Abstract
Background
Heterogeneity in COVID-19 morbidity and mortality is often associated with a country's health-services structure and social inequality. This study aimed to characterize social inequalities in COVID-19 mortality in São Paulo, the most populous city in Brazil and Latin America.
Methods
We conducted a population-based study, including COVID-19 deaths among São Paulo residents from March to September 2020. Age-standardized mortality rates and unadjusted rate ratios (RRs) [with corresponding 95% confidence intervals (CIs)] were estimated by race, sex, age group, district of residence, household crowding, educational attainment, income level and percentage of households in subnormal areas in each district. Time trends in mortality were assessed using the Joinpoint model.
Results
Males presented an 84% increase in COVID-19 mortality compared with females (RR = 1.84, 95% CI 1.79–1.90). Higher mortality rates were observed for Blacks (RR = 1.77, 95% CI 1.67–1.88) and mixed (RR = 1.42, 95% CI 1.37–1.47) compared with Whites, whereas lower mortality was noted for Asians (RR = 0.63, 95% CI 0.58–0.68). A positive gradient was found for all socio-economic indicators, i.e. increases in disparities denoted by less education, more household crowding, lower income and a higher concentration of subnormal areas were associated with higher mortality rates. A decrease in mortality over time was observed in all racial groups, but it started earlier among Whites and Asians.
Conclusion
Our results reveal striking social inequalities in COVID-19 mortality in São Paulo, exposing structural inequities in Brazilian society that were not addressed by the governmental response to COVID-19. Without an equitable response, COVID-19 will further exacerbate current social inequalities in São Paulo.
Keywords: COVID-19, mortality, social determinants of health, race factors
Introduction
In December 2019, a cluster of pneumonia of unknown cause was registered in Wuhan, China and its etiologic agent, a new coronavirus, was named by the World Health Organization (WHO) as SARS-CoV-2 and the disease as COVID-19.1 Given the spread of the disease worldwide, a pandemic was declared on 11 March.2
Key Messages
According to WHO data, as of 12 October 2020, Brazil was the country with the third-highest number of cases, with 5 082 637 registered cases and 150 198 deaths.3 The city of São Paulo is the most populous in the country (12.2 million inhabitants) and the first to report a COVID-19 case in Brazil. As of 14 October 2020, 302 223 cases and 13 196 deaths had been confirmed in the city, corresponding to crude incidence and mortality rates of 2467 and 108 per 100 000 inhabitants, respectively.4
Males presented an 84% increase in COVID-19 mortality compared with females [relative risk (RR) = 1.84, 95% CI 1.79–1.90]. Higher mortality rates were observed for Blacks (RR = 1.77, 95% CI 1.67–1.88) and mixed (RR = 1.42, 95% CI 1.37–1.47) compared with Whites, whereas lower mortality was noted for Asians (RR = 0.63, 95% CI 0.58–0.68).
A positive gradient was found for all socio-economic indicators, i.e. increases in disparities denoted by less education, more household crowding, lower income and a higher concentration of subnormal areas were associated with higher mortality rates.
A decrease in mortality over time was observed in all racial groups, but it started earlier among Whites and Asians.
Our finding of a higher percentage of deaths occurring in primary-care or isolated emergency-care units observed for Black and mixed populations may be related to barriers in healthcare access.
Our results reveal striking social inequalities in COVID-19 mortality in São Paulo, exposing structural inequities in Brazilian society that were not addressed by the governmental response to COVID-19. Without an equitable response, COVID-19 will further exacerbate current social inequalities in São Paulo.
Health inequities are recognized worldwide, with most vulnerable populations presenting higher incidence and mortality for communicable and non-communicable diseases. During the COVID-19 pandemic, socio-economic gradients were reported in Brazil,5,6 the USA7–9 and the UK.10Racial and ethnic disparities in infection and disease outcomes were described for many countries, with Blacks, Asians and minority ethnic groups emerging as more vulnerable than the White population.5,7–9,11
Heterogeneity in high rates of COVID-19 morbidity and mortality is often associated with a country's health structure and social inequality. Brazil is a highly unequal country, with substantial health and socio-economic differences observed between and within cities.12 Previous studies have shown racial and socio-economic differences on COVID-19 mortality in Brazil,5,6 but there are still gaps in knowledge regarding the interplay between race and socio-economic factors. This study addresses this issue and aims to characterize mortality patterns from COVID-19 in the city of São Paulo, Brazil and to identify risk factors of mortality considering various socio-demographic indicators.
Methods
Study design: population-based descriptive study
Data source
Data on COVID-19 mortality among residents in the city of São Paulo were extracted from the Mortality Information System.13 These include all deaths classified with a confirmed code B34.2 or a suspected code U04.9, based on the International Classification of Diseases version 10 (ICD-10). We included deaths that occurred between March and September 2020. Each record had information on age (<4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75+ years), sex (male, female), race (White, Black, mixed, Asian, indigenous), educational level (years of study: 0, 1–3, 4–7, 8–11, 12+, unknown), district of residence, place of death, date of death, type of hospital administration (public state government, public city government, private/non-profit) and cause of death.
We also gathered information on population estimates from SEADE (Statewise System for Data Analysis Foundation) and on the following socio-economic indicators: household crowding (calculated as the average number of persons/household),14 educational attainment (calculated as the percentage of the population with a university degree),15 income level (calculated as the percentage of persons living with less than one-quarter of the minimum wage in 2018)16 and percentage of households located in subnormal areas (irregular settlements, also known as ‘favelas’).17 All variables were collected for each district (n = 96) of the city and categorized based on their quintile distribution.
Analytical methods
Age-standardized mortality rates (ASRs) (2010 Brazilian Census population) and crude rate ratios (RRs) [with corresponding 95% confidence intervals (95% CIs)] were calculated. Mortality rates were expressed as deaths per 100 000 person-years and analysed by race, sex, age group and residential district. Also, to investigate the possible influence of socio-economic factors on COVID-19 mortality, we calculated ASR by household crowding, educational attainment, income level and percentage of subnormal areas in each district. We further split age into two categories (<60 years = young/adult population and ≥60 years = elderly population), calculating ASR for each group according to race, sex and all socio-economic indicators. We have also evaluated the association between place of death, type of hospital administration and race. We calculated odds ratios (ORs) and 95% CIs. We have tested whether age modifies these associations using the Breslow-Day test for the homogeneity of ORs. These analyses were performed using Stata 15.1 (Stata Corp., College Station, TX, USA).
We also analysed time trends in COVID-19 mortality in São Paulo's city according to race using a Joinpoint Regression Model with the age-standardized mortality rate as the dependent epidemiological week (from Week 12: 15–21 March to Week 39: 20–26 September) as the independent variable. We estimated the weekly percent changes (WPCs) in the mortality rate and corresponding 95% CIs. The number of join points was obtained using a permutation test via Monte Carlo resampling. Once the number k of join points was obtained, the different models were compared by estimating their Bayesian Information Criterion (BIC).18 Analyses were performed using the Joinpoint Regression Program version 4.7.0.0 (Joinpoint Regression Program, Version 4.7.0.0. February 2019; Statistical Research and Applications Branch, National Cancer Institute).
Results
Between March and September 2020, the mortality system recorded 19 498 deaths due to COVID-19, corresponding to 37.1% of all deaths due to diseases that occurred in the city in the same period (n = 52 519). Overall, the age-adjusted mortality rate observed in the period was 123.2 deaths/100 000 for all ages.
The highest mortality rate was observed in the district of Brás (192.3 deaths/100 000 inhabitants) and the lowest in Jardim Paulista (48.1 deaths/100 000 inhabitants) (Supplementary Table 1, available as Supplementary data at IJE online). The distribution of the deaths according to age, sex, race, month of death, place of death, ICD-10 code and type of hospital administration is shown in Table 1.
Table 1.
Distribution of COVID-19 deaths according to age, sex, race school attainment, place of death, month of death, ICD-10 code and hospital administration
| Variables | Number of deaths (%) |
|---|---|
| Age (years) | |
| 0–19 | 80 (0.5) |
| 20–39 | 600 (3.8) |
| 40–59 | 2801 (17.5) |
| 60–74 | 5163 (32.3) |
| ≥75 | 7331 (45.9) |
| Sex | |
| Male | 10 670 (54.7) |
| Female | 8824 (45.3) |
| Unknown | 4 (0.0) |
| Race | |
| White | 11 946 (61.3) |
| Black | 1793 (9.2) |
| Asian | 444 (2.3) |
| Mixed | 4644 (23.8) |
| Indigenous | 13 (0.1) |
| Unknown | 658 (3.4) |
| School attainment (years) | |
| 0 (illiterate) | 1515 (7.8) |
| 1–7 | 9413 (48.3) |
| ≥8 | 6250 (32.0) |
| Unknown | 2320 (11.9) |
| Place of death | |
| Hospital | 18 253 (93.6) |
| Other health facilities | 447 (2.3) |
| Home | 709 (3.6) |
| Other places | 89 (0.5) |
| Month of death | |
| March | 511 (2.6) |
| April | 3529 (18.1) |
| May | 5000 (25.7) |
| June | 4061 (20.8) |
| July | 2929 (15.0) |
| August | 2268 (11.6) |
| September | 1200 (6.2) |
| ICD-10 code | |
| B34.2 (confirmed) | 13 801 (70.8) |
| U04.9 (suspect) | 5697 (29.2) |
| Type of hospital administrationa | |
| Public/state government | 5144 (29.3) |
| Public/city government | 5196 (29.6) |
| Private/non-profit | 7210 (41.1) |
Only for deaths that occurred in hospitals/other health facilities located in São Paulo city (n = 17 550).
Following guidance from the Ministry of Health of March 2020, code U04.9 (Severe acute respiratory syndrome, unspecified) was used as a marker for suspected cases of COVID-19 and code B34.2 (Coronavirus infection, unspecified site) was used for confirmed cases of the disease (SARS-CoV-2 detected by RT-PCR).
Males presented an 84% increase in mortality due to COVID-19 compared with females (RR = 1.84, 95% CI 1.79–1.90). Analysis by age group revealed that sex differences were similar in the age groups (young/adult population, RR = 2.00, 95% CI 1.88–2.13; elderly population, RR = 1.76, 95% CI 1.70–1.82) (Table 2).
Table 2.
Number of deaths, age-standardized mortality rates (/100 000 hab) and rate ratios (95% CI) of COVID-19 according to education attainment, sex, race, household density, household income, percentage of households in subnormal areas and age group, São Paulo city, March–September 2020
| Variables | Age group |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All ages |
<60 |
≥60 |
||||||||||
| n | ASR | RR | 95% CI | n | ASR | RR | 95% CI | n | ASR | RR | 95% CI | |
| Population with university degree (%) | ||||||||||||
| <8.61 | 5301 | 151.6 | 2.23 | 2.13–2.34 | 1477 | 40.2 | 4.02 | 3.42–4.72 | 3824 | 1072.3 | 1.96 | 1.86–2.06 |
| 8.61–13.20 | 4812 | 130.8 | 1.92 | 1.83–2.02 | 1127 | 34.5 | 3.45 | 2.92–4.08 | 3685 | 926.5 | 1.69 | 1.61–1.78 |
| 13.21–19.20 | 3308 | 122.2 | 1.73 | 1.65–1.82 | 610 | 32.5 | 3.25 | 2.75–3.84 | 2698 | 863.2 | 1.58 | 1.49–1.66 |
| 19.21–34.80 | 2940 | 101.8 | 1.57 | 1.48–1.67 | 436 | 22.7 | 2.27 | 1.89–2.72 | 2504 | 755.3 | 1.38 | 1.31–1.46 |
| >34.80 | 2277 | 68.0 | Ref. | Ref. | 169 | 10.0 | Ref. | Ref. | 2108 | 547.7 | Ref. | Ref. |
| Sex | ||||||||||||
| Male | 10 670 | 173.4 | 1.84 | 1.79–1.90 | 2579 | 72.2 | 2.00 | 1.88–2.13 | 8086 | 1713.3 | 1.76 | 1.70–1.82 |
| Female | 8824 | 94.2 | Ref. | Ref. | 1494 | 36.1 | Ref. | Ref. | 7329 | 974.3 | Ref. | Ref. |
| Race a | ||||||||||||
| White | 11 946 | 106.2 | Ref. | Ref. | 1979 | 25.5 | Ref. | Ref. | 9964 | 772.7 | Ref. | Ref. |
| Black | 1793 | 188.1 | 1.77 | 1.67–1.88 | 475 | 54.0 | 2.12 | 1.85–2.42 | 1317 | 1296.2 | 1.68 | 1.56–1.80 |
| Asian | 444 | 66.7 | 0.63 | 0.58–0.68 | 42 | 12.0 | 0.47 | 0.38–0.59 | 402 | 518.5 | 0.67 | 0.62–0.73 |
| Mixed | 4644 | 150.8 | 1.42 | 1.37–1.47 | 1416 | 41.4 | 1.62 | 1.51–1.75 | 3226 | 1055.2 | 1.37 | 1.31–1.43 |
| Indigenous | 13 | 96.2 | 0.91 | 0.54–1.52 | 5 | 46.2 | 1.81 | 0.56–5.81 | 8 | 509.7 | 0.66 | 0.38–1.15 |
| Mean household density (persons/room) | ||||||||||||
| <2.71 | 2340 | 69.9 | Ref. | Ref. | 182 | 10.4 | Ref. | Ref. | 2158 | 561.3 | Ref. | Ref. |
| 2.71–2.88 | 3720 | 107.7 | 1.54 | 1.46–1.62 | 618 | 26.5 | 2.55 | 2.18–2.97 | 3102 | 778.7 | 1.39 | 1.31–1.47 |
| 2.89–2.99 | 3918 | 112.0 | 1.60 | 1.52–1.69 | 845 | 30.8 | 2.96 | 2.57–3.41 | 3073 | 857.2 | 1.53 | 1.45–1.61 |
| 3.00–3.07 | 4235 | 141.5 | 2.02 | 1.92–2.13 | 994 | 38.1 | 3.66 | 3.21–4.18 | 3241 | 995.7 | 1.77 | 1.68–1.87 |
| >3.07 | 4425 | 146.8 | 2.10 | 2.00–2.21 | 1180 | 39.4 | 3.79 | 3.55–4.28 | 3245 | 1034.2 | 1.84 | 1.74–1.95 |
| Population with monthly income <1/4 minimum wage (%) | ||||||||||||
| <5.34 | 2780 | 88.6 | Ref. | Ref. | 330 | 17.2 | Ref. | Ref. | 2450 | 678.9 | Ref. | Ref. |
| 5.34–6.77 | 3456 | 107.1 | 1.21 | 1.15–1.27 | 574 | 26.8 | 1.56 | 1.37–1.77 | 2882 | 770.9 | 1.14 | 1.08–1.20 |
| 6.78–8.28 | 2838 | 99.3 | 1.12 | 1.06–1.18 | 466 | 24.7 | 1.44 | 1.26–1.64 | 2372 | 715.6 | 1.05 | 1.00–1.12 |
| 8.29–10.65 | 4965 | 133.8 | 1.51 | 1.44–1.58 | 1131 | 34.1 | 1.98 | 1.78–2.21 | 3834 | 958.2 | 1.41 | 1.34–1.48 |
| >10.65 | 4599 | 149.6 | 1.69 | 1.61–1.77 | 1318 | 40.4 | 2.35 | 2.12–2.60 | 3281 | 1052.0 | 1.55 | 1.47–1.63 |
| Percentage of households in subnormal areas (%) | ||||||||||||
| <0.65 | 2501 | 83.1 | Ref. | Ref. | 274 | 16.5 | Ref. | Ref. | 2227 | 633.5 | Ref. | Ref. |
| 0.65–3.05 | 2579 | 100.0 | 1.20 | 1.14–1.27 | 388 | 25.2 | 1.53 | 1.31–1.79 | 2191 | 718.2 | 1.13 | 1.07–1.20 |
| 3.06–6.67 | 4249 | 129.2 | 1.55 | 1.48–1.63 | 915 | 35.0 | 2.12 | 1.87–2.40 | 3334 | 907.9 | 1.43 | 1.36–1.51 |
| 6.68–12.56 | 3918 | 138.7 | 1.67 | 1.59–1.76 | 970 | 37.9 | 2.30 | 2.04–2.59 | 2948 | 971.9 | 1.45 | 1.37–1.51 |
| >12.56 | 5391 | 126.9 | 1.53 | 1.46–1.60 | 1272 | 31.7 | 1.92 | 1.72–2.15 | 4119 | 913.4 | 1.44 | 1.37–1.52 |
Deaths with unknown age = 3 White, 1 Black, 2 mixed, 1 unknown race.
ASR, age-standardized rate; RR, rate ratio.
Higher mortality rates were observed for Blacks (RR = 1.77, 95% CI 1.67–1.88) and mixed (RR = 1.42, 95% CI 1.37–1.47) compared with Whites, whereas lower mortality was noted for Asians (RR = 0.63, 95% CI 0.58–0.68). A stratified analysis by age revealed that differences in COVID-19 mortality according to race were more pronounced among persons <60 years old (Blacks, RR = 2.12, 95% CI 1.85–2.42; mixed, RR = 1.62, 95% CI 1.51–1.75; Asians, RR = 0.47, 95% CI 0.38–0.59) than among the elderly (Blacks, RR = 1.68, 95% CI 1.56–1.80; mixed, RR = 1.37, 95% CI 1.31–1.43; Asians, RR = 0.67, 95% CI 0.62–0.73) (Table 2).
A positive gradient was found for all indicators of socio-economic status (SES), i.e. increases in disparities denoted by less education, more household crowding, lower income and a higher concentration of subnormal areas were associated with higher mortality rates. However, a ‘dose–response’ effect was only observed for education and household density. Among all indicators, the educational level was the one showing the most substantial disparity between the categories. In the young/adult population, among those living in areas with the lowest percentage of the population with a university degree, mortality was four times higher compared with that in the most educated group (RR = 4.02, 95% CI 3.42–4.72); in the elderly population, the same comparison denoted a 96% increased risk of death (Table 2).
Stratified analysis examining racial disparities in mortality according to area-based measures of school attainment revealed that differences between Whites and Blacks were higher in areas with a better educational level. In areas with the lowest percentages of persons with a university degree, Blacks had a risk of death 32% higher than that for Whites, whereas, for those living in the group of districts with the highest percentages of persons with a university degree, this risk was 49% higher than that observed for the White population. Differences between White and mixed populations also persisted in all groups, except in the second and fifth quintiles. Similarly, stratified analysis by household density denoted that higher risk of deaths for Blacks compared with Whites were present in all strata, with relative risks ranging between 1.39 and 1.77; accordingly, the same pattern was observed when comparing mixed to White populations, although with increases in the risk of death ranging from 6% to 39% (Table 3).
Table 3.
Number of deaths, age-standardized mortality rates (/100,000 hab) and rate ratios (95% CI) of COVID-19 according to race, stratified by education attainment and household density, São Paulo city, March–September 2020
| Variables | Quintiles | Race |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White |
Black |
Asian |
Mixed |
Indigenous |
||||||||||||||||
| n | ASR | RR | n | ASR | RR | 95% CI | n | ASR | RR | 95% CI | n | ASR | RR | 95% CI | n | ASR | RR | 95% CI | ||
| Percentage of the population with university degree | <8.61 | 2508 | 139.2 | Ref. | 647 | 183.3 | 1.32 | 1.20-1.45 | 24 | 58.9 | 0.42 | 0.33-0.55 | 1913 | 147.5 | 1.06 | 1.00-1.13 | 6 | 129.1 | 0.93 | 0.43-2.01 |
| 8.61-13.20 | 2708 | 119.3 | Ref. | 537 | 186.5 | 1.56 | 1.40-1.74 | 60 | 99.1 | 0.83 | 0.66–1.05 | 1365 | 110.9 | 0.93 | 0.87–0.99 | 5 | 108.8 | 0.91 | 0.39–2.11 | |
| 13.21–19.20 | 2182 | 106.7 | Ref. | 275 | 187.4 | 1.75 | 1.49–2.04 | 110 | 102.0 | 0.96 | 0.79–1.15 | 643 | 117.4 | 1.10 | 1.01–1.20 | 1 | 58.8 | 0.55 | 0.13–2.31 | |
| 19.21–34.80 | 2121 | 91.5 | Ref. | 208 | 178.5 | 1.95 | 1.61–2.36 | 115 | 65.1 | 0.71 | 0.60–0.84 | 395 | 125.8 | 1.37 | 1.22–1.55 | 1 | 38.4 | 0.42 | 0.12–1.44 | |
| >34.80 | 1909 | 64.4 | Ref. | 51 | 96.1 | 1.49 | 1.07–2.09 | 124 | 56.9 | 0.88 | 0.74–1.05 | 98 | 69.4 | 1.08 | 0.87–1.33 | 0 | 0.0 | – | – | |
| Household density (persons/household) | <2.71 | 1931 | 65.2 | Ref. | 64 | 111.5 | 1.71 | 1.24–2.35 | 129 | 59.9 | 0.92 | 0.77–1.09 | 119 | 77.5 | 1.19 | 0.97–1.45 | 0 | 0.00 | – | – |
| 2.71–2.88 | 2648 | 97.1 | Ref. | 256 | 172.0 | 1.77 | 1.50–2.09 | 129 | 67.5 | 0.70 | 0.60–0.81 | 583 | 135.3 | 1.39 | 1.26–1.54 | 1 | 46.5 | 0.48 | 0.13–1.81 | |
| 2.89–2.99 | 2283 | 106.6 | Ref. | 373 | 163.0 | 1.53 | 1.35–1.74 | 83 | 74.1 | 0.70 | 0.58–0.84 | 1030 | 133.3 | 1.25 | 1.16–1.35 | 2 | 53.9 | 0.51 | 0.19–1.33 | |
| 3.00–3.07 | 2341 | 141.9 | Ref. | 511 | 209.6 | 1.48 | 1.32–1.65 | 62 | 88.7 | 0.63 | 0.51–0.76 | 1183 | 156.5 | 1.10 | 1.03–1.18 | 3 | 104.4 | 0.74 | 0.28–1.94 | |
| >3.07 | 2225 | 163.6 | Ref. | 514 | 226.7 | 1.39 | 1.24–1.54 | 30 | 69.4 | 0.42 | 0.34–0.54 | 1499 | 172.8 | 1.06 | 0.99–1.13 | 7 | 195.6 | 1.20 | 0.53–2.69 | |
ASR, age-standardized rate; RR, rate ratio.
We observed an association between race and place of death. Whereas Asians were the group with the highest percentage of home deaths (6.8%), Blacks (3.2%) and mixed populations (3.1%) presented the highest percentages of deaths in health facilities other than hospitals, i.e. primary-care units and isolated emergency-care units. Compared with Whites, the Black and mixed populations presented higher risks of dying in other health facilities (67% and 64%, respectively), whereas Asians had a higher probability of dying at home (OR = 1.67, 95% CI 1.14–2.45). The distribution of deaths according to the type of health-facility administration showed that Blacks and mixed individuals died predominantly in public institutions (75.5% and 76.5%, respectively). In comparison, deaths among Whites (49.4%) and Asians (72.2%) were concentrated in private or non-profit health facilities. We observed that age modifies this association, with larger effects being observed for the groups of elderly Blacks and mixed (Table 4).
Table 4.
Number and percentage of COVID–19 deaths and odds ratios according to race, place of death, type of hospital administration and age group, São Paulo city, March–September, 2020
| Race | Age group (years) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All ages |
<60 |
≥60 |
|||||||||||||
| Place of deatha (%) | |||||||||||||||
| Hospital (H) | Other health facilities (OHF) | Home (HO) | Crude OR H vs OHF (95% CI) | Crude OR H vs HO (95% CI) | Hospital (H) | Other health facilities (OHF) | Home (HO) | Crude OR H vs OHF (95% CI) | Crude OR H vs HO (95% CI) | Hospital (H) | Other health facilities (OHF) | Home (HO) | Crude OR H vs OHF (95% CI) | Crude OR H vs HO (95% CI) | |
| White |
11 170 (94.0) |
227 (1.9) | 491 (4.1) | Ref. | Ref. | 1863 (94.8) | 35 (1.8) | 68 (3.4) | Ref. | Ref. | 9305 (93.8) | 192 (1.9) | 422 (4.3) | Ref. | Ref. |
| Black | 1676 (93.8) | 57 (3.2) | 53 (3.0) | 1.67 (1.25–2.25) | 0.72 (0.54––0.96) | 445 (94.1) | 13 (2.7) | 15 (3.2) | 1.56 (0.82–2.96) | 0.92 (0.52–1.63) | 1230 (93.8) | 44 (3.3) | 38 (2.9) | 1.73 (1.24–2.42) | 0.68 (0.49–0.95) |
| Asian | 408 (92.5) | 3 (0.7) | 30 (6.8) | 0.36 (0.11–1.13) | 1.67 (1.14–2.45) | 39 (92.9) | 0 (0.0) | 3 (7.1) | NC – | 2.11 (0.63–6.99) | 369 (92.5) | 3 (0.7) | 27 (6.8) | 0.39 (0.12–1.24) | 1.61 (1.08–2.41) |
| Mixed | 4353 (94.1) | 145 (3.1) | 127 (2.8) | 1.64 (1.33–2.02) | 0.66 (0.54–0.81) | 1311 (93.4) | 49 (3.5) | 44 (3.1) | 1.99 (1.28––3.09) | 0.92 (0.62–1.35) | 3040 (94.4) | 96 (3.0) | 83 (2.6) | 1.53 (1.19–1.96) | 0.60 (0.47–0.76) |
|
|
|||||||||||||||
|
Type of hospital administrationb (%) |
|||||||||||||||
| Public/ State Gov. (PSG) | Public/ City Gov. (PCG) | Private (P) | Crude OR P vs PSG (95% CI) | Crude OR P vs PCG (95% CI) | Public/ State Gov. (PSG) | Public/ City Gov. (PCG) | Private (P) | Crude OR P vs PSG (95% CI) | Crude OR P vs PCG (95% CI) | Public/ State Gov. (PSG) | Public/ City Gov. (PCG) | Private (P) | Crude OR P vs PSG (95% CI) | Crude OR P vs PCG (95% CI) | |
|
|
|||||||||||||||
| White | 2845 (26.5) | 2587 (24.1) | 5301 (49.4) | Ref. | Ref. | 540 (31.0) | 541 (31.0) | 662 (38.0) | Ref. | Ref. | 2306 (25.6) | 2046 (22.8) | 4636 (51.6) | Ref. | Ref. |
| Black | 586 (36.4) | 631 (39.1) | 395 (24.5) | 2.76 (2.41–3.16) | 3.27 (2.86–3.74) | 144 (34.7) | 169 (40.7) | 102 (24.6) | 1.73 (1.31–2.28) | 2.03 (1.55–2.66) | 442 (37.0) | 461 (38.5) | 293 (24.5) | 3.03 (2.59–3.54) | 3.56 (3.05–4.16) |
| Asian | 64 (16.2) | 46 (11.6) | 286 (72.2) | 0.42 (0.32–0.55) | 0.33 (0.24–0.45) | 8 (22.2) | 7 (19.4) | 21 (58.4) | 0.47 (0.20–1.06) | 0.41 (0.17–0.97) | 56 (15.6) | 39 (10.8) | 265 (73.6) | 0.42 (0.32–0.57) | 0.33 (0.24–0.47) |
| Mixed | 1486 (35.5) | 1713 (41.0) | 984 (23.5) | 2.81 (2.56–3.09) | 3.57 (3.26–3.91) | 412 (33.0) | 524 (41.9) | 314 (25.1) | 1.61 (1.34–1.94) | 2.04 (1.70–2.44) | 1073 (36.6) | 1188 (40.5) | 670 (22.9) | 3.22 (2.89–3.59) | 4.02 (3.61–4.48) |
OR, odds ratio.
Excludes deaths that occurred in other places and deaths with indigenous or unknown race (n = 758).
Includes only deaths that occurred in São Paulo city in hospitals or other facilities (n = 17 550); excludes deaths with indigenous or unknown race (n = 638).
Breslow-Day test for interactions between race and age.
Place of death (hospital vs other health facilities): Blacks = chi-square = 0.089, P = 0.769; Asians = not calculated; Mixed = chi-square = 1.037, P = 0.310.
Place of death (hospital vs home): Blacks = chi-square = 0.814, P = 0.367; Asians = chi-square = 0.171, P = 0.679; Mixed = chi-square = 3.348, P = 0.067.
Type of hospital administration (private vs public state government): Blacks = chi-square = 11.9, P = 0.001; Asians = chi-square = 0.045, P = 0.832; Mixed = chi-square = 39.97, P < 0.001.
Type of hospital administration (private vs public city government): Blacks = chi-square = 12.56, P < 0.001; Asians = chi-square = 0.181, P = 0.670; Mixed = chi-square = 39.86, P < 0.001.
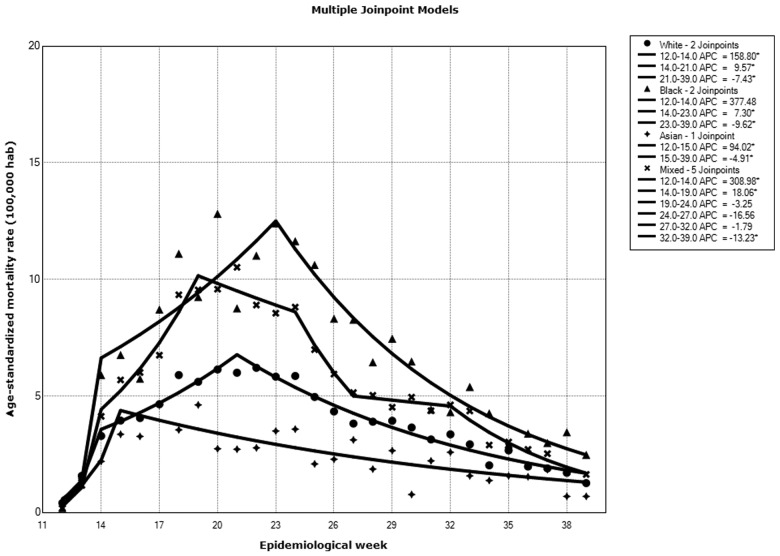
In the White population, we observed a significant increase in COVID-19 mortality between Weeks 12 and 14 (WPC = 158.8%, 95% CI 37.7–386.4), followed by another increase between epidemiological Weeks 14 and 21 (WPC = 9.6%, 95% CI 5.3–14.0) and a decrease between Weeks 21 and 39 (WPC = –7.4%, 95% CI –8.3 to –6.5). Overall, for the entire period, there was an average weekly increase of 4.4% in COVID-19 mortality in this group (AWPC = 4.4, 95% CI –0.3 to 9.2). Among Blacks, a non-significant increase in COVID-19 mortality between Weeks 12 and 14 was also noted (WPC = 377.5%, 95% CI –34.7 to 3392.4), followed by an increase between epidemiological Weeks 14 and 23 (WPC = 7.3%, 95% CI 2.5–12.3) and a decrease between Weeks 23 and 39 (WPC = –9.6%, 95% CI –11.4 to –7.8). Overall, there was an average weekly increase of 16.8% in COVID-19 mortality in this group (AWPC = 8.3, 95% CI –5.9 to 24.5). For the mixed group, the model identified more join points, with a large increase in COVID-19 mortality between Weeks 12 and 14 (WPC = 309.0%, 95% CI 13.3–1376.9), followed by another increase between epidemiological Weeks 14 and 19 (WPC = 18.1%, 95% CI 9.3–27.5) and decreases in rates between Weeks 19 and 24 (WPC = –3.2%, 95% CI –9.6 to 3.6), Weeks 24 and 27 (WPC = –16.6%, 95% CI –35.8 to 8,5), Weeks 27 and 32 (WPC = –1.8%, 95% CI –10.8 to +8.1) and Weeks 32 and 39 (WPC = –13.2%, 95% CI –17.9 to -8.3); the average weekly percentage change in this population was 7.1% (95% CI –2.3 to 17.5). Asians showed the lowest increase in mortality in the study period (WPC 1: Weeks 12–15 = 94.0%, 95% CI –0.1 to 276.2; WPC 2: Weeks 15–39 = –4.6%, 95% CI –7.4 to –1.7; AWPC = 6.5%, 95% CI –4.2 to 18.3) (Figure 1).
Figure 1.
Trends in COVID-19 mortality according to epidemiological week and race, São Paulo city, March–July 2020
Discussion
To our knowledge, this is the first population-based study addressing racial disparities and examining time trends in COVID-19 mortality in the most populous city in Latin America. We showed that Blacks and mixed individuals observed mortality rates 81% and 45% higher than Whites, respectively, and disparities were more pronounced in the young/adult population. It is also noteworthy that Asians presented a decreased risk of death compared with Whites. Besides, the analysis of mortality rates by other indicators such as income, education and housing revealed a social gradient, i.e. worsening in the indicators increases mortality rates. Our results corroborate previous findings indicating sex, race and socio-economic disparities in COVID-19 mortality worldwide.19–21
A previous Brazilian study using data from 11 321 patients admitted to hospitals included in the SIVEP-Gripe data set (Influenza Epidemiological Surveillance System) has shown that mixed and Blacks with COVID-19 presented a higher risk of death compared with Whites (45% and 32%, respectively), with disparities likely explained by differences in exposure to infection by SARS-Cov-2 and access to healthcare.5 In an ecological study, Martins-Filho et al. found that, in Aracaju, the capital of Sergipe state (Northeast region), case-fatality rates increased concomitantly with decreasing values of a living-condition index (LCI) (including area-based information on education, income and housing); whereas the case-fatality rate was 0.8% in the neighbourhoods with high LCI, those areas with very low LCI had a value of 2%.22 Also, Santos et al. described that, in Brazil, maternal mortality due to COVID-19 among Blacks was almost twice the value observed for White women. Although Black and White women were similar in age and co-morbidities burden, they differed in the clinical status at hospital admission, with Black women being hospitalized in worse conditions, suggesting that these disparities could be linked to a context outside of the hospitals.23
Using data from 27 states in the USA and the city of New York, Gross et al. reported a strong association between Black race, Hispanic ethnicity and COVID-19 mortality. Overall, the mortality rate among blacks was 3.6 times higher than that among Whites, whereas Hispanics presented a 88% increase in mortality.24 Results from a national survey conducted in the USA demonstrated that, compared with Whites, African Americans, younger individuals and men reported more COVID-19 exposure, less social isolation and had less detailed knowledge about the disease.25
Our results show that COVID-19 mortality was 84% higher among males than that among females, with an effect persisting in both young/adults and elderly groups. This finding is consistent with several previous reports showing an excess of COVID-19 mortality in men.26–28 It is still debatable whether these disparities are attributable to biologic factors such as sex hormones and expression of ACE2 receptor,29 differences in lifestyle that could result in men having more co-morbidities,30–31 differences in the exposure to SARS-CoV-2 due to behavioural and occupational factors29 or symptoms awareness,32 and differences in adherence to preventive health measures.33 Elucidating whether the mechanisms behind these differences are linked to social or biological factors is crucial to designing better strategies for COVID-19 prevention and treatment.34
Differences in COVID-19 mortality according to education, income and housing conditions described in our study are concordant with findings from a previous ecological study that has used a composite socio-economic index and spatio-temporal analyses to show that socio-economic conditions and COVID-19 mortality were associated in the city of São Paulo.6 In the UK, COVID-19 mortality rates in the most deprived areas were almost 100% higher than those observed in the least deprived regions, and the effect was not modified after controlling for ethnicity. Similarly to our study, authors reported that, after adjustment for age, geographic region, sex and ethnicity, social disparities were more pronounced among people of working age (most deprived compared with least deprived, 93% higher risk of death) than among elderly persons (most deprived compared with least deprived, 9% higher risk of death).10 Evidence suggests that social disparities in COVID-19 mortality may reflect a combination of factors resulting in an increased vulnerability to being exposed to the virus, such as household crowding, underlying medical conditions, occupation and modes of transportation.35
We found a higher difference between mortality rates among Blacks and Whites in more affluent areas (higher education and lower household density). According to Telles, residential segregation by race in Brazil is lowest for the low-income groups and increases with increasing income level.36 We hypothesized that our findings are related to inequalities in job opportunities, as Blacks are disproportionally overrepresented as domestic employees,37 with these deaths denoting people working and living in the same place. Data from the 2015 National Household Sample Survey (PNAD) show that, in the Southeast region, 0.7% of all mixed and Black females working as domestic employees lived in the same household in which they worked, but only 52.2% were legally registered; this may reflect lower income and difficulties in immediate access to health services.38
Our finding of a higher percentage of deaths occurring in primary-care or isolated emergency-care units observed for Black and mixed populations may be related to barriers in healthcare access. Racial disparities in medical care, both quantitatively and qualitatively, are well documented, being described as a possible contributor to racial disparities in health outcomes.39 The city of São Paulo has 4.09 intensive-care-unit (ICU) beds/10 000 inhabitants, but this rate is 5.27 in the private sector and only 1.58 ICU beds/10 000 inhabitants for the public one [Sistema Único de Saúde (SUS)].40 Data from SIVEP-Gripe for the same period of our study show that Asians (39.0%) and Whites (35.7%) were more frequently admitted to ICUs than Blacks (29.8%), mixed (30.4%) and indigenous (24.7%). One could argue that differences in disease severity could explain this, but an analysis of a very high-risk group (persons with diabetes, O2 saturation <95% and symptoms of respiratory distress) also revealed these disparities in ICU admission (Whites = 46.2%, Blacks = 40.2%, Asians = 47.0%, mixed = 39.9).41 In New York City, which has an extensive hospital system, a study assessing racial-area composition based on zip codes has demonstrated a striking disparity in hospital resources during pandemics, with predominantly White areas having almost nine times more beds/1000 persons than predominantly Black/Hispanic areas.42
Despite being the group with the lowest COVID-19 mortality, a higher proportion of home deaths was found among Asians. Further exploration of our data shows that this effect was even more substantial when we restricted the analysis to COVID-19 suspected deaths. This finding could be explained by the fear of seeking medical care in the context of the pandemics (misinterpreting the symptoms) or cultural differences/preferences on facing a severe disease and the risk of death, particularly among elderly persons.43
Time-trend analyses showed an earlier decrease in mortality among Asians and Whites compared with that among Blacks and mixed. Possible explanations include sustained higher incidence and mortality rates among blacks and mixed due to difficulties in adhering to social-distancing measures. Coelho et al. reported the challenges of implementing social-distancing and adequate hygiene measures in areas with high social vulnerability due to household crowding and poor living conditions.44 In the USA, the disparate impact of COVID-19 in the Latino community has been linked to work and living conditions and financial burden, as paid sick leave and working from home are not options for many Hispanic workers.7 In Brazil, the situation is not different. Pires et al. reported that we should expect that more vulnerable Brazilians will be disproportionally affected by COVID-19, not only because of the previously cited reasons, but also because there is a high incidence of co-morbidities among persons with less education.45
Limitations of our study include the use of area-based SES measures for relatively large geographical units (districts) and the lack of detailed information about co-morbidities. Regarding ‘ecological fallacy’, it is important to emphasize that we are aware of this bias and the limitations of using area-based measures for relatively large geographic units (districts). However, data from the literature suggest that heterogeneity in the neighbourhood would probably produce underestimation and not overestimation of the effects.46–47 Also, it is essential to mention the possibility of racial misclassification, as, in Brazil, this is self-reported mostly based on skin colour and it is strongly affected by the context and social status.48 The strengths of the study lie in the quality of the mortality data and its completeness. In the state of São Paulo, the coverage and completeness of death registration are equal to 100%49 and only 11.4% of the deaths were reported as ‘garbage codes’ (unpublished observation).
In conclusion, our results show social inequalities in COVID-19 mortality in São Paulo, Brazil. Unfortunately, the relationship between more vulnerable = less health is not a new phenomenon, and we believe that pandemics have exacerbated existing structural inequalities. Stiglitz correctly states that ‘COVID-19 is not an equal opportunity virus’, emphasizing that differences in the success (or not) in the management of the pandemics in each country are linked to several factors including the healthcare system, pre-existing health inequalities, economy's resilience, reliance on science and citizens’ trust in government guidance.50 In Brazil, the governmental response to COVID-19 has been marked by the lack of leadership at the federal level, distrust of science, denial of the importance of the virus and progressive cuts to health and research funding. ‘So what?’51–52 We want politicians to remember what is written in our Constitution: ‘Health is a right for all Brazilian citizens and a duty of the State, guaranteed through social and economic policies designed to reduce the risk of disease and promote universal and equal access to healthcare.’53 Until equity and trust in science become essential elements in the Brazilian response, COVID-19 will continue to highlight the sorrowful shades of social disparities in the largest city of Latin America.
Supplementary Data
Supplementary data are available at IJE online.
Funding
None.
Supplementary Material
Acknowledgements
Data derived from a source in the public domain (Sistema de Informações sobre Mortalidade—SIM/PRO-AIM/CEInfo-SMS/SP) available at http://tabnet.saude.prefeitura.sp.gov.br/cgi/deftohtm3.exe?secretarias/saude/TABNET/SIM_PROV/obitop.def. Ethics approval was not necessary because this study analysed only mortality publicly available data, not including identifiable information.
Conflict of Interest
None declared.
References
- 1. Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Coronavirus Disease 2019 (COVID-19)—Situation Report 51. Geneva: World Health Organization. [Google Scholar]
- 3. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/ (12 October 2020, date last accessed).
- 4. Ministério da Saúde. COVID-19 NO BRASIL. https://covid.saude.gov.br/ (14 October 2020, date last accessed).
- 5. Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Glob Health 2020;8:e1018–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bermudi PMM, Lorenz C, Aguiar BS, Failla MA, Barrozo LV, Chiaravalloti-Neto F. Spatiotemporal ecological study of COVID-19 mortality in the city of São Paulo, Brazil: shifting of the high mortality risk from areas with the best to those with the worst socio-economic conditions. Travel Med Infect Dis 2021;39:101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Macias Gil R, Marcelin JR, Zuniga-Blanco B, Marquez C, Mathew T, Piggott DA. COVID-19 pandemic: disparate health impact on the Hispanic/Latinx population in the United States. Journal of Infectious Diseases 2020;222:1592–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baidal JW, Wang AY, Zumwalt K et al. Social determinants of health and COVID-19 among patients in New York City. Res Sq 2020; doi: 10.21203/rs.3.rs-70959/v1. [Google Scholar]
- 9. Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet 2020;395:1243–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Public Health England. Disparities in the Risk and Outcomes of COVID-19. London. https://www.gov.uk/government/publications/covid-19-review-of-disparities-in-risks-and-outcomes (5 October 2020, date last accessed).
- 11. Amengual-Moreno M, Calafat-Caules M, Carot A et al. [Social determinants of the incidence of Covid-19 in Barcelona: a preliminary ecological study using public data.]. Rev Esp Salud Publica 2020;94:e202009101. [PMC free article] [PubMed] [Google Scholar]
- 12. Góes C, Karpowicz I. Inequality in Brazil: A Regional Perspective, International Monetary Fund Working Papers 2017. https://www.imf.org/en/Publications/WP/Issues/2017/10/31/Inequality-in-Brazil-A-Regional-Perspective-45331 (10 October 2020, date last accessed).
- 13. PRO-AIM/CEINFO-SMS-SP. Sistema de Informações sobre Mortalidade. https://www.prefeitura.sp.gov.br/cidade/secretarias/saude/tabnet/mortalidade/index.php?p=6529 (5 October 2020, date last accessed).
- 14. SEADE—Fundação Sistema Estadual de Análise de Dados. Sistema SEADE de Projeções Populacionais. http://produtos.seade.gov.br/produtos/projpop/ (2 October 2020, date last accessed).
- 15. Companhia do Metropolitano de São Paulo. Pesquisa Origem Destino—2017. http://www.metro.sp.gov.br/pesquisa-od/ (30 September 2020, date last accessed).
- 16. SEADE—Fundação Sistema Estadual de Análise de Dados. Informações dos municípios paulistas. http://www.imp.seade.gov.br/frontend/#/ (2 October 2020, date last accessed).
- 17. Secretaria Municipal de Desenvolvimento Urbano de São Paulo. Observatório de Indicadores da Cidade de São Paulo. http://observasampa.prefeitura.sp.gov.br/ (30 September 2020, date last accessed).
- 18. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statist Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 19. Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Res Sq 2020; doi: 10.21203/rs.3.rs-61444/v1, preprint: not peer-reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Golestaneh L, Neugarten J, Fisher M et al. The association of race and COVID-19 mortality. E Clinical Medicine 2020;25:100455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martins-Filho PR, de Souza Araujo AA, Quintans-Junior LJ, Santos VS. COVID-19 fatality rates related to social inequality in Northeast Brazil: a neighborhood-level analysis. J Travel Med 2020;27:taaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santos DS, Menezes MO, Andreucci CB et al. Disproportionate impact of COVID-19 among pregnant and postpartum Black Women in Brazil through structural racism lens. Clin Infect Dis 2020. Published online 2020 Jul 28. doi: 10.1093/cid/ciaa1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gross CP, Essien UR, Pasha S, Gross JR, Wang SY, Nunez-Smith M. Racial and ethnic disparities in population-level Covid-19 mortality. J Gen Intern Med 2020;35:3097–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alsan M, Stantcheva S, Yang D, Cutler D. Disparities in coronavirus 2019 reported incidence, knowledge, and behavior among US adults. JAMA Netw Open 2020;3:e2012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen T, Wu D, Chen H et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li LQ, Huang T, Wang YQ et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jin JM, Bai P, He W et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and gender-based differences in COVID-19. Front Public Health 2020;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Elgendy IY, Pepine CJ. Why are women better protected from COVID-19: clues for men? Sex and COVID-19. Int J Cardiol 2020;315:105–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burstrom B, Tao W. Social determinants of health and inequalities in COVID-19. Eur J Public Health 2020;30:617–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun Z, Yang B, Zhang R, Cheng X. Influencing factors of understanding COVID-19 risks and coping behaviors among the elderly population. Ijerph 2020;17:5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okten IG, Oettingen G. Gender differences in preventing the spread of coronavirus. PsyArXiv 2020; doi: 10.31234/osf.io/ch4jy, preprint: not peer-reviewed. [Google Scholar]
- 34. Islam N, Khunti K, Dambha-Miller H, Kawachi I, Marmot M. COVID-19 mortality: a complex interplay of sex, gender, and ethnicity. Eur J Public Health 2020;30:847–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blundell R, Costa Dias M, Joyce R, Xu X. COVID-19 and inequalities. Fisc Stud 2020; doi: 10.1111/1475-5890.12232 [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Telles EE. Residential segregation by skin color in Brazil. Am Sociological Rev 1992;57:186–97. [Google Scholar]
- 37. Lovell PA. Race, gender and regional labor market inequalities in Brazil. Rev Social Econ 2000;58:277–93. [Google Scholar]
- 38. Instituto de Pesquisa Econômica Aplicada. Retrato das Desigualdades de Gênero e Raça. https://www.ipea.gov.br/retrato/apresentacao.html (23 October 2020, date last accessed).
- 39. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev 2000;21:75–90. [PMC free article] [PubMed] [Google Scholar]
- 40. Conselho Federal de Medicina. Menos de 10% dos municípios brasileiros possuem leito de UTI. https://portal.cfm.org.br/index.php?option=com_content&view=article&id=27828:2018-09-04-19-31-41&catid=3 (2 October 2020, date last accessed).
- 41. Secretaria Municipal de Saúde de São Paulo. SIVEP-Gripe. São Paulo. http://tabnet.saude.prefeitura.sp.gov.br/cgi/deftohtm3.exe?secretarias/saude/TABNET/RSRAG/sragh.def (23 October 2020, date last accessed).
- 42. Douglas JA, Subica AM. COVID-19 treatment resource disparities and social disadvantage in New York City. Prev Med 2020;141:106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leite AKF, Ribeiro KB. Older adults with cancer in the city of Sao Paulo: what factors determine the place of death? Rev Saúde Pública 2018;52:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Coelho FC, Lana RM, Cruz OG et al. Assessing the spread of COVID-19 in Brazil: Mobility, morbidity and social vulnerability. PLoS One 2020;15:e0238214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pires LN, Carvalho L, Xavier LL. COVID-19 e Desigualdade no Brasil. CEBES. http://cebes.org.br/2020/04/covid-19-e-desigualdade-no-brasil/ (20 October 2020, date last accessed).
- 46. Hyndman JC, Holman CD, Hockey RL, Donovan RJ, Corti B, Rivera J. Misclassification of social disadvantage based on geographical areas: comparison of postcode and collector's district analyses. Int J Epidemiol 1995;24:165–76. [DOI] [PubMed] [Google Scholar]
- 47. Krieger N. Women and social class: a methodological study comparing individual, household, and census measures as predictors of Black/White differences in reproductive history. J Epidemiol Community Health 1991;45:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miranda M. Classificação de Raça/Cor e Etnia: conceitos, Terminologia e Métodos Utilizados Nas Ciências da Saúde No Brasil. Thesis. Escola Nacional de Saúde Pública, Rio de Janeiro: 2010. [Google Scholar]
- 49. Queiroz BL, Freire F, Gonzaga MR, Lima EEC. Completeness of death-count coverage and adult mortality (45q15) for Brazilian states from 1980 to 2010. Rev Bras Epidemiol 2017;20:21–33. [DOI] [PubMed] [Google Scholar]
- 50. Stiglitz J, Conquering the Great Divide. Finance & Development. 2020. September. International Monetary Fund. https://www.imf.org/external/pubs/ft/fandd/2020/09/COVID19-and-global-inequality-joseph-stiglitz.htm (20 October 2020, date last accessed).
- 51. The Lancet. COVID-19 in Brazil: ‘so what?’. Lancet 2020;395:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barberia LG, Gómez EJ. Political and institutional perils of Brazil's COVID-19 crisis. Lancet 2020;396:367–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Presidency of the Republic - Civil House. Constitution of the federative Republic of Brazil of 1988. In: Presidency of the Republic—Civil House, editor. Brasília-DF; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.