Abstract
During the COVID-19 outbreak in the French overseas department Mayotte, 11 children developed multisystem inflammatory syndrome (MIS-C). They all had a fever and gastrointestinal symptoms. Six patients were admitted to intensive care unit; management included intravenous immunoglobulin and corticosteroid. Severe acute respiratory syndrome coronavirus 2 was documented in all patients. The risk of developing MIS-C was much higher than in all of France.
Keywords: Indian Ocean, Mayotte, MIS-C, SARS-CoV-2
Multisystem inflammatory syndrome in children (MIS-C) has been described as an emerging condition associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections [1–3].
Mayotte is an overseas department of France situated in the northern Mozambique Channel in the Indian Ocean off the coast of Southeast Africa; the first case of COVID-19 was documented on March 13, 2020, with a peak of positive cases between April and June 2020.
Starting mid-April 2020, children with fever and multisystem inflammation were admitted to the only hospital of the island; some patients were critically ill with myocarditis and severe arterial hypotension and required intensive care. Herein, we aim to describe the characteristics, management, and evolution of 11 hospitalized children who met the criteria of MIS-C.
PATIENTS AND METHODS
This retrospective, descriptive study was conducted at the medical center in Mamoudzou, a 400-bed care center situated in the main city of Mayotte. We included all children and adolescents (aged ≤18 years) who were admitted during 6 months since the beginning of the SARS-CoV-2 outbreak and met the criteria for MIS-C. Patients were followed up until September 13, 2020. We reviewed the medical files of all the patients to collect personal and clinical data, laboratory test results, imaging, and echocardiographic findings using a standardized study form.
For the purposes of this study, we used the criteria of the World Health Organization (WHO) to define the SARS-CoV-2-associated MIS-C [4]. Kawasaki disease (KD) was defined according to the criteria of the American Heart Association (AHA) [5]. Clinical myocarditis was defined as cardiac dysfunction on echocardiography with an elevated troponin level.
From each patient, we obtained a nasopharyngeal swab to test for SARS-CoV-2 using reverse transcription-polymerase chain reaction (RT-PCR) (SARS-CoV-2 R-GENE, Argene; bioMérieux, France) within the first 3 days of hospital admission. Cardiology investigations included regular electrocardiography and echocardiography. Continuous variables were expressed with medians and range values and categorical variables with frequencies and percentages. Statistical analyses were performed with IBM SPSS Statistics 25.0.
The study was approved by the local ethics committee; oral informed consent was obtained from the parents of the children studied.
RESULTS
Epidemiological Features of MIS-C in Mayotte
Eleven children with MIS-C were admitted between April 14 and August 14, 2020. They were all native of Mayotte or the neighbor Comoro Islands. The incidence of MIS-C in Mayotte during the study period was estimated at 8 cases for 100 000 children (population under 18 years: 129 805, INSEE 2017). Among the SARS-CoV-2 confirmed cases younger than 18 years (302 between March 13, 2020, and September 13, 2020), the proportion of patients who developed MIS-C was 3%.
Clinical, Laboratory, and Echocardiographic Features of MIS-C Patients
None of the included patients had known congenital heart disease or previous cardiovascular conditions; 1 child was obese. The demographic, clinical, and biological data of the 11 children are summarized in Table 1.
Table 1.
Demographic, Clinical, and Biological Characteristics of 11 Children With MIS-C
| Value | |
|---|---|
| Age, median, years (range) | 9 (5-17) |
| Male, no. (%) | 8/11 (73%) |
| Underlying conditions | 1/11 (9%) |
| Admission delay from first symptoms, days, median (range) | 3 (1-11) |
| Clinical description | |
| Fever, no. (%) | 10/11 (91%) |
| Bilateral non-exudative conjunctivitis, no. (%) | 6/11 (55%) |
| Changes of mouth or lips, no. (%) | 3/11 (27%) |
| Lymphadenopathy, no. (%) | 3/11 (27%) |
| Erythema of hands or feet, no. (%) | 2/11 (18%) |
| Skin rash with desquamation, no. (%) | 1/11 (9%) |
| Vomiting, no. (%) | 10/11 (91%) |
| Abdominal pain, no. (%) | 9/11 (82%) |
| Diarrhea, no. (%) | 3/11 (27%) |
| Headache, no. (%) | 6/11 (55%) |
| Diffused myalgia, no. (%) | 3/11 (27%) |
| Dry cough, no. (%) | 2/11 (18%) |
| Chest pain, no. (%) | 2/11 (18%) |
| Hemodynamic and echocardiographic features | |
| Systemic arterial hypotension, no. (%) | 11/11 (100%) |
| Left ventricular ejection fraction in the first week of hospital stay, median (range), % | 40% (35%-59%) |
| Mitral valve regurgitation | |
| Mild, no. (%) | 4/11 (36%) |
| Moderate, no. (%) | 2/11 (18%) |
| Severe, no. (%) | 1/11 (9%) |
| Pericardial effusion | 4/11 (11%) |
| Wall motion abnormalities | 6/11 (55%) |
| Laboratory values, median (range) | |
| Hemoglobin, g/dL | 9,5 (6,3-14,1) |
| White cells count, ×109/L | 17,9 (6,8-44,4) |
| Thrombocytes in the first week, ×109/L | 216 (72-511) |
| Thrombocytes in the second week, ×109/L | 467 (395-992) |
| C-reactive protein, mg/L | 290 (<5-444) |
| Procalcitonin, ng/mLa | 19,14 (0,02-98) |
| Fibrinogen, g/L | 6,7 (3,74-10,91) |
| D-dimer, µg/mLb | 4,78 (1,2-6,22) |
| Ferritin, µg/mLb | 470 (210-1580) |
| Na, mmol/L | 127 (121-137) |
| Albumin, g/Lc | 26 (21-43) |
| Alanine Aminotransferase, UI/L | 52 (10-179) |
| Brain Natriuretic Peptide, ng/Lc | 926 (45-5444) |
| Troponin I, µg/L | 0,22 (0,004-22) |
| Venous lactate, mmol/L | 1,95 (1,2-4,1) |
| Evidence of SARS-CoV-2 infection | |
| SARS-CoV-2 RT-PCR on nasopharyngeal swabs | 6/11 (55%) |
| SARS-CoV-2 RT-PCR in stool samplesd | 1/11 (9%) |
| SARS-CoV-2 Immunoglobulin G (IgG) antibodiesa | 9/11 (82%) |
| Management | |
| Fluid resuscitation, no. (%) | 8/11 (73%) |
| ICU, no. (%) | 6/11 (55%) |
| Length of ICU stay, days, median (range) | 5 (2-7) |
| Inotropic/vasoactive drug support, no. (%) | 5/11 (45%) |
| Norepinephrine, no. (%) | 5/11 (45%) |
| Dobutamine, no. (%) | 1/11 (9%) |
| Length of inotropic/vasoactive drug support, days, median (range) | 2 (1-5) |
| Invasive ventilation, no. (%) | 0 |
| High-flow nasal oxygen, no. (%) | 1/11 (9%) |
| Intravenous immunoglobulin, no. (%) | 4/11 (36%) |
| Acetylsalicylic acid, no. (%) | 2/11 (18%) |
| Methylprednisolone, no. (%) | 3/11 (27%) |
| Antibiotic treatment, no. (%) | 10/11 (91%) |
| Length of antibiotic treatment, days, median (range) | 5 (3-12) |
| Outcome | |
| Death, no. (%) | 0 |
| Length of hospital stay, days, median (range) | 8 (3-21) |
| Length of follow-up, days, median (range) | 30 (6-50) |
| Lost to follow-up after discharge, no. (%) | 3/11 (27%) |
Abbreviations: ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome in children; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription-polymerase chain reaction.
aAvailable for 9 patients.
bAvailable for 8 patients.
cAvailable for 10 patients.
dAvailable for 4 patients.
Among the 10 children presenting with fever, 5 had a fever over 40°C. All patients suffered from acute gastrointestinal problems. Arterial systemic hypotension occurred in all patients and was associated with cold extremities, decreased peripheral pulse, capillary refill time >3 seconds, and increased blood lactate (>2 mmol/L) in severe patients (5/11, 45%). Five patients fulfilled the criteria of the AHA for incomplete KD, and 6 patients presented clinical myocarditis. One patient had a late cardiac involvement after a febrile phase without hyperinflammation.
All children but one had elevated inflammatory values, which decreased during the hospital stay. Ferritin was abnormal in 8 of the 8 tested patients and highly elevated (>500 µg/L) in 50% of the cases.
Children with acute myocarditis had left ventricular (LV) systolic dysfunction; echocardiography showed reduced left ventricular ejection fraction (LVEF) (40%), with transient valve regurgitation and regional wall motion abnormalities. None of the children presented coronary arteries dilatation. Pericardial effusion was observed in 4 children (36%). Electrocardiographic ST segment and T wave (ST-T) changes occurred in 3 patients (27%).
Evidence of SARS-CoV-2 Infection
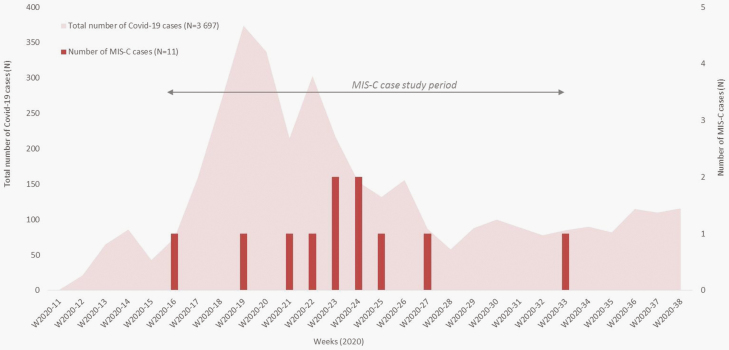
MIS-C cases occurred with a delay of 4–6 weeks after the peak of SARS-CoV-2 cases in Mayotte (Figure 1). SARS-CoV-2 infection was confirmed in 6 of the 11 patients in nasopharyngeal secretion by RT-PCR. SARS-CoV-2 antibodies turned positive for all the patients screened (n = 9). In 4 patients, both RT-PCR and serology turned positive.
Figure 1.
Cases of multisystem inflammatory syndrome in children during the severe acute respiratory syndrome coronavirus 2 outbreak in Mayotte.
Therapeutic Management and Intensive Care Unit Requirement
All patients experienced arterial hypotension, 8 patients needed fluid resuscitation, 6 patients (55%) required admission to intensive care unit, and 5 children needed an inotropic/vasoactive drug support. No patient required venoarterial extracorporeal membrane oxygenation. None was intubated, and 1 child needed high-flow nasal oxygen.
Four patients were treated with intravenous immunoglobulin (IVIG) (2 g/kg), and 2 patients received a second infusion (2 g/kg). Two patients were treated with additional acetylsalicylic acid at anti-inflammatory dose (40–60 mg/kg/day) during the acute phase, followed by low-dose aspirin (5 mg/kg/day) for 8 weeks. Intravenous methylprednisolone at 2 mg/kg/day, followed by a weaning course of oral prednisolone, was administered in 3 patients. Ten patients received an empiric antibiotic treatment with a third-generation cephalosporin.
Outcome
Blood cultures remained sterile in all patients. RT-PCR for the detection of dengue virus in blood turned negative in all 11 patients. No child died and all were discharged home. All patients had a full recovery of the LV function prior to discharge. No echocardiographic abnormalities were detected during the follow-up.
DISCUSSION
We describe here a case series of 11 hospitalized pediatric patients with a febrile systemic inflammation and gastrointestinal manifestations, associated with cardiac dysfunction and documented SARS-CoV-2 infection.
To our knowledge, this is the first case series from a tropical setting with patients from Southeast Africa exclusively. People living in Mayotte are mainly native of Mayotte or other Comoro Islands, and only a small minority are born in the rest of the French Republic or other countries [6]. Previous reports described a high proportion of MIS-C in patients of African ethnicity or ancestry [7–10], suggesting some favoriting genetic or environmental conditions. In the pediatric population from Mayotte, we found a higher incidence of MIS-C than in all of France, where the incidence of MIS-C was estimated at 12.5 cases/1 000 000 children [11] and the risk of developing MIS-C, based on confirmed, probable, and possible cases, was estimated at less than 0.02% [12].
Due to the scarce knowledge of MIS-C and missing established approach at the time point of our patients’ inclusion, management of MIS-C patients was extrapolated from our experience with acute myocarditis and KD. All patients had a favorable evolution and did not show late cardiac complications even without IVIG treatment.
Limitations of this report include presumed underestimation of SARS-CoV-2 infections and MIS-C, possibly due to asymptomatic cases or mild cases of MIS-C that did not involve hospitalization. During the initial period, serology testing was not available in our laboratory, and PCR testing of stool was not routinely undertaken. Inclusion occurred during the development of the case definition, and a standardized approach for the management of patients was missing.
CONCLUSIONS
We reported a case series of 11 hospitalized children from Mayotte who developed MIS-C. Identification of IgG and a delay by 4–6 weeks after the beginning of COVID-19 outbreak suggest a postinfection manifestation. Few patients were treated with IVIG, but all patients had a favorable evolution.
Notes
Financial support: The authors received no specific funding for this work.
Authors’ contributions: C. C., M. D., A. C., and T. S. made substantial contributions to the conception or design of the work. C. C. and M. D. acquired data for the article. M. S. performed epidemiological analyses. S. A. gave a substantial contribution to cardiological investigations and follow-up. P. C. performed the laboratory studies. C. C. and T. S. drafted the work. M. D., M. S., A. C., and T. S. revised the article critically for important intellectual content. C. C., M. D., S. M., A. C., S. A., P. C., and T. S. gave final approval for the article to be published.
Potential conflicts of interests: We declare no competing interests and no financial support. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ahmed M, Advani S, Moreira A, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine 2020; 26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis 2020; 20:e276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J 2020; 39:e340–e346. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization, Scientific Brief. Multisystem inflammatory syndrome in children and adolescent with COVID-19. 2020. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed 15 May 2020. [Google Scholar]
- 5. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–99. [DOI] [PubMed] [Google Scholar]
- 6. Chaussy C, Merceron S, and Genay V. À Mayotte, près d’un habitant sur deux est de nationalité étrangère. 2019. Insee première n°1737.
- 7. Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Whittaker E, Bamford A, Kenny J, et al. ; PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated With SARS-CoV-2. JAMA 2020; 324:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramcharan T, Nolan O, Lai CY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2020; 41:1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santé Publique France. COVID-19 – Point épidémiologique hebdomadaire du 05 novembre 2020. Sante Publique France. https://www.santepubliquefrance.fr
- 12. Belot A, Antona D, Renolleau S, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill 2020; 25:pii=2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]