Abstract
Background
Activation of the vasopressin system plays a key role for the maintenance of osmotic, cardiovascular, and stress hormone homeostasis during disease. We investigated levels of copeptin, the C-terminal segment of the vasopressin prohormone, that mirrors the production rate of vasopressin in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Methods
We measured levels of copeptin on admission and after days 3/4, 5/6, and 7/8 in 74 consecutive hospitalized adult COVID-19 patients and compared its prognostic accuracy to that of patients with community-acquired pneumonia (n = 876) and acute or chronic bronchitis (n = 371) from a previous study by means of logistic regression analysis. The primary endpoint was all-cause 30-day mortality.
Results
Median admission copeptin levels in COVID-19 patients were almost 4-fold higher in nonsurvivors compared with survivors (49.4 pmol/L [iterquartile range (IQR) 24.9–68.9 pmol/L] vs 13.5 pmol/L [IQR 7.0–26.7 pmol/L]), resulting in an age- and gender-adjusted odds ratio of 7.0 (95% confidence interval [CI] 1.2–40.3), p < 0.03 for mortality. Higher copeptin levels in nonsurvivors persisted during the short-term follow-up. Compared with the control group patients with acute/chronic bronchitis and pneumonia, COVID-19 patients did not have higher admission copeptin levels.
Conclusions
A pronounced activation of the vasopressin system in COVID-19 patients is associated with an adverse clinical course in COVID-19 patients. This finding, however, is not unique to COVID-19 but similar to other types of respiratory infections.
Keywords: biomarker, copeptin, COVID-19, 30-day mortality, prognostic markers, SARS-CoV-2
Since the beginning of this pandemic, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a major impact on health systems worldwide. The high fatality rate of hospitalized and especially ventilated patients with coronavirus disease 2019 (COVID-19) [1], and the continuing daily rise of confirmed cases are still alarming. Global research efforts are currently investigating both the physiopathology of SARS-CoV-2 as well as safe and effective treatment strategies and improved risk stratification in COVID-19 patients. The pulmonary manifestations of SARS-CoV-2 range from asymptomatic infection to severe pneumonitis and acute respiratory distress syndrome [2]. Various pathophysiological pathways such as direct viral toxicity, endothelial cell damage, and thrombo-inflammation have been discussed [3–5]. Also, dysregulation of the immune response and the renin-angiotensin-aldosterone-system has been identified as an important mechanism [6], since Angiotensin-converting-enzyme 2 receptors—the entry mechanism of the virus—are expressed on multiple tissues [6]. In addition, a dysregulation of the osmotic system with hyponatremia has been described in some cases [7, 8]. Arginine vasopressin (AVP), also known as antidiuretic hormone, plays a key role in different physiologic and pathologic processes by inducing water conservation by the kidney, thereby contributing to osmotic and cardiovascular homeostasis. Arginine vasopressin is a potent stimulator of the adreno-corticotrophic hormone and pivotal for the endocrine stress response [9]. It also has hemostatic and central nervous system effects and could be implicated in the course of disease caused by SARS-CoV-2. However, to our knowledge, there is a lack of studies systematically investigating the role of copeptin in predicting worse prognosis in patients with COVID-19, as already investigated in other cohorts.
Herein, we aimed to compare levels of copeptin—the stable prehormone of vasopressin—in patients with COVID-19 with those of patients with other types of respiratory infections and to investigate its association with 30-day mortality.
Materials and Methods
Study design and setting
This prospective observational study included consecutively hospitalized adult patients (≥18 years) admitted during the 1st wave due to confirmed SARS-CoV-2 infection at the Cantonal Hospital Aarau, a tertiary care medical center in Switzerland, between February 26, 2020, and April 30, 2020. Written general informed consent was provided from all analyzed patients. The study was approved by the local ethical committee (EKNZ, 2020-01306) and performed in conformance with the Declaration of Helsinki ethical guidelines.
Baseline characteristics of is cohort has been published recently, including a detailed description of the study methodology [10]. In brief, COVID-19 was defined by a positive real-time reverse transcription polymerase chain reaction taken from nasopharyngeal swabs or lower respiratory tract specimens, according to the World Health Organization (WHO) guidelines [11]. Most patients presented with typical clinical symptoms (eg, respiratory symptoms with or without fever, and/or pulmonary infiltrates, and/or anosmia/dysgeusia). All data of interest was assessed as part of the clinical routine during hospitalization.
Data collection
Clinical information, including demographics and comorbidities, pre-existing medical prescriptions, and COVID-19-specific in-patient medication were assessed until hospital discharge or death and extracted from the electronic health records. Experimental antiviral treatment was recorded if given, and included at this time was hydroxychloroquine (alone or in combination with azithromycin) and occasionally tocilizumab. Comorbidities were also assessed via chart review and based on International Statistical Classification of Diseases and Related Health Problems codes (ICD-10). Subsequently, patient outcomes, including admission to the intensive care unit (ICU), length of hospital stay, and length of ICU stay were collected via chart review. Thirty-day mortality was collected by abstraction of hospital records. Laboratory test results were available according to clinical routine. Copeptin was batch-tested later and was therefore not available to the treating team during the index hospitalization.
Control group
We used patients with confirmed community-acquired pneumonia or acute and chronic bronchitis included in a previous prospective study as a control group [12–14]. In brief, from October 2006 to March 2008, consecutive patients with respiratory infection from 6 different hospitals located in the northern part of Switzerland were included and prospectively followed for the assessment of mortality and other endpoints. Within this previous study, copeptin levels were measured on admission in all patients to understand its prognostic significance regarding 30-day mortality.
Endpoint and study objective
The primary endpoint of the current study was all cause 30-day mortality. For both the COVID-19 patients and the control group, we assessed vital status 30 days after admission by abstraction of hospital records and systematic telephone interviews with patients, their families, or their primary care physicians.
Measurements of copeptin
Following hospital admission, plasma and serum samples were collected in BD Vacutainer Heparin and serum-separating tube (SST) tubes, and leftover samples were stored at -80°C until analysis. Results from routine laboratory tests were recorded. Copeptin was assessed in batch using a commercially available automated fluorescent sandwich immunoassay (BRAHMS KRYPTOR; Thermo Fisher Scientific, Hennigsdorf, Germany), as described in detail elsewhere [15, 16]. The immunoassay has a limit of detection of 0.9 pmol/L, and the functional assay sensitivity—defined as concentration with an interassay coefficient of variation of <20%—was 2.0 pmol/L. Values for the analytes followed Gaussian distribution in healthy individuals without significant difference between males and females, as listed in the assay documentation. Laboratory technicians measuring copeptin were blinded to patient characteristics and study details.
For the SARS-CoV-2-affected patients, different timepoints during hospitalization were analyzed, namely T0 (initial blood draw upon hospital admission), T1 (day 3/4), T2 (day 5/6), and T3 (day 7/8). For the control group, blood samples for biomarker measurement were upon hospital admission. Copeptin levels for these patients were batch-measured in plasma with sandwich immunoassays (KRYPTOR; Thermo Scientific) [13].
Statistical analyses
Discrete variables are expressed as frequency (%) and continuous variables are expressed as medians with interquartile ranges (IQR) or mean with standard deviation (SD). A multivariable logistic regression model was used to examine the association of copeptin levels with the primary endpoint. As predefined, regression models were adjusted for age, gender (Table 3, Model 1), and, additionally, sodium concentration at admission, cancer, coronary artery disease, chronic kidney disease, diabetes and glucose concentration at admission (Table 3, Model 2). The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were reported as a measure of association and C-statistics (area under the operating receiver curve [ROC-AUC]) as a measure of discrimination. We also validated the prognostic value of different predefined copeptin cutoffs to predict all-cause 30-day mortality, which were based on distribution in the analyzed study population. A 2-sided p-value of <0.05 was considered significant. Statistical analysis was performed using Stata 15.1 (StataCorp, College Station, TX, USA).
Table 3.
Univariable and multivariable logistic regression analyses for initial copeptin levels and 30-day mortality in the different respiratory infections
| Community-acquired Pneumonia (n = 876) | Acute or Chronic Bronchitis (n = 371) | SARS-CoV-2 (n = 74) | |
|---|---|---|---|
| Regression analysis, OR (95% CI), p-value | |||
| Unadjusted model | 6.1 (3.3–11.2), p < 0.01 | 17.9 (4.6–70.5), p < 0.01 | 14.1 (3.1–63.4), p < 0.01 |
| Multivariate model 1a | 4.0 (2.0–7.7), p < 0.01 | 20.7 (3.8–111.9), p < 0.01 | 7.0 (1.2–40.3), p < 0.03 |
| Multivariate model 2b | 2.7 (1.3–5.8), p < 0.01 | 26.8 (3.1–231.9), p < 0.01 | 5.3 (0.7–40.8), p = 0.11 |
| Discrimination statistics | |||
| AUC (95% CI) | 0.73 (0.6–0.81) | 0.89 (0.82–0.96) | 0.81 (0.70–0.93) |
Abbreviations: AUC, area under the curve; CI, confidence interval; OR, odd ratio; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2;
aAdjusted for age and gender.
bAdjusted for age, gender, sodium concentration at admission, cancer, coronary artery disease, chronic kidney disease, diabetes, and glucose concentration at admission.
Results
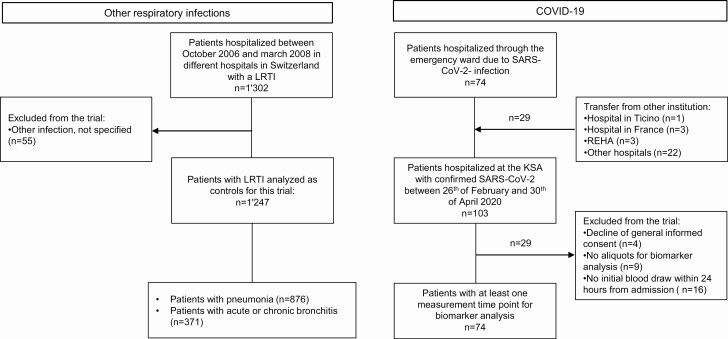
Overall, 103 patients with confirmed COVID-19 (74 were admitted directly and 29 were transferred from other hospitals) were eligible. Four patients were excluded from the analysis (decline of general consent). A further 25 cases had to be excluded because of missing aliquots for biomarker analysis (n = 9), or due to missing initial blood sampling 24 hours from admission (n = 16). Thus, in total, 74 COVID-19 patients were included in the final analysis. In addition, 1247 control patients with a final diagnosis of pneumonia (n = 876) and acute or chronic bronchitis (n = 371) were included. Figure 1 provides an overview of the study flow.
Figure 1.
Flow chart of the study. Abbreviations: COVID-19, coronavirus disease 2019; KSA, Kantonsspital Aarau (cantonal hospital Aarau); LRTI, Lower Respiratory Tract Infections; REHA, Rehabilitation; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
Baseline characteristics
Table 1 shows patient demographics and comorbidities, as well as vital signs, laboratory findings, and outcomes within 30 days in all patients and stratified by type of respiratory infection. Overall, patients with a diagnosis of COVID-19 were younger and more often male compared with the control groups. Further, patients with COVID-19 had higher initial blood pressure values with median systolic blood pressure of 141.5 mmHg (IQR 126.0–156.0 mmHg) and a diastolic blood pressure of 81.5 mmHg (IQR 72.0–88.0 mmHg) p < 0.01). COVID-19 patients also had a higher prevalence of obesity (p = 0.01) but a lower prevalence of chronic heart failure (p < 0.01), as well as of chronic obstructive pulmonary disease (p < 0.01) compared with non-COVID-19 patients, while no significant differences could be observed when comparing other comorbidities. Initial laboratory findings showed more severe deterioration in blood gas analysis, especially regarding PCO2- values in COVID-19 patients at ambient air as well as with initial O2-supply and for FiO2-value compared with the other control patients (p < 0.01, each). Regarding 30-day outcomes, COVID-19 patients had higher rates of all-cause mortality and admission to the ICU (p < 0.01, each). Length of stay was slightly longer in COVID-19 patients (p = 0.09).
Table 1.
Baseline characteristic and 30-day endpoints for the analyzed study population stratified by respiratory infections
| Non-SARS-CoV-2 | SARS-CoV-2 | p-value a | ||
|---|---|---|---|---|
| Pneumonia | Acute or Chronic Bronchitis | |||
| n = 876 | n = 371 | n = 74 | ||
| Sociodemographics | ||||
| Age [years] median (IQR) | 73.0 (59.0, 82.0) | 73.0 (60.0, 81.0) | 64.5 (57.0, 74.0) | <0.01 |
| Male gender, n (%) | 361 (41.2) | 168 (45.3) | 47 (63.5) | <0.01 |
| Pre-existing risk factors and medication | ||||
| Active smoker, n (%) | 219 (25.7) | 123 (34.1) | 4 (7.0) | <0.01 |
| Corticosteroid use, n (%) | 69 (8.1) | 69 (18.9) | 1 (1.4) | <0.01 |
| Immunosuppressant, n (%) | 14 (1.6) | 3 (0.8) | 3 (4.1) | 0.07 |
| Comorbidities | ||||
| Diabetes, n (%) | 149 (17.0) | 57 (15.4) | 18 (24.3) | 0.08 |
| Cancer, n (%) | 110 (12.6) | 42 (11.3) | 6 (8.1) | 0.29 |
| Coronary artery disease, n (%) | 176 (20.1) | 85 (22.9) | 18 (24.3) | 0.49 |
| Chronic heart failure, n (%) | 151 (17.2) | 55 (14.8) | 2 (2.7) | <0.01 |
| Solid organ transplant recipient, n (%) | 2 (0.2) | 1 (0.3) | 0 (0.0) | 0.67 |
| Chronic kidney disease, n (%) | 196 (22.4) | 79 (21.3) | 18 (24.3) | 0.65 |
| Liver cirrhosis, n (%) | 19 (2.2) | 4 (1.1) | 0 (0.0) | 0.24 |
| Obesity (BMI > 30 kg/m2), n (%) | 35 (17.7) | 32 (18.4) | 23 (31.1) | 0.01 |
| Initial vital signs | ||||
| Blood pressure, systolic [mmHg], median (IQR) | 132.0 (119.0, 148.0) | 138.0 (120.0, 150.0) | 141.5 (126.0, 156.0) | <0.01 |
| Blood pressure, diastolic [mmHg], median (IQR) | 74.0 (65.0, 82.0) | 78.0 (66.2, 85.0) | 81.5 (72.0, 88.0) | <0.01 |
| Pulse [bpm], median (IQR) | 95.0 (82.0, 107.0) | 90.0 (80.0, 101.0) | 87.5 (77.5, 97.5) | <0.01 |
| Respiratory rate [breaths/min], median (IQR) | 20.0 (16.0, 25.0) | 20.0 (16.0, 26.0) | 22.0 (18.0, 27.0) | 0.27 |
| Temperature [°C], median (IQR) | 38.1 (37.2, 38.9) | 37.3 (36.7, 38.1) | 37.7 (37.2, 38.3) | 0.47 |
| Temperature > 38°C, n (%) | 446 (50.9) | 105 (28.3) | 37 (50.0) | 0.33 |
| SpO2 [%], median (IQR) | 93.0 (89.0, 96.0) | 93.0 (89.0, 96.0) | 93.0 (87.8, 95.3) | 0.90 |
| Initial laboratory findings | ||||
| Blood gas analysis for ambient air, n (%) | 455 (54.9%) | 198 (53.4%) | 54 (73.0%) | |
| PO2 [mmHg], median (IQR) | 65 (57, 76) | 66 (58, 76) | 68 (61, 72) | 0.99 |
| PCO2 [mmHg], median (IQR) | 35 (31, 38) | 36 (32, 41) | 31 (29, 33) | <0.01 |
| Lactate [mmol/l], median (IQR) | 1.6 (1.0, 2.3) | 1.4 (1.1, 1.6) | 1.3 (0.9, 1.7) | 0.05 |
| FiO2 [%], median (IQR) | 21 | 21 | 21 | N/A |
| Blood gas analysis for initial O2-supply, n (%) | 421 (48.1%) | 173 (46.6%) | 20 (27.0%) | |
| PO2 [mmHg], median (IQR) | 60 (53, 70) | 62 (53, 74) | 65 (56, 75) | 0.16 |
| PCO2 [mmHg], median (IQR) | 35 (31, 39) | 38 (34, 47) | 32 (31, 35) | <0.01 |
| Lactate [mmol/l], median (IQR) | 1.3 (1.0, 1.8) | 1.0 (0.9, 1.4) | 1.2 (1.0, 1.5) | 0.44 |
| FiO2 [%], median (IQR) | 32 (28, 36) | 28 (28, 36) | 47 (32, 95) | <0.01 |
| Blood tests | ||||
| Leukocytes [G/L], median (IQR) | 12.0 (9.0, 16.4) | 9.7 (7.5, 13.3) | 7.3 (4.6, 8.7) | <0.01 |
| Sodium [mmol/L], median (IQR) | 136.0 (133.0, 138.0) | 136.0 (134.0, 139.0) | 136.0 (133.0, 139.0) | 0.87 |
| Hyponatremia (<135 mmol/L), n (%) | 422 (48.2%) | 135 (36.4%) | 32 (43.8%) | 0.35 |
| Normonatremia (136–143 mmol/L), n (%) | 432 (49.3%) | 225 (60.6%) | 41 (56.2%) | |
| Hypernatremia (>143 mmol/L), n (%) | 22 (2.5%) | 11 (3.0%) | 0 (0.0%) | |
| Osmolality [mosmol/kg], median (IQR) | 287.1 (280.2, 294.1) | 287.9 (282.3, 293.9) | 285.8 (280.6, 292.0) | 0.36 |
| Hypoosmolar (<280 mosmol/kg), n (%) | 214 (24.4%) | 53 (14.3%) | 16 (21.6%) | 0.79 |
| Normoosmolar (280–300 mosmol/kg) n (%) | 563 (64.3%) | 285 (76.8%) | 52 (70.3%) | |
| Hyperosmolar (>300 mosmol/kg), n (%) | 99 (11.3%) | 33 (8.9%) | 6 (8.1%) | |
| Glucose [mmol/L], median (IQR) | 7.1 (6.0, 8.5) | 6.7 (5.8, 7.9) | 6.7 (5.9, 8.6) | 0.70 |
| Urea [mmol/L], median (IQR) | 7.1 (4.9, 10.5) | 6.5 (4.4, 9.6) | 5.9 (4.4, 9.4) | 0.34 |
| CRP [mg/L], median (IQR) | 154.5 (74.2, 251.9) | 41.0 (14.0, 98.4) | 94.3 (49.9, 150.0) | 0.10 |
| PCT [µg/L], median (IQR) | 0.46 (0.15, 2.66) | 0.12 (0.08, 0.20) | 0.11 (0.05, 0.26) | <0.01 |
| Creatinine[µmol/L], median (IQR) | 89.0 (69.0, 113.0) | 84.0 (66.0, 106.0) | 91.0 (77.0, 115.0) | 0.24 |
| Copeptin [pmol/L], median (IQR) | 25.0 (12.7, 51.05) | 14.9 (6.9, 35.9) | 18.6 (8.1, 40.0) | 0.17 |
| Outcomes within 30 days | ||||
| ICU care, n (%) | 80 (9.1%) | 13 (3.5%) | 22 (29.7%) | <0.01 |
| 30-day mortality, n (%) | 49 (5.6%) | 10 (2.7%) | 17 (23.0%) | <0.01 |
| Length of stay [day], median (IQR) | 8.0 (5.0, 13.0) | 7.0 (3.0, 11.0) | 9.0 (5.0, 14.0) | 0.09 |
Abbreviations: BMI, body mass index; bpm, beats per minute; CRP, C-reactive protein; FiO2, fraction of inspired oxygen; ICU, intensive care unit; IQR, interquartile range; min, minute; mmHg, millimeter of mercury; N/A, not applicable; PCO2, partial pressure of carbon dioxide; PCT, procalcitonin; PO2, partial pressure of oxygen; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SpO2, oxygen saturation.
a P-values are referring to SARS-CoV-2 vs non-SARS-CoV-2 (pneumonia + acute or chronic bronchitis).
Association of admission copeptin levels and mortality at 30 days
Median admission copeptin levels in COVID-19 patients were almost 4-fold higher in nonsurvivors compared with survivors (49.4 pmol/L [IQR 24.9–68.9 pmol/L] vs 13.5 pmol/L [IQR 7.0–26.7 pmol/L], p < 0.01). This was similar also among the other patient groups, where again nonsurvivors had significantly higher copeptin levels at admission compared with survivors. However, regarding admission copeptin, median levels were not higher in COVID-19 patients compared with non-COVID-19 patients (pneumonia and acute/chronic bronchitis patients) (18.6 pmol/L vs 22.1 pmol/L, p = 0.17). Table 2 gives an overview of copeptin median levels as well as groups stratified according to copeptin among the different types of infections.
Table 2.
Copeptin values and different cutoffs stratified by the analyzed respiratory infections and by survivors and nonsurvivors
| Pneumonia (n = 876) | Acute or Chronic Bronchitis (n = 371) | SARS-CoV-2 (n = 74) | ||||
|---|---|---|---|---|---|---|
| Survivors (n = 827) | Nonsurvivors (n = 49) | Survivors (n = 361) | Nonsurvivors (n = 10) | Survivors (n = 57) | Nonsurvivors (n = 17) | |
| Copeptin values | ||||||
| Copeptin overall [pmol/lL], median (IQR) | 24.0 (12.4, 47.7) | 70.4 (30.9, 152.0) | 13.9 (6.9, 34.1) | 78.9 (55.4, 155.0) | 13.5 (7.0, 26.7) | 49.4 (24.9, 68.9) |
| Copeptin cutoffs, n (%) | ||||||
| ≥8.0 pmol/L | 724 (87.5%) | 46 (94%) | 250 (69.3%) | 10 (100%) | 40 (70.2%) | 16 (94%) |
| ≥20.0 pmol/L | 468 (56.6%) | 41 (84%) | 152 (42.1%) | 10 (100%) | 20 (35.1%) | 15 (88%) |
| ≥40.0 pmol/L | 262 (31.7%) | 33 (67%) | 73 (20.2%) | 9 (90%) | 8 (14.0%) | 10 (59%) |
| Copeptin median cutoff, n (%) | ||||||
| ≥22.0 pmol/L | 433 (52.4%) | 41 (84%) | 142 (39.3%) | 10 (100%) | 19 (33.3%) | 14 (82%) |
Abbreviations: IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
For COVID-19 patients, the resulting age- and gender-adjusted OR of admission copeptin was 7.0 (95% CI 1.2–40.3, p < 0.03) and 5.3 (95% CI 0.7–40.8, p = 0.11) when additionally adjusted for sodium concentration at admission and different comorbidities (Table 3) for predicting 30-day mortality. For patients with pneumonia and acute or chronic bronchitis, copeptin, the association of copeptin levels, and mortality was even stronger and remained significant in the fully adjusted multivariate analyses. Regarding discrimination, copeptin had accuracy for all groups, with highest AUC values for acute or chronic bronchitis (AUC 0.89) and for COVID-19 (AUC 0.81), and lower for pneumonia (AUC 0.73).
Prognostic accuracy of copeptin in COVID-19 at specific cutoff levels
In addition, the prognostic accuracy of copeptin for 30-day mortality was analyzed for different cutoffs (Table 4). Based on the Youden’s index, we found an optimal cutoff at 20.0 pmol/L, which produced a sensitivity to correctly predict 30-day mortality of 88.2% (95% CI 63.6–98.5%), and a specificity of 64.9% (95% CI 51.1–77.1%). Furthermore, the cutoff at 20.0 pmol/L revealed the highest negative predictive value of 94.9 (95% CI 82.7–99.4%). The use of a lower cutoff at 8.0 pmol/L showed a higher sensitivity of 94.1% (95% CI 71.3–99.9%) but a very low specificity with 29.8% (95% CI 18.4–43.4%). In contrast, a higher cutoff at 40.0 pmol/L reached a high specificity of 86.0% (95% CI 74.2–93.7%) but only a low sensitivity of 58.8% (95% CI 32.9–81.6%).
Table 4.
Prognostic accuracy of different copeptin cutoffs at baseline for patients with COVID-19
| Copeptin Cut-off Value Based on Distribution in the Study Population [pmol/L] | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive vValue (95% CI) | Negative Predictive Value (95% CI) |
|---|---|---|---|---|
| 8.0 pmol/L | 94.1 (95% CI 71.3–99.9) | 29.8 (95% CI 18.4–43.4) | 28.6 (95% CI 17.3–42.2) | 94.4 (95% CI 72.7–99.9) |
| 20.0 pmol/L | 88.2 (95% CI 63.6–98.5) | 64.9 (95% CI 51.1–77.1) | 42.9 (95% CI 26.3–60.6) | 94.9 (95% CI 82.7–99.4) |
| 40.0 pmol/L | 58.8 (95% CI 32.9–81.6) | 86.0 (95% CI 74.2–93.7) | 55.6 (95% CI 30.8–78.5) | 87.5 (95% CI 75.9–94.8) |
| Copeptin median [pmol/L] | ||||
| 22.0 pmol/L | 82.4 (95% CI 56.6–96.2) | 66.7 (95% CI 52.9–78.6) | 42.4 (95% CI 25.5–60.8) | 92.7 (95% CI 80.1–98.5) |
Abbreviation: CI, confidence interval.
Kinetics of serial measurement of copeptin in SARS-CoV-2 infections
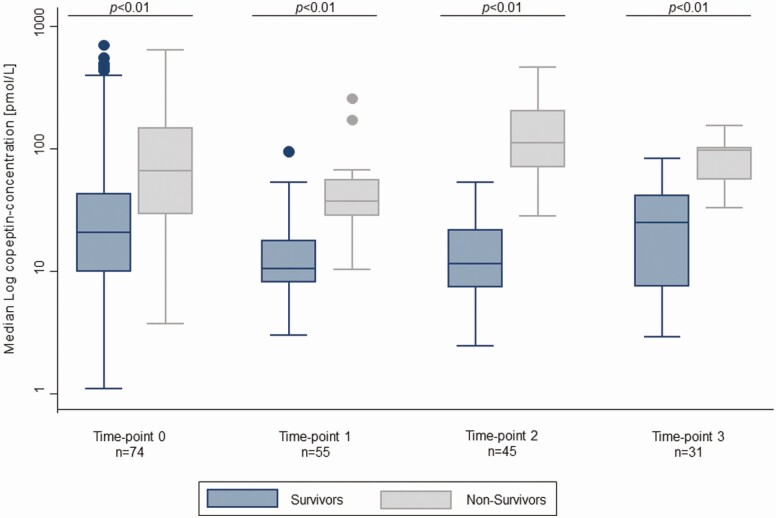
Figure 2 illustrates the kinetics of copeptin levels during the follow-up period stratified by survival status. Copeptin values were significantly higher in nonsurvivors at every timepoint measured compared with patients who survived. Notably, levels of copeptin in survivors remained in the lower range during the whole follow-up period, but were still in the nonphysiological range, whereas in nonsurvivors, values were high and reached the peak in the second half of hospitalization at days 5 and 6.
Figure 2.
Median copeptin values at different measurement time-points for survivors and nonsurvivors.
Discussion
The results of this prospective study comparing the levels of copeptin in patients with COVID-19 with those of patients with other types of respiratory infections, and among survivors and nonsurvivors has 2 main findings. First, we observed that admission levels of copeptin—a marker which mirrors activation of the vasopressin system and thus the individual stress response—were increased 4-fold in patients with a COVID-19 diagnosis who had a fatal outcome compared with survivors and were, therefore, strongly associated with 30-day mortality. This result also remained significant in statistical models adjusted for age and gender but not in the fully adjusted model also including hyponatremia, as shown for pneumonia and acute or chronic bronchitis. This suggests that the activation of the vasopressin system plays an important role in COVID-19 disease particularly regarding a severe course of disease. Second, this finding was not specific for COVID-19 but was similar to other types of viral and bacterial respiratory tract infections. Therefore, these results suggest that the activation of the vasopressin system is not specific to COVID-19 but a more general adaptation of severe respiratory disease. In turn, copeptin may allow for individual and early risk stratification and the monitoring of patients with respiratory infections including COVID-19.
Although survivors had significantly lower levels of copeptin at admission and during the follow-ups, the levels were not in the normal low range, but they were still elevated compared with healthy persons with a median concentration of 4.2 pmol/L [17].
Our data is in line with earlier studies demonstrating that infectious diseases such as pneumonia and other respiratory infections can markedly increase circulating levels of copeptin [18–23]. Several mechanisms may be responsible for this increase. Similar to other respiratory infections, severe disease and thus physiological stress caused by SARS-CoV-2 may trigger the release of the stress marker copeptin, aiming to increase free water resorption in the kidneys and thus maintaining blood pressure homeostasis through V2 receptors and producing vasoconstriction of blood vessels through V1 receptors [24–26]. According to our data septic conditions such as pneumonia had a higher increase in copeptin, which may reflect more blood pressure disturbances and shock compared with viral infections. Copeptin levels may go in parallel with the inflammatory cytokine response, which is quite strong in SARS-CoV-2 disease [27]. Pneumonia and other respiratory diseases are associated with the syndrome of (in)appropriate antidiuretic hormone secretion (SIADH), which in turn leads to hyponatremia [28], especially if associated with a salt-restricted diet and disease-related malnutrition. In our sample, hyponatremia was a frequent finding in patients. A recently published case series indeed confirmed that 3 patients with confirmed COVID-19 and who were suffering from hyponatremia showed an SIADH constellation with euvolemic hyponatremia, a high urine osmolality [8]. Besides intravascular volume depletion due to the infection [29, 30], emotional, physical, or psychological stress, as well as pain and certain medication like opiates, which can be associated with an infection like COVID-19, can stimulate the release of antidiuretic hormone (ADH) [31]. This, in turn, correlates with the severity of the disease and with individual stress levels found in lower respiratory tract infections [21].
Our findings suggest that copeptin can mirror disease severity and differentiate patients with a fatal outcome from survivors. This was true on admission but also during follow-up. Similar findings were also shown in our study investigating the association of prognostic accuracy of initial and follow-up midregional proadrenomedullin (MR-proADM) levels with in-hospital mortality in patients with confirmed SARS-CoV-2 infection [32]. In fact, our findings confirm that increased levels of MR-proADM on admission and during hospital stays were independently associated with in-hospital mortality and may allow for better risk stratification, and, in particular, rule out a fatal outcome in COVID-19 patients [32].
Our analysis is in line with copeptin data in other populations such as sepsis patients [33, 34], respiratory infection patients [22, 23], and also stroke patients [35–40]. In addition, copeptin was also found to be of prognostic importance in ventilator-associated pneumonia [41], and community acquired pneumonia [42]. We found an optimal cutoff at 20.0 pmol/L in COVID-19 patients. At this levels, copeptin provides additional information regarding disease progression and risk of 30-day mortality. Interestingly, our optimal cutoff (20.0 pmol/L) differs from the cutoffs proposed by Elham et al (58.1 pmol/L) [43] and by Zhang et al (86.3 pmol/L) [44]. These differences could be due to different methodologies of copeptin measurement, which made a direct comparison difficult. However, when comparing with the cutoff found by Battista et al (23.2 pmol/L) [45], who used the same methodology as in this study, the described cutoff levels are comparable.
Limitations
This study has several limitations. First, the number of analyzed COVID-19 patients was small with a single-center design. Second, not all clinical and laboratory parameters and characteristics were available for all patients, resulting in some missing data. Third, we have not collected the needed clinical data for the current WHO disease criteria. Thus, a proper stratification of the disease severity was not possible. Fourth, we were not able to give more detail on the pathophysiology behind the increase of copeptin in COVID-19 and whether therapeutic modulation may have any advantages in patients.
Conclusion
To the best of our knowledge, this is the 1st study analyzing copeptin levels in patients with confirmed COVID-19. Our data indicate that a pronounced activation of the vasopressin system is associated with an adverse clinical course in COVID-19 patients. This finding, however, is not unique to COVID-19 but similar to other types of respiratory infections.
Acknowledgments
We thank all participating patients and their families, and all health care workers at the Cantonal Hospital Aarau for their great dedication to reducing the burden of this severe disease. We thank Erica Holt for the native English review.
Financial Support: Costs for biomarker measurement (reagents) were externally sponsored by ThermoFisher Scientific, BRAHMS, Henningsdorf (Germany). This study was funded by Research Council KSA (Kantonsspital Aarau). The funding agencies have no bearing on the study design, data collection and analysis, or writing of the manuscript.
Author Contributions: CG, AK, and PS had the idea, wrote the protocol, and initiated the study. CG managed the trial and collected data. LB and AHL managed the laboratory investigations. CG, DK, and SH performed the statistical analyses, and CG, AM, AK, and PS drafted the manuscript. EH, SH, AC, LB, AHL, CAF, and BM amended and commented on the manuscript. PS and BM provided funding. All authors approved the final version. CG and AM are equally contributing first authors.
Glossary
Abbreviations
- ACE2
angiotensin-converting-enzyme 2
- ACTH
adreno-corticotrophic hormone
- ADH
antidiuretic hormone
- ARDS
acute respiratory distress syndrome
- AUC
area under the curve
- AVP
arginine vasopressor
- BMI
body-mass-index
- bpm
beats per minute
- CI
confidence intervals
- COVID-19
coronavirus disease 2019
- CRP
c-reactive protein
- ICD10
international statistical classification of diseases and related health problems codes
- ICU
intensive care unit
- IQR
interquartile ranges
- KSA
cantonal hospital Aarau
- LOD
limit of detection
- LOS
length of hospital stay
- mmHg
millimeter of mercury
- MR-proADM
midregional proadrenomedullin
- NS
nonsurvivors
- OR
odds ratio
- PCT
procalcitonin
- RAAS
renin-angiotensin-aldosterone-system
- ROC-AUC
area under the operating receiver curve
- RT-PCR
reverse transcription polymerase chain reaction
- S
survivors
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
- SIADH
syndrome of (in)appropriate antidiuretic hormone secretion
- SpO2
oxygen saturation
- VAP
ventilator-associated pneumonia
- WHO
World Health Organization
Additional Information
Disclosures: PS and BM received research support paid to the Cantonal Hospital Aarau from Thermofisher, bioMerieux, Roche Diagnostics, Nestle Health Science, and Abbott Nutrition. PS received sponsoring for biomarker-measurement costs (reagents) from BRAHMS. All other authors reported no conflicts of interest.
Data Availability
Some or all datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581(7809):465-469. [DOI] [PubMed] [Google Scholar]
- 3. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaiser UB, Mirmira RG, Stewart PM. Our response to COVID-19 as endocrinologists and diabetologists. J Clin Endocrinol Metab. 2020;105(5):1299-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yousaf Z, Al-Shokri SD, Al-Soub H, Mohamed MFH. COVID-19-associated SIADH: a clue in the times of pandemic! Am J Physiol Endocrinol Metab. 2020;318(6):E882-E885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr Physiol. 2016;6(2):603-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316. [DOI] [PubMed] [Google Scholar]
- 11. WHO. Clinical management of severe acute respiratory infection when novel conovirus (nCov) infection is supsected; interim guidance, 25 January 2020. Published January 25, 2020. [Google Scholar]
- 12. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group . Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-1066. [DOI] [PubMed] [Google Scholar]
- 13. Kutz A, Grolimund E, Christ-Crain M, et al. ; ProHOSP Study Group . Pre-analytic factors and initial biomarker levels in community-acquired pneumonia patients. BMC Anesthesiol. 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuetz P, Christ-Crain M, Wolbers M, et al. ; ProHOSP study group . Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC Health Serv Res. 2007;7:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Müller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9(6):R816-R824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823-1829. [DOI] [PubMed] [Google Scholar]
- 17. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52(1):112-119. [DOI] [PubMed] [Google Scholar]
- 18. Schuetz P, Wolbers M, Christ-Crain M, et al. ; ProHOSP Study Group . Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care. 2010;14(3):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krüger S, Ewig S, Kunde J, et al. ; CAPNETZ study group . Assessment of inflammatory markers in patients with community-acquired pneumonia–influence of antimicrobial pre-treatment: results from the German competence network CAPNETZ. Clin Chim Acta. 2010;411(23-24):1929-1934. [DOI] [PubMed] [Google Scholar]
- 20. Struck J, Morgenthaler NG, Bergmann A. Copeptin, a stable peptide derived from the vasopressin precursor, is elevated in serum of sepsis patients. Peptides. 2005;26(12):2500-2504. [DOI] [PubMed] [Google Scholar]
- 21. Katan M, Christ-Crain M. The stress hormone copeptin: a new prognostic biomarker in acute illness. Swiss Med Wkly. 2010;140:w13101. [DOI] [PubMed] [Google Scholar]
- 22. Stolz D, Christ-Crain M, Morgenthaler NG, et al. Copeptin, C-reactive protein, and procalcitonin as prognostic biomarkers in acute exacerbation of COPD. Chest. 2007;131(4):1058-1067. [DOI] [PubMed] [Google Scholar]
- 23. Müller B, Morgenthaler N, Stolz D, et al. Circulating levels of copeptin, a novel biomarker, in lower respiratory tract infections. Eur J Clin Invest. 2007;37(2):145-152. [DOI] [PubMed] [Google Scholar]
- 24. Michell RH, Kirk CJ, Billah MM. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979;7(5):861-865. [DOI] [PubMed] [Google Scholar]
- 25. Penit J, Faure M, Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983;244(1):E72-E82. [DOI] [PubMed] [Google Scholar]
- 26. Thibonnier M. Use of vasopressin antagonists in human diseases. Kidney Int Suppl. 1988;26:S48-S51. [PubMed] [Google Scholar]
- 27. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together? J Endocrinol Invest. 2020;43(8):1137-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dhawan A, Narang A, Singhi S. Hyponatraemia and the inappropriate ADH syndrome in pneumonia. Ann Trop Paediatr. 1992;12(4):455-462. [DOI] [PubMed] [Google Scholar]
- 29. Anderson RJ. Hospital-associated hyponatremia. Kidney Int. 1986;29(6):1237-1247. [DOI] [PubMed] [Google Scholar]
- 30. Share L, Levy MN. Cardiovascular receptors and blood titer of antidiuretic hormone. Am J Physiol. 1962;203(3):425-428. [DOI] [PubMed] [Google Scholar]
- 31. Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771(1):192-203. [DOI] [PubMed] [Google Scholar]
- 32. Gregoriano C, Koch D, Kutz A, et al. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: an observational study. Clin Chem Lab Med. 2021;59(5):995-1004. [DOI] [PubMed] [Google Scholar]
- 33. Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M. Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock. 2007;28(2):219-226. [DOI] [PubMed] [Google Scholar]
- 34. Lindner KH, Strohmenger HU, Ensinger H, Hetzel WD, Ahnefeld FW, Georgieff M. Stress hormone response during and after cardiopulmonary resuscitation. Anesthesiology. 1992;77(4):662-668. [DOI] [PubMed] [Google Scholar]
- 35. Katan M, Fluri F, Morgenthaler NG, et al. Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol. 2009;66(6):799-808. [DOI] [PubMed] [Google Scholar]
- 36. Zweifel C, Katan M, Schuetz P, et al. Copeptin is associated with mortality and outcome in patients with acute intracerebral hemorrhage. BMC Neurol. 2010;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blum CA, Winzeler B, Nigro N, et al. Copeptin for risk stratification in non-traumatic headache in the emergency setting: a prospective multicenter observational cohort study. J Headache Pain. 2017;18(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Marchis GM, Katan M, Weck A, et al. Copeptin adds prognostic information after ischemic stroke: results from the CoRisk study. Neurology. 2013;80(14):1278-1286. [DOI] [PubMed] [Google Scholar]
- 39. De Marchis GM, Weck A, Audebert H, et al. Copeptin for the prediction of recurrent cerebrovascular events after transient ischemic attack: results from the CoRisk study. Stroke. 2014;45(10):2918-2923. [DOI] [PubMed] [Google Scholar]
- 40. Urwyler SA, Schuetz P, Fluri F, et al. Prognostic value of copeptin: one-year outcome in patients with acute stroke. Stroke. 2010;41(7):1564-1567. [DOI] [PubMed] [Google Scholar]
- 41. Seligman R, Papassotiriou J, Morgenthaler NG, Meisner M, Teixeira PJ. Copeptin, a novel prognostic biomarker in ventilator-associated pneumonia. Crit Care. 2008;12(1):R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krüger S, Ewig S, Kunde J, Hartmann O, Suttorp N, Welte T; CAPNETZ Study Group . Pro-atrial natriuretic peptide and pro-vasopressin for predicting short-term and long-term survival in community-acquired pneumonia: results from the German Competence Network CAPNETZ. Thorax. 2010;65(3):208-214. [DOI] [PubMed] [Google Scholar]
- 43. Elham M, Sobhya MMN, Hammada MG, Rashedb LA. Prognostic value of the biomarker copeptin in critically ill patients with sepsis. Kasr Al Ainy Med J. 2016;22(3):123-128. [Google Scholar]
- 44. Zhang Q, Dong G, Zhao X, Wang M, Li CS. Prognostic significance of hypothalamic-pituitary-adrenal axis hormones in early sepsis: a study performed in the emergency department. Intensive Care Med. 2014;40(10):1499-1508. [DOI] [PubMed] [Google Scholar]
- 45. Battista S, Audisio U, Galluzzo C, et al. Assessment of diagnostic and prognostic role of copeptin in the clinical setting of sepsis. Biomed Res Int. 2016;2016:3624730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.