Abstract
Background
While secondary pneumococcal pneumonia occurs less commonly after coronavirus disease 2019 (COVID-19) than after other viral infections, it remains unclear whether other interactions occur between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and Streptococcus pneumoniae.
Methods
We probed potential interactions between these pathogens among adults aged ≥65 years by measuring associations of COVID-19 outcomes with pneumococcal vaccination (13-valent conjugate vaccine [PCV13] and 23-valent pneumococcal polysaccharide vaccine [PPSV23]). We estimated adjusted hazard ratios (aHRs) using Cox proportional hazards models with doubly robust inverse-propensity weighting. We assessed effect modification by antibiotic exposure to further test the biologic plausibility of a causal role for pneumococci.
Results
Among 531 033 adults, there were 3677 COVID-19 diagnoses, leading to 1075 hospitalizations and 334 fatalities, between 1 March and 22 July 2020. Estimated aHRs for COVID-19 diagnosis, hospitalization, and mortality associated with prior PCV13 receipt were 0.65 (95% confidence interval [CI], .59–.72), 0.68 (95% CI, .57–.83), and 0.68 (95% CI, .49–.95), respectively. Prior PPSV23 receipt was not associated with protection against the 3 outcomes. COVID-19 diagnosis was not associated with prior PCV13 within 90 days following antibiotic receipt, whereas aHR estimates were 0.65 (95% CI, .50–.84) and 0.62 (95% CI, .56–.70) during the risk periods 91–365 days and >365 days, respectively, following antibiotic receipt.
Conclusions
Reduced risk of COVID-19 among PCV13 recipients, transiently attenuated by antibiotic exposure, suggests that pneumococci may interact with SARS-CoV-2.
Keywords: COVID-19, SARS-CoV-2, Streptococcus pneumoniae, pneumococcal conjugate vaccine, older adults, polymicrobial infection
After adjusting for risk factors and other exposures, adults aged ≥65 years who received 13-valent pneumococcal conjugate vaccine (PCV13) experienced lower incidence of COVID-19 diagnosis, hospitalization, and death. Antibiotic receipt transiently attenuated the association between PCV13 receipt and COVID-19 diagnosis.
Bacterial–viral interactions in the upper and lower airways influence the natural history of numerous respiratory virus infections. A substantial proportion of influenza-associated respiratory disease involves the bacterium Streptococcus pneumoniae (pneumococcus; [1]) likewise, incidence of severe pneumococcal infections closely tracks the incidence of influenza and other respiratory virus infections [2]. A canonical explanation for this observation is that virus-induced inflammation in the upper airway impairs innate (monocyte or cytokine) responses to pneumococci [3], facilitating the acquisition of pneumococcal carriage and a loss of control over progression to secondary bacterial pneumonia [4].
However, both epidemiological and experimental studies reveal viral–bacterial interactions arising at earlier stages of the clinical course. Individuals shedding respiratory viruses—including influenza virus, respiratory syncytial virus (RSV), adenoviruses, endemic human coronaviruses (HCoVs), and human rhinoviruses [5–11]—have higher-density pneumococcal carriage than individuals without respiratory virus infection. Additionally, pneumococcal carriers have diminished mucosal antibody responses to influenza virus challenge [12], and higher likelihood of both acquiring respiratory viruses and thereafter experiencing acute respiratory symptoms [13]. Whereas these interactions have been shown to promote transmission of both pneumococci and viruses [14, 15], clinical implications including impacts on viral disease pathogenesis remain less clearly understood [16].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing coronavirus disease 2019 (COVID-19), has rapidly achieved pandemic spread and has caused substantial morbidity and mortality in the United States (US) and worldwide. While available data have shown limited burden of secondary bacterial pneumonia among individuals with severe COVID-19 illness [17], the contribution of respiratory microbiota to SARS-CoV-2 infection and pathogenesis has not been widely investigated [18, 19]. In 1 study of patients with invasive pneumococcal disease (IPD), concomitant SARS-CoV-2 infection and IPD was associated with 7-fold higher odds of death in comparison to IPD without SARS-CoV-2 infection; furthermore, SARS-CoV-2 infection within 28 days after IPD was associated with 4-fold higher mortality [20].
Among adults, 13-valent pneumococcal conjugate vaccine (PCV13) reduces nasopharyngeal carriage acquisition and density [21, 22] for targeted pneumococcal serotypes, in addition to preventing invasive disease and nonbacteremic pneumonia [23, 24] involving these serotypes. In contrast, 23-valent pneumococcal polysaccharide vaccine (PPSV23), also recommended for US adults aged ≥65 years to prevent IPD, has not been found to confer strong protection against mucosal endpoints such as pneumococcal carriage and nonbacteremic pneumonia [25]. Randomized controlled trials among children and adults [26–29] have found PCVs to confer 23%–49% protection against pneumonia associated with respiratory viruses, including HCoVs [27, 28], supporting the etiologic involvement of pneumococci in virus-associated respiratory disease. To probe the potential for similar interactions between SARS-CoV-2 and pneumococci, we compared COVID-19 outcomes in a cohort of US older adults who had received and had not received PCV13.
METHODS
Cohort
The Kaiser Permanente Southern California (KPSC) healthcare system provides comprehensive care to roughly 19% of the Southern California population. Members are enrolled through employer-provided health insurance plans, prepaid plans, and state- and federally sponsored insurance programs. Electronic health records including member demographics, services (including vaccination), and diagnoses are tracked from outpatient, emergency department, and hospital settings. Care received out of network is captured through insurance claim reimbursements, enabling near-complete medical care ascertainment for KPSC members. We included individuals aged ≥65 years as of 1 March 2020 and thus eligible for PCV13 receipt, who were KPSC members for at least 1 year before this date. The KPSC Institutional Review Board provided ethical approval.
Outcomes and Exposures
We assessed time to each of the following endpoints: any COVID-19 diagnosis, defined as a positive result of a molecular test for SARS-CoV-2 infection or a clinically confirmed COVID-19 diagnosis; COVID-19 hospitalization, defined as a new inpatient admission between 7 days before and 28 days after a COVID-19 diagnosis; and fatal COVID-19 hospitalization, defined as death within 60 days of the admission date for a COVID-19 hospitalization. We excluded observational admissions lasting <24 hours from our definition of COVID-19 hospitalization.
The primary exposure of interest for our study was receipt of PCV13 (with or without PPSV23) concordant with Advisory Committee on Immunization Practices (ACIP) guidelines at age ≥65 years. We excluded cohort members who received PCV13 and PPSV23 at intervals discordant with 2015 ACIP guidelines [30], which stipulated the timing of PCV13 dosing as follows: ≥1 year before PPSV23 for immunocompetent individuals or ≥8 weeks before PPSV23 for immunocompromised persons; or ≥1 year after the most recent PPSV23 dose for individuals previously vaccinated with PPSV23 (owing to the reduced immunogenicity of PCV13 when administered shortly after PPSV23 [31]). We updated vaccination status for individuals who received PPSV23 or PCV13 during the follow-up period, excluding person-time contributed during the first 30 days after receipt of any PCV13 or PPSV23 dose to allow for time to onset of immunity.
To mitigate confounding due to differences in risk status, contact patterns and SARS-CoV-2 exposure, and healthcare utilization associated with vaccination, covariates included in our analyses were age group (defined in 5-year bins), sex, race/ethnicity, current or former smoking, body mass index, history of comorbid conditions, prior year healthcare utilization (across outpatient, emergency, and inpatient settings), median household income within individuals’ residential census tract, and prior receipt of any zoster vaccine as well as 2019–2020 seasonal influenza vaccine. Comorbid conditions were defined by International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes for prior diagnoses of myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, hypertension, hyperlipidemia, diabetes, chronic obstructive pulmonary disease, renal disease, moderate or severe liver disease, asthma, obstructive sleep apnea, human immunodeficiency virus (HIV)/AIDS, immunocompromising conditions, organ transplant, malignancy or metastatic solid tumor, rheumatologic or inflammatory disorders, and depression. We encoded missing information on race/ethnicity, body mass index, smoking, and median census tract household income as a distinct exposure category, as the occurrence of missing data was informatively associated with healthcare utilization and history of vaccination. To assess effect modification, we also recorded dates of any oral or intravenous antibiotic receipt among cohort members.
Statistical Analysis
We measured the association between PCV13 receipt and COVID-19 outcomes via the adjusted hazard ratio (aHR), estimated via Cox proportional hazards regression with doubly-robust inverse propensity weighting for PCV13 exposure [32]. We computed stabilized weights for cohort members that were inversely proportional to their propensity of having received PCV13, based on demographic and clinical attributes, using a logistic regression model defining any PCV13 receipt as the outcome. Covariates listed above were the predictors. We controlled for all covariates included the treatment model in the outcome models.
Because covariate adjustment may not completely remove bias in the presence of unmeasured confounding [33], we corrected for the association of COVID-19 outcomes with receipt of zoster vaccination (defined as receipt of any live or recombinant zoster vaccine dose, for which there are similar age-based recommendations among US older adults) as a negative control exposure. While not hypothesized to confer specific protection against COVID-19 outcomes, we expected that zoster vaccine receipt could be associated with COVID-19 outcomes through unmeasured confounding pathways that would also affect analyses of PCV13, such as an association between vaccine uptake and personal protective measures against COVID-19 (eg, mask wearing, avoidance of social gatherings). Using the method of Sanderson and colleagues [33], we adjusted estimates for the association of zoster vaccine receipt with COVID-19 outcome via the formula , where and were adjusted hazard ratio estimates for PCV13 and zoster vaccine exposures, respectively. We considered findings to be statistically significant if the 95% confidence interval (CI) around excluded 1; here, statistical significance indicated that the magnitude of effect associated with pneumococcal vaccination exceeded the estimated negative control effect size.
Effect Modification by Antibiotic Exposure
Finally, to assess the hypothesis that associations with PCV13 receipt were mediated by presence of pneumococci in the respiratory tract, we assessed differences in effect size estimates across strata defined according to individuals’ history of antibiotic exposure. While direct verification of individuals’ pneumococcal carriage status was not possible in this retrospective study, we hypothesized that no effects of PCV13 would be evident during risk periods immediately following antibiotic treatment, as persistence of pneumococci in the upper airway following antibiotic treatment would be unlikely [34]. Our analyses used a case-control framework, defining cases as individuals who experienced COVID-19 diagnosis over the duration of the study period, and controls as individuals who did not. We estimated adjusted odds ratios (aORs) for predictors of case status using logistic regression models applying stabilized inverse propensity weights (as defined above) for PCV13 receipt. We quantified the negative control-corrected association of PCV13 with COVID-19 outcomes as consistent with our primary analyses, for the aORs associated with receipt of PCV13 (P) and zoster (Z) vaccines. Owing to the low incidence of COVID-19 (<1% in the study cohort over the follow-up period), the odds ratio provided a suitable approximation for the hazard ratio [35].
We defined separate strata for cases who experienced COVID-19 diagnoses within 1–30, 31–60, or 61–90 days following receipt of any antibiotic (defined by the date of receipt for antibiotics with a single administration, or the last day of antibiotic supply for multiday prescriptions); cases who never received antibiotics during the study period; and cases who received antibiotics in a remote risk period 91–365 days before their COVID-19 diagnosis. Stratification of controls for antibiotic receipt was unnecessary as the risk ratio of control status among unvaccinated and vaccinated persons—a factor in the odds ratio—approaches 1 at low risk of infection ():
where a1 and a0 are the prevalence of antibiotic receipt among controls who received and did not receive PCV13, respectively, is the risk ratio of COVID-19 given PCV13 receipt (and conditioned on antibiotic exposure status), andis the risk ratio of COVID-19 diagnosis given antibiotic receipt.
RESULTS
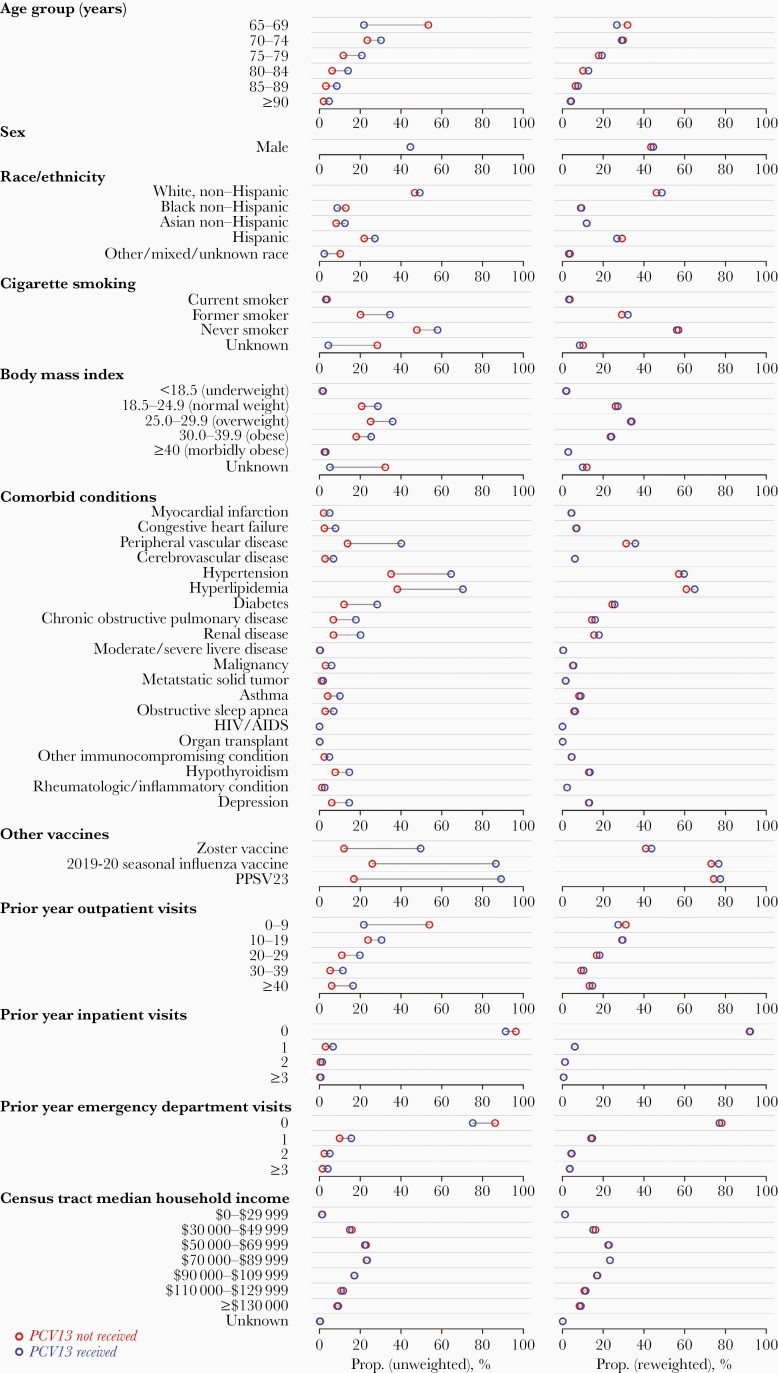
The study cohort comprised 531 033 individuals aged ≥65 years enrolled in KPSC health plans, among whom there were 3677 COVID-19 cases, 1075 COVID-19 hospitalizations, and 334 fatal COVID-19 hospitalizations from 1 March to 22 July 2020. In total, 451 068 cohort members received PCV13 at age ≥65 years. On average, recipients of PCV13 were older than nonrecipients, had higher prevalence of comorbid conditions associated with risk of pneumonia and COVID-19, and had higher rates of healthcare utilization in the preceding year (Table 1; Supplementary Table 1). Incidence rates of COVID-19 diagnosis, hospitalization, and fatal hospitalization were higher among individuals with each comorbid condition included in analyses, with the exception of malignancy, metastatic solid tumor, and HIV infection (for which the sample size was small; Supplementary Table 1). Reweighting of the cohort according to the inverse propensity of PCV13 receipt balanced the distribution of confounding variables between recipients and nonrecipients (Figure 1; Supplementary Table 2).
Table 1.
Descriptive Attributes of the Study Cohort
| Characteristic | No. (%) of Individuals | |
|---|---|---|
| PCV13 Received per ACIP Guidelinesa | PCV13 Not Received | |
| (n = 451 068) | (n = 80 600) | |
| Age on 1 March 2020 | ||
| 65–69 y | 96 812 (21.5) | 42 898 (53.2) |
| 70–74 y | 136 556 (30.3) | 18 982 (23.6) |
| 75–79 y | 94 209 (20.9) | 9482 (11.8) |
| 80–84 y | 63 650 (14.1) | 5031 (6.2) |
| 85–89 y | 38 473 (8.5) | 2569 (3.2) |
| ≥90 y | 21 368 (4.7) | 1638 (2.0) |
| Sex | ||
| Female | 250 005 (55.4) | 44 662 (55.4) |
| Male | 201 063 (44.6) | 35 938 (44.6) |
| Race/ethnicity | ||
| White, non-Hispanic | 222 100 (49.2) | 37 664 (46.7) |
| Black, non-Hispanic | 39 483 (8.8) | 10 415 (12.9) |
| Asian or Pacific Islander, non-Hispanic | 56 331 (12.5) | 6585 (8.2) |
| Hispanic (any race) | 122 540 (27.2) | 17 664 (21.9) |
| Other, mixed, or unknown race | 10 614 (2.4) | 8272 (10.3) |
| Tobacco smoking | ||
| Current smoker | 14 080 (3.1) | 2982 (3.7) |
| Former smoker | 156 177 (34.6) | 16 196 (20.1) |
| Never smoker | 261 103 (57.9) | 38 383 (47.6) |
| Unknown | 19 708 (4.4) | 23 039 (28.6) |
| Body mass index, kg/m2 | ||
| <18.5 (underweight) | 8253 (1.8) | 1112 (1.4) |
| 18.5–24.9 (normal weight) | 129 518 (28.7) | 16 711 (20.7) |
| 25.0–29.9 (overweight) | 162 049 (35.9) | 20 200 (25.1) |
| 30.0–39.9 (obese) | 114 448 (25.4) | 14 532 (18.0) |
| ≥40.0 (morbidly obese) | 13 786 (3.1) | 1954 (2.4) |
| Unknown | 23 014 (5.1) | 26 091 (32.4) |
| Comorbid conditions | ||
| Myocardial infarction | 22 589 (5.0) | 1664 (2.1) |
| Congestive heart failure | 35 647 (7.9) | 1977 (2.5) |
| Peripheral vascular disease | 181 756 (40.3) | 11 123 (13.8) |
| Cerebrovascular disease | 31 028 (6.9) | 2240 (2.8) |
| Hypertension | 291 730 (64.7) | 28 304 (35.1) |
| Hyperlipidemia | 317 967 (70.5) | 30 758 (38.2) |
| Diabetes | 127 981 (28.4) | 9761 (12.1) |
| COPD | 80 680 (17.9) | 5487 (6.8) |
| Renal disease | 91 274 (20.2) | 5543 (6.9) |
| Moderate or severe liver disease | 1682 (0.4) | 94 (0.1) |
| Malignancy | 26 844 (6.0) | 2398 (3.0) |
| Metastatic solid tumor | 8184 (1.8) | 803 (1.0) |
| Asthma | 45 130 (10.0) | 3249 (4.0) |
| Obstructive sleep apnea | 31 469 (7.0) | 2270 (2.8) |
| HIV/AIDS | 174 (<0.1) | 7 (<0.1) |
| Organ transplant | 556 (0.1) | 33 (<0.1) |
| Other immunocompromising condition | 22 059 (4.9) | 1894 (2.3) |
| Hypothyroidism | 66 156 (14.7) | 6238 (7.7) |
| Rheumatologic/inflammatory condition | 11 579 (2.6) | 964 (1.2) |
| Depression | 66 030 (14.6) | 4844 (6.0) |
| Zoster vaccinationb | ||
| Any zoster vaccine dose received | 224 317 (49.7) | 9625 (11.9) |
| Influenza vaccination | ||
| Vaccinated in 2019–2020 season | 390 322 (86.5) | 20 617 (25.6) |
| PPSV23 vaccination | ||
| PPSV23 ever received | 404 730 (89.7) | 13 743 (17.1) |
| Prior year outpatient visits | ||
| 0–9 | 98 005 (21.7) | 43 533 (54.0) |
| 10–19 | 137 344 (30.4) | 19 172 (23.8) |
| 20–29 | 89 508 (19.8) | 8839 (11.0) |
| 30–39 | 51 813 (11.5) | 4241 (5.3) |
| ≥40 | 74 398 (16.5) | 4815 (6.0) |
| Prior year inpatient visits | ||
| 0 | 411 875 (91.3) | 77 679 (96.4) |
| 1 | 30 152 (6.7) | 2391 (3.0) |
| 2 | 6186 (1.4) | 361 (0.4) |
| ≥3 | 2855 (0.6) | 169 (0.2) |
| Prior year emergency department visits | ||
| 0 | 339 108 (75.2) | 69 482 (86.2) |
| 1 | 70 527 (15.6) | 7954 (9.9) |
| 2 | 22 915 (5.1) | 1945 (2.4) |
| ≥3 | 18 518 (4.1) | 1219 (1.5) |
| Census tract median household income | ||
| $0–$29 999 | 5460 (1.2) | 1173 (1.5) |
| $30 000–$49 999 | 67 197 (14.9) | 12 848 (15.9) |
| $50 000–$69 999 | 100 439 (22.3) | 18 458 (22.9) |
| $70 000–$89 999 | 105 416 (23.4) | 18 570 (23.0) |
| $90 000–$109 999 | 77 593 (17.2) | 13 705 (17.0) |
| $110 000–$129 999 | 52 644 (11.7) | 8538 (10.6) |
| ≥$130 000 | 41 645 (9.2) | 6965 (8.6) |
| Unknown | 674 (0.1) | 343 (0.4) |
| Receipt of antibiotics | ||
| At any point during study period | 2064 (0.5) | 232 (0.3) |
| ≥1 d before COVID-19, if ever diagnosed | 1406 (0.3) | 169 (0.2) |
Values show the number of individuals with each risk factor who belonged at any point during follow-up to the exposure groups. We indicate incidence of COVID-19 outcomes associated with each attribute in Supplementary Table 1. We present reweighted estimates in Figure 1 and Supplementary Table 2.
Abbreviations: ACIP, Advisory Committee on Immunization Practices; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
aReceipt of PCV13 ≥1 year before PPSV23 for immunocompetent individuals, or ≥8 weeks before PPSV23 for immunocompromised individuals; or, for individuals who had previously received PPSV23, receipt of PCV13 ≥1 year after the most recent PPSV23 dose.
bReceipt of ≥1 dose of live or recombinant zoster vaccine.
Figure 1.
We illustrate the distribution of demographic and clinical attributes of individuals within the cohort by receipt (blue) or nonreceipt (red) of 13-valent pneumococcal vaccine (PCV13). Plots in the left-hand column illustrate cohort characteristics, as observed; individuals receiving PCV13 tended to be older, had higher prevalence of comorbid conditions, and had higher rates of healthcare utilization in the preceding year than individuals who did not receive PCV13. Plots in the right-hand column illustrate cohort characteristics after reweighting of individuals according to the inverse of their propensity of PCV13 receipt (as estimated in logistic regression models defining PCV13 receipt as the outcome variable and factors listed in the figure as covariates) [32]; this approach reduced confounding based on differences in measured risk factors between PCV13 recipients and nonrecipients. We indicate sample characteristics in Supplementary Table 1 (with reweighted estimates in Supplementary Table 3) and indicate differences in incidence of COVID-19 endpoints associated with each risk factor in Supplementary Table 2. Abbreviations: HIV, human immunodeficiency virus; PCV13, 13-valent pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
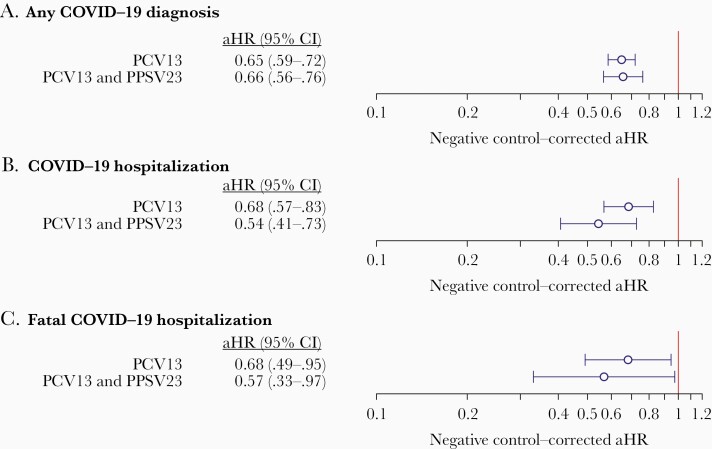
Accounting for zoster vaccination as a negative-control exposure, the estimated aHR for COVID-19 among PCV13 recipients, vs nonrecipients, was 0.65 (95% CI, .59–.72; Figure 2; Supplementary Table 3). For hospitalization and fatal hospitalization endpoints, aHR estimates were 0.68 (95% CI, .57–.83) and 0.68 (95% CI, .49–.95), respectively. In contrast, receipt of PPSV23 was not independently associated with clear differences in risk of the 3 COVID-19 outcomes (Figure 2; Supplementary Table 4); estimates suggested a reduction in risk of hospitalization associated with PPSV23 receipt, although this effect size did not differ significantly from the negative control association with zoster vaccination (aHR, 1.02 [95% CI, .78–1.29]). Individuals receiving only PCV13 experienced lower risk of each outcome than individuals receiving only PPSV23 (Supplementary Table 5).
Figure 2.
We present estimates of the effectiveness of any 13-valent pneumococcal vaccine (PCV13) receipt, and receipt of PCV13 and 23-valent pneumococcal polysaccharide vaccine (relative to no pneumococcal vaccination) according to 2015 guidelines of the Advisory Committee on Immunization Practices [30], against outcomes of any coronavirus disease 2019 (COVID-19) diagnosis (A), COVID-19 hospitalization (B), and fatal COVID-19 hospitalization (C). We obtain estimates using doubly robust Cox proportional hazards models applying inverse weights for the propensity of PCV13 receipt, correcting for the association of each endpoint with prior zoster vaccination as a negative control exposure. Lines (and numbers in parentheses) signify 95% confidence intervals around maximum likelihood estimates (points). Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; COVID-19, coronavirus disease 2019; PCV13, 13-valent pneumococcal vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
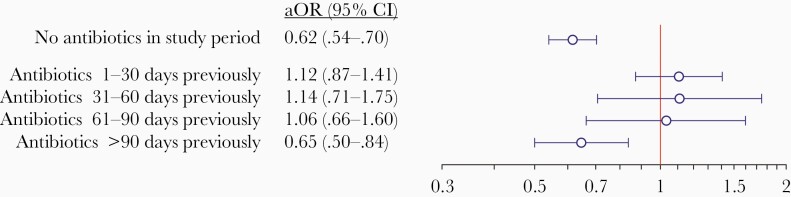
In analyses distinguishing risk periods according to history of antibiotic receipt, the aOR for COVID-19 among PCV13 recipients, vs nonrecipients, was 0.62 (95% CI, .54–.70) among individuals who never received antibiotics over the study period (Figure 3; owing to the low incidence of COVID-19, the aOR is directly comparable to the aHR for the same outcome). In contrast, estimates of the aOR over the time periods 1–30 days, 31–60 days, and 61–90 days after antibiotic receipt were 1.12 (95% CI, .87–1.41), 1.14 (95% CI, .71–1.75), and 1.06 (95% CI, .66–1.60), respectively. Over the period 90–365 days after antibiotic receipt, the aOR for COVID-19 associated with PCV13 receipt was 0.65 (95% CI, .50–.84), suggesting that differences in effect estimates shortly after antibiotic administration were not attributable to fundamental differences between individuals who received and did not receive antibiotics.
Figure 3.
We present estimates of the effectiveness of any 13-valent pneumococcal vaccine (PCV13) receipt for risk periods defined by recent antibiotic exposure: no antibiotics received in the preceding 30 days (top), antibiotics received within the preceding 1–30 days (middle), and antibiotics received >60 days previously (and not within the ensuing 60 days; bottom). We obtain estimates using doubly robust Cox proportional hazards models applying inverse weights for the propensity of PCV13 receipt, correcting for the association of coronavirus disease 2019 diagnosis with prior zoster vaccination as a negative control exposure. Lines (and numbers in parentheses) signify 95% confidence intervals around maximum likelihood estimates (points). Abbreviations: aOR, odds ratio; CI, confidence interval.
We indicate negative-control (ie, noncausal) associations of zoster vaccine receipt with COVID-19 outcomes, used to correct for residual confounding, in Supplementary Table 6. In contrast to the lower observed incidence of COVID-19 among zoster vaccine recipients than nonrecipients, 2019–2020 seasonal influenza vaccination was not independently associated with COVID-19 outcomes in the study cohort (Supplementary Tables 7 and 8), illustrating that our findings were not sensitive to the choice of zoster vaccination as the negative control exposure.
DISCUSSION
Within a cohort of US adults aged ≥65 years, receipt of PCV13 was associated with lower incidence of any COVID-19 diagnosis, COVID-19 hospitalization, and fatal COVID-19 hospitalization after correction for multiple potential sources of confounding. These results are consistent with previous findings of interactions between pneumococci and respiratory viruses [5–9], contributing to PCV efficacy against virus-associated pneumonia among both children and older adults [26–29]. Although exploratory in nature as our study used an observational design, our findings support the hypothesis that interactions with pneumococci in the upper airway contribute to SARS-CoV-2 pathogenesis in 3 respects. First, similarity of PCV13 effect estimates across all 3 outcomes suggests that protection arose from the prevention of early stages of COVID-19 pathogenesis rather than prevention of severe postinfection sequelae, which would have led to higher effectiveness estimates against hospitalization and death. This finding is externally consistent with previous studies, suggesting a low burden of secondary pneumococcal pneumonia following SARS-CoV-2 infection, in contrast to experience with other viral pathogens such as influenza [16]. Second, receipt of PPSV23—which, unlike PCV13, would not be expected to prevent pneumococcal colonization—showed little association with protection against COVID-19 outcomes. Third, recent antibiotic receipt was a modifier of the PCV13 effect estimate. Individuals who recently received antibiotics, and who would therefore not be expected to carry pneumococci, did not experience PCV13-associated protection against subsequent COVID-19 diagnosis.
Improved understanding of viral–bacterial interactions during SARS-CoV-2 infection remains necessary to validate the mechanistic basis for our findings. However, our results are in agreement with other data, suggesting that the pathogenicity of respiratory viruses may be modified by bacterial carriage [36]. Evidence that upper respiratory commensal bacteria promote viral infection dates at least to 1987 [37], with studies demonstrating that enzymes expressed by bacteria (including pneumococci [38]) enhance influenza virus replication and pathogenicity. More recently, blunting of innate immune responses to influenza virus during pneumococcal colonization has been demonstrated in human challenge studies [12]. Such mechanisms of interaction may account for epidemiologic observations of enhanced virus acquisition and symptom risk among carriers of pneumococci and other respiratory commensal pathogenic bacteria [9, 13]. To substantiate our findings, it remains crucial to determine whether risk of SARS-CoV-2 infection and adverse clinical outcomes differ among individuals who carry or do not carry pneumococci. Several classes of observational studies would be helpful to test this hypothesis. Epidemiological studies should evaluate interactions between pneumococci and SARS-CoV-2 by comparing the prevalence and density of pneumococcal carriage among COVID-19 cases and uninfected controls, or among individuals with SARS-CoV-2 infection who experience or do not experience symptoms. Such studies will also provide an opportunity to assess whether interactions between pneumococci and SARS-CoV-2 are serotype specific, as reported in prior laboratory and epidemiologic studies of pneumococcal interactions with influenza and RSV [36, 39–41]. Studies assessing whether preexisting or concomitant pneumococcal colonization is associated with differential immune responses to SARS-CoV-2 could further establish whether host-mediated mechanisms of interaction between pneumococci and influenza also apply contribute to the observed association in our study [12].
Counter-arguments to the above hypothesis merit consideration as well. First, prevalence of pneumococcal carriage among older adults has historically been reported at very low levels. However, recent studies using sensitive techniques of nasal wash or saliva sampling with molecular pneumococcal detection methods have found that low-density carriage is much more common than previously thought among adults [42]. Notably, roughly one-third of serotypes carried by adults are PCV13 types in settings with well-established pediatric vaccination programs [43–45], indicating that vaccination of young children reduces but does not eliminate PCV13-serotype circulation. While short-term effects on adult carriage were reported in in a large-scale randomized trial of PCV13 in the Netherlands [46], use of traditional nasopharyngeal sampling methods was a limitation of this study. Challenge experiments have provided further demonstration of PCV13-conferred protection against vaccine-serotype carriage acquisition among adults [12, 22]. Second, among children, reductions in vaccine-serotype carriage are largely offset by increases in carriage of nonvaccine serotypes following PCV13 receipt. Whether this is also true of adult carriage is uncertain, in particular because adults may be exposed to a lower force of infection than children. Previous studies have reported reduced risk of virus-associated symptoms among children receiving PCVs despite serotype replacement in carriage [13, 26, 28, 29], further suggesting that nonvaccine serotypes may differ in their propensity for viral interaction. It remains unclear to what extent our findings could be explained by lower pneumococcal carriage prevalence among adults receiving PCV13, if serotype replacement is minimal, or by serotype-specific virus interaction, if serotype replacement is substantial.
Our study has limitations. Laboratory confirmation was recorded for 81.2% of COVID-19 diagnoses; clinically confirmed cases may have been tested by other providers. While nonrandomized PCV13 exposure was a limitation, use of inverse propensity weighting helped to mitigate confounding in our analyses. Moreover, accounting for other adult vaccine exposures reduces risk that unmeasured confounding pathways—such as an association between vaccine access and compliance with social distancing—would explain our findings. As PCV13 receipt was more common among older individuals and those with high-risk conditions, bias would be expected to suggest greater risk among PCV13 recipients than nonrecipients, contrary to our results. Our findings that PPSV23 did not prevent COVID-19 outcomes, and that PCV13 effects were not evident following antibiotic receipt, are consistent with the hypothesis that protection was mediated by prevention of pneumococcal carriage. Hospitalizations and death within the specified time window surrounding COVID-19 diagnosis may have been due to other causes. Such outcome misclassification, however, would be expected to obscure vaccine effects against COVID-19 hospitalization and fatality endpoints, biasing outcomes in favor of the null hypothesis. We assumed that associations of COVID-19 endpoints with zoster vaccination provided an indicator of bias due to residual confounding. Although we are unaware of data demonstrating nonspecific effects of zoster vaccination against COVID-19, this possibility merits fuller investigation. Exposure to children and living in group housing are key risks factor for pneumococcal carriage among older adults that were not assessed in this study, although our analyses did address other risk factors including age group, smoking status, receipt of antibiotics and PCV13, and comorbid chronic diseases [47–49]. Finally, periods immediately surrounding the timing of antibiotic receipt may have been associated with differential risk of SARS-CoV-2 infection for adults in the study, although we are unaware of a reason why this would differentially modify risk among PCV13 recipients and nonrecipients.
Despite longstanding pediatric PCV13 use, circulation of vaccine-targeted serotypes continues to occur in the United States, accounting for 28% of invasive pneumococcal disease cases in 2015–2016 [50]. Reductions in childhood vaccine coverage associated with the COVID-19 pandemic may accentuate the relative importance of vaccine-targeted serotypes in pneumococcal carriage and in diseases for which pneumococci play a role in the causal pathway. Efforts to understand bacterial accentuation of viral infection or pathogenicity are needed, both in the context of the COVID-19 pandemic and for other important viral pathogens such as RSV and influenza.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. The study was funded by Pfizer, Inc. J. A. L. received funding from the National Institute of General Medical Sciences (grant number MIDASNI2020-3 under U24GM132013-02S2); the National Institute of Allergy and Infectious Diseases (grant number R01-AI14812701A1); and the Berkeley Population Center, on behalf of the National Institute of Child Health and Human Development (grant number P2CHD073964).
Potential conflicts of interest. J. A. L. and S. Y. T. have received research grants from Pfizer, Inc, to their respective institutions. J. A. L. has received consulting fees from Pfizer, Inc. L. R. G., L. J., and B. D. G. are employees of Pfizer, Inc. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Morens DM, Taubenberger JK, Fauci AS.Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinberger DM, Grant LR, Steiner CA, et al. Seasonal drivers of pneumococcal disease incidence: impact of bacterial carriage and viral activity. Clin Infect Dis 2014; 58:188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jochems SP, Marcon F, Carniel BF, et al. Inflammation induced by influenza virus impairs human innate immune control of pneumococcus. Nat Immunol 2018; 19:1299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCullers JA, McAuley JL, Browall S, Iverson AR, Boyd KL, Henriques Normark B. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J Infect Dis 2010; 202:1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karppinen S, Terasjarvi J, Auranen K, et al. Acquisition and transmission of Streptococcus pneumoniae are facilitated during rhinovirus infection in families with children. Am J Respir Crit Care Med 2017; 196:1172–80. [DOI] [PubMed] [Google Scholar]
- 6. Thors V, Christensen H, Morales-Aza B, et al. High-density bacterial nasal carriage in children is transient and associated with respiratory viral infections—implications for transmission dynamics. Pediatr Infect Dis J 2019; 38:533–8. [DOI] [PubMed] [Google Scholar]
- 7. De Steenhuijsen Piters WAA, Heinonen S, Hasrat R, et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med 2016; 194:1104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baggett HC, Watson NL, Knoll MD, et al. Density of upper respiratory colonization with Streptococcus pneumoniae and its role in the diagnosis of pneumococcal pneumonia among children aged <5 years in the PERCH study. Clin Infect Dis 2017; 64:S317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demuri GP, Gern JE, Eickhoff JC, Lynch SV, Wald ER. Dynamics of bacterial colonization with Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis during symptomatic and asymptomatic viral upper respiratory tract infection. Clin Infect Dis 2018; 66:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miellet WR, van Veldhuizen J, Nicolaie MA, et al. Influenza-like illness exacerbates pneumococcal carriage in older adults [manuscript published online ahead of print 30 October 2020]. Clin Infect Dis 2020. doi:10.1093/cid/ciaa1551. [DOI] [PubMed] [Google Scholar]
- 11. de Steenhuijsen Piters WA, Jochems SP, Mitsi E, et al. Interaction between the nasal microbiota and Streptococcus pneumoniae in the context of live-attenuated influenza vaccine. Nat Commun 2019; 10:2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carniel BF, Marcon F, Rylance J, et al. Pneumococcal colonization impairs nasal and lung mucosal immune responses to live attenuated influenza vaccination in adults. JCI Insight 2021; 6:141088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howard LM, Zhu Y, Griffin MR, et al. Nasopharyngeal pneumococcal density during asymptomatic respiratory virus infection and risk for subsequent acute respiratory illness. Emerg Infect Dis 2019; 25:2040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowe HM, Livingston B, Margolis E, et al. Respiratory bacteria stabilize and promote airborne transmission of influenza A virus. mSystems 2020; 5:e00762-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diavatopoulos DA, Short KR, Price JT, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB J 2010; 24:1789–98. [DOI] [PubMed] [Google Scholar]
- 16. Howard LM. Is there an association between SARS-CoV-2 and Streptococcus pneumoniae? Clin Infect Dis 2021; 72:e76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26:1622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cox MJ, Loman N, Bogaert D, O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe 2020; 1:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pirofski LA, Casadevall A. Pathogenesis of covid-19 from the perspective of the damage-response framework. mBio 2020; 11:e01175–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the COVID-19 pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with SARS-CoV-2: prospective national cohort study, England. Clin Infect Dis 2020; 72:e65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins AM, Wright AD, Mitsi E, et al. First human challenge testing of a pneumococcal vaccine: double-blind randomized controlled trial. Am J Respir Crit Care Med 2015; 192:853–8. [DOI] [PubMed] [Google Scholar]
- 22. German EL, Solórzano C, Sunny S, et al. Protective effect of PCV vaccine against experimental pneumococcal challenge in adults is primarily mediated by controlling colonisation density. Vaccine 2019; 37:3953–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonten MJM, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med 2015; 372:1114–25. [DOI] [PubMed] [Google Scholar]
- 24. McLaughlin JM, Jiang Q, Isturiz RE, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: a test-negative design. Clin Infect Dis 2018; 67:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pilishvili T, Bennett NM. Pneumococcal disease prevention among adults: strategies for the use of pneumococcal vaccines. Vaccine 2015; 49(6 Suppl 4); S383–90. [DOI] [PubMed] [Google Scholar]
- 26. Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med 2004; 10:811–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huijts SM, Coenjaerts FEJ, Bolkenbaas M, van Werkhoven CH, Grobbee DE, Bonten MJM; CAPiTA Study Team . The impact of 13-valent pneumococcal conjugate vaccination on virus-associated community-acquired pneumonia in elderly: exploratory analysis of the CAPiTA trial. Clin Microbiol Infect 2018; 24:764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nunes M, Cutland C, Klugman KP, Madhi SA. Pneumococcal conjugate vaccine protection against coronavirus-associated pneumonia hospitalization in children living with and without HIV. mBio 2021; 12:e02347-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karppinen S, Toivonen L, Schuez-Havupalo L, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against all respiratory tract infections in children under two years of age. Vaccine 2019; 37:2935–41. [DOI] [PubMed] [Google Scholar]
- 30. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2015; 64:944–7. [DOI] [PubMed] [Google Scholar]
- 31. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60–64 years of age. Vaccine 2014; 32:2364–74. [DOI] [PubMed] [Google Scholar]
- 32. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008; 168:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanderson E, Macdonald-Wallis C, Smith GD. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol 2018; 47:587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewnard JA, Tähtinen PA, Laine MK, et al. Impact of antimicrobial treatment for acute otitis media on carriage dynamics of penicillin-susceptible and penicillin-nonsusceptible Streptococcus pneumoniae. J Infect Dis 2018; 218:1356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Symons MJ, Moore DT. Hazard rate ratio and prospective epidemiological studies. J Clin Epidemiol 2002; 55:893–9. [DOI] [PubMed] [Google Scholar]
- 36. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 2014; 12:252–62. [DOI] [PubMed] [Google Scholar]
- 37. Tashiro M, Ciborowski P, Klenk HD, Pulverer G, Rott R. Role of Staphylococcus protease in the development of influenza pneumonia. Nature 1987; 325:536–7. [DOI] [PubMed] [Google Scholar]
- 38. Nishikawa T, Shimizu K, Tanaka T, et al. Bacterial neuraminidase rescues influenza virus replication from inhibition by a neuraminidase inhibitor. PLoS One 2012; 7:e45371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weinberger DM, Harboe ZB, Viboud C, et al. Serotype-specific effect of influenza on adult invasive pneumococcal pneumonia. J Infect Dis 2013; 208:1274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hament JM, Aerts PC, Fleer A, et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res 2005; 57:1198–203. [DOI] [PubMed] [Google Scholar]
- 41. Greenberg D, Givon-Lavi N, Faingelernt Y, et al. Nasopharyngeal pneumococcal carriage during childhood community-acquired alveolar pneumonia: relationship between specific serotypes and coinfecting viruses. J Infect Dis 2017; 215:1111–6. [DOI] [PubMed] [Google Scholar]
- 42. Arguedas A, Trzciński K, O’Brien KL, et al. Upper respiratory tract colonization with Streptococcus pneumoniae in adults. Expert Rev Vaccines 2020; 19:353–66. [DOI] [PubMed] [Google Scholar]
- 43. Adler H, Nikolaou E, Gould K, et al. Pneumococcal colonization in healthy adult research participants in the conjugate vaccine era, United Kingdom, 2010–2017. J Infect Dis 2019; 219:1989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krone CL, Wyllie AL, van Beek J, et al. Carriage of Streptococcus pneumoniae in aged adults with influenza-like illness. PLoS One 2015; 10:e0119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wyllie AL, Wijmenga-Monsuur AJ, van Houten MA, et al. Molecular surveillance on Streptococcus pneumoniae carriage in non-elderly adults; little evidence for pneumococcal circulation independent from the reservoir in children. Sci Rep 2016; 6:34888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Deursen AMM, van Houten MA, Webber C, et al. The impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal carriage in the community acquired pneumonia immunization trial in adults (CAPiTA) study. Clin Infect Dis 2018; 67:42–9. [DOI] [PubMed] [Google Scholar]
- 47. Regev-Yochay G, Raz M, Dagan R, et al. Nasopharyngeal carriage of Streptococcus pneumoniae by adults and children in community and family settings. Clin Infect Dis 2004; 38:632–9. [DOI] [PubMed] [Google Scholar]
- 48. Almeida ST, Paulo AC, Froes F, de Lencastre H, Sa-Leão R. Dynamics of pneumococcal carriage in adults: a new look at an old paradigm [manuscript published online ahead of print 2 September 2020]. J Infect Dis 2020. doi:10.1093/infdis/jiaa558. [DOI] [PubMed] [Google Scholar]
- 49. Almeida ST, Nunes S, Santos Paulo AC, et al. Low prevalence of pneumococcal carriage and high serotype and genotype diversity among adults over 60 years of age living in Portugal. PLoS One 2014; 9:e90974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beall B, Chochua S, Gertz RE, et al. A population-based descriptive atlas of invasive pneumococcal strains recovered within the U.S. during 2015–2016. Front Microbiol 2018; 9:2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.