1+, and Ki-67+ CD8+ T cells. To analyze the function of CD8+ T cells, PBMCs were stimulated with PMA/ionomycin for 4.5 h in the presence of BFA and monensin. (D–H) The production of IL-2 (D, left panel), GZMB (D, right panel), IFN-γ (E, left panel), IFN-γ/IL-2, IFN-γ/GZMB (F), IL-4 (G), and IL-17A (H) by CD8+ T cells were analyzed by intracellular staining. T-bet+ cells among CD8+ T cells without stimulation (E, middle panel) and the correlation of T-bet+ with IFN-γ+ (E, right panel) are shown. Data were obtained from 55 healthy donors and 55 COVID-19 recovered individuals, except that data on IL-2 (D) and IFN-γ/IL-2 (F) were obtained from 55 healthy donors and 36 COVID-19 recovered individuals. ns, nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Significant reductions in the Tcm, PD-1+, Th1, Th2, Th17, and cTfh subsets of CD4+ T cells in the CR cohort
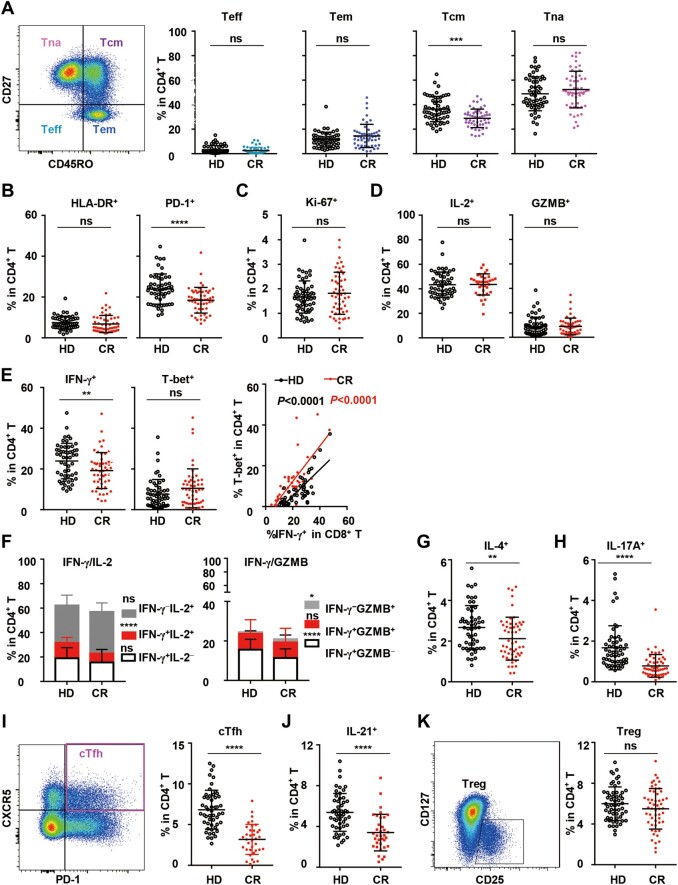
In the context of CD4+ T cells, the frequencies of Teff, Tem, or Tna cells in the CR cohort showed no change, but the frequency of Tcm cells was remarkably reduced compared to that in the HD cohort (Figure 3A). In terms of activation, the frequencies of HLA-DR+ CD4+ T cells in the CR and HD cohorts also showed no difference, although the frequency of HLA-DR+ CD4+ Tna cells in the CR cohort was significantly reduced (Figure 3B;Supplementary Figure S2A). However, the frequency of PD-1+ cells in all CD4+ T cell subsets was remarkably reduced in the CR cohort (Figure 3B;Supplementary Figure S2B), indicating the significant repression of CD4+ T cell activation in the CR cohort. Proliferation, as indicated by Ki-67 expression, showed no difference between the CR and HD cohorts (Figure 3C), although the frequency of Ki-67+ cells in the Tcm subset in the CR cohort was slightly higher (Supplementary Figure S2C). Functional analysis under polyclonal stimulation showed that the frequency of IFN-γ+ CD4+ T cells (Th1) in the CR cohort was notably lower than that in the HD cohort (Figure 3E, left panel), although the frequencies of IL-2+ and GZMB+ CD4+ T cells showed no difference between the CR and HD cohorts (Figure 3D). Similar to that of CD8+ T cells, the frequency of T-bet+ CD4+ T cells in the CR cohort was not reduced, and the correlation between T-bet+ and IFN-γ+ cells was still observed (Figure 3E, middle and right panels). In more detail, the proportions of IFN-γ+IL-2+ and IFN-γ+GZMB− CD4+ T cells in the CR cohort were significantly reduced, but the proportion of IFN-γ−GZMB+ CD4+ T cells was remarkably increased (Figure 3F). In addition, the frequencies of IL-4+ CD4+ T cells (Th2) and IL-17A+ CD4+ T cells (Th17) in the CR cohort were both reduced significantly (Figure 3G and H), indicating that the functions of Th1, Th2, and Th17 cells were somehow repressed.
Figure 3.
Differentiation, activation, proliferation, and function of CD4+ T cells in peripheral blood in the HD and CR cohorts. (A) Frequencies of Teff (CD45RO−CD27−), Tem (CD45RO+CD27−), Tcm (CD45RO+CD27+), and Tna (CD45RO−CD27+) subsets among CD4+ T cells in PBMCs. (B and C) Frequencies of HLA-DR+, PD-1+, and Ki-67+ cells in CD4+ T cells. To analyze the function of CD4+ T cells, PBMCs were stimulated with PMA/ionomycin for 4.5 h in the presence of BFA and monensin. (D–H and J) The production of IL-2 (D, left panel), GZMB (D, right panel), IFN-γ (E, left panel), IFN-γ/IL-2, IFN-γ/GZMB (F), IL-4 (G), IL-17A (H), and IL-21 (J) by CD4+ T cells in the HD and CR cohorts was analyzed by intracellular staining. T-bet+ cells in CD4+ T cells without stimulation (E, middle panel) and the correlation of T-bet+ with IFN-γ+ (E, right panel) are shown. (I and K) Gating strategy and the frequency of cTfhs (CXCR5+PD-1+) and Tregs (CD25+CD127+) in CD4+ T cells in the HD and CR cohorts. Data were obtained from 55 healthy donors and 55 COVID-19 recovered individuals, except that data on IL-2 (D), IFN-γ/IL-2 (F), and in I and J were obtained from 55 healthy donors and 36 COVID-19 recovered individuals. ns, nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Follicular helper T (Tfh) cells, which express CXCR5 as a marker, are a specialized subset of CD4+ T cells necessary for germinal centers and related B cell responses (Crotty, 2019). The frequency of circulating Tfh cells (cTfhs, PD-1+CXCR5+) in the peripheral blood in the CR cohort was distinguishably lower than that in the HD cohort, and the percentage of IL-21-expressing CD4+ T cells in the CR cohort was also lower than that in the CR cohort (Figure 3I and J). cTfhs can be further classified into three subsets with different capabilities: cTfh1 (CCR6−CXCR3+), cTfh2 (CCR6−CXCR3−), and cTfh17 cells (CCR6+CXCR3−) (Morita et al., 2011). The percentage of cTfh1 cells in the CR cohort was remarkably lower than that in the HD cohort, whereas the relative proportions of cTfh2 and cTfh17 cells in the CR cohort were correspondingly higher (Supplementary Figure S3A). Similar to that in the whole CD4+ T population, activation, as indicated by inducible T cell costimulator (ICOS) expression, and proliferation, as indicated by Ki-67 expression, in the cTfh subset showed no differences between the CR and HD cohorts (Supplementary Figure S3B and C). Moreover, Th1, Th2, Th17, and cTfh cells in the CR cohort consistently deviated from those in the CR cohort and showed no tendency to recover within 4–11 weeks post clinical discharge (Supplementary Figure S6D‒G).
In contrast to the Th effector subsets, the frequencies of regulatory T cells (Tregs, CD3+CD8−CD4+CD127−CD25+) in the CR and HD cohorts were not significantly different (Figure 3K). For Treg subsets, the frequency of activated Tregs (CD45RA−FoxP3hi, aTreg) in the CR cohort was not different from that in the HD cohort, although resting Tregs (CD45RA+FoxP3lo, rTreg) in the CR cohort showed a slightly lower frequency (Supplementary Figure S4A). cytotoxic T lymphocyte-associated antigen 4 (CTLA4) is one of the most important coinhibitory molecules expressed by Tregs. As shown in Supplementary Figure S4B, the frequencies of CTLA4+ Tregs and rTregs in the CR cohort were similar to those in the HD cohort, except for CTLA4+ aTregs, which showed a relatively lower frequency in the CR cohort.
Significant reduction in IgM− memory B cells in the CR cohort
The frequencies of B cell subsets, including plasmablasts (CD27+CD38hi), naïve B cells (CD27−CD38lo), and memory B cells (MBCs, CD27+CD38lo), were not different between the CR and HD cohorts (Figure 4A). However, the percentage of isotype-switched MBCs (IgM−CD20hi) in the CR cohort was significantly lower than that in the HD cohort (Figure 4B). More detailed analysis of the B cell activation marker CD71 and the costimulatory molecule ICOS ligand (ICOSL) was carried out. The frequencies of CD71+ cells among both IgM+ MBCs and IgM− MBCs but not naïve B cells in the CR cohort were higher than those in the HD cohort (Figure 4C). The frequencies of ICOSL+ cells were similar among IgM+ MBCs, IgM− MBCs, and naïve B cells in the HD and CR cohorts (Figure 4C). SARS-CoV-2 infection had an effect on isotype-switched MBCs that appeared to be long lasting (Figure 4B) because MBCs in the CR cohort were still in the process of active proliferation.
Figure 4.
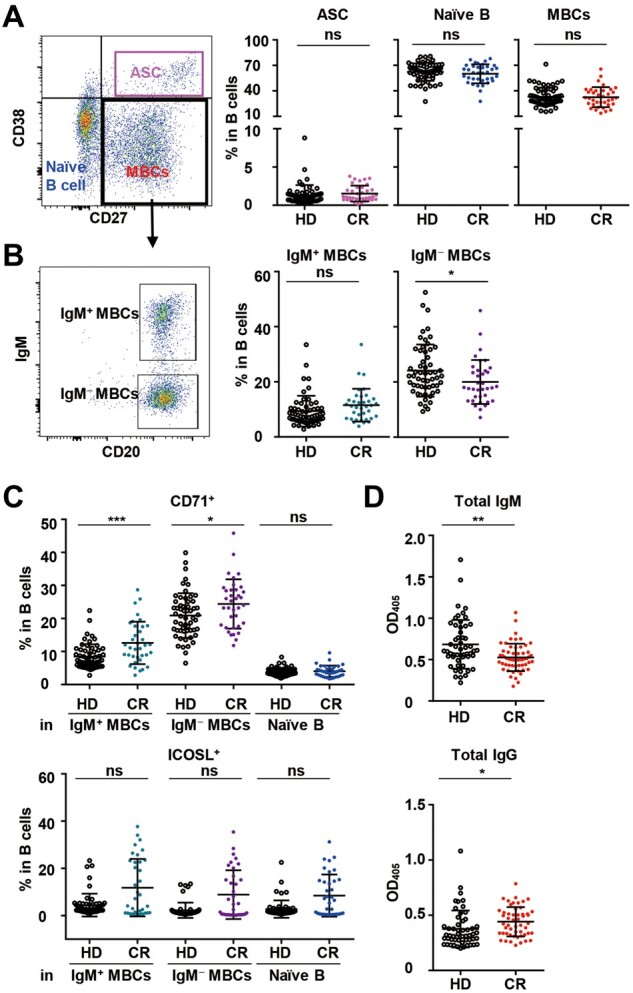
Differentiation and activation of B cells in PBMCs and total IgM and IgG in plasma in the HD and CR cohorts. (A–C) Phenotypic analysis of B cells in PBMCs. (A) Gating strategy and the frequencies of the naïve B cell (CD38loCD27−), plasmablast (CD38hiCD27−), and memory B cell (MBC, CD38loCD27+) subsets in B cells. (B) The frequencies of nonisotype-switched IgM+ MBCs (IgM+CD20hi) and isotype-switched IgM− MBCs (IgM−CD20hi) in B cells. (C) The frequencies of CD71+ and ICOSL+IgM+ MBCs, IgM− MBCs, and naïve B cells. (D) Total IgM and IgG in plasma were assayed by ELISA. A dilution of 1:25600 was used for IgM and 1:512000 for IgG. Data in A–C were obtained from 55 healthy donors and 36 COVID-19 recovered individuals, while data in D were obtained from 55 healthy donors and 55 COVID-19 recovered individuals. ns, nonsignificant; *P < 0.05; **P < 0.01; ***P < 0.01.
To further characterize the functional potential of B cells, total IgM and IgG levels in plasma were assayed by ELISA. Preliminary experiments were carried out by serial dilution of a small number of plasma samples to obtain the optimal dilutions. Finally, a dilution of 1:25600 was used for IgM and of 1:512000 for IgG. As shown in Figure 4D, the total IgM level in the CR cohort was lower than that in the HD cohort, whereas the total IgG level in the CR cohort was higher than that in the HD cohort.
High potential for dysfunction of CD3−HLA-DR− lymphocytes in the CR cohort
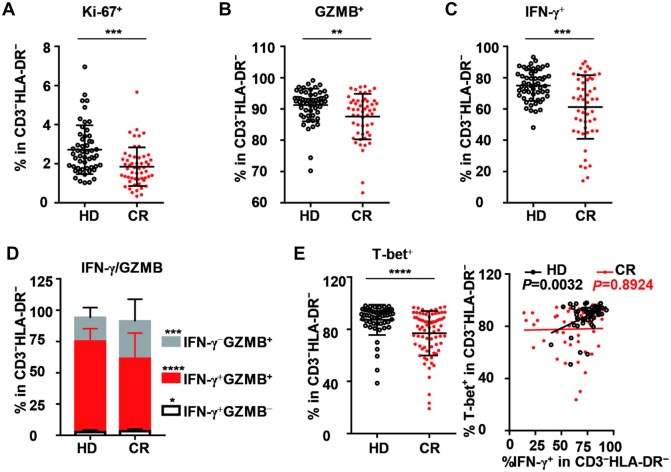
Among CD3−HLA-DR− lymphocytes in the CR cohort, the frequency of Ki-67+ cells was significantly lower than that in the HD cohort (Figure 5A). Furthermore, under polyclonal stimulation, the frequencies of GZMB+ and IFN-γ+CD3−HLA-DR− cells in the CR cohort were both significantly lower than those in the HD group (Figure 5B and C). Among CD3−HLA-DR− cells, the proportion of IFN-γ+GZMB+ cells in the CR cohort was remarkably reduced, while the proportion of IFN-γ−GZMB+ cells was markedly increased compared to that in the HD cohort (Figure 5D). On the other hand, the frequency of T-bet-expressing cells among CD3−HLA-DR− cells in the CR cohort was also reduced. Furthermore, the correlation between T-bet+ and IFN-γ+ disappeared in CD3−HLA-DR− cells in the CR cohort, whereas a good correlation was observed between them in the HD cohort (Figure 5E). Further analysis showed that ∼85% of cells in the CD3−HLA-DR− population were CD56+ NK cells in both the CR and HD cohorts (Supplementary Figure S5A and B), suggesting that the phenotypes of CD3−HLA-DR− cells described above were mostly ascribed to NK cells. Similar to that of CD8+ and CD4+ T cells, the IFN-producing potential of CD3−HLA-DR− lymphocytes in the CR cohort showed no tendency toward recovery with an increase in the number of days post clinical discharge (Supplementary Figure S6H).
Figure 5.
Proliferation and function of CD3−HLA-DR− lymphocytes in peripheral blood in the HD and CR cohorts. (A) Frequency of Ki-67+ cells in CD3−HLA-DR− lymphocytes of PBMCs. To analyze the function of CD3−HLA-DR− lymphocytes, PBMCs were stimulated with PMA/ionomycin for 4.5 h in the presence of BFA and monensin. (B–D) The production of GZMB (B), IFN-γ (C), and IFN-γ/GZMB (D) by CD3−HLA-DR− lymphocytes were analyzed by intracellular staining. (E) T-bet+ cells in CD3−HLA-DR− lymphocytes without stimulation (left panel) and correlation of T-bet+ with IFN-γ+ (right panel) in CD3−HLA-DR− lymphocytes. All data were obtained from 55 healthy donors and 55 COVID-19 recovered individuals. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
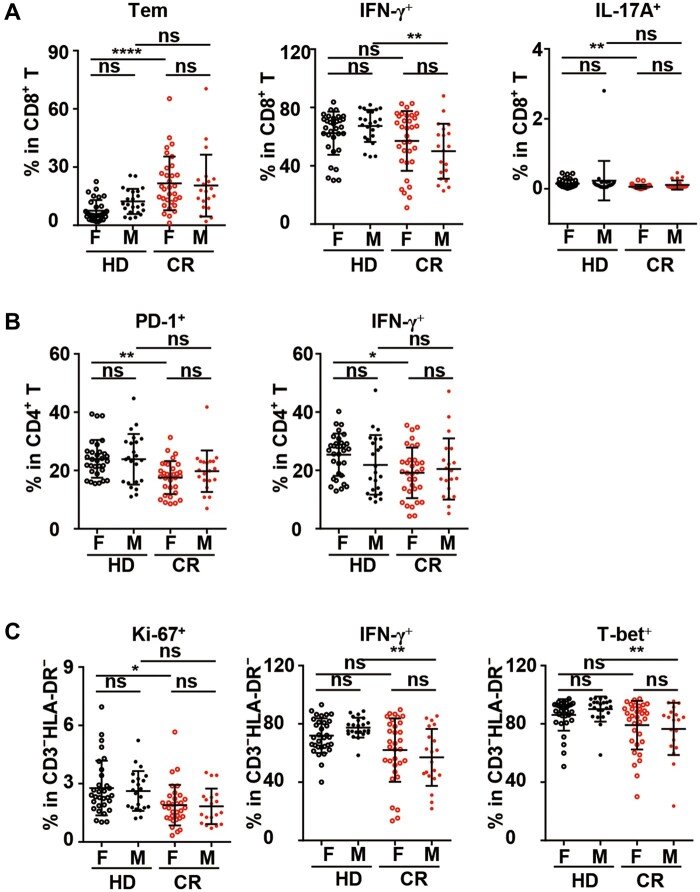
Males were more vulnerable to potential dysfunction of CD8+ and CD3−HLA-DR− lymphocytes
Sex has been reported to be a factor in COVID-19, with higher numbers of cases, greater disease severity, and higher death rates being observed among men than women across the lifespan (Gadi et al., 2020; Guan et al., 2020). Therefore, based on sex, we reanalyzed the data that were significantly different between the two cohorts at the total cohort level. First, in both the HD and CR cohorts, no significant difference existed between females and males.
Among CD8+ T cells, unlike the analysis at the total cohort level, the frequencies of Teff, Tcm, Ki-67+, and Tc2 cells in the female or male sub-CR cohort were not significantly different from those in their counterparts in the HD cohort. Moreover, a substantial increase in Tem cells and a significant decrease in Tc17 cells in the CR cohort were only observed in females, while a remarkable decrease in Tc1 cells was found in males alone (Figure 6A;Supplementary Figure S7A). For CD4+ T cells, similar to the statistical results at the total cohort level, decreases in the Tcm, Th17, IL-21-producing, and cTfh cell subsets were also observed in the female or male sub-CR cohorts compared with the respective subcohorts in the HD cohort. However, unlike the results of the analysis at the total cohort level, the frequency of Th2 cells in the female and male sub-CR cohorts was not significantly different from that in their counterparts in the HD cohort. Moreover, significant decreases in the PD-1+ and Th1 subsets were only observed in females in the CR cohort (Figure 6B;Supplementary Figure S7B). For B cells, unlike the results of the analysis at the total cohort level, the frequencies of IgM− MBCs, CD71+IgM+ MBCs, and CD71+IgM− MBCs in the female and male sub-CR cohorts were not significantly different from those in their counterparts in the HD cohort (Supplementary Figure S7C). Among CD3−HLA-DR− lymphocytes in the CR cohort, the frequencies of GZMB+ cells in female and male sub-CR cohorts were not significantly different from that in their counterparts in the HD cohort. Moreover, a significant decrease in Ki-67+ cells was only observed in females in the CR cohort, while a remarkable decrease in IFN-γ-producing and T-bet+ cells was found in males alone (Figure 6C;Supplementary Figure S7D).
Figure 6.
Sex-based differences in phenotypic alterations and potential dysfunction of lymphocytes in the CR cohort. To elucidate the sex-based differences in lymphocyte changes in the CR cohort, the data from Figures 2−5, which were significantly different between the HD and CR cohorts at the total cohort level, were reanalyzed. (A) Frequencies of Tem, IFN-γ+, and IL-17A+ CD8+ T cells in PBMCs. (B) Frequencies of PD-1+ and IFN-γ+ cells in CD4+ T cells in PBMCs. (C) Frequencies of Ki-67+, IFN-γ+, and T-bet+ cells in CD3−HLA-DR− lymphocytes. ns, nonsignificant; *P < 0.05; **P < 0.01; ****P < 0.0001.
Discussion
Upon SARS-CoV-2 infection, a comprehensive understanding of the host immune response, the immunopathological characteristics, and the immune protective effect in COVID-19 patients and recovered individuals is very important for developing effective therapeutic treatment and prophylactic vaccine. Because of the critical role of T cells in defending against viruses, studies on specific T cell responses against SARS-CoV-2, as performed previously (Sekine et al., 2020; Weiskopf et al., 2020), are undoubtedly extremely important. Nevertheless, it is also essential to characterize the changes in the general immune response, including the immune phenotype and functional potential of lymphocytes, over time (Mathew et al., 2020). In this study, we generated cross-sectional lymphocyte response data from a COVID-19 CR cohort in Wuhan. By using flow cytometry with different molecule marker sets, we generated an overview picture of the CD8+ T, CD4+ T, B, and CD3−HLA-DR− populations in terms of differentiation, proliferation, activation, and functional potential. In CR individuals, a significant loss in CD4+ T cells was still observed after clinical recovery. Specifically, the loss was reflected to a significant degree in the decreased percentages of CD4+ Tcm cells in the CD4+ T cell population (Figure 1B) of the CR cohort.
What is even more remarkable is that profoundly decreased function was observed in almost all T cell subsets we tested from the CR cohort, including Tc1, Tc2, Tc17, Th1, Th2, Th17, and cTfh cells. The decrease in function was persistent even 11 weeks after the CR cohort had clinically recovered. This suggests that COVID-19 patients experienced long-lasting repression of the function of both CD4+ and CD8+ T cells. The long-lasting dysfunction of T lymphocytes is common in patients infected by viruses that could induce chronic infection, such as HIV and HCV, or cancer patients (Douek et al., 2002; Ahmed et al., 2019; Hodgins et al., 2019) but is rarely reported in patients infected by viruses that only induce acute infection, except for the reported loss of Th17 cells in influenza-infected individuals (Jiang et al., 2010). To the best of our knowledge, there is currently no report regarding whether this kind of long-lasting reduction in function occurs in patients infected with the highly pathogenic coronavirus, Middle East respiratory syndrome coronavirus or SARS-CoV. Our findings in the present study suggested that SARS-CoV-2 infection may uniquely affect lymphocytes and maintain suppression of the functions of lymphocytes for a long time. The mechanism underlying specific lymphocyte loss in COVID-19 patients warrants further investigation.
In the present study, we observed a profound reduction in CXCR5+PD-1+ cTfhs in the CR cohort. Kuri-Cervantes et al. (2020) and Mathew et al. (2020) both reported that the proportions of cTfhs were similar in healthy donors, acute COVID-19 patients, and recovered individuals. The discrepancy in our study compared with the other two studies might be due to, at least in part, confounding demographic factors such as age, sex, race, and the size of the cohorts. Moreover, Mathew et al. (2020) also documented that the expression of CXCR5 was significantly decreased on cTfhs from both COVID-19 patients and recovered donors. Additionally, the loss of Bcl-6+ Tfh cells and germinal centers and a striking reduction in Bcl-6+ germinal center B cells were found in the thoracic lymph nodes and spleens of deceased COVID-19 patients (Duan et al., 2020; Kaneko et al., 2020). Moreover, parallel peripheral blood studies revealed the loss of follicular B cells in severe disease, which indicated the consistency of the lymphocyte response between the draining lymph nodes and peripheral blood. Together, these data identify defective Tfh cell generation in COVID-19 patients and suggest the possibility of a decrease in cTfhs, the circulating counterpart of Tfh cells, in peripheral blood.
It should be noted that the frequencies of cTfhs were reduced significantly in the CR cohort compared to the HD cohort, but the expression levels of both Ki-67 and ICOS in cTfhs in the CR and HD cohorts were similar, suggesting that there was no suppression of the proliferation and activation of cTfhs in the CR cohort (Figure 3I;Supplementary Figure S3). This lack of suppression is in line with the elevated activation indicated by expression of CD71 in memory B cells in the CR cohort (Weinstein et al., 2018). The lower frequency of IgM isotype-switched MBCs in the CR cohort than in the HD cohort indicated that the induction of isotype switching in MBCs might be repressed by SARS-CoV-2 infection. This repression of isotype switching might be related to the reduced frequency of cTfhs, which are critical for the differentiation and isotype switching of germinal center B cells (Weinstein et al., 2018). In a recent report on the antibody responses of individuals who recovered from SARS-CoV-2 infection, SARS-CoV-2-specific antibody responses rapidly waned within 8 weeks (Long et al., 2020). As IgM− isotype-switched MBCs are more responsive than IgM+ MBCs (Marasco et al., 2017), the decrease in IgM− isotype-switched MBCs might be one reason for the sharp decrease in SARS-CoV-2-specific antibody responses. Moreover, the total IgM level in the CR cohort was less than that in the HD cohort (Figure 4D), which indicated that SARS-CoV-2 also reduced the production of nonspecific IgM.
We further found that the proportions of IFN-γ−GZMB+ cells were significantly higher among CD4+ T cells, CD8+ T cells, and CD3−HLA-DR− lymphocytes in the CR cohort than among those in the HD cohort, in contrast to the reduced proportions of IFN-γ+ cells in the corresponding cell populations (Figures 2E and F, 3E and F, and 5C and D). In the CD3−HLA-DR− cell population, the correlation between T-bet and IFN-γ disappeared in the CR cohort, whereas a good correlation was observed in the HD cohort (Figure 5E). These data suggested that perturbations were also observed in T cells and CD3−HLA-DR− lymphocytes from COVID-19 CR individuals in addition to repression. However, further classification of CD3−HLA-DR− lymphocytes was not performed in this study due to the lack of corresponding antibodies during the COVID-19 crisis in Wuhan. Although ∼85% of the CD3−HLA-DR− lymphocytes might be NK cells, as we further determined in either the CR or HD cohort, there is another important cell population, innate lymphocytes (ILCs), which are also CD3−HLA-DR− cells. The responses and statuses of NK cells and ILCs in the CR cohort should be of high interest for understanding COVID-19.
Since a growing body of studies have reported greater disease severity and higher death rates among men than women (Gadi et al., 2020; Guan et al., 2020; Shi et al., 2020), we also analyzed the association of sex with the long-term phenotype and dysfunction potential of lymphocytes. Although the statistical analysis results at the sex-based subcohort level were somewhat different from those at the total cohort level, it was also suggested that long-term decreases in function were also observed in the female and male sub-CR cohorts, especially in CD8+ T, CD4+ T, and CD3−HLA-DR− lymphocytes. For CD4+ T cells, the susceptibility of females and males were very similar, which was exemplified by decreases in both Th17 and cTfh cells. However, in terms of CD8+ T and CD3−HLA-DR− lymphocytes, males were more vulnerable, as shown by the lack of differentiation of CD8+ Tem cells and a decrease in IFN-γ-producing CD8+ T and CD3−HLA-DR− lymphocytes. Therefore, in agreement with the sex-based susceptibility to COVID-19 described in previous studies, a higher probability of long-term lymphocyte dysfunction was found among men than women in our cohort. The female sex hormones and the immune stimulatory genes, including Toll-like receptors, interleukins, and microRNAs, present on the X chromosome (Chanana et al., 2020) may result in reduced infectivity and mortality due to SARS-CoV-2, as described in previous studies, and a lower probability of long-term lymphocyte dysfunction in females than in males in our study.
After infection with SARS-CoV-2, patients experience asymptomatic to severe disease or even death. Numerous studies have reported that COVID-19 severity impacted the lymphocyte count. Lymphopenia, i.e. a reduction in lymphocytes, especially T and NK cells, was reported in individuals with COVID-19, and decreased frequencies of these cells were associated with more severe disease (Chen et al., 2020; Kuri-Cervantes et al., 2020; Mathew et al., 2020; Sekine et al., 2020). Moreover, disease severity also affected the activation of T and NK cells. By deep immune profiling of 125 COVID-19 patients with various disease severities, Mathew et al. (2020) found that HLA-DR+CD38+ CD8+ T cells as well as both Ki-67+ and HLA-DR+CD38+ CD4+ T cells were increased in patients with more severe disease. By further employing uniform manifold approximation and projection embedding, they suggested a link between CD4+ T cell activation and an increased severity score (Mathew et al., 2020). Kuri-Cervantes et al. (2020) observed that CD16, an activation marker of NK cells, was markedly downregulated in patients with severe disease. However, disease severity had different impacts on the formation of SARS-CoV-2-specific CD4+ and CD8+ T cells. A higher frequency of SARS-CoV-2-specific CD4+ T cells was seen in convalescent donors with severe disease, whereas the production of SARS-CoV-2-specific CD8+ T cells was reduced (Sekine et al., 2020). Collectively, the results indicated that COVID-19 severity impacted the count and activation of T and NK cells, but the impact was not uniform. However, in our CR cohort, there were only 2 patients (#30 and #32) with a history of severe COVID-19, and the remaining 53 patients had a history of mild to moderate COVID-19. Therefore, it is not possible to divide the patients into two subcohorts because of the limited number of subjects with a medical history of severe disease.
Protein kinase C (PKC) is a key enzyme in T lymphocytes, where it plays an important role in signal transduction downstream of the activated T cell antigen receptor and the CD28 costimulatory receptor (Isakov and Altman, 2012). Phorbol 12-myristate 13-acetate (PMA), a phorbol ester, is a PKC activator, while ionomycin is a calcium ionophore. Therefore, cells can be activated by stimulation with optimal concentrations of PMA/ionomycin in vitro. In this study, we used the polyclonal stimulator PMA/ionomycin, which is widely applied by immunologists for detecting T cell functional potential in infections by viruses such as respiratory syncytial virus (Knudson et al., 2015) and hepatitis B virus (Chang et al., 2019) as well as SARS-CoV-2 (MazzoNi et al., 2020; Zheng et al., 2020), to evaluate the functional potential of CD8+ T, CD4+ T, and CD3−HLA-DR− lymphocytes. Since they act as the first and second activation signals of T cells, αCD3 and αCD28 are also frequently used as polyclonal stimulators of T cells. In a recent work, Mathew et al. (2020) used αCD3 plus αCD28 to stimulate PBMCs in vitro. They found that PBMCs from COVID-19 patients produced more cytokines and chemokines than those from healthy donors, and the concentrations of some chemokines were correlated in the matched supernatants from stimulated PBMCs and plasma samples, which supported the notion that PBMCs produce proinflammatory cytokines and leukocyte-recruiting chemokines (Mathew et al., 2020).
Our studies of the phenotypic alterations and potential dysfunctions of lymphocytes in COVID-19 recovered patients focused on peripheral blood. Since Kaneko et al. (2020) found that the loss of follicular B cells in peripheral blood in severely ill COVID-19 patients was consistent with the striking reduction in Bcl-6+ germinal center B cells in the parallel thoracic lymph nodes and spleen, it is quite possible that the changes in the respiratory tract or secondary lymphoid compartments in the CR cohort may reflect similar changes in blood. Nevertheless, a deeper understanding will likely await the acquisition of more knowledge and the development of suitable animal models. For example, human ACE2-expressing mice will be very helpful in addressing how lymphocyte responses in the blood are related to lung-infiltrating lymphocytes during the acute and convalescence phases of SARS-CoV-2 infection (Chen and John Wherry, 2020; Jiang et al., 2020).
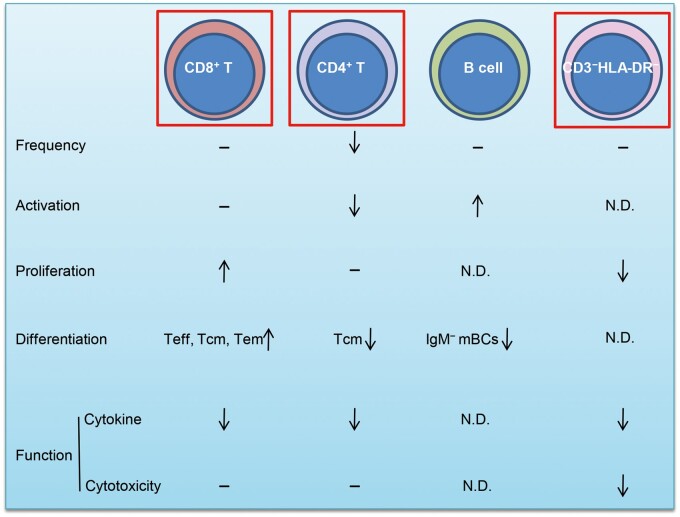
In summary, we provided a cross-sectional profile of lymphocyte responses in a COVID-19 CR cohort and found significant long-term phenotype alterations and potential dysfunctions of lymphocytes in this cohort (Figure 7). We still do not know how long the phenotypic alterations and potential dysfunctions of lymphocytes will last. As reported, CD4+ T cells, CD8+ T cells, and NK cells are all critical for the control of intracellular pathogen infections and tumors (Douek et al., 2002; Yang et al., 2016; Molgora et al., 2017) and can coordinate with each other (Kelly et al., 2013; Bai et al., 2019). CD4+ T cells, especially Tfh and B cells, are also critical for the processes that lead to long-term humoral immunity (Morita et al., 2011). The broad long-term dysfunction of these lymphocyte subsets might profoundly impair immune surveillance and protection by lymphocytes in individuals clinically recovered from COVID-19, although SARS-CoV-2-specific memory CD4+ T cells and CD8+ T cells with broad and strong effects could be detected in COVID-19 patients (Peng et al., 2020; Sekine et al., 2020; Weiskopf et al., 2020). A recent study on SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals highlighted that antiviral T cells may not be maintained at high numbers in PBMCs in recovered patients (Ni et al., 2020). This finding alerts us more to concerns regarding the prognosis of COVID-19 patients. Considering that the SARS-CoV-2-specific antibody response also sharply wanes over time (Long et al., 2020), it is hard to speculate whether the clinically recovered population could resist reinfection for a long period. If lymphocyte dysfunction is sustained for a long time, this population might be even more susceptible to SARS-CoV-2 infection or other viral infections. Hence, more comprehensive longitudinal and cross-sectional profiling studies of the relationship of immune responses and SARS-CoV-2 infection in different cohorts are urgently needed.
Figure 7.
The phenotypic alterations and potential dysfunction of lymphocytes in individuals clinically recovered from COVID-19 compared with those in healthy controls. −, without significant change; ↓, reduction; ↑, upregulation; N.D., not detected. The CD8+ T, CD4+ T, and CD3−HLA-DR− cells contained in the red rectangles warrant further study.
Materials and methods
Study approval
This study was reviewed and approved by the Medical Ethical Committee of Wuhan Jinyintan Hospital (approval number KY-2020-47.01). Written informed consent was obtained from individuals recovered from COVID-19 and healthy donors.
Study design and participants
We performed a cohort study on individuals aged 25–70 years in Wuhan Jinyintan Hospital in April 2020. We collected peripheral blood from 72 individuals who had clinically recovered from documented COVID-19 and were recruited for physical re-examination after discharge for at least 4 weeks and from 61 healthy donors who were recruited for regular physical examination.
Clinically recovered individuals with redetection of virus positivity (3 persons) and those with both SARS-CoV-2 receptor-binding domain (RBD)-specific IgM and IgG negativity in plasma (13 persons) were excluded. Healthy donors without ongoing or past SARS-CoV-2 infection were enrolled and confirmed based on nucleotide acid assays of nasopharyngeal swab samples and SARS-CoV-2 RBD-specific IgM and IgG assays of plasma (two persons excluded). Individuals who had chronic conditions, such as HIV infection, HCV infection, cerebrovascular disease, and kidney disease, were also excluded (one clinically recovered individual was excluded). To ensure age- and sex-matching of the CR and HD cohorts, four young male individuals were further excluded.
After exclusion, a cohort of 55 healthy donors (HD, 23 male and 32 female, mean age 49.1, median age 51) and a cohort of 55 COVID-19 clinically recovered patients (CR, 21 male and 34 female, mean age 48.8, median age 51) were involved in the study (Supplementary Table S1), except for the assay of cTfh and B cells and the cytokine IL-2, for which a subcohort of only 36 clinically recovered individuals (14 male and 22 female, mean age 50.6, median age 52) was used due to a lack of antibody reagents.
Clinical laboratory measurements
The clinical laboratory measurements, including the complete blood count test, SARS-CoV-2 RBD-specific antibody detection using colloidal gold strips, and SARS-CoV-2-specific nucleotide detection, were performed at Wuhan Jinyintan Hospital in April 2020. Nasopharyngeal swab samples were collected on the day of peripheral blood collection and were tested by quantitative real-time polymerase chain reaction for amplification of the E gene, RdRp gene, and N gene of SARS-CoV-2 as described by our previous study (Cao et al., 2020).
Lymphocyte response evaluation by flow cytometry
Plasma and cell pellets were separated from fresh peripheral blood from clinically recovered subjects and healthy donors. Plasma was used for the detection of SARS-CoV-2-binding IgM or IgG and total IgM and total IgG. PBMCs were separated from the cell pellets after resuspension in phosphate-buffered saline by density gradient centrifugation and used for lymphocyte response evaluation by flow cytometry as described in Supplementary Materials and methods.
Measurement of total IgM and IgG in plasma
Total IgM and IgG in plasma were determined by ELISA as described in Supplementary Materials and methods.
Statistical analysis
Data are presented as mean ± SD. To analyze the differences between the CR and HD cohorts, unpaired two-tailed Student’s t-test was used for normally distributed data with homogeneous variance, and the Mann–Whitney U test was used for nonnormally distributed data. To analyze the tendency of the functional recovery of lymphocytes or the association of T-bet and IFN-γ, linear regression was used. Statistical analysis was carried out with InStat, version 8.0 (GraphPad Software). P < 0.05 was considered significant.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Supplementary Material
Acknowledgements
We thank the individuals who recovered from COVID-19 and healthy donors involved in this study and the staff at Wuhan Jinyintan Hospital.
Funding
This work was supported by the Major Projects of Technological Innovation in Hubei Province (2019ABA089) and the Kunming Science and Technology Department (2020-1-N-037).
Conflict of interest: none declared.
References
- Ahmed F., Ibrahim A., Cooper C.L., et al. (2019). Chronic hepatitis C virus infection impairs M1 macrophage differentiation and contributes to CD8+ T-cell dysfunction. Cells 8, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L., Peng H., Hao X., et al. (2019). CD8+ T cells promote maturation of liver-resident NK cells through the CD70‒CD27 axis. Hepatology 70, 1804–1815. [DOI] [PubMed] [Google Scholar]
- Callaway E. (2020). Coronavirus vaccines: five key questions as trials begin. Nature 579, 481. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., et al. (2020). A trial of lopinavir‒ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 382, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanana N., Palmo T., Sharma K., et al. (2020). Sex-derived attributes contributing to SARS-CoV-2 mortality. Am. J. Physiol. Endocrinol. Metab. 319, E562–E567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.M., Traum D., Park J.J., et al. (2019). Distinct phenotype and function of circulating Vδ1+ and Vδ2+ γδT-cells in acute and chronic hepatitis B. PLoS Pathog. 15, e1007715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., et al. (2020). Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., John Wherry E. (2020). T cell responses in patients with COVID-19. Nat. Rev. Immunol. 20, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Mascola J.R., Fauci A.S., et al. (2020). A strategic approach to COVID-19 vaccine R&D. Science 368, 948–950. [DOI] [PubMed] [Google Scholar]
- Crotty S. (2019). T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek D.C., Brenchley J.M., Betts M.R., et al. (2002). HIV preferentially infects HIV-specific CD4+ T cells. Nature 417, 95–98. [DOI] [PubMed] [Google Scholar]
- Duan Y.Q., Xia M.H., Ren L., et al. (2020). Deficiency of Tfh cells and germinal center in deceased COVID-19 patients. Curr. Med. Sci. 40, 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadi N., Wu S.C., Spihlman A.P., et al. (2020). What's sex got to do with COVID-19? Gender-based differences in the host immune response to coronaviruses. Front. Immunol. 11, 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., et al. (2020). Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., et al. (2020). Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins J.J., Khan S.T., Park M.M., et al. (2019). Killers 2.0: NK cell therapies at the forefront of cancer control. J. Clin. Invest. 129, 3499–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N., Altman A. (2012). PKC-θ-mediated signal delivery from the TCR/CD28 surface receptors. Front. Immunol. 3, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R.D., Liu M.Q., Chen Y., et al. (2020). Pathogenesis of SARS-CoV-2 in transgenic mice expressing human angiotensin-converting enzyme 2. Cell 182, 50–58.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T.J., Zhang J.Y., Li W.G., et al. (2010). Preferential loss of Th17 cells is associated with CD4 T cell activation in patients with 2009 pandemic H1N1 swine-origin influenza A infection. Clin. Immunol. 137, 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N., Kuo H.H., Boucau J., et al. (2020). Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 183, 143–157.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M.N., Zheng M., Ruan S., et al. (2013). Memory CD4+ T cells are required for optimal NK cell effector functions against the opportunistic fungal pathogen Pneumocystis murina. J. Immunol. 190, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson C.J., Hartwig S.M., Meyerholz D.K., et al. (2015). RSV vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 11, e1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Cervantes L., Pampena M.B., Meng W., et al. (2020). Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 5, eabd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie M. (2020). T cells found in coronavirus patients ‘bode well’ for long-term immunity. Science 368, 809–810. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., et al. (2020). Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 26, 1200–1204. [DOI] [PubMed] [Google Scholar]
- Marasco E., Farroni C., Cascioli S., et al. (2017). B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur. J. Immunol. 47, 131–143. [DOI] [PubMed] [Google Scholar]
- Mathew D., Giles J.R., Baxter A.E., et al. (2020). Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369, eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A., Salvati L., Maggi L., et al. (2020). Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J. Clin. Invest. 130, 4694–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgora M., Bonavita E., Ponzetta A., et al. (2017). IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature 551, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R., Schmitt N., Bentebibel S.E., et al. (2011). Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 34, 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.L., et al. (2020). Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 52, 971–977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Yin J., Wang W., et al. (2020). Downregulated gene expression spectrum and immune responses changed during the disease progression in patients with COVID-19. Clin. Infect. Dis. 71, 2052–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., et al. (2020). Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 21, 1336–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., et al. (2020). Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 71, 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., et al. (2020). Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183, 158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang Y., Shao C., et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 27, 1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J.S., Laidlaw B.J., Lu Y., et al. (2018). STAT4 and T-bet control follicular helper T cell development in viral infections. J. Exp. Med. 215, 337–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf D., Schmitz K.S., Raadsen M.P., et al. (2020). Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 5, eabd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zhang E., Zhong M., et al. (2020). Longitudinal characteristics of T cell responses in asymptomatic SARS-CoV-2 infection. Virol. Sin. 35, 838–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Bai Y., Xiong Y., et al. (2016). Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., et al. (2020). Viral and host factors related to the clinical outcome of COVID-19. Nature 583, 437–440. [DOI] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., et al. (2020). Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 17, 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.