Abstract
A cohort consisting of asymptomatic healthcare workers donated temporal serum samples after infection with severe acute respiratory syndrome coronavirus 2. Analysis shows that all asymptomatic healthcare workers had neutralizing antibodies, that these antibodies persist for ≥60 days, and that anti-spike receptor-binding domain immunoglobulin G levels were correspondingly durable over the same time period.
Keywords: COVID-19, immunity, serologic test
Asymptomatic healthcare workers infected with severe acute respiratory syndrome coronavirus 2 show durable viral neutralization titers from baseline to 60 days with a BSL-3 viral plaque reduction neutralization test. Anti–receptor-binding domain immunoglobulin G is also durable during this time.
The reported decay of anti-spike antibodies [1] and the temporal reduction in viral neutralization titers [2] have led to debate on the longevity of protection after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. To address this question, we report a study enrolling asymptomatic healthcare workers (HCWs) without prior coronavirus disease 2019 (COVID-19) symptoms but with detectable SARS-CoV-2 antibodies, as well as mildly symptomatic patients. Current evidence in animal models and natural experiments suggests that the presence of neutralizing antibodies predicts protection from SARS-CoV-2 [3–5]. While correlates of protection in COVID-19 are as yet unknown, including how cellular responses may provide protection apart from, or in conjunction with, antibody responses [6, 7], the prophylactic protection provided by administered neutralizing antibodies supports the hypothesis that neutralizing antibodies can prevent infection [8]. Therefore, analyzing the antibody immune response of asymptomatic or mildly symptomatic patients may shed light on protection from reinfection.
METHODS
All participants were consented and samples collected under institutional review board approval from the appropriate institution.
Identification of Asymptomatic HCW Cohort Participants
HCWs previously infected with to SARS-CoV-2 were identified by means of an Ortho Diagnostics Vitros anti-S1 total immunoglobulin enzyme-linked immunosorbent assay (ELISA) serology screening program during April and May 2020 at Inova Hospital in Northern Virginia. HCWs were asked whether they had developed symptoms before or during enrollment. During follow-up, each participant had to answer the same set of questions of whether he or she had experience symptoms, including, but not limited to, fever, cough, chest tightness or shortness of breath, throat pain, loss of taste, loss of smell, or other respiratory symptoms related to COVID-19 disease.
The follow-up questionnaire was performed at 2- and 6-month intervals. Individuals identified as positive with the Ortho Diagnostics assay provided consent and donated serum samples, which are denoted as baseline. The same individuals returned at 60 days after the initial donation and provided a second temporal serum sample, denoted as 60 days. Of 17 matched serum samples, 2 (1 baseline and 1 60-day sample) were evaluated only with ELISA and not with the 90% plaque reduction neutralization test (PRNT90) owing to volume constraints, leaving 16 baseline samples evaluated with PRNT90 and 15 matched pairs evaluated with PRNT90.
Identification of Symptomatic Cohort Participants
Symptomatic, convalescent participants with previous SARS-CoV-2 infection were recruited from the George Mason University community. Participants were either positive with reverse-transcription polymerase chain reaction (RT-PCR) or had a symptomatic episode consistent with COVID-19, including otherwise unexplained fever, chills, loss of taste and/or smell, or cough (n = 12). Symptomatic participants were not screened with the Ortho Diagnostics anti-S1 total antibody assay.
ELISAs were conducted under College of American Pathologists/Clinical Laboratory Improvement Amendments certification, as follows: ELISA plates (Immulon 1B; Thermo Fisher no. 3355) were coated with 2 µg of SARS-CoV-2 receptor-binding domain (RBD)–mFc protein (Sino Biological no. 40592-V05H). Serum dilutions (10-fold dilutions from 1:2 to 1:20 000) were incubated on coated plates for 2 hours; binding was detected using 1:5000 goat anti–human immunoglobulin (Ig) G–horseradish peroxidase)–conjugated secondary antibody (Jackson ImmunoResearch no. 109-035-098) with TMB (3,3’,5,5′-tetramethylbenzidine; Fisher no. 34028) with 2 mol/L sulfuric acid stop solution. Samples were normalized to the geometric mean of 4 calibrator values (3 positive control serum samples, 1 negative control) added to each plate.
The PRNT90 was conducted as follows: serum incubated with SARS-CoV-2 (BEI Resources no. NR-52281) for 1 hour was used to inoculate Vero cells (American Type Culture Collection CCL-81; density, 2 × 105 cells per well) for 1 hour (37°C; 5% carbon dioxide). After infection, a 1:1 overlay of 0.6% agarose and cell medium (2× Eagle's minimal essential medium without phenol red, 10% fetal bovine serum, nonessential amino acids, 1 mmol/L sodium pyruvate, 2 mmol/L L-glutamine, and 1% penicillin-streptomycin) was added to each well. Plates were incubated at 37°C for 48 hours, fixed with 10% formaldehyde for 1 hour, and stained with crystal violet. Neutralizing titers are expressed as the reciprocal of the highest dilution of serum that neutralized ≥90% of plaques. Statistical analysis was completed with GraphPad Prism software, version 8.0.
RESULTS
We used 2 participant cohorts to probe the relationship between neutralizing antibodies and anti-spike-RBD antibody assays, which are thought to be predictive of neutralizing responses. The asymptomatic cohort (n = 17) consists of HCWs identified as SARS-CoV-2 infected by an anti-spike total antibody binding assay (Ortho Diagnostics) during a serology screening program of 1853 total asymptomatic HCWs at a large quaternary hospital in Northern Virginia. A total of 21 HCWs were identified as infected (1%), and 17 returned for follow-up 60 days later, at which time all remained antibody positive. The mildly symptomatic cohort (n = 26; all nonhospitalized) were either RT-PCR-positive (n = 14) or had a symptomatic episode consistent with COVID-19, based on interviews with the participants (n = 12).
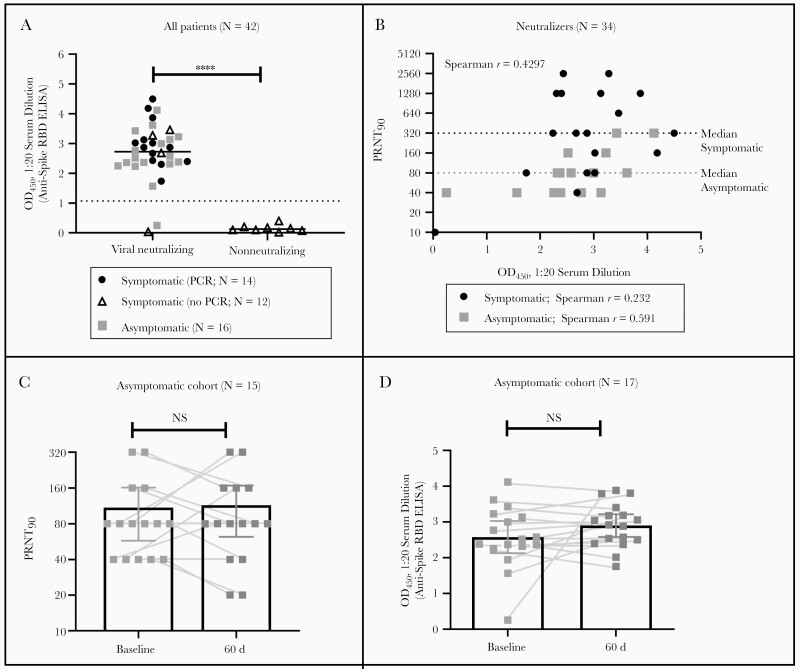
As shown in Figure 1A, the presence of anti-spike RBD IgG was predictive of viral neutralizing activity in a standard PRNT90 in both symptomatic and asymptomatic cohorts. For the symptomatic cohort, those without confirmatory PCR findings were generally nonneutralizing, indicating that symptoms were likely due to an illness other than COVID-19. However, as shown in Figure 1B, we do not see a strong correlation between anti-RBD titers and viral neutralization titers in our cohort, even in a subgroup analysis separating symptomatic from asymptomatic patients, although this correlation is reported by some other groups [9, 10].
Figure 1.
Patients with mild or asymptomatic coronavirus disease 2019 produce neutralizing antibodies, which for asymptomatic patients are shown to be durable over the course of 60 days in viral neutralization assays with authentic severe acute respiratory syndrome coronavirus 2, yet not strongly correlated with anti-receptor-binding domain (RBD) immunoglobulin G titers. A, Anti-spike RBD enzyme-linked immunosorbent assay (ELISA) optical density at 450 nm (OD450) values at 1:20 dilution are predictive of the presence of viral neutralizing antibodies in patient serum samples. Differences between medians were evaluated with unpaired t test and were significant (****P < .0001). For asymptomatic patients, baseline data are shown. B, All patients with neutralizing antibody titers (n = 34) were evaluated for correlation between neutralizing titers and signal intensity in the anti-spike RBD ELISA. For both cohorts, Spearman’s r is 0.4297 (P = .01). Subgroup analysis with neutralizers from the symptomatic cohort (n = 18) give a poorer correlation; Spearman’s r for this subgroup is 0.232 (P = .35). Subgroup analysis with neutralizers from the asymptomatic cohort show an improved but still weak correlation; Spearman’s r for this subgroup is 0.591 (P = .02). C, Differences between 90% plaque reduction neutralization test (PRNT90) values at baseline and at 60 days for patients in the asymptomatic healthcare worker cohort were analyzed using paired t tests; differences in PRNT90 values were not significant (P > .99). D, Differences between anti-spike RBD ELISA OD450 values at 1:20 dilution at baseline and at 60 days for patients in the asymptomatic healthcare worker cohort were analyzed with paired t test; differences in anti-spike RBD ELISA OD450were not significant (P = .18). Abbreviations: NS, not significant; PCR, polymerase chain reaction.
For the asymptomatic cohort, we were able to obtain an additional serum samples 60 days after their initial sample. We determined the longitudinal PRNT90 values for the asymptomatic participants (n = 15; 2 samples unavailable [see Methods]) to evaluate the persistence of neutralizing antibodies for this cohort. As shown in Figure 1C, it is clear that all asymptomatic HCWs had detectable neutralizing antibodies at baseline. Furthermore, these titers are largely unchanged at 60 days after baseline. Given that it is unknown how long after infection an asymptomatic participant’s baseline sample was obtained, protection could easily last >60 days. Because baseline samples were acquired in April or May 2020, and the first confirmed SARS-CoV-2 case in the state of Virginia was reported on 7 March [11], it is unlikely that any participant had been infected for >2 months before donation of the baseline sample.
Notably, consistent neutralizing titers over 60 days are mirrored by consistent positivity via our anti-RBD ELISA over the same time period, as shown in Figure 1D. One participant showed very low anti-RBD titers at baseline that rose significantly by 60 days, suggesting that this participant may have provided a baseline sample very early in the convalescent phase. Given the unknown timeline of exposure or infection before donation at baseline, it is unclear whether the titers are “stable” over the 2-month window observed here, as peak antibody concentrations may have been missed; however, the durability of such a response over the time frame is clear.
DISCUSSION
We conclude that anti-RBD ELISAs, while excellent for determining previous infection and predicting the presence of neutralizing antibodies, may be a poor surrogate for the level of viral neutralization activity, depending on the composition and size of the cohort, based on the results shown in Figure 1B. Our cohort of asymptomatic HCWs may differ immunologically from primarily symptomatic cohorts [9, 10], for which a strong correlation between anti-RBD and the neutralizing antibody titers is reported.
Previous findings suggest that mildly affected cohorts, such as children, make distinct antibody responses [12], and 1 report has shown reduced anti-spike IgG generation specifically in an asymptomatic cohort, as well as reduced correlation between anti-RBD titers and neutralizing titers, as we report here [13]. It is worth noting that the lack of correlation between anti-RBD IgG and neutralization antibody could be explained in part by the fact that neutralization titers in this study population of asymptomatic participants are at the low end of the spectrum, which could make their association less linear. Furthermore, our PRNT90 has differences from the assays used by some other groups [9, 10]. Our measured end point is a plaques, rather than quantification of virus present through RT-PCR, luminesce assays, or anti-N antibody staining. While anti-RBD responses may be predictive of previous infection, further research into additional epitopes targeted by neutralizing antibodies in this cohort may shed light on other epitopes relevant to protection in asymptomatic or mildly affected cohorts.
We eagerly report that asymptomatic HCWs mount viral-neutralizing responses that are durable over ≥60 days. Previous reports have suggested that asymptomatic patients mount neutralizing titers at reduced intensity compared with symptomatic cohorts [13], a finding we replicate here with median PRNT90 titers of 320 and 80 in the symptomatic and asymptomatic cohorts, respectively (Figure 1B). However, we did not find marked reduction in neutralizing titers in short periods of time among asymptomatic patients, as feared after reports of reduced IgG titers in asymptomatic patients by others [14]. While we cannot rule out the possibility of subsequent asymptomatic reinfection of subjects during the 60 days, data suggest that this is unlikely [15], and particularly unlikely that it would apply to significant numbers of cohort participants.
Sustained viral neutralization titers are an independent metric indicating protection of asymptomatic patients, regardless of whether anti-RBD IgG decays or persists. The durability of neutralizing responses even in asymptomatic participants, as reported here, has clinical implications for public health and society as a whole as vaccination or other immune therapy for COVID-19 disease becomes available in clinical practice and serologic assays are used to guide treatment [16]. While there are data to support potentially giving only a single messenger RNA vaccine dose for symptomatic, previously infected patients with COVID-19 [17], it is unclear whether this research is translatable to those who remain asymptomatic. Our data suggests some level of durable protection even in asymptomatic infections, suggesting that single-dose messenger RNA vaccinations constitute a worthy area of further study in asymptomatic individuals. Additional follow-up for durability of neutralizing antibodies beyond 60 days in asymptomatic, but infected individuals is sorely needed.
Notes
Financial support. This work was supported by the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (research support to A. A. D.), which receives funding from the National Institute on Aging (grant P30-AG021334) and the National Heart, Lung, and Blood Institute Mentored Patient-Oriented Research Career Development Award (grant K23-HL153771-01; and by the National Center for Advancing Translational Science of the National Institutes of Health Award (grant UL1TR003015 to C. R. d.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med 2020; 383:1085–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 2020; 26:845–8. [DOI] [PubMed] [Google Scholar]
- 3. Rogers TF, Zhao F, Huang D, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020; 369:956–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol 2020; 58:e02107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang S, Peng Y, Wang R, et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat Commun 2020; 11:5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schwarzkopf S, Krawczyk A, Knop D, et al. Cellular immunity in COVID-19 convalescents with PCR-confirmed infection but with undetectable SARS-CoV-2–specific IgG. Emerg Infect Dis2021; 27. [DOI] [PubMed] [Google Scholar]
- 7. Sattler A, Angermair S, Stockmann H, et al. SARS–CoV-2–specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest 2020; 130:6477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Regeneron Pharmaceuticals. Regeneron reports positive interim data with REGEN-COVTM antibody cocktail used as passive vaccine to prevent COVID-19/ https://investor.regeneron.com/news-releases/news-release-details/regeneron-reports-positive-interim-data-regen-covtm-antibody/. Accessed 26 March 2021.
- 9. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370:1227–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol 2020; 5:eabc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyer, J. Timeline: a look at how coronavirus news developed and quickly escalated in Virginia [Internet]. Richmond Times-Dispatch. 24 March 2020. Available from: https://richmond.com/special-report/coronavirus/timeline-a-look-at-how-coronavirus-news-developed-and-quickly-escalated-in-virginia/article_bcdd6def-41d7-56d5-a936-316709704d17.html. Accessed 26 March 2021.
- 12. Weisberg SP, Connors TJ, Zhu Y, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol2021; 22:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther 2020; 5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 15. Harvey RA, Rassen JA, Kabelac CA, et al. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med doi:10.1001/jamainternmed.2021.0366. Published 24 February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Damluji Abdulla A, Christenson Robert H, deFilippi C. Clinical application of serologic testing for coronavirus disease 2019 in contemporary cardiovascular practice. J Am Heart Assoc 2021; 10:e019506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wise J. Covid-19: people who have had infection might only need one dose of mRNA vaccine. BMJ 2021; 372:n308. [DOI] [PubMed] [Google Scholar]