Abstract
It is currently unknown how post-COVID-19 syndrome (PCS) may affect those infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This longitudinal study includes healthcare staff who tested positive for SARS-CoV-2 between March and April 2020, with follow-up of their antibody titers and symptoms. More than half (21 of 38) had PCS after 7–8 months. There was no statistically significant difference between initial reverse-transcription polymerase chain reaction titers or serial antibody levels between those who did and those who did not develop PCS. This study highlights the relative commonality of PCS in healthcare workers and this should be considered in vaccination scheduling and workforce planning to allow adequate frontline staffing numbers.
Keywords: SARS-CoV-2, Post–COVID-19 Syndrome, COVID-19, Healthcare workers, multidisciplinary team, Imperial Hybrid DABA, Long-COVID
In this study, 55% of healthcare workers with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) had Post-COVID-19 Syndrome at 7-8 months following symptom onset. Developing the syndrome had no association with antibodies against the receptor binding domain of SARS-CoV-2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the ongoing coronavirus disease 2019 (COVID-19) pandemic. Disease severity ranges from asymptomatic to fatal. As of November 2020, the pandemic was responsible for 1.2 million deaths worldwide [1]. Mortality risk factors include increased age, male sex, comorbid conditions, black, Asian, or minority ethnic (BAME) status, and socioeconomic deprivation [2].
Most work on SARS-CoV-2 has focused on the immediate presentation and sequelae of COVID-19. However, there is a growing appreciation that symptoms can persist after infection, resulting in post–COVID-19 syndrome (PCS), otherwise known as long COVID [3, 4]. The National Institute for Health and Care Excellence (NICE) defines PCS as “[s]igns and symptoms that develop during or following an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis” [4]. The symptoms of PCS include fatigue, headache, anosmia and lower respiratory tract symptoms. More than 5 symptoms within a week of diagnosis of SARS-CoV-2 increase the chances of developing PCS [5].
Although an estimated 60 000 people in the United Kingdom have PCS, there are limited peer-reviewed data on the subject, and longitudinal observational studies are needed [6]. The unprecedented global reach of the COVID-19 pandemic means that the impact of PCS is profound. Thus, identifying risk factors for developing PCS is crucial for planning rehabilitation.
Antibody response to SARS-CoV-2 may inform on immunity to the virus. Establishing the longevity of antibody response to SARS-CoV-2 could predict reinfection risk, the necessity of vaccination in infected individuals and the need for vaccine boosters. We hypothesized that it may affect the risk of developing PCS.
Antibody response to SARS-CoV-2 infection has been analyzed up to 94 [7], 98 [8], 152 [9] and 210 days after symptom onset [10], with antibodies being maintained for ≥6 months [10]. However, it is established that humoral immunity to other coronaviruses decreases over time [11]. Antibody levels to SARS-CoV-2 were initially higher in patients with greater disease severity [7], before falling to the same level as lower disease severity or asymptomatic patients at 3–4 months [9]. This study of 42 healthcare workers considers potential associations between PCS and (1) initial viral titers and (2) serial antibody levels.
METHODS
This descriptive study of healthcare workers was conducted at a hospital in North West London. Ethical approval was granted from the hospital review board. All participants gave written informed consent. Serial blood samples were processed, and serum was stored at −80°C at the local University Communicable Disease Research Tissue Bank (NRES SC/20/0226).
Cohort Description
All hospital staff at the hospital who tested positive for SARS-CoV-2 with nasopharyngeal swab samples were eligible to volunteer for the study and were invited by e-mail to do so. Information collected at enrollment included sex, age, job, comorbid conditions, regular medications, and ethnicity.
Study Timing
The first serum sample for anti-SARS-CoV2 antibody testing was taken between 27 and 69 days after symptom onset. Three staff members were asymptomatic, meaning diagnosis date was used instead of symptom onset date. Serum samples were taken at weekly intervals for the first month, followed by monthly samples.
To assess symptoms of PCS, participants were followed up with a questionnaire that was completed 7–8 months after symptom onset. The authors believed that this represented enough time to have recovered from the initial viral infection, clearly differentiating PCS from ongoing symptomatic COVID-19.
PCS was diagnosed based on using the NICE definition, with screening questions based on the commonly reported symptoms in the NICE guidelines [4]. These included breathlessness, cough, chest tightness/pain, palpitations, fatigue, fever, pain, cognitive impairment, headache, sleep disturbance, peripheral neuropathic symptoms, dizziness, delirium, abdominal pain, diarrhea, anorexia/reduced appetite, joint/muscle pain, depression, anxiety, tinnitus, earache, sore throat, dizziness, loss of appetite, anosmia, and rashes.
SARS-CoV-2 Diagnosis
Diagnostic testing for SARS-CoV-2 was undertaken by Micropathology (University of Warwick) using the method described previously [12]. Samples were analyzed within a day of testing, and viral RNA was extracted via the Maxwell HT 96 NA kit of the KingFisher FLEX platform (Thermo Fisher Scientific).
As outlined in the US Centers for Disease Control and Prevention recommendations for SARS-CoV-2 testing [13], specific primers and probes were used for the reverse-transcription (RT) polymerase chain reaction (PCR). The primer and probe sequences are detailed elsewhere [13]. The probe anneals to a specific target sequence located between the forward and reverse primers. RT-PCR was performed with a Roche LightCycler 480. The following cycles were used: RT (50°C for 10 minutes) and polymerase activation (95°C for 2 minutes), followed by PCR (45 cycles of 95°C for 5 seconds and 55°C for 20 seconds). Results were expressed as cycle threshold (Ct) values. The internal control used was the DNA from the baculovirus, Adoxophyes orana granulovirus. This was added to the samples at a fixed concentration, meaning its amplification in each sample should be the same (Ct, approximately 33). If the internal control failed (ie, no amplification was seen) or amplification was delayed (Ct, >37) the sample was retested.
Antibody Detection
Antibody testing for SARS-CoV-2 was undertaken using the “in-house” double antigen-binding enzyme-linked immunosorbent assay (Imperial Hybrid Double antigen-binding assay (DABA); Imperial College London). This detects total antibodies against the SARS-CoV-2 receptor-binding domain and has a specificity of 100% (95% confidence interval, 99.6%–100%) and a sensitivity of 98·9% (96.8%–99.8%) [14]. As described by Rosadas et al [14], the Imperial Hybrid DABA uses S1 antigen and enzyme-labeled receptor-binding domain in the solid and fluid phase respectively. As the Hybrid DABA detects total immunoglobulin, confounding seroconversion from immunoglobulin M to immunoglobulin G is avoided. The assay cutoff was calculated from receiver operating characteristic curve analysis. Serum reactivity was normalized by using the binding ratio, the ratio of optical density values generated in a sample to the cutoff optical density value. Antibody-positive samples were determined by a binding ratio of ≥1.
Samples with a binding ratio >20 were diluted further and retested using the Imperial Hybrid DABA, to correct for a loss of linearity when the binding ratio exceeded 20. This was necessary in only a minority of samples (29 of 291). To quantify the antibody level in each sample, an indicative value, measured in arbitrary units was calculated from the standard curve, using a method described elsewhere by Tedder et al [15].
Statistical Analysis
Analyses were carried out using Python software, version 3.7. A 2-sided Man-Whitney test was used to compare Ct values and antibody levels between the non-PCS (NPCS) and PCS groups. Fisher exact test (2 tailed) was used to compare the likelihood of PCS in men versus women and in BAME vs non-BAME participants. Spearman rank correlation coefficient was used to correlate Ct values and Hybrid DABA antibody-binding ratios.
RESULTS
Demographics
A total of 42 staff members were enrolled into the study (36 female). All staff were RT-PCR positive for SARS-CoV-2 and were either asymptomatic (n = 3) or symptomatic but not requiring hospitalization (n = 39). The average age was 43 years (range, 23–67 years) and 37% (n = 14) were from a BAME group. Full demographic information is detailed in Table 1, excluding the 4 staff members who did not complete the PCS questionnaire. Diagnoses were made between 21 March and 5 May 2020 with symptom onset ranging from 16 March to 28 April 2020.
Table 1.
Demographic Characteristics of Healthcare Workers Participating in Study
| Characteristic | Overall (N = 38) | NPCS (n = 17) | PCS (n = 21) |
|---|---|---|---|
| Male sex, no. (%) | 6 (16) | 5 (29) | 1 (5) |
| Age, mean, y | 43 | 44 | 43 |
| BAME group, no. (%) | 14 (37) | 4 (24) | 10 (48) |
| Comorbid condition present, no. (%) | 12 (32) | 5 (29) | 7 (33) |
| Specific condition, no. | |||
| Pulmonary disease | 2 | 0 | 2 |
| Cardiovascular disease | 1 | 0 | 1 |
| Active oncological disease | 1 | 1 | 0 |
| Endocrine disease | 6 | 2 | 4 |
| Taking immunosuppressants | 1 | 1 | 0 |
| On regular medication at time of diagnosis, no. | 9 | 4 | 5 |
| Initial no. of symptoms, mean | 6 | 5 | 7 |
| Asymptomatic, no. | 3 | 3 | 0 |
| Staff position, no. | |||
| Administrator | 8 | 2 | 6 |
| Dietician | 1 | 0 | 1 |
| Housekeeping staff | 3 | 1 | 2 |
| Physician | 4 | 3 | 1 |
| Nursing staff | 12 | 6 | 6 |
| Occupational therapist or physiotherapist | 7 | 4 | 3 |
| Pharmacists | 2 | 0 | 2 |
| Phlebotomist | 1 | 1 | 0 |
Abbreviations: BAME, black, Asian, or minority ethnic; NPCS, non-PCS; PCS, post-COVID-19 syndrome.
PCS Symptoms
The questionnaire response rate was 90% (38 of 42). Of participants who completed the questionnaire, 55% (21 of 38) reported ≥1 ongoing symptom. The most common was fatigue (57%), followed by loss of smell (29%), breathlessness (24%), and difficulty concentrating (24%). Of the 21 staff members with PCS, 38% had 1, 29% had 2, and 33% had ≥3 ongoing symptoms. Sixteen percent (6 of 38) reported that they were no longer able to participate in a sport or recreational activity because of their ongoing symptoms. PCS developed in a higher percentage of BAME individuals (10 of 14), although this difference was not statistically significant (P = .18). PCS appeared more likely to develop in women (in 63% vs 17% of men), but again this difference was not significant (P = .07), and only 6 participants in the study were male.
Antibody Response Over Time
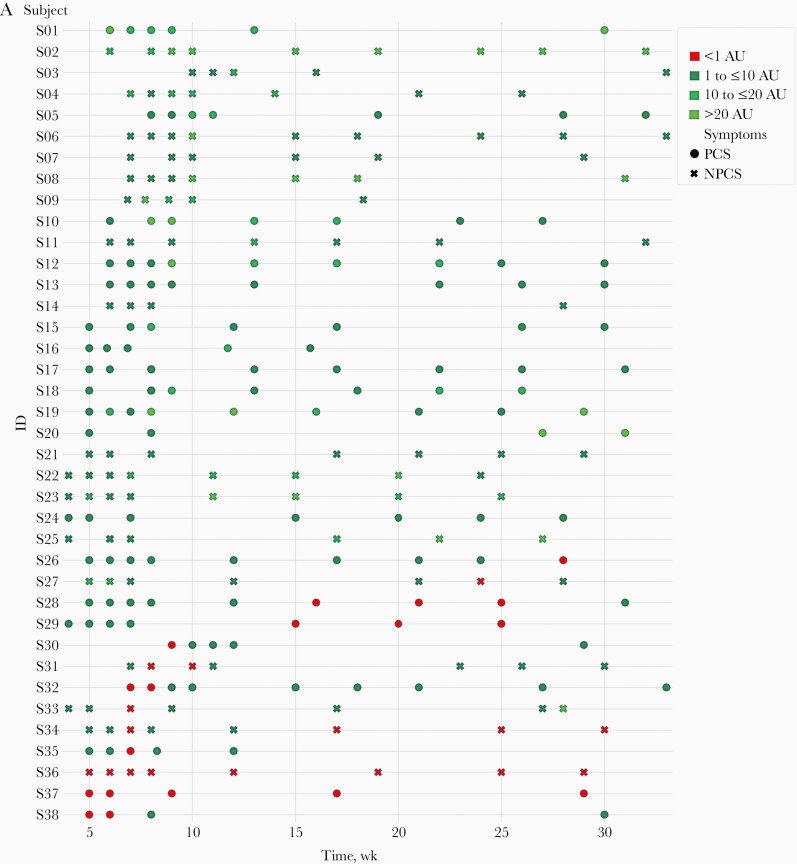
Antibody response over time is shown in Figure 1A. Five staff members (12%) elected to stop donating samples before 6 months. Excluding these participants, serum samples were taken for a mean of 205 days after symptom onset (range, 177–231 days), with a mean of 7 sequential samples per person.
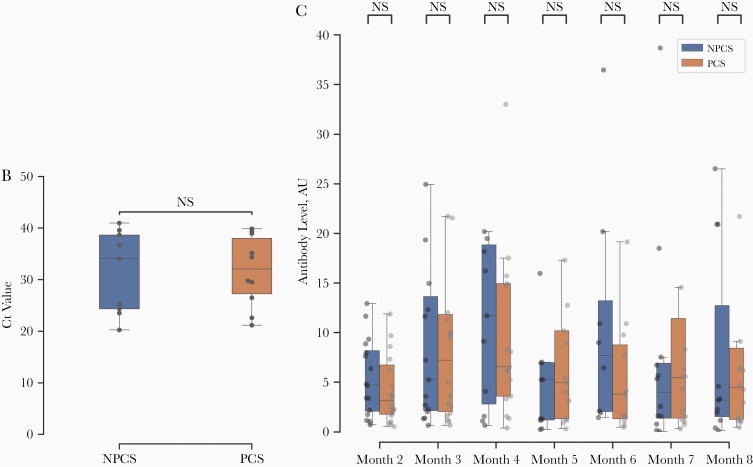
Figure 1.
A, Time course of antibody levels (Hybrid DABA results) in staff with or without post-COVID-19 syndrome (PCS), with levels represented in arbitrary units (AU), in 4 groupings ranging from <1 to >20 AU. B, Reverse-transcription polymerase chain reaction cycle threshold (Ct) values for severe acute respiratory syndrome coronavirus 2 at week 0 in PCS versus non-PCS (NPCS) groups. Abbreviation: NS, not significant. C, Hybrid Double antigen-binding assay (DABA) results over 8 months in PCS versus NPCS groups. Note omissions of outliers with antibody levels >40 AU. If >1 level was recorded in the same month for the same participant, the mean is displayed.
Of the 37 staff members who continued giving serum samples, 89% had detectable antibodies at 6 months. Of those whose antibody levels were negative at 6 months, 3 were initially seropositive and then became seronegative, while detectable antibodies never developed in 1. There was no significant correlation between Ct values (which indicate viral load) and antibody response over time.
PCS and Antibody Response
There was no significant difference in Ct values for SARSCoV-2 RT-PCR between the PCS and NPCS groups at diagnosis, as shown in Figure 1B, or in weeks 1–4. The median Ct value was 32.0 for the PCS group (interquartile range, 27.2–38.0) and 34.0 for the NPCS group (24.3–38.7). Similarly, there was no significant difference in serial antibody-binding ratios between the PCS and NPCS groups (Figure 1C). At month 2, the median antibody-binding ratios were 3.14 (interquartile range, 1.8–6.8) for PCS and 4.7 (2.1–8.2) for NPCS, while at month 8 the median antibody-binding ratios were 4.5 (1.3–8.4) for PCS and 3.2 (1.5–12.7) for NPCS.
DISCUSSION
This study shows that, in a cohort of SARSCov-2-infected healthcare workers who did not require hospitalization, antibody levels were detectable for 89% of tested staff for ≥6 months from symptom onset (33 of 37). This is in line with a study on a larger cohort [10]. Of the 4 subjects who were seronegative at 6 months, 3 started seropositive and became seronegative and antibodies never developed in 1. One of the staff who was initially antibody seropositive and later became seronegative was asymptomatic and on immunosuppressants. The other 3 staff who were seronegative at 6 months were symptomatic at the time of diagnosis.
This is the first study to our knowledge to examine the relationship between initial viral titers and antibody measurements with development of PCS. Our results show that PCS rates are high among healthcare workers (55%) at 7–8 months. This is higher than in studies with shorter follow-up times, where rates of PCS fell to 2.3% at >12 weeks [5].
In the current study, neither initial viral titers at diagnosis nor serial antibody measurements up to 8 months from symptom onset differentiated between NPCS and PCS groups. This was unexpected, because it may suggest that prolonged effects are not directly related to the severity of the initial infection or the host response to the virus.
Our study had certain limitations. which include the small group size (resulting in wide confidence limits on the measured values) and the fact that PCS information was collected only once; therefore, our results should be interpreted with caution. It is possible that some participants had PCS symptoms that had resolved by the time of the questionnaire, potentially underestimating the scale of PCS. Completing the questionnaire after 7–8 months may have resulted in recall bias for the initial symptoms developed. It is noteworthy that a diagnosis of PCS was made based on self-reported symptoms, the most common of which was relatively nonspecific (fatigue). There was no comparison with an uninfected group of healthcare workers, and no investigations were undertaken to identify alternative causes. Finally, we comment only on the humoral response, while it is likely that non–B-cell responses also play a role in physiological defenses.
These findings highlight the high incidence of symptoms consistent with PCS among healthcare workers. They should be considered by those involved in workforce planning and vaccination scheduling to minimize the impacts of PCS on the welfare of healthcare workers and the provision of health care. The lack of difference in (1) viral load and (2) serial antibody reactivity between staff with and without PCS suggests that this is a complex disease process. Our knowledge of PCS remains in its infancy, with an urgent need to understand its etiology and treatment.
Notes
Acknowledgments. We would like to thank all participants who agreed to take part in this study. We also thank those involved in recruitment and sample processing, including Vasileios Pastarmatzis, Scott Lister, Jelyne Jesusa, Paul Andrew Tiamzon, Andy Taylor, Ronan Calvez, and Mohamed Zuhair.
Financial support. This work was supported by the Department of Infectious Disease, Imperial College London.
Potential conflicts of interest. C. R., R. S. T., and M. O. M. declare an interest in the Imperial Hybrid Double antigen-binding assay (DABA) (patent file IRN.FID4816059). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) global epidemiological situation. 2020. https://www.who.int/publications/m/item/weekly-epidemiological-update---10-november-2020. Accessed 10 November 2020. [Google Scholar]
- 2. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perego E, Callard F, Stras L, Melville-Jóhannesson B, Pope R, Alwan NA. Why the patient-made term “Long Covid” is needed. Wellcome Open Res 2020; 5:224. [Google Scholar]
- 4. National Institute for Health and Care Excellence. (2020). COVID-19 guideline scope: management of the long-term effects of COVID-19. https://www.nice.org.uk/guidance/ng188. Accessed 18 December 2020.
- 5. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long-COVID: analysis of COVID cases and their symptoms 1 collected by the Covid Symptoms Study App. medRxiv [Preprint: not peer reviewed]. 19 December 2020. medRxiv. doi: 10.1101/2020.10.19.20214494 [DOI] [Google Scholar]
- 6. Greenhalgh T, Ladds E, Knight M, Ravindran D. “Long Covid”: evidence, recommendations and priority research. 2020; 1 -11. - Written evidence (COV0050). House of Lords Select Committee; 2020 https://committees.parliament.uk/writtenevidence/12345/pdf. Accessed 25 November 2020.
- 7. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol 2020; 5:1598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao XY, Liu W, Li Z-Y, et al. Neutralizing and binding antibody kinetics of COVID-19 patients during hospital and convalescent phases. medRxiv [Preprint: not peer reviewed]. 21 July 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.07.18.20156810v1.
- 9. Crawford KHD, Dingens AS, Eguia R, et al. Dynamics of neutralizing antibody titers in the months after severe acute respiratory syndrome coronavirus 2 infection. J Infect Dis 2021; 223:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lumley SF, O’Donnell D, Stoesser NE, et al. Antibodies to SARS-CoV-2 are associated with protection against reinfection. medRxiv [Preprint: not peer reviewed]. 19 November 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.11.18.20234369v1.
- 11. Kellam P, Barclay W. The dynamics of humoral immune responses following SARS-CoV-2 infection and the potential for reinfection. J Gen Virol 2020; 101:791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris BHL, Zuhair M, Di Giovannantonio M, et al. Asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a rehabilitation facility: evolution of the presence of nasopharyngeal SARS-CoV-2 and serological antibody responses. J Infect Dis 2021; 223:192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Real-time RT-PCR primers and probes for COVID-19.2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html. Accessed 4 December 2020.
- 14. Rosadas C, Randell P, Khan M, McClure MO, Tedder RS. Testing for responses to the wrong SARS-CoV-2 antigen? Lancet 2020; 396:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tedder RS, Samuel D, Dicks S, et al. ; Ebola_CP Consortium Investigators . Detection, characterization, and enrollment of donors of Ebola convalescent plasma in Sierra Leone. Transfusion 2018; 58:1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]