Abstract
Invasive pulmonary aspergillosis (IPA) is a severe infection caused by aspergillus sp. that usually develops in patients with severe immunosuppression. IPA has been recently described in critically ill COVID-19 patients (termed as COVID-associated pulmonary aspergillosis, or CAPA) that are otherwise immunocompetent. In order to describe the characteristics of patients with CAPA, we conducted a retrospective cohort study in a tertiary care center in Mexico City. We included all patients with confirmed COVID-19 admitted to the intensive care unit that had serum or bronchoalveolar lavage galactomannan measurements. We used the criteria proposed by Koehler et al. to establish the diagnosis of CAPA. Main outcomes were the need for invasive mechanical ventilation (IMV) and in-hospital mortality. Out of a total of 83 hospitalized patients with COVID-19 in the ICU, 16 (19.3%) met the criteria for CAPA. All patients diagnosed with CAPA required IMV whereas only 84% of the patients in the non-IPA group needed this intervention (P = 0.09). In the IPA group, 31% (n = 5) of the patients died, compared to 13% (n = 9) in the non-CAPA group (P = 0.08). We conclude that CAPA is a frequent co-infection in critically ill COVID-19 patients and is associated with a high mortality rate. The timely diagnosis and treatment of IPA in these patients is likely to improve their outcome.
Lay Summary
We studied the characteristics of patients with COVID-19-associated invasive pulmonary aspergillosis (CAPA). Patients with CAPA tended to need invasive mechanical ventilation more frequently and to have a higher mortality rate. Adequate resources for its management can improve their outcome
Keywords: COVID-19, invasive pulmonary aspergillosis, tocilizumab, glucocorticoids
Introduction
Since its emergence in December 2019, SARS-CoV-2 has spread all over the world. By October 16, 2020 the coronavirus disease 2019 (COVID-19) has caused the death of more than one million people, with mortality rates reaching up to 12% in some countries.1,2 COVID-19 causes a wide variety of clinical manifestations, ranging from an asymptomatic disease to a severe form with acute respiratory distress syndrome (ARDS) and multiorgan dysfunction.3
Invasive pulmonary aspergillosis (IPA) is a severe infection caused by Aspergillus Sp, which is an opportunistic fungal pathogen. IPA usually develops in patients with severe immunosuppression due to a variety of conditions such as hematological malignancies, neutropenia of any cause and HIV infection.4 Transplant recipients or chronic users of corticosteroids and other immunosuppressive therapies are at increased risk of presenting IPA.5,6 Interestingly, IPA has been extensively described in patients with severe viral pneumonia without the aforementioned typical risk factors. In some series of patients with severe influenza pneumonia the incidence of IPA can be as high as 19% and is associated with a mortality rate of 51%.7 IPA has also been described in critically ill COVID-19 patients, and has been termed COVID-associated pulmonary aspergillosis (CAPA), with a similar clinical presentation to that of influenza-associated IPA.8,9
At the beginning of the pandemic several non-standardized immunosuppressive therapies were used widely for the treatment of COVID-19, which could represent an important risk factor associated with the development of IPA. One of them, tocilizumab which is a monoclonal antibody that targets the IL-6 receptor, was increasingly being used with questionable results to treat the cytokine storm that develops in critically ill COVID-19 patients. Tocilizumab has been associated with the development of opportunistic infections caused by mycobacteria, Pneumocystis carinii and fungi such as Candida albicans and Aspergillus.10–12 Another extensively prescribed treatment for COVID-19 were high-dose glucocorticoids. Before the results of the RECOVERY13 trial became public, steroids were used in over 80% of COVID-19 patients with ARDS. Glucocorticoids impair the function of neutrophils and lymphocytes and are also considered a traditional risk factor for the development of IPA when used for prolonged periods of time.14
Influenza-associated pulmonary aspergillosis (IAPA) criteria proposed by Verweij et al.7 were originally used to confirm or rule out CAPA, however, recent criteria, developed specifically for CAPA, have been proposed by Koehler et al. The case definitions are divided in proven tracheobronchitis or other pulmonary form, probable tracheobronchitis, other probable pulmonary forms and other possible pulmonary forms.15 We carried out the present study to ascertain the incidence and clinical characteristics of IPA in critically ill COVID-19 patients in our population, as well as the impact of the use of high dose steroids and tocilizumab in the development of IPA.
Materials and methods
Study design and setting
We conducted a cohort study using the COVID-19 registry of the American British Cowdray Medical Center, a private teaching hospital in Mexico City. This registry constitutes an ongoing, prospective database, which gathers data on all hospitalized adult patients with COVID-19 diagnosed and treated at our institution since March 12, 2020. The diagnosis of COVID-19 was established based on the presence of the typical clinical manifestations (fever, cough, myalgias, and headache), the detection of SARS-CoV2 RNA on pharyngeal swabs, as well as a chest CT scan with the characteristic findings (atypical pneumonia with ground glass appearance). The database includes demographic variables, past medical history, clinical variables upon hospital admission, initial and follow-up laboratory studies, treatments received during hospitalization, documented nosocomial infections, and outcomes. The main outcomes were the need for invasive mechanical ventilation (IVM) and in-hospital mortality.
Study participants
We analyzed the data from all patients registered between March 15 and July 10, 2020, who were admitted to the intensive unit (ICU) and who had a serum or bronchoalveolar lavage fluid galactomannan measurement during hospitalization. We excluded all patients with missing data on critical variables (diagnostic criteria and outcomes). The case group consisted of patients who fulfill the case definitions for proven or probable CAPA proposed by Koehler et al.15 and the control group comprised patients that had IPA ruled out with a negative serum galactomannan. Patients with further clinical suspicion also had a bronchoalveolar lavage (BAL) performed to rule out IPA.
Study procedures
Samples for SARS-CoV-2 PCR testing were obtained according to the Center for Disease Control guidelines and processed with the kit RNeasy Mini Kit (Qiagen). Galactomannan (either on serum or bronchoalveolar lavage fluid samples) was determined using the EUROIMMUN Analyzer 1-2P system ELISA.
Statistical analysis
We used mean and standard deviations, or medians and interquartile ranges (IQR), as appropriate, depending on data distribution to summarize continuous variables. For categorical variables, we used frequencies and simple proportions. We compared patients’ characteristics with confirmed CAPA and without CAPA using the chi-square test, Student's t-test, or the Mann–Whitney U test as applicable. Time until death was evaluated using a Kaplan-Meier curve and the log-rank test. Statistical significance was considered to be present when the P value was < 0.05. As statistical software, we used SAS, University Edition version 9.4 (SAS Institute, Cary, NC, USA).
Results
Out of a total of 198 patients, we included 83 patients that met the study criteria. We identified 16 patients (19.2%) that were diagnosed with CAPA during hospitalization, meeting the IAPA criteria. These 16 patients met the criteria for a probable case with a positive galactomannan determination (87.5%), but only 2 patients were diagnosed with a proven case with positive cultures of Aspergillus sp. Five out of 16 patients (31.2%) had a positive serum galactomannan while other seven had a positive galactomannan from BAL with two patients having both positive serum and BAL galactomannan. As mentioned before, only two patients had a positive culture, both from sterile bronchoalveolar aspirations. Twenty-seven patients of the control group (35.8%) had a negative galactomannan from BAL while on IMV, no bronchoalveolar lavage in this group was done in non-intubated patients. The rest of the control group had a negative serum galactomannan. The performance of the serum and BAL galactomannan for the diagnosis of IPA was of 43.7 and 77.7%, respectively. The median time from COVID-19 onset to a positive galactomannan result was 13 days (IQR 9-20). The median time elapsed from intubation to a positive galactomannan result was 6 days (IQR 4-9).
Table 1 depicts the baseline characteristics of COVID-19 patients with and without proven IPA. Patients with CAPA were older (64 vs. 55, P < 0.001), more frequently had a history of cancer and tended to have higher scores in the NEWS scale. Patients with IPA had microbiological isolates found in their CVCs more often than patients without IPA. We found no other significant differences among both groups.
Table 1.
Patient characteristics.
| Variable | CAPA (n = 16) | Controls (n = 67) | P-value |
|---|---|---|---|
| Age (years), mean (SD) | 64 (10) | 55 (15) | <0.001 |
| Female sex | 5(31.25) | 15 (22) | 0.47 |
| PaFIO2, median (IQR) | 122 (84–166) | 108 (82–160) | 0.76 |
| Comorbidities | |||
| Overweight | 8 (50) | 33 (49) | 0.39 |
| Obesity | 6 (38) | 19 (28) | 0.39 |
| COPD | 1 (6) | 3 (4) | 0.77 |
| Hypertension | 4 (25) | 22 (33) | 0.57 |
| Diabetes mellitus | 5 (31) | 13 (19) | 0.30 |
| Cancer | 3 (19) | 3 (4) | 0.05 |
| Laboratory data, median (IQR) | |||
| Leucocytes | 9 (6–10) | 8 (6–12.5) | 0.94 |
| Lymphocytes | 0.93 (0.62–1.27) | 0.88 (0.54–1.10) | 0.65 |
| Platelets | 205 (135–257) | 192 (139–298) | 0.66 |
| D-Dimer | 1088 (714–2795) | 1072 (648–2085) | 0.68 |
| C reactive protein | 18 (5–26) | 17 (9–30) | 0.39 |
| Interleukin 6 | 15 (9–88) | 44 (12.5–77) | 0.60 |
| Ferritin | 1756 (1027–2387) | 1178 (722–2089) | 0.18 |
| Severity scales | |||
| NEWS, mean (SD) | 7.1 (2.47) | 6.84 (2.45) | <0.001 |
| CALL, median (IQR) | 10 (8.5–11.5) | 9 (7–11) | 0.21 |
| MuLBSTA, median (IQR) | 12 (11–12) | 9 (7–12) | 0.26 |
| Positive bacterial isolates | |||
| CVC culture | 5 (31) | 4 (6) | 0.01 |
| Endotracheal tube culture | 3 (19) | 7 (10) | 0.39 |
| Sputum culture | 1 (6) | 3 (4) | 1.00 |
Here we show the comparison of the baseline characteristics among patients with and without CAPA. All values are N(%) unless otherwise noted.
COPD: Chronic obstructive pulmonary disease, CRP: C-reactive protein, CVC: Central venous catheter, IL-6: Interleukin-6.
Table 2 depicts the different treatments received by the patients. There were no significant differences between the two groups in regard to the proportion or dose of glucocorticoids (1.3 mg/kg of prednisone or equivalent), tocilizumab, lopinavir/ritonavir, azithromycin or hydroxychloroquine. Patients with a positive galactomannan tended to receive treatment with azithromycin more often that those with a negative galactomannan, although this did not reach statistical significance (50 vs. 29.8%, P = 0.24).
Table 2.
COVID-19 treatment received.
| COVID-19 treatment | CAPA (n = 16) | Controls (n = 67) | P-value |
|---|---|---|---|
| Hydroxychloroquine | 14 (88) | 56 (84) | 0.71 |
| Lopinavir/ritonavir | 11 (69) | 45 (67) | 0.91 |
| Azithromycin | 12 (75) | 56 (84) | 0.43 |
| Steroids | 2 (13) | 22 (33) | 0.34 |
| Tocilizumab | 12 (75) | 43 (64) | 0.52 |
Here we show the COVID-19 treatment received by each group. There were no significant differences in the frequencies in which COVID-19 treatment was administered among both groups. All values are N (%).
All patients diagnosed with CAPA required invasive mechanical ventilation whereas that was the case for 84% of the patients in the non-CAPA group (P = 0.09). At the end of the follow up, 31% (n = 5) of the patients in the CAPA group died, compared to 13% (n = 9) in the non-CAPA group (P = 0.08) (Figure 1). When assessing time to in-hospital mortality, the CAPA group showed a peak for in-hospital mortality at approximately 30 days into their stay in the ICU. This did not prove to be statistical significant (P = 0.1279) (Figure 2).
Figure 1.
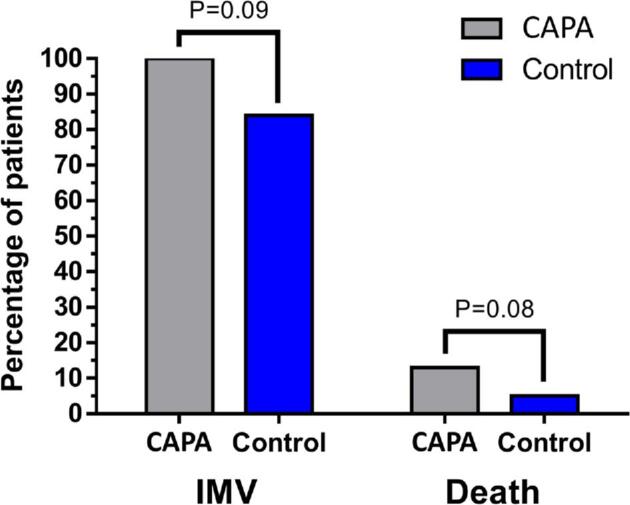
Clinical outcomes. Here we show the comparison of both clinical outcomes of interest (invasive mechanical ventilation and in-hospital death) among both groups. Both outcomes tended to be more frequent in the CAPA group, although neither reached statistical significance.
Figure 2.
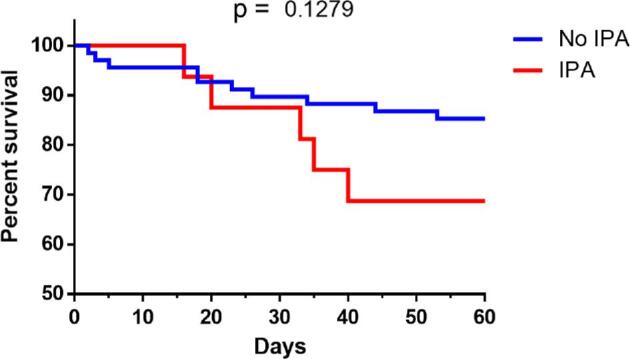
Kaplan-Meier curve for in-hospital mortality. Comparison of survival curves in the CAPA group and control group. Mortality tended to be more frequent in the invasive pulmonary aspergillosis, showing a peak in incidence at approximately 30 days of hospitalization. There was no statistical significance in in-hospital mortality (P = 0.1279).
Discussion
IPA has been described in multiple settings as an important infection in COVID-19 patients. CAPA may significantly contribute to the morbidity and mortality of these critically ill individuals.16,17 In our study, patients with IPA were older and were more likely to have a history of malignancy. Consistent with most COVID-19 reports, diabetes and obesity were frequent comorbidities in the whole cohort. There were no other clear risk factors among the analyzed baseline characteristics or administered COVID-19 treatments for presenting CAPA, which is consistent with published reports.17 Although there was an important trend towards worse clinical outcomes in the CAPA group (including the need for invasive mechanical ventilation), both univariate and multivariate analysis showed no significant difference between groups, which probably is a consequence of our small sample size and subsequently of a lack of statistical power.
The incidence of CAPA found in the present study (19%) was similar to that found in other reports, however our mortality rate was significantly lower (16.86%) than that previously reported in case series and systematic reviews (44–54.1%).17–20 In fact, unlike previously reported series, the difference in mortality rate among our patients with and without CAPA did not reach statistical significance. A possible explanation of the lower mortality rate reported in our study is the readily accessible resources for the treatment of COVID and IPA in our center. We have had no shortage of equipment or human resources and we have been able to offer IVM to all patients that have required it. Galactomannan processing time in our center is <48 h, and it is a standard practice in our ICU that enables us to promptly initiate the appropriate antifungal therapy as soon as the diagnosis of CAPA is suspected. Some studies have reported the early use of antifungal therapies (like voriconazole) as likely factors associated with an increased survival rate in these patients.20 All these factors emphasize the importance of constant re-evaluation and prompt detection of complications by the involved medical team.
IPA usually develops in patients with traditional risk factors such as neutropenia, due to cancer chemotherapy or immunosuppressive therapy.21 Interestingly, in practically none of our patients were these risk factors present prior to the diagnosis of SARS-CoV-2 infection, and only one of them had received high-dose steroids for 3 weeks before the diagnosis. This lack of traditional risk factors for IPA has also been observed in most other studies.22 and it is similar to what happens with influenza-associated IPA, whereby most patients do not have a clear history of immunosuppression.23 Viral infection by itself seems to be the risk factor predisposing to the development of CAPA.24 The pathophysiology of CAPA is not completely understood, but some of the proposed mechanisms are the disruption of natural lung barriers by respiratory viruses, including the damage to the respiratory epithelium and the overexpression of cytokines and the consequent dysregulation of the immune response.25–27
Another important proposed risk factor for the development of CAPA is the use of immunosuppressive therapy for the treatment of COVID-19. IPA has been previously described in patients with SARS-CoV-1 infection using high dose corticosteroids,28 but this relationship is still not clearly described in SARS-CoV-2. Interestingly, in our cohort the proportion of patients receiving high-dose glucocorticoid therapy was lower among the patients who developed CAPA than among those who did not, although this did not reach statistical significance (13 vs. 33%). Both groups of patients received similar doses of glucocorticoids for similar periods of time, and almost no patients met EORTC criteria of steroid use as a risk factor for IPA. Another therapy that poses an important risk factor for the development of IPA is tocilizumab. There was no apparent risk factor for CAPA, with both groups having very similar proportion of patients treated with tocilizumab (P = 0.75). Azithromycin, has been widely used during the pandemic because of its immunomodulatory properties, however, a recent study by Delliere, shows that a dose >1500 mg may increase the risk of developing IPA. In our cohort, there was no difference in the dose of azithromycin in both groups.29
Two main limitations in our study are its retrospective nature and the small sample size. Nevertheless, this remains one of the bigger cohorts of CAPA when compared with other reports.9,16–19,21 The major bias in our study relates to the comparison between patients with invasive and noninvasive mechanical ventilation. Although there was a similar proportion of BAL galactomannan measurements among patients that required IMV in the non-CAPA and CAPA groups (48.21 vs 56.25%, P = 0.570), none of the non-intubated patients in the control group had bronchoalveolar lavage specimens taken. Furthermore, all non-intubated patients in the control group had a favorable clinical course (and were discharged while stable) without the addition of an antifungal treatment, which makes the diagnosis of CAPA unlikely even in the absence of BAL investigations. Another important limitation is that most patients were diagnosed as probable CAPA and only a few patients had a proven diagnosis documented by a culture. Of important note, most previously published studies have used these same diagnostic criteria, even though they have not been extensively validated. A recently published study by Mohamed et al., have proposed new diagnostic criteria, which includes a more extensive documentation of the presence of IPA using other markers such as Beta-D-Glucan, but still need to be validated.28 Finally, our study was carried out in a teaching hospital in Latin America, where medical residents give the vast majority of care and provide most interventions in COVID-19 patients.
We conclude that IPA is a frequent co-infection in critical COVID-19 patients. Settings with the adequate resources for the diagnosis and treatment of this clinical entity have the ability to propitiate good clinical outcomes and to decrease the mortality rate of these patients. This study represents a tertiary care center's real-life experience and may constitute the basis for future investigations.
Acknowledgements
To all of our residents and friends from the ICU, for their amazing labor and commitment during the pandemic, which has allowed us to have a minimum mortality rate. To all of the ARMII study group, who made this work possible: Renzo Pérez-Dórame, María Fernanda Coss-Rovirosa, Rodolfo Jiménez-Soto, Alejandra Kerbel Laiter, Guillermo Bracamontes-Castelo, Cecilia Nehmad Misri, Carlos Andrés Rodríguez-Toledo, Alma Nelly Rodríguez-Alcocer, Stefany Jacob Kuttothara, Ana Paula Landeta-Sa, Mariana Covadonga Ansoleaga-García, Andrea Romo López, Santiago Montiel-Romero, José Carlos Krause Marún, Juan Pablo Guillermo-Durán, Victor José Leal Alcántara, María Luisa Montes de Oca-Loyola, Adolfo Díaz Cabral, Laura Crespo-Ortega, Walter Valle-Uitzil,, Víctor Hugo Gomez-Johnson, Gina Gonzalez Calderón, José Antonio Garcia-Gordillo, Arturo Cadena-Fernández, Latife Salame Khouri, Jesica Naanous Rayek, Isabel Gutiérrez-Lozano, Jorge Carlos Salado-Burbano, Mariana Rotzinger-Rodríguez, Rodrigo Sánchez Magallán, Tábata Cano-Gámez. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Mariana Vélez Pintado, Department of Medicine, Centro Médico ABC, Mexico City, Mexico. Sur 136 No. 116, Col. Las Américas, Álvaro Obregón, 01120.
Antonio Camiro-Zúñiga, Department of Medicine, Centro Médico ABC, Mexico City, Mexico. Sur 136 No. 116, Col. Las Américas, Álvaro Obregón, 01120.
Mercedes Aguilar Soto, Department of Medicine, Centro Médico ABC, Mexico City, Mexico. Sur 136 No. 116, Col. Las Américas, Álvaro Obregón, 01120.
Dalia Cuenca, Department of Medicine, Centro Médico ABC, Mexico City, Mexico. Sur 136 No. 116, Col. Las Américas, Álvaro Obregón, 01120.
Moisés Mercado, Research Unit in Endocrine Diseases, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City. Av. Cuauhtémoc 330, Doctores, Cuauhtémoc, 06720.
Brenda Crabtree-Ramirez, Department of Infectious Diseases, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Vasco de Quiroga 15, Belisario Domínguez Secc 16, Tlalpan, 14080 Ciudad de México, CDMX.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- 1. Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020; 382: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO . Situation Report. Coronavirus Disease (COVID-19). 2020. Consulted February 12th. [Google Scholar]
- 3. Adhikari SP, Meng S, Wu YJ et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quindós G. Epidemiología de las micosis invasoras: un paisaje en continuo cambio. Rev Iberoam Micol. 2018; 35: 171–178. [DOI] [PubMed] [Google Scholar]
- 5. Balloy V, Huerre M, Latgé J-P, Chignard M. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005; 73: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015; 70: 270–277. [DOI] [PubMed] [Google Scholar]
- 7. Verweij PE, Rijnders BJA, Brüggemann RJM et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020; 46: 1524–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blaize M, Mayaux J, Nabet C, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020; 26: 1636–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koehler P, Cornely OA, Böttiger BW et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020; 63: 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020; 55: 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alzghari SK, Acuña VS. Supportive treatment with tocilizumab for COVID-19: a systematic review. J Clin Virol. 2020; 127: 104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kherani I, Chin C, Kherani RB, Kherani F. Refractory giant cell arteritis on prednisone and tocilizumab: improvement with subsequent tuberculosis reactivation. Can J Ophthalmol. 2019; 54: 192–194. [DOI] [PubMed] [Google Scholar]
- 13. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020:NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp: implications for pathogenesis. Microbiology. 1994; 140: 2475–2479. [DOI] [PubMed] [Google Scholar]
- 15. Koehler P, Basseti M, Chakrabarti A et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020;S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arastehfar A, Carvalho A, van de Veerdonk FL et al. COVID-19 associated pulmonary aspergillosis (CAPA)-From immunology to treatment. J Fungi (Basel). 2020; 6: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salmanton-García J, Sprute R, Stemler J et al. COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis. 2021 Feb 4; 27(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020; 202: 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020; 8: e48–e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apostolopoulou A, Esquer Garrigos Z, Vijayvargiya P, Lerner AH, Farmakiotis D. Invasive pulmonary aspergillosis in patients with SARS-CoV-2 infection: A systematic review of the literature. Diagnostics (Basel). 2020; 10: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koehler P, Salmanton-García J, Gräfe SK et al. Baseline predictors influencing the prognosis of invasive aspergillosis in adults. Mycoses. 2019; 62: 651–658. [DOI] [PubMed] [Google Scholar]
- 22. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: An observational study from Pakistan. Mycoses. 2020; 63: 766–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018; 31: 471–480. [DOI] [PubMed] [Google Scholar]
- 24. Gu X, Zhou F, Wang Y, Fan G, Cao B. Respiratory viral sepsis: epidemiology, pathophysiology, diagnosis and treatment. Eur Respir Rev. 2020; 29: 200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cunha C, Goncalves SM, Duarte-Oliveira C et al. IL-10 overexpression predisposes to invasive aspergillosis by suppressing antifungal immunity. J Allergy Clin Immunol. 2017; 140: 867–870. [DOI] [PubMed] [Google Scholar]
- 26. McCullers J. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014; 12: 252–262. [DOI] [PubMed] [Google Scholar]
- 27. Rutsaert L, Steinfort N, Van Hunsel T et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care 2020; 10: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohamed A, Rogers TR, Talento AF. COVID-19 associated invasive pulmonary aspergillosis: diagnostic and therapeutic challenges. J Fungi (Basel). 2020; 6: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dellière S, Dudoignon E, Fodil S et al. Risk factors associated with Covid-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020;S1198-743X(20)30756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


