Abstract
Background
Among the many medical challenges presented by the COVID-19 pandemic, management of the majority of patients in community outpatient settings is crucial. The aim of this study was to describe the characteristics and outcomes among confirmed COVID-19 cases who were managed at three settings: two outpatient settings and one inpatient.
Methods
A retrospective database cohort study was conducted in a large Israeli Health Maintenance Organization. All COVID-19 cases diagnosed between 28 February 2020 and 20 July 2020 were included. Cases in the community settings were managed through a nationwide remote monitoring center, using preliminary telehealth triage and 24/7 virtual care. Outcome parameters included hospital admission, disease severity, need for respiratory support and mortality.
Results
About 5448 cases, aged range 0–97 years, were enrolled; 88.7% were initially managed as outpatient either at home or in designated hotels, 3.1 and 2.1% of them, respectively, later required hospitalization. The main reason for hospitalization was dyspnea; 12 were diagnosed with severe disease; 56 patients (1.3%) died, five (0.1%) of whom were initially allocated to the outpatient settings.
Conclusions
Care for appropriately selected COVID-19 patients in the community provides a safe and effective option. This can contribute to reducing the hospitalization burden, with no evidence of increased morbidity or mortality.
Keywords: community, COVID-19, healthcare system, outpatient management, telehealth
Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic poses a major threat to healthcare systems. As medical resources such as hospital beds and ventilators are limited, particularly during an emerging pandemic, a solution for mild or moderate cases or after hospital discharge is required outside the hospital setting. The World Health Organization recommends keeping mildly ill COVID-19 patients in non-traditional facilities such as repurposed hotels, or at home, until symptoms resolve and laboratory tests for COVID-19 virus are negative.1 However, limited data informing outpatient management strategies are available.
Following the first reported case and hospitalization of a coronavirus patient in Israel, with the accompanying forecast of an overflow of inpatients, a process was established for admitting and monitoring patients in a community setting. The Israel Ministry of Health (MoH) defined three options for COVID-19 patients’ care: hospital, home or 12 hotels designated by the Israeli government.2 Parameters for management took into account the individual patient’s clinical and social circumstances as well as available local resources. All community treatment was based on remote monitoring and telehealth services.
Understanding the clinical course of the disease in community outpatient care, where the vast majority of COVID-19 patients are managed3,4 is crucial in planning for ongoing and subsequent phases of the pandemic.
This study aimed to evaluate the clinical course, and outcomes of our community COVID-19 patients in one inpatient and two outpatient modalities.
Methods
In this retrospective study, in the second-largest Health Maintenance Organization (HMO) in Israel, Maccabi Healthcare Services (MHS) serving over 2.4 million members in the community. Early in the COVID-19 outbreak, in March 2020, MHS set up a remote management center for COVID-19 outpatients. This comprehensive, nationwide coordinated outpatient care program called ‘Maccabi COVID-19 Care’ is staffed by a multidisciplinary team that includes physicians, most of them primary care physicians, nurses, social workers and other health care professionals.
The program included initial telephone contact and triage for all MHS SARS-COV-2 positive cases. The triage evaluation enabled allocation of each patient to either hospital or outpatient care,2 based upon individual patient risk, severity of symptoms and course of disease, and was guided by national and internal organization criteria (see Supplementary data). The initial decision to transfer the patient to a designated hotel as versus home follow-up was based on social and epidemiologic criteria regarding the ability to isolate at home (see Supplementary data). Outpatients were treated by a protocol using telehealth visits; 24 hours a day, 7 days a week telephone medical response by a dedicated line. Daily remote monitoring of symptoms and signs—including temperature, heart rate and oxygen saturation by home pulse oximetry5—was carried out by telephone report or electronic questionnaire. This remote medical supervision was used to identify any deterioration of the patients’ condition as early as possible and refer them to hospitals, if needed according to national criteria (see Supplementary data) and the physicians’ clinical judgement. Monitoring enabled the staff to treat other medical exacerbations and to provide support for isolation-related social and medical adversities. Post-discharge care either at home or at a hotel was also provided by the outpatient care program. In addition, the staff instructed the patients about the importance of infection control, self-isolation until recovery (recovery criteria evolved according to changing Israeli MoH policy2) and the duration of quarantine for their household contacts.
All MHS SARS-COV-2 positive cases diagnosed nationwide between 28 February and 20 June, including the initial peak of infection in March–April and the beginning of the resurgence which started in late May, were included in this retrospective study. The criteria for conducting the test were in accordance with the Israeli MoH guidelines and included both epidemiological and clinical criteria.2 A patient was considered positive for SARS-COV-2 following a positive result of real-time reverse-transcription polymerase chain reaction (RT-PCR) testing of a nasopharyngeal and throat swab. The kits for RT-PCR were validated by the Israeli Central Virology lab and varied according to availability. All tests were approved by the Israeli MoH. Tests were performed and interpreted according to the manufacturer’s instructions.
Data from the medical records were retrieved from the nationwide centralized database of MHS, spanning over 20 years. Additional information was retrieved by one of the authors (SBBD), who manually reviewed records of SARS-COV-2 positive cases. All hospitalized patients’ medical records were reviewed as well as randomly selected outpatients by their first letter of surname. Data collected included demographics smoking status, body mass index (BMI), immunosuppression status, comorbidities, hospitalizations over the past year (not including maternity) and pregnancy status. Medical records were reviewed for self-reported symptoms and epidemiologic data.
Outcome parameters included: hospital admission, disease severity in the hospital setting according to the Israeli MoH’s definition (mild, moderate and severe),2 the need for oxygen support and ventilation, and mortality.
The study was approved by the local ethics committee and IRB (approval number: 0023-20MHS). Data were analyzed anonymously using SPSS 25, using descriptive statistics and student t-test.
Results
From the beginning of the outbreak until 20 June 2020, 150 622 MHS members carried out RT-PCR tests for SARS-CoV-2; 5525 of them (3.7%) were positive. After excluding 77 members who live in long-term health care or geriatric facilities, our national sample included 5448 members (Figure 1); 76.7% were initially treated at home, 12% in hotels and 11.3% in hospitals.
Fig. 1.
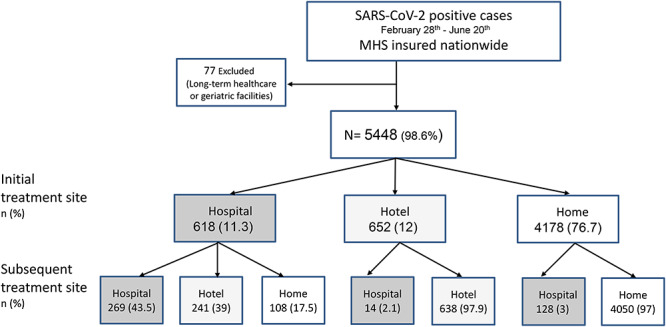
Flowchart of cohort creation, cohort population—All SARS-CoV-2 PCR positive cases insured in MHS members who live in excluding patient in long-term health care or geriatric facilities, between 28 February 2020 and 20 June 2020. Patients were treated in three modalities: home, hotel or hospital; initial treatment site—follow telephone triage after SARS-CoV-2 diagnosis; subsequent treatment site—some were transferred to different modality due to deterioration or clinical improvement.
Cases’ age ranged from birth to 97 years (Table 1). Compared with the general MHS population, a larger proportion of positive SARS-CoV-2 cases was in the third decade of life and a lower proportion was in the first decade (23.5 versus 14% and 8 versus 19%, respectively, P < 0.05; Figure 2). The most common comorbidities were obesity (BMI ≥ 30 Kg/cm2), hypertension and diabetes. While the prevalence of hypertension in the general MHS population was similar to its proportion in the COVID-19 patients (12.7 versus 13.0%, SD 0.336, respectively, P < 0.001), the prevalence of diabetes and obesity were significantly higher among the COVID-19 patients (5.8 versus 7.4%, SD 0.262, P < 0.001, and 18.3 versus 26.3%, SD 0.440, P < 0.001, respectively).
Table 1.
Baseline characteristics and symptoms of 5448 outpatients and inpatients with COVID-19, MHS, 28 February—20 June 2020
| Total N = 5448 | Home n = 4178 | Hotel n = 652 | Hospital n = 618 | P1–2-3 | P1–2 | P1–3 | |
|---|---|---|---|---|---|---|---|
| Age (Yr)—median (IQR) | 33 {21,52} | 32 {20, 50} | 23 {18, 37} | 58.5 {44, 72} | 0.000 | 0.000 | |
| Gender, Male—no. (%) | 3002 (55.1) | 2235 (53.5) | 389 (59.7) | 377 (61.0) | 0.000 | 0.003 | 0.001 |
| SES ‡ (n = 5440)—mean, SD | 5.09 ± 2.03 | 5.15 ± 2.04 | 4.33 ± 1.69 | 5.52 ± 2.09 | 0.000 | 0.000 | |
| BMI (n = 4146)—mean, SD | 26.86 ± 5.76 | 26.36 ± 5.69 | 25.69 ± 5.64 | 28.93 ± 5.76 | 0.001 | 0.000 | |
| Current Smoker § (n = 4469)—no. (%) | 321 (7.2) | 225 (6.6) | 51 (10.6) | 45 (7.6) | 0.000 | 0.000 | 0.000 |
| Past Smoker § (n = 4606)—no. (%) | 990 (21.4) | 699 (19.8) | 111 (22.0) | 180 (30.0) | 0.000 | 0.553 | 0.000 |
| Pregnancy no. | 90 | 75 | 8 | 7 | |||
| Comorbidities (n = 5446)—no. (%) | |||||||
| Diabetes | 404 (7.4) | 211 (5.1) | 24 (3.7) | 169 (27.4) | 0.000 | 0.130 | 0.000 |
| Hypertension | 706 (13) | 442 (10.6) | 35 (5.4) | 229 (37.1) | 0.000 | 0.000 | 0.000 |
| COPD | 46 (0.8) | 24 (0.6) | 3 (0.5) | 19 (3.1) | 0.000 | 0.715 | 0.000 |
| Cancer | 223 (4.1) | 127 (3) | 10 (1.5) | 86 (13.9) | 0.000 | 0.031 | 0.000 |
| Cardiovascular disease | 185 (3.4) | 86 (2.1) | 9 (1.4) | 90 (14.6) | 0.000 | 0.246 | 0.000 |
| Immunosuppress. status I | 92 (1.7) | 48 (1.1) | 3 (0.5) | 41 (6.6) | 0.000 | 0.109 | 0.000 |
| CKD II | 25 (0.5) | 6 (0.1) | 0 | 19 (3.1) | 0.000 | 0.333 | 0.000 |
| Dementia | 57 (1.0) | 17 (0.4) | 2 (0.3) | 38 (6.2) | 0.000 | 0.704 | 0.000 |
| IBD | 44 (0.8) | 32 (0.8) | 4 (0.6) | 10 (1.6) | 0.076 | 0.674 | 0.000 |
| Asplenia | 4 (0.1) | 3 (0.1) | 0 | 1 (0.2) | 0.565 | 0.494 | 0.033 |
| Obesity III | 1089 (26.3) | 761 (24.4) | 100 (22) | 228 (39.8) | 0.000 | 0.260 | 0.469 |
| Underweight IV | 53 (1.3) | 41 (1.3) | 9 (2) | 3 (0.5) | 0.109 | 0.258 | 0.000 |
| No. of Comorbidities Mean, SD | 0, 0.53 ± 0.96 | 0, 0.42 ± 0.81 | 0, 0.29 ± 0.64 | 1, 1.51 ± 1.50 | |||
| Hospitalization in past year, Mean, SD | 1, 1.88 ± 2.11 | 1, 1.37 ± 0.75 | 1, 2.45 ± 2.34 | 2, 3.5 ± 3.94 | |||
| Symptoms (n = 3521)—no. (%) | |||||||
| No symptoms | 546 (15.5) | 417 (16.7) | 90 (21.7) | 39 (6.4) | 0.000 | 0.014 | 0.000 |
| Fever† | 1569 (45.3) | 1061 (42.5) | 132 (31.8) | 403 (65.8) | 0.000 | 0.000 | 0.000 |
| Cough | 1551 (44) | 1033 (41.4) | 170 (41) | 348 (57) | 0.000 | 0.854 | 0.000 |
| Weakness | 754 (21.4) | 502 (20.1) | 80 (19.3) | 172 (28.1) | 0.000 | 0.708 | 0.000 |
| Myalgia | 522 (14.8) | 392 (15.7) | 45 (10.8) | 85 (13.9) | 0.024 | 0.010 | 0.000 |
| Smell disturbances | 493 (14) | 397 (15.9) | 68 (16.4) | 28 (4.6) | 0.000 | 0.821 | 0.214 |
| Taste disturbances | 440 (12.5) | 354 (14.2) | 55 (13.3) | 30 (4.9) | 0.000 | 0.599 | 0.000 |
| Rhinitis | 410 (11.6) | 305 (12.2) | 53 (12.8) | 52 (8.5) | 0.020 | 0.754 | 0.000 |
| Sore throat | 404 (11.4) | 295 (11.8) | 52 (12.5) | 58 (9.5) | 0.135 | 0.679 | 0.007 |
| Headache | 353 (11.2) | 25 (11.4) | 56 (13.5) | 54 (8.8) | 0.043 | 0.226 | 0.062 |
| Subjective shortness of breath | 296 (8.4) | 130 (5.2) | 27 (6.5) | 139 (22.7) | 0.000 | 0.279 | 0.000 |
| Diarrhea¶ | 271 (7.7) | 177 (7.1) | 27 (6.5) | 65 (1.6) | 0.009 | 0.645 | 0.004 |
| Nausea | 63 (1.8) | 37 (6) | 6 (1.4) | 20 (3.3) | 0.026 | 0.906 | 0.009 |
1—Home, 2—Hotel, 3—Hospital
SES ‡—Socio-economic status—defined by the Israel Central Bureau of Statistics. Low: 1–3. Medium 4–6, High 7–10, § ≥15 years old.
IImmunosuppression status—Congenital or acquired immunodeficiency, HIV infection, iatrogenic immunosuppression, immunosuppressive therapy including chemotherapy and radiation therapy.
IICKD—Chronic kidney disease, estimated glomerular filtration rate, eGFR < 30
IIIObesity—BMI kg/cm2 ≥ 30
IVUnderweight—BMI < 17
†Fever: 38.0°C or more.
¶Diarrhea: 3 or more loose stools per day.
Fig. 2.
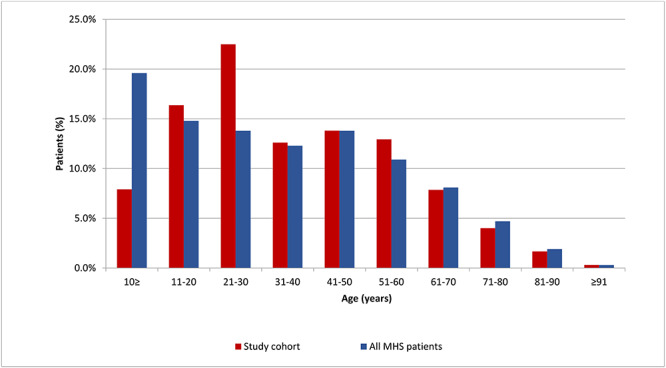
Age distribution of MHS SARS-CoV-2 positive cases (Study cohort) compared with the general MHS population by 10-year age intervals, MHS—Maccabi Healthcare Services Study cohort: All SARS-CoV-2 PCR positive cases insured in MHS, excluding patients in long-term institutions between 28 February 2020 and 20 June 2020: n = 5448; MHS general population: n = 2 403 018.
Reflecting the use of triage protocol, when COVID-19 patients were compared by site of initial treatment allocation, the patients who required hospital admission were older (median age 56 years [interquartile range {IQR}, 36, 76]). All comorbidities were much more frequent in the hospitalized patients. In general, the population who stayed in a hotel was younger (median age 27 years, [IQR, 17–43]), with lower SES (4.33 ± 1.69) and more likely to smoke (10.6%).
Of the 3521 records reviewed for symptoms, 546 (15.5%) cases were asymptomatic (Table 1). Thirty-nine hospital inpatients were asymptomatic; most (90%) of them were diagnosed by screening during a hospital admission for other reasons, and the others were admitted due to social circumstances (such as accompanying a SARS-COV-2 positive family member). Dyspnea was much more common in the population who required hospital admission (138, 22.7%) than the other modalities (129, 5.3%; 27, 6.5% for home and hotel, respectively, P < 0.01). Smell and taste disturbances were 3-fold more likely to be reported by outpatients (Table 1).
Outcomes
A total of 760 of 5448 hospitalized at any point in the course of illness. Among 4178 patients originally allocated to home stay and 652 patients originally allocated to a hotel, 128 (3.1) and 14 (2.1%) patients, respectively, needed hospital admission at some point during the course of the disease (Figure 1). The main reasons for hospital admission among the 142 patients were: subjective shortness of breath (45, 31.7%), chest/pleuritic pain (25, 17.6%), desaturation (oxygen saturation less than 94% in room air, 20, 14.1%), prolonged fever (18, 12.7%), dehydration/vomiting/diarrhea (12, 8.4%) and weakness (8, 5.6%).
Most of the 760 patients who were admitted to the hospital had mild disease (550, 72.4%) (Table 2). Respiratory support (including any need for supplementary oxygen) was needed in 204 (26.8%) patients, among them 79 (10.4%) required mechanical ventilation. Among the 142 patients who were transferred to hospital from the community setting, 12 were diagnosed with severe disease and four died. Their median age was 70 years (range 49–90), their median BMI was 28.2 kg/cm2 (range 20.2–52.2), none of them were smokers, 11 (91.7%) of them had chronic hypertension and only one (8.3%) was immunosuppressed due to chemotherapy.
Table 2.
Main outcomes: disease severity, respiratory support and death among hospitalized patients
| Hospital first (n = 618) | Home to hospital (n = 128) | Hotel to hospital (n = 14) | All hospitalized patients (n = 760) | |
|---|---|---|---|---|
| Disease severity † —no. (%) | ||||
| Mild | 444 (71.8) | 95 (74.2) | 11 (78.5) | 550 (72.4) |
| Moderate | 81 (13.1) | 14 (10.9) | 3 (21.5) | 98 (12.9) |
| Severe | 83 (13.4) | 12 (9.3) | 0 | 95 (12.5) |
| Missing data | 10 (1.6) | 7 (5.4) | 0 | 17 (2.2) |
| Respiratory support—no. (%) | ||||
| None | 435 (70.4) | 96 (75) | 11 (78.6) | 542 (71.3) |
| Oxygen Support | 98 (15.8) | 17 (13.3) | 2 (14.3) | 117 (15.4) |
| High-flow Oxygen | 6 (1) | 1 (0.7) | 1 (7.1) | 8 (1) |
| Mechanical Ventilation | 69 (11.2) | 10 (7.8) | 0 | 79 (10.4) |
| Missing data | 10 (1.6) | 4 (3.2) | 0 | 14 (1.8) |
| Death—no. (%) | ||||
| 51 (8.2) | 4 (3) | 55 (7.2) | ||
†Disease severity according to Israeli MoH criteria that were in force at the time of hospitalization:2 • Mild disease: Upper respiratory tract infection or pneumonia which does not comply with the definition of severe pneumonia.• Moderate disease: Pneumonia with one of the following: Respiratory rate of more than 30 per minute, respiratory distress, oxygen saturation less than 90% in room air.• Severe disease: Respiratory failure/acute respiratory distress syndrome, sepsis or shock.
The cumulative number of deaths in Israel due to COVID-19 until 20 June was 305 and death rate was 1.48% (n = 20 633).6 The death rate in our nationwide cohort was 1% with a total of 56 deceased (P < 0.05, n = 5448); 55 patients died in the hospital; 51 of them were originally allocated to hospital and four were transferred from home due to deterioration (Table 2). One 93-year-old bedridden patient died at home, a few hours after the SARS-CoV-2 test was performed.
Discussion
This retrospective descriptive study evaluates the characteristics and clinical course of COVID-19 patients, most of them outpatients, in a large HMO during the first and the beginning of the second waves. The patients were assigned to three healthcare locations during the illness, either in hospital and/or in one of two community options: home or a designated hotel. Our COVID-19 outpatient care was based on the performance of preliminary triage by a physician, taking into account risk factors, clinical status and social context; as well as a comprehensive telemedicine monitoring center, based on consistent protocols.
Patients who were treated in the community settings by the rapidly implemented centralized remote COVID-19 care program were younger with less comorbidities, as would be expected when using a triage protocol. Their overall prognosis was good; 142 patients (2.9%) were admitted to the hospital following referral by the care center for clinical deterioration; 12 of them had severe disease and five (0.1% of total COVID-19 outpatients) died. This low mortality rate would seems to indicate a safe approach to outpatient management.
While a great number of published studies focus on inpatient COVID-19 clinical presentation and outcome,7,8 far fewer address the symptoms and disease course of the community outpatient population.9 Although the number of symptoms did not differ between the outpatient and hospital populations in our study, diarrhea, rhinitis as well as taste and smell disturbances were much more common in outpatients than in the hospital—similar to what was reported in a study from the USA.10 A possible reason could be a lack of questioning for minor symptoms at hospital admission or their omission in the discharge records, as versus the MHS record of daily telephone questioning about symptoms in the outpatient setting. Another explanation is a lack of awareness of those symptoms in the early days of the pandemic,11 when milder or even asymptomatic cases were admitted to hospital for monitoring. Subjective dyspnea and desaturation (94% or below) were much more common in that proportion of outpatients who required hospital care. These symptoms should be considered as ‘red flags’ (warning of potential deterioration) for hospital admission from the community settings, as also presented in a study from Minnesota.12
Among the two community outpatient settings, 12 designated hotels were repurposed for accommodation of SARS-CoV-2 cases as an alternative for home care, with supervision by the same centralized program. The most noticeable differences between the population at home and hotel were age and socioeconomic status. The hotel population was younger with lower socioeconomic status, representing a population that was unable to maintain adequate isolation at home, mostly due to crowded living conditions and/or the lack of a local support network, and therefore needed to be sent to designated hotels. As the outcome for the two outpatient modalities was similar, the option of hotel accommodation seems to be a good alternative for home care.
Patient care involving remote monitoring by a multidisciplinary healthcare team who are able to use appropriate tools to monitor many patients efficiently for vital parameters and symptoms has already been used for other respiratory diseases13 and COVID-19.14,15 Efficient outpatient monitoring has the potential to reduce unnecessary emergency department visits16 and outpatient care is much more convenient for the patient.17 As we observed very few cases of patients assigned to either the home or hotel settings who were subsequently transferred to the hospital due to deterioration, the question arises as to the optimal frequency of outpatient monitoring to maximize the effective use of resources in an overburdened healthcare system while retaining the ability to detect the warning signs of incipient deterioration. We conclude that COVID-19 outpatient management strategies should be geared to enable more frequent monitoring of those with risk factors since they are the ones most likely to deteriorate.
Strengths and limitations
Our study has several limitations: this is a retrospective descriptive database study, and as such, has several limitations related to coding and possible level of detail. Therefore, in this study, one of the authors manually reviewed the medical records of a major random sample of the patients. Secondly, the allocation to inpatient hospital care, and outpatient—home or hotel was not randomized and was determined by the national and HMO policies as well as the individual judgement of the triaging physician. It was mainly based on social rather than medical considerations for hotel versus home, and more medical than social considerations for hospital versus home or hotel allocation. Therefore, any comparative conclusion may be due to the triage policy based on patient characteristics rather than random allocation.
Our study has important strengths. To our knowledge, this study represents one of the largest community cohort studies of COVID-19 patients. Our results represent real-world data, which describes systematically a large population with mild-to-severe COVID-19 patients with extensive information from various healthcare settings. That may be generalizable to other healthcare systems dealing with the COVID-19 pandemic. The detailed clinical course of each patient due to extensive monitoring gives reliable follow-up data, which have eliminated the recall bias often found in retrospective studies.
Conclusions
Among the many medical challenges presented by the COVID-19 pandemic, management of the majority of patients in community outpatient settings is crucial. The aim of this study was to describe the characteristics and outcomes among confirmed COVID-19 cases who were managed at three settings: two outpatient settings and one inpatient. A retrospective database cohort study was conducted in a large Israeli HMO. All COVID-19 cases diagnosed between 28 February 2020 and 20 July 2020 were included. Cases in the community settings were managed through a nationwide remote monitoring center, using preliminary telehealth triage and 24/7 virtual care. Outcome parameters included hospital admission, disease severity, need for respiratory support and mortality; 5448 cases, aged range 0–97 years, were enrolled; 88.7% were initially managed as outpatient either at home or in designated hotels, 3.1 and 2.1% of them, respectively, later required hospitalization. The main reason for hospitalization was dyspnea; 12 were diagnosed with severe disease; 56 patients (1.3%) died, five (0.1%) of whom were initially allocated to the outpatient settings. As the COVID-19 pandemic has highlighted a need for outpatient care in the community setting, our study demonstrates that care for appropriately selected COVID-19 patients with remote monitoring techniques, when implemented in a systematic manner involving clinical personnel, provide a safe and effective option for treating COVID-19 positive patients, allowing a reduction in hospitalization burden with no evidence of increased morbidity or mortality.
Funding
No funding was received.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics approval
The study was approved by the local ethics committee and IRB (approval number: 0023-20MHS).
Consent to participate and for publication
Not applicable.
Availability of data and material
The data that support the findings of this study are available from Maccabi Healthcare Services (MHS) but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Access to the data is, however, available upon reasonable request and signing an MTA agreement with MHS.
Code availability
Not applicable.
Authors’ contributions
Shirley Shapiro Ben David, Josef and Nachman Ash perceived and designed the study. Material preparation, data collection and analysis were performed by Shirley Shapiro Ben David, Joseph Azuri, Angela Irony, Gili Ofer-Bialer and Orly Greenfeld. The manuscript was written by Shirley Shapiro Ben David, Daniella Cohen, Rebekah Karplus and Israel Potasman and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Shirley Shapiro Ben David, MD
Daniella Cohen, MD
Rebekah Karplus, MD
Angela Irony, PhD, MPH, R.N
Gili Ofer-Bialer, MD
Israel Potasman, MD
Orly Greenfeld, MD
Joseph Azuri, MD, MHA
Nachman Ash, MD, MS
Contributor Information
Shirley Shapiro Ben David, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel.
Daniella Cohen, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel.
Rebekah Karplus, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel.
Angela Irony, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel.
Gili Ofer-Bialer, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel.
Israel Potasman, Internal Medicine and Infectious Diseases, Maccabi Healthcare Services, Haifa 3474407, Israel.
Orly Greenfeld, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel.
Joseph Azuri, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel; Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv 6997801, Israel.
Nachman Ash, Health Division, Maccabi Healthcare Services, Tel Aviv 6812509, Israel; Health Systems Management Department, Ariel University, Ariel 4077625, Israel.
References
- 1. World Health Organization . Home care for patients with COVID-19 presenting with mild symptoms and management of their contacts: interim guidance. https://www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts (30 March 2020, date last accessed).
- 2. Israel Ministry of Health (MoH) . COVID-19 Guidelines. 2020. Hebrew. https://govextra.gov.il/ministry-of-health/corona/corona-virus/medical-guidelines-corona/ (15 March 2020, date last accessed). [Google Scholar]
- 3. Stokes EK, Zambrano LD, Anderson KN et al. Coronavirus disease 2019 case surveillance-United States January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69(24):759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19); 2020. https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (09 July 2020, date last accessed).
- 5. Shah S, Majmudar K, Stein A et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med 2020;27(8):681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coronavirus (COVID-19) mortality rate. 2020. https://www.worldometers.info/coronavirus/ (01 July 2020, date last accessed).
- 7. Docherty AB, Harrison EM, Green CA et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richardson S, Hirsch JS, Narasimhan M et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pullen MF, Skipper CP, Hullsiek KH et al. Symptoms of COVID-19 outpatients in the United States. Open Forum Infect Dis 2020;7(7):ofaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tenforde MW, Billig Rose E, Lindsell CJ et al. Characteristics of adult outpatients and inpatients with COVID-19-11 academic medical Centers United States, March-May 2020. MMWR Morb Mortal Wkly Rep 2020;69(26):841–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giacomelli A, Pezzati L, Conti F et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 2020;71(15):889–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Annis T, Pleasants S, Hultman G et al. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc 2020;27(8):1326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sul AR, Lyu DH, Park DA. Effectiveness of telemonitoring versus usual care for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare 2020;26:189–99. [DOI] [PubMed] [Google Scholar]
- 14. Xu H, Huang S, Qiu C et al. Monitoring and Management of Home-Quarantined Patients with COVID-19 using a WeChat-based telemedicine system: retrospective cohort study. J Med Internet Res 2020;22(7):e19514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu L, Gu J, Shao F et al. Application and preliminary outcomes of remote diagnosis and treatment during the COVID-19 outbreak: retrospective cohort study. JMIR Mhealth Uhealth 2020;8(7):e19417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nascimento BR, Brant LC, Castro ACT et al. Impact of a large-scale telemedicine network on emergency visits and hospital admissions during the coronavirus disease 2019 pandemic in Brazil: data from the UNIMED-BH system. J Telemed Telecare 2020;25:1357633X20969529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Groeger JL, Starrels JL, Ehrlich AR. Older adults with COVID-19 can choose care at home: lessons learned from new York City. J Gen Intern Med 2020;35(9):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Maccabi Healthcare Services (MHS) but restrictions apply to the availability of these data, which were used under license for the current study and so are not publicly available. Access to the data is, however, available upon reasonable request and signing an MTA agreement with MHS.


