Abstract
Monocyte to lymphocyte ratio (MLR) has been confirmed as a novel marker of poor prognosis in patients with coronary heart disease (CAD). However, the prognosis value of MLR for patients with CAD after percutaneous coronary intervention (PCI) needs further studies. In present study, we aimed to investigate the correlation between MLR and long-term prognosis in patients with CAD after PCI. A total of 3,461 patients with CAD after PCI at the First Affiliated Hospital of Zhengzhou University were included in the analysis. According to the cutoff value of MLR, all of the patients were divided into 2 groups: the low-MLR group (<0.34, n = 2338) and the high-MLR group (≥0.34, n = 1123). Kaplan–Meier curve was performed to compare the long-term outcome. Multivariate COX regression analysis was used to assess the independent predictors for all-cause mortality, cardiac mortality and MACCEs. Multivariate COX regression analysis showed that the high MLR group had significantly increased all-cause mortality (ACM) [hazard ratio (HR) = 1.366, 95% confidence interval (CI): 1.366-3.650, p = 0.001] and cardiac mortality (CM) (HR = 2.379, 95%CI: 1.611-3,511, p < 0.001) compared to the low MLR group. And high MLR was also found to be highly associated with major adverse cardiovascular and cerebrovascular events (MACCEs) (HR = 1.227, 95%CI: 1.003-1.500, p = 0.047) in patients with CAD undergoing PCI. MLR was an independent predictor of ACM, CM and MACCEs in CAD patients who underwent PCI.
Keywords: monocyte to lymphocyte ratio, long-term prognosis, coronary artery disease, percutaneous coronary intervention
Introduction
During the past several decades, extensive observations have supported the importance of inflammation in the development and destabilization of atherosclerosis, which means inflammation plays a causal role in atherosclerosis.1 Recently, CANTOS trial has revealed that modulation of inflammation decreases the risk of cardiovascular events further.2 Several inflammation biomarkers, such as high-sensitivity C-reactive protein (hs-CRP) total white blood cell (WBC) count, neutrophil, lymphocyte and monocyte count, have been confirmed to be independent predictors of death in patients with CAD.3,4 There is an evidence that the monocyte count is significantly correlated with peripheral endothelial dysfunction, which is an important part in the development of atherosclerosis, in CAD patients and plays a key role in the formation, development and rupture of plaque.5,6 Meanwhile, a prospective study suggested that increased numbers of monocytes were contributed to the development of atherosclerosis or plaque disruption and the peak monocyte counts during hospitalization and increased monocyte counts during follow-up period were related to plaque progression.7 High monocyte count and low lymphocyte count were reported as independent prognostic factors of long-term mortality and MACCEs in triple-vessel coronary artery disease.8 Meanwhile, there is a significant correlation between high monocyte count and low lymphocyte count and long-term outcome in patients with heart failure.9,10 Increased MLR, as a combination of 2 inflammation biomarkers has been confirmed as a novel marker of poor prognosis in patients with lung cancer.11 Low lymphocyte to monocyte ratio (LMR) was regarded as a high risk for peripheral arteriosclerotic occlusive disease (PAOD) and significantly associated with the poor 5-year overall survival rate in patients with classical Hodgkin lymphoma.12,13 Recently, MLR also has been demonstrated to be significantly associated with the lesion severity, and have better performance to reflect the severity of coronary lesion, compared to neutrophil to lymphocyte ratio (NLR).9 However, there are few studies about the relationship between MLR and the long-term outcomes in CAD patients after PCI. The aim of this study was to investigate the correlation between MLR and the long-term prognosis among patients with CAD who underwent PCI.
Methods
Study Population and Protocol
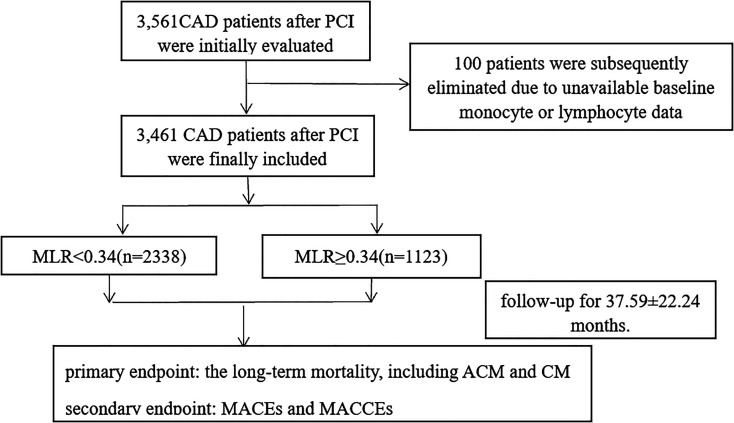
All of the patients were from the Clinical Outcomes and Risk Factors of Patients with Coronary Heart Disease after PCI, the details of which could be browsed on http://www.chictr.org.cn(registration number: ChiCTR1800019699). The CORFCHD-ZZ study, which was a large, retrospective cohort study, aimed to evaluate the clinical outcomes and factors affecting patients with CAD after PCI. 3561 post-PCI patients with CAD admitted to the First Affiliated Hospital of Zhengzhou University from January 2013 to December 2017 were included in the study and its data were obtained from case records and follow-ups. CAD was defined as at least 1 coronary artery stenosis ≥50% confirmed by coronary angiography with 1 or more drug-eluting stents implanted. Exclusion criteria included severe valvular heart disease, hyperthyroid heart disease, congenital heart disease, cardiomyopathy, chronic cor pulmonale, sever disfunction of liver and kidney, rheumatic autoimmune disease, tumor, hematologic disease, acute and chronic infectious disease, the history of PCI or coronary artery bypass graft (CABG) and the missing of clinical data and follow-up data. Finally, only 3,461 were eligible for study inclusion and 100 patients were excluded for the absent laboratory or follow-up data. Based on receiver operating characteristics (ROC) curve analysis, the best critical value for predicting long-term mortality of patients with coronary heart disease after PCI was 0.34. According to the critical value, patients were divided into 2 groups, the low MLR group (MLR < 0.34, n = 2.338) and the high MLR group (MLR ≥0.34, n = 1,123). Figure 1 shows the flowchart of participants enrollment.
Figure 1.
The flowchart of patient’s enrollment.
This study is in line with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University.
Study Procedures and Clinical Data Collection
The demographic data, laboratory data, and cardiovascular risk factors were collected and recorded for all study population. The cardiovascular risk factors included the prevalence of diabetes mellitus (DM) and hypertension, the history of smoking and the history of drinking. DM was defined by pre-existing condition diagnosed before admission (patients on insulin, oral glucose-lowering drugs or on a diet), or newly diagnosed DM based on fasting plasma glucose (FPG) ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L during an oral glucose tolerance test (OGTT). Hypertension was defined as the patient with blood pressure measurements ≥140/90 mmHg on at least 3 resting measurements on 3 mornings or receiving treatment of antihypertensive drugs. The classifications of smoking status were current smokers, former smokers, and never-smokers. Current smokers were considered as persons regularly using tobacco in the last 6 months. Persons who ingested alcohol in the previous 6 months were considered as alcohol users.
Patients were advised to fast at least for 12 hours before taking blood samples after admission. The laboratory data includes monocyte count, lymphocyte count, WBC, high density lipoprotein (HDL-C), low density lipoprotein (LDL-C), triglyceride (TG), total cholesterol (TC), blood urea nitrogen (BUN), uric acid (UA), and creatinine (Cr). Measurement of the blood parameters were measured using a standard method in accordance to the central laboratory standard of the First Affiliated Hospital of Zhengzhou University. MLR was calculated by dividing monocyte count with lymphocyte count.
Endpoints
The primary endpoint of the study was long-term mortality, including all-cause mortality (ACM) and cardiac mortality (CM). The secondary endpoint was major adverse cardiovascular events (MACEs) and MACCEs. MACEs were defined as the combination of cardiovascular death, recurrent myocardial infarction (MI) and target vessel reconstruction, while MACCEs were defined as MACEs plus stroke. Deaths were considered as a cardiac condition unless a non-cardiac definite cause was identified. Recurrent MI was defined as a new Q wave, and an increased concentration of creatine kinase MB (CK-MB) to greater than five-times the upper limit of the normal range within 48 h after procedure or new Q waves or an increase in CK-MB concentration to greater than the upper limit of the normal range plus ischemic symptoms or signs, if occurring more than 48 h after the procedure.14 Target vessel revascularization was defined as any repetitive revascularization in a treated vessel where there was stenosis of at least 50% diameter in the presence of ischemic signs or symptoms or stenosis of at least 70% in the absence of ischemic signs or symptoms.14 Stroke was characterized as a neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause, including cerebral infarction, intracerebral hemorrhage and subarachnoid hemorrhage and lasting for >24 hours.15 Bleeding events were determined in line with the Bleeding Academic Research Consortium (BARC) standard.15 Readmission was defined as patients admitted to the hospital again after discharge, due to symptoms or signs of angina pectoris. All incidents were determined by an adjudication committee that was blinded to the group of patients. All events were adjudicated by an event adjudication committee blinded to the group of the patients.
Followed-Up
The follow-up of the study participants was done through telephone contact or office visits. Median follow-up time was 37.59 ± 22.24 months. All the patients were followed up in a minimum 1.5 years and a maximum of 7 years. During the follow-up, the compliance of the drugs and adverse events were assessed by trained clinical physicians carefully.
Statistical Analyses
SPSS 22.0 statistical software was used for analysis. Continuous variables were defined as mean ± standard deviation (SD); categorical variables were expressed as percentages. ROC curve was used to evaluate MPVLR’s predictive value of mortality after PCI. According to the best critical value of the ROC curve, the patients were divided into 2 groups (<0.34 or ≥0.34). For continuous variables, sample T test was applied to test the normality of distribution. For categorical variables, the chi-square test was used. Kaplan–Meier curve analysis was performed and comparisons of long-term mortality was performed using the log-rank test among the 2 groups. Multivariate Cox proportional hazards regression model was used to detect the predictors of endpoint events. Variables with a significant influence on mortality in univariate analysis were entered into the multivariate model, including of age, sex, WBC, Cr, UA, TG, TC, HDL-C, L-LDL, the history of smoking and drinking, the prevalence of DM and hypertension. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated, and a 2-sided P value < 0.05 was considered statistically significant.
Result
Baseline Data
A total of 3,461 patients with CAD who underwent PCI at the First Affiliated Hospital of Zhengzhou University were included in the final analysis. According to the cutoff value of MLR, all of the patients were divided into 2 groups: the low-MLR group (<0.34, n = 2338) and the high-MLR group (≥0.34, n = 1123). As showed in Table 1, patients in the high MLR group were older and more female, and tended to have higher WBC, Cr, UA and a higher proportion of smoking and drinking; while patients in the low MLR group had higher TG, TC, HDL-C, LDL-C. There was no significant difference in BUN and the prevalence of DM and hypertension.
Table 1.
Characteristics of Participants in Groups.
| Variables | MLR < 0.34 (n = 2338) | MLR ≥0.34 (n = 1123) | X 2 or t | p-value |
|---|---|---|---|---|
| Age (years) | 62.53 ± 10.45 | 64.74 ± 10.70 | -5.781 | <0.001 |
| Sex, female [n (%)] | 847 (36.2) | 231 (20.6) | 86.723 | <0.001 |
| Smoking | 661 (28.3) | 393 (35.0) | 16.192 | <0.001 |
| Drinking | 353 (15.1) | 204 (18.2) | 5.285 | 0.022 |
| Heart rate (times/min) | 74.27 ± 19.97 | 75.43 ± 12.10 | -1.777 | 0.076 |
| Hypertension, n (%) | 1298 (55.5) | 620 (55.2) | 0.029 | 0.864 |
| Diabetes, n (%) | 558 (23.9) | 261 (23.2) | 0.164 | 0.685 |
| WBC (*109/L) | 6.66 ± 1.84 | 7.72 ± 2.76 | -13.273 | <0.001 |
| BUN (mmol/L) | 5.71 ± 4.83 | 5.72 ± 3.55 | -0.044 | 0.965 |
| Cr (μmol/L) | 69.95 ± 24.36 | 78.73 ± 51.38 | -6.746 | <0.001 |
| UA (μmol/L) | 295.60 ± 82.17 | 305.85 ± 93.53 | -3.245 | 0.001 |
| TG (mmol/L) | 1.73 ± 1.17 | 1.52 ± 0.97 | 5.001 | <0.001 |
| TC (mmol/L) | 3.95 ± 1.02 | 3.79 ± 1.02 | 4.200 | <0.001 |
| HDL-C (mmol/L) | 1.05 ± 0.30 | 1.02 ± 0.28 | 2.860 | 0.004 |
| LDL-C (mmol/L) | 2.42 ± 0.84 | 2.34 ± 0.84 | 2.815 | 0.005 |
| ACM, n (%) | 53 (2.3) | 76 (6.8) | 42.824 | <0.001 |
| CM, n (%) | 33 (1.4) | 48 (4.3) | 27.202 | <0.001 |
| MACEs, n (%) | 249 (10.7) | 143 (12.7) | 3.279 | 0.070 |
| MACCEs, n (%) | 320 (13.7) | 191 (17.0) | 6.649 | 0.010 |
| Stroke, n (%) | 82 (3.5) | 50 (4.5) | 1.847 | 0.174 |
| Bleeding events, n (%) | 62 (2.7) | 31 (2.8) | 0.034 | 0.853 |
| readmission, n (%) | 702 (30.0) | 321 (28.6) | 0.757 | 0.384 |
| Re-infarction, n (%) | 70 (3.0) | 27 (2.4) | 0.969 | 0.325 |
Abbreviations: MLR, monocyte to lymphocyte ratio; WBC: white blood cell; Cr, creatinine; UA, uric acid; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ACM: all-cause mortality; CM, cardiac mortality; MACEs, major adverse cardiovascular events; MACCEs, major adverse cardiovascular and cerebrovascular events.
Clinical Outcomes
As showed in Table 1, the high MLR group had significantly higher ACM (6.8% vs. 2.3% p < 0.001) and CM (4.3% vs. 1.4%, p < 0.001) compared with the low MLR group. For the secondary endpoints, there was a significant difference between the 2 groups in the incidence of MACCEs (17.0% vs. 13.7%s, p = 0.010), while there was no significant difference in MACEs (12.7% vs. 10.7%, p = 0.070). Besides, stroke (4.5% vs. 3.5%, p = 0.174), bleeding events (2.8% vs. 2.7%, p = 0.853), readmission (28.6% vs. 30.0%, 0.384) and re-infarction (2.4% vs. 3.0%, p = 0.325) did not significantly differ among the 2 groups.
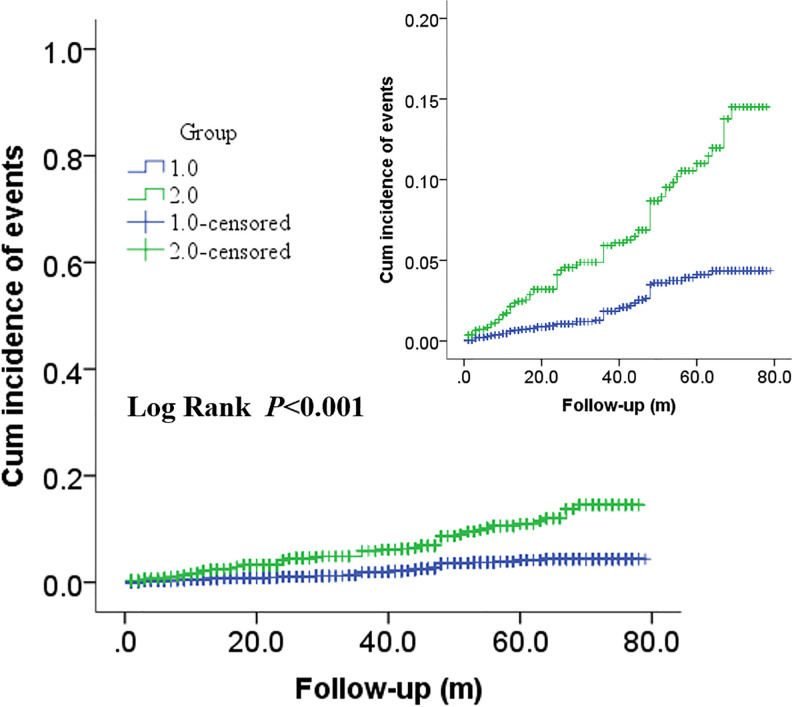
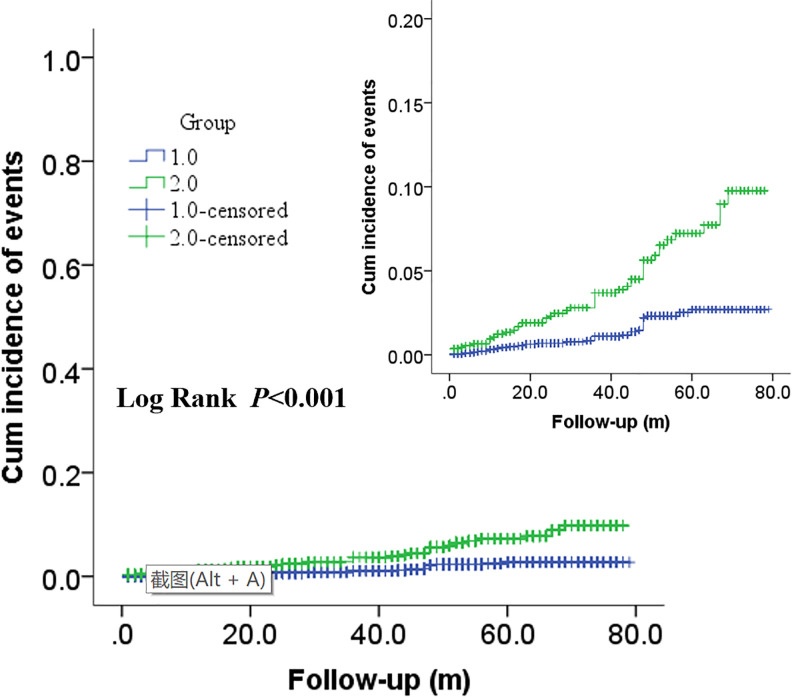
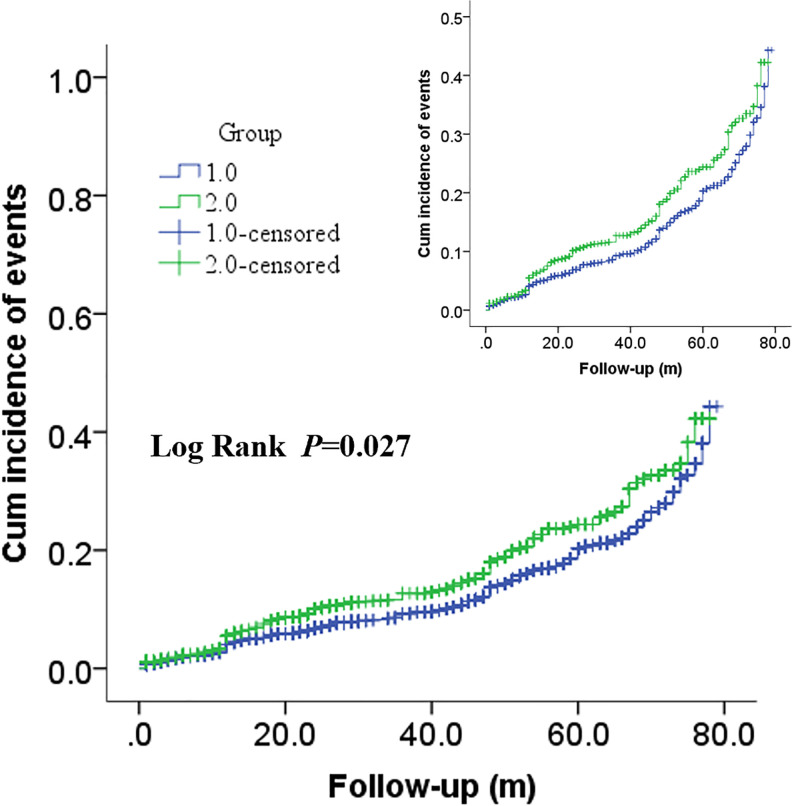
In Figures 2 and 3, Kaplan–Meier survival analysis revealed that patients in the high MLR group showed a significantly increased risk of ACM (log rank P < 0.001) and CM (log rank p < 0.001) compared with patients in the low MLR group. Moreover, significantly increased long-term MACCEs rates were also observed in patients with high MLR (long rank p = 0.002) in Figure 4.
Figure 2.
Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of ACM. The X axis represents the follow-up time, and the Y axis represents the cumulative incidence of ACM. The green line indicates the higher MLR, and the blue line indicates the lower MLR.
Figure 3.
Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of CM. The X axis represents the follow-up time, and the Y axis represents the cumulative incidence of ACM. The green line indicates the higher MLR, and the blue line indicates the lower MLR.
Figure 4.
Cumulative Kaplan–Meier estimates of the time to the first adjudicated occurrence of ACM. The X axis represents the follow-up time, and the Y axis represents the cumulative incidence of MACCEs. The green line indicates the higher MLR, and the blue line indicates the lower MLR.
Multivariate COX regression analyses were used to detect the correlation between MLR and long-term prognosis in patients with CAD after PCI. All above significant correlation variables were taken into multivariate Cox regression, including of age, sex, WBC, Cr, UA, TG, TC, HDL-C, LDL-C, the status of smoking and drinking. After adjusting for covariates, patients in the high MLR group had significantly increased ACM (HR = 1.366, 95%CI: 1.366-3.650, p = 0.001) and CM (HR = 2.379, 95%CI: 1.611-3,511, p ≤ 0.001) compared to the low MLR group (Tables 2 and 3). And high MLR was also found to be highly associated with MACCEs (HR = 1.227, 95%CI: 1.003-1.500, p = 0.047) in patients with CAD after PCI (Table 4).
Table 2.
Multivariable Cox Regression Analysis for All-Cause Mortality.
| Variables | B | SE | Wald | p-value | HR (95%CI) |
|---|---|---|---|---|---|
| Sex | -0.220 | 0.334 | 0.434 | 0.510 | 0.802 (0.416 1.545) |
| Smoking | 0.232 | 0.307 | 0.572 | 0.449 | 1.261 (0.691 2.300) |
| Drinking | -0.154 | 0.367 | 0.176 | 0.675 | 0.857 (0.418 1.760) |
| Age | 0.059 | 0.012 | 23.470 | <0.001 | 1.061 (1.036 1.087) |
| WBC | 0.028 | 0.052 | 0.296 | 0.589 | 1.028 (0.929 1.139) |
| Cr | 0.006 | 0.001 | 21.155 | <0.001 | 1.006 (1.003 1.008) |
| UA | 0.002 | 0.001 | 1.689 | 0.194 | 1.002 (0.999 1.004) |
| TG | 0.032 | 0.130 | 0.060 | 0.807 | 1.032 (0.800 1.332) |
| TC | 0.065 | 0.280 | 0.054 | 0.817 | 1.067 (0.616 1.849) |
| HDL-C | -0.990 | 0.558 | 3.144 | 0.076 | 0.372 (0.124 1.110) |
| LDL-C | -0.076 | 0.300 | 0.064 | 0.800 | 0.927 (0.515 1.668) |
| MLR | 0.803 | 0.251 | 10.254 | 0.001 | 1.366 (1.366 3.650) |
Abbreviations: MLR, monocyte to lymphocyte ratio; WBC: white blood cell; Cr, creatinine; UA, uric acid; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 3.
Multivariable Cox Regression Analysis for Cardiac Mortality.
| Variables | B | SE | Wald | p-value | HR (95%CI) |
|---|---|---|---|---|---|
| Sex | 0.088 | 0.224 | 0.131 | 0.718 | 1.092 (0.677 1.763) |
| Smoking | -0.078 | 0.268 | 0.084 | 0.771 | 0.925 (0.548 1.563) |
| Drinking | 0.312 | 0.301 | 1.075 | 0.300 | 1.366 (0.758 2.462) |
| Age | 0.066 | 0.010 | 44.374 | <0.001 | 1.068 (1.047 1.089) |
| WBC | 0.049 | 0.040 | 1.503 | 0.220 | 1.050 (0.971 1.135) |
| Cr | 0.006 | 0.001 | 49.621 | <0.001 | 1.006 (1.005 1.008) |
| UA | <0.001 | 0.001 | 0.058 | 0.809 | 1.000 (0.998 1.002) |
| TG | -0.006 | 0.096 | 0.004 | 0.947 | 0.994 (0.823 1.200) |
| TC | 0.297 | 0.169 | 3.102 | 0.078 | 1.346 (0.967 1.873) |
| HDL-C | -0.702 | 0.409 | 2.948 | 0.086 | 0.495 (0.222 1.104) |
| LDL-C | -0.302 | 0.189 | 2.888 | 0.090 | 0.726 (0.501 1.051) |
| MLR | 0.867 | 0.199 | 19.024 | <0.001 | 2.379 (1.611 3,511) |
Abbreviations: MLR, monocyte to lymphocyte ratio; WBC: white blood cell; Cr, creatinine; UA, uric acid; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Table 4.
Multivariable Cox Regression Analysis for MACCEs.
| Variables | B | SE | Wald | p-value | HR (95%CI) |
|---|---|---|---|---|---|
| Sex | 0.040 | 0.122 | 0.109 | 0.741 | 1.041(0.819 1.323) |
| Smoking | -0.037 | 0.132 | 0.080 | 0.778 | 0.963(0.743 1.249) |
| Drinking | -0.081 | 0.156 | 0.269 | 0.604 | 0.922(0.679 1.252) |
| Age | 0.009 | 0.005 | 4.053 | 0.044 | 1.009(1.000 1.019) |
| WBC | 0.020 | 0.022 | 0.766 | 0.382 | 1.020(0.976 1.066) |
| Cr | 0.004 | 0.002 | 14.708 | <0.001 | 1.004(1.002 1.005) |
| UA | <0.001 | 0.002 | 0.001 | 0.980 | 1.000(0.999 1.001) |
| TG | 0.096 | 0.050 | 3.662 | 0.056 | 1.101(0.998 1.216) |
| TC | -0.281 | 0.122 | 5.273 | 0.022 | 0.755(0.595 0.960) |
| HDL-C | -0.010 | 0.199 | 0.003 | 0.959 | 0.990(0.669 1.463) |
| LDL-C | 0.376 | 0.131 | 8.285 | 0.004 | 1.456(1.127 1.882) |
| MLR | 0.204 | 0.103 | 3.955 | 0.047 | 1.227(1.003 1.500) |
Abbreviations: MLR, monocyte to lymphocyte ratio; WBC: white blood cell; Cr, creatinine; UA, uric acid; TG, triglyceride; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol.
Discussion
In the present study, we revealed that ACM, CM and MACCEs of CAD patients after PCI in the high MLR group significantly increased compared with patents in the low MLR group. Therefore, MLR was an independent predictor of ACM, CM and MACCEs in patients with CAD who underwent PCI.
Inflammation plays a critical role throughout the process of atherosclerosis, from the initiation and development of atherosclerosis to plaque rupture leading to acute cardiovascular events.1 There is growing evidence that leukocyte subsets, including monocytes and lymphocytes, obviously regulate and participate in the initiation of atherosclerosis and atherothrombotic complications.16 Circulating monocytes and resident vascular macrophages are the first leukocytes to be recruited to the early atheromatous plaque and play an essential role in atherosclerotic development in response to inflammation and infection.17 Recruited monocytes are an important systemic source for renewal of tissue macrophages. Then, overabundance of inflammatory macrophages in the infarct compromises repair and promotes heart failure.2 In addition most cases of ACS occur from disruption of unstable plaque. One of the characteristic features of a vulnerable plaque is a higher predominance of lipid-laden macrophages which are derived from circulating monocytes in the cap.18 That is to say monocytes and monocytes-derived macrophages contribute to the initiation development and rupture of plaques in CAD patients. A large of studies have demonstrated circulating monocytes play an important role in the progression of coronary plaque and are associated with major adverse cardiac events in patients with CAD.7,19 Lymphocytes play a crucial role in the regulation of inflammatory responses at all levels of the atherosclerotic process as well. Low lymphocyte count is a common manifestation of systemic inflammation, and has shown to be related to accelerate atherosclerosis.20 There is an evidence that low lymphocyte could aggravate plaque load and cause acute plaque rupture.21 Low lymphocyte has been reported as an independent predictor for ACS on admission and is associated with MACE during clinical follow-up in CAD patients.22 Meanwhile, there is an important correlation between decreased lymphocyte count and re-infraction in STEMI and non-STEMI patients.23,24
In USTEMI patients, MLR has been demonstrated to be significantly associated with the lesion severity and slow coronary flow, and has better performance to reflect the severity of coronary lesion compared to neutrophil to lymphocyte ratio (NLR).25,26 In STEMI patients, there are also numerous evidences that suggest MLR is an independent risk factor associated with MACEs and no reflow after PCI.27,28 CAD patients with increased LMR not only have better-developed coronary collateral circulation and better clinical outcomes, but also have a lower proportion of in-stent restenosis if they were treated with PCI.29,30 To our knowledge, this is the first study to suggest that increased MLR is significantly associated with ACM, CM and MACCEs during long follow-up period in patients with CAD who underwent PCI.
In addition, there was a positive relation between CD14++, CD16+ monocyte numbers and plasma lipids and lipoproteins,31 which was consistent with our findings that patients in the high-MLR group had higher TC and TG than patients in the low-MLR group. Wu TT et al reported that HDL-C functioned as a reversal factor during the monocytes’ pro-inflammatory and pro-oxidant processes.32 In our study, our results showed that there was a significant difference in TC, HDL-C and LDL-C between the high-MLR and the low-MLR groups. After including the confounding factors into multivariate COX regression analyses, the results indicated that MLR was an independent predictor of MACCEs along with TC and LDL-C in CAD patients after PCI. The relationship between monocytes and lipids may need to be further demonstrated by prospective trials in the future.
Limitation
There were several limitations in the present study. First, this was a single-center and retrospective study. A multicenter and prospective study is needed to verify the findings of our study further. Secondly, we only measured MLR once at admission. It would be more interesting to take the blood samples more times during follow-up period. Thirdly, we didn’t measure other inflammatory markers, for example hs-CRP. Finally, we couldn’t rule out the possibility of residual confounding or even more likely, the lack of adjustment for unknown or unmeasured covariates. However, the sample size for the study was relatively large and may improve the statistical power of the results.
Conclusion
In present study, we found that patients with CAD after PCI in the high-MLR group had a significant increase in ACM, CM and MACCEs. MLR could be an independent predictor of long-term prognosis in patients with CAD who underwent PCI. However, more prospective trials are needed to demonstrate the prognostic value of MLR in CAD patients further.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 81870328, 81760043, 81800267).
ORCID iD: Feng-Hua Song  https://orcid.org/0000-0001-6006-4928
https://orcid.org/0000-0001-6006-4928
Ying-Ying Zheng  https://orcid.org/0000-0003-0003-4270
https://orcid.org/0000-0003-0003-4270
References
- 1. Raggi P, Genest J, Giles JT, et al. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. [DOI] [PubMed] [Google Scholar]
- 2. Ridker PM, MacFadyen JG, Everett BM, et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. [DOI] [PubMed] [Google Scholar]
- 3. Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. [DOI] [PubMed] [Google Scholar]
- 4. Tayefi M, Tajfard M, Saffar S, et al. hs-CRP is strongly associated with coronary heart disease (CHD): a data mining approach using decision tree algorithm. Comput Methods Programs Biomed. 2017, 141:105–109. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto E, Sugiyama S, Hirata Y, et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis. 2016;255:210–216. [DOI] [PubMed] [Google Scholar]
- 6. Flynn MC, Pernes G, Lee MKS, et al. Monocytes, macrophages, and metabolic disease in atherosclerosis. Front Pharmacol. 2019;10:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nozawa N, Hibi K, Endo M, et al. Association between circulating monocytes and coronary plaque progression in patients with acute myocardial infarction. Circ J. 2010;74(7):1384–1391. [DOI] [PubMed] [Google Scholar]
- 8. Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–882. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Liu N, Ren L, Lei L, Ye H, Peng J. Association of monocyte count on admission with the angiographic thrombus burden in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Arq Bras Cardiol. 2018;110(4):333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carubelli V, Bonadei I, Castrini AI, et al. Prognostic value of the absolute lymphocyte count in patients admitted for acute heart failure. J Cardiovasc Med (Hagerstown). 2017;18(11):859–865. [DOI] [PubMed] [Google Scholar]
- 11. Chen XL, Wu JB, Zhang FR, et al. Prognostic significance of pre-operative monocyte-to-lymphocyte ratio in lung cancer patients undergoing radical surgery. Lab Med. 2018;49(2):e29–e39. [DOI] [PubMed] [Google Scholar]
- 12. Koh YW, Jung SJ, Yoon DH, et al. The absolute lymphocyte to monocyte ratio is associated with poor prognosis in classical Hodgkin lymphoma patients younger than 60 years of age. Hematol Oncol. 2015; 33:133–140. [DOI] [PubMed] [Google Scholar]
- 13. Gary T, Pichler M, Belaj K, et al. Lymphocyte-to-monocyte ratio: a novel marker for critical limb ischemia in PAOD patients. Int J Clin Pract. 2014,68(12):1483–1487. [DOI] [PubMed] [Google Scholar]
- 14. Hicks KA, Mahaffey KW, Mehran R, et al. 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71(9):1021–1034. [DOI] [PubMed] [Google Scholar]
- 15. Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125(16):2015–2026. [DOI] [PubMed] [Google Scholar]
- 16. Shimada K. Immune system and atherosclerotic disease: heterogeneity of leukocyte subsets participating in the pathogenesis of atherosclerosis. Circ J. 2009;73(6):994–1001. [DOI] [PubMed] [Google Scholar]
- 17. Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. [DOI] [PubMed] [Google Scholar]
- 18. Ghattas A, Griffiths HR, Devitt A, et al. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. [DOI] [PubMed] [Google Scholar]
- 19. Höpfner F, Jacob M, Ulrich C, et al. Subgroups of monocytes predict cardiovascular events in patients with coronary heart disease. The PHAMOS trial (Prospective Halle Monocytes Study). Hellenic J Cardiol. 2019;60(5):311–321. [DOI] [PubMed] [Google Scholar]
- 20. Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–3233. [DOI] [PubMed] [Google Scholar]
- 21. Campbell KA, Lipinski MJ, Doran AC, et al. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012;110(6):889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bian C, Wu Y, Shi Y, et al. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessels. 2010;25(6):469–473. [DOI] [PubMed] [Google Scholar]
- 23. Núñez J, Sanchis J, Bodí V, et al. Therapeutic implications of low lymphocyte count in non-ST segment elevation acute coronary syndromes. Eur J Intern Med. 2009; 20(8):768–774. [DOI] [PubMed] [Google Scholar]
- 24. Núñez J, Núñez E, Bodí V, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis. 2010;21(1):1–7. [DOI] [PubMed] [Google Scholar]
- 25. Yayla Ç, Akboğa MK, Gayretli YK, et al. A novel marker of inflammation in patients with slow coronary flow: lymphocyte-to-monocyte ratio. Biomark Med. 2016;10(5):485–493. [DOI] [PubMed] [Google Scholar]
- 26. Chen H, Li M, Liu L, Dang X, Zhu D, Tian G. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine (Baltimore). 2019;98(26):e16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurtul A, Yarlioglues M, Celik IE, et al. Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2015;26(8):706–712. [DOI] [PubMed] [Google Scholar]
- 28. Wang Q, Ma J, Jiang Z, et al. Association of lymphocyte—to—monocyte ratio with in-hospital and long-terms major adverse cardiac and cerebrovascular events in patients with ST-elevated myocardial infarction. Medicine Baltimore. 2017;96(34):e7897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Murat SN, Yarlioglues M, Celik IE, et al. The relationship between lymphocyte-to-monocyte ratio and bare-metal stent in-stent restenosis in patients with stable coronary artery disease. Clin Appl Thromb Hemost. 2017;23(3):235–240. [DOI] [PubMed] [Google Scholar]
- 30. Kurtul A, Duran M. The correlation between lymphocyte/monocyte ratio and coronary collateral circulation in stable coronary artery disease patients. Biomark Med. 2017;11(1):43–52. [DOI] [PubMed] [Google Scholar]
- 31. Kim JE, Lin G, Zhou J, et al. Weight loss achieved using an energy restriction diet with normal or higher dietary protein decreased the number of CD14CD16 proinflammatory monocytes and plasma lipids and lipoproteins in middle-aged, overweight, and obese adults. Nutr Res. 2017;40:75–84. [DOI] [PubMed] [Google Scholar]
- 32. Wu TT, Zheng YY, Chen Y, et al. Monocyte to high-density lipoprotein cholesterol ratio as long-term prognostic marker in patients with coronary artery disease undergoing percutaneous coronary intervention. Lipids Health Dis. 2019;18(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]