Abstract
Background
The coronavirus disease 2019 (COVID-19) has quickly spread across the world. However, the nutritional status of COVID-19 patients has not yet been extensively examined.
Objectives
The aim of this study was to evaluate the nutritional status of COVID-19 patients and to identify factors independently associated with malnutrition risk.
Methods
In this single-center, cross-sectional study, we analyzed data from 760 hospitalized COVID-19 patients between 29 January 2020 and 15 March 2020. Based on the Nutrition Risk Screening (NRS) 2002 score, we divided patients into the normal nutrition group (NRS score <3) and the malnutrition risk group (NRS score ≥3). The associations of age, gender, symptoms, comorbidities, BMI, serum albumin and prealbumin concentrations, disease severity, activities of daily living (ADL) score, and clinical outcomes with malnutrition risk were analyzed. Multivariable logistic regression analysis was used to identify independent factors associated with malnutrition risk.
Results
Of patients with COVID-19, 82.6% were at risk of malnutrition. There were statistical differences in the age, incidence of fever, BMI, serum albumin and prealbumin concentrations, ADL score, and disease severity between the 2 groups. Multivariable logistic regression analysis revealed that age ≥65 y (vs. <65 y; OR: 5.40; P < 0.001), serum albumin <35 g/L (vs. ≥35 g/L; OR: 3.61; P < 0.001), serum prealbumin <150 mg/L (vs. ≥150 mg/L; OR: 2.88; P = 0.042), critical cases (vs. moderate cases; OR: 4.46; P < 0.001), ADL score 41–60 (vs. ADL score 100; OR: 4.50; P = 0.012), and ADL score ≤40 (vs. ADL score 100; OR: 9.49; P < 0.001) were significantly associated with the risk of malnutrition in COVID-19 patients.
Conclusions
This study showed that prevalence of malnutrition risk was high in COVID-19 patients. Older age, low serum albumin and prealbumin concentrations, ADL score <60, and disease severity were independent factors associated with malnutrition risk.
Key words: COVID-19, nutrition, malnutrition, risk of malnutrition, risk factors
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues unabated, posing serious threats to global health. Although the disease primarily affects the respiratory tract, it may also cause significant damage to other target organs, including the liver, heart, and kidneys, which may lead to death due to multiple organ failure (1., 2., 3.). Patients with COVID-19 typically present with severe inflammation, fever, and reduced food intake.
The decreased food intake in COVID-19 patients increases the risk of malnutrition, impacts the quality of life, and may lead to unfavorable clinical outcomes. Nutritional treatment is recommended by numerous experts and guidelines, including the Chinese Society of Parenteral and Enteral Nutrition of the Chinese Medical Association (4), the European Society for Clinical Nutrition and Metabolism (ESPEN) (5), and the Infectious Diseases Society of America (6). However, the clinical relevance of the nutritional status of patients with COVID-19 and the prevalence of malnutrition remain unknown. A recent cross-sectional study in 182 elderly COVID-19 patients reported malnutrition [Mini Nutritional Assessment (MNA) score <17] in 52.7% of patients and malnutrition risk (MNA score 17–23.5) in 27.5% of patients (7). However, the nutritional status of COVID-19 patients in different age groups has not been extensively reported.
In the present study, we retrospectively evaluated the nutritional status of hospitalized COVID-19 patients and identified the independent factors associated with malnutrition risk in patients infected with SARS-CoV-2.
Methods
Study design and participants
This cross-sectional study included 760 adult COVID-19 patients admitted to the West campus of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (Wuhan, China), from 29 January 2020 to 15 March 2020. The clinical outcomes were monitored until 28 April 2020, by which date all of the patients either had died or were discharged. This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (ECUH 2020–0211).
COVID-19 diagnosis was based on the sixth edition of the guidelines issued by China's National Health Commission (8). Specifically, we used the following diagnostic criteria: 1) a history of epidemiological exposure; 2) clinical symptoms, such as fever (≥37.3°C), cough, fatigue, dyspnea, muscle soreness, headache, or diarrhea; 3) laboratory tests indicating that the total number of white blood cells was normal (4.0–10.0 × 109/L) or decreased (<4.0 × 109/L) and the lymphocyte count was decreased (<1.0 × 109/L); 4) chest computed tomography showing multiple patchy shadows, ground-glass shadows, infiltration, or consolidation changes; and 5) positive detection of SARS-CoV-2 nucleic acid by qRT-PCR using pharyngeal swab specimens. If a patient had a history of epidemiological exposure, he/she should meet the criterion number 5 and any 2 of criteria 2, 3, and 4. If a patient did not have a history of epidemiological exposure, he/she should meet all of the criteria 2, 3, 4, and 5.
Hospitalized COVID-19 patients aged >18 y old were included in the study. Patients with one of the following conditions were excluded from the study: pregnancy, obnubilation, linguistic difficulties, incomplete medical records (e.g., transfer to any other hospital), incomplete questionnaires, severe hepatic dysfunction, and severe renal dysfunction ( Figure 1).
Figure 1.
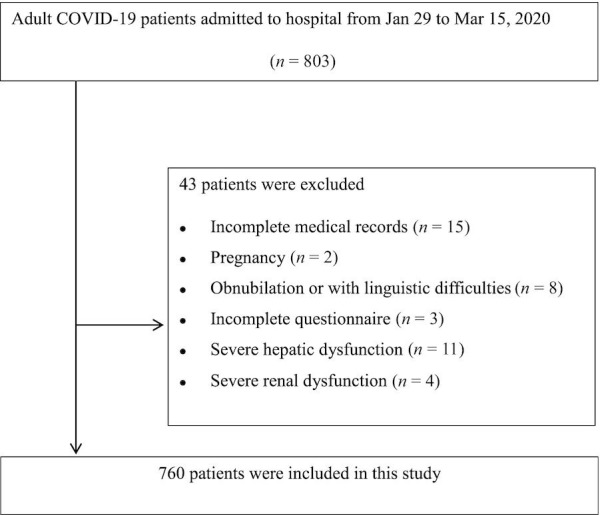
Patient flowchart. COVID-19, coronavirus disease 2019.
Data collection
Patient characteristics, including age, gender, symptoms, BMI, comorbidities, disease severity, and clinical outcomes, were extracted from the hospital's electronic medical records. Laboratory data, such as serum albumin and serum prealbumin concentrations, were collected from the hospital's laboratory information system; laboratory evaluations were performed using an Olympus AU-5400 automatic analyzer (Olympus). The reference levels for serum albumin were 35–55 g/L and for prealbumin were 150–400 mg/L. Barthel score was used to assess the performance in activities of daily living (ADL). ADL scores ranged from 0 to 100, and ADL performance was classified as normal (ADL score, 100), mild impairment (ADL score, 61–99), moderate impairment (ADL score, 41–60), and severe impairment (ADL score, ≤40). The Nutrition Risk Screening (NRS) 2002 (5) was used to assess the risk of malnutrition. Malnutrition risk was defined as an NRS score of ≥3. COVID-19 cases were classified as mild, moderate, severe, or critical cases according to the clinical course. Adult mild cases were considered those with clinical symptoms but without abnormal pulmonary imaging findings; moderate cases were considered those with both clinical symptoms and abnormal pulmonary imaging findings. Severe cases were considered those with any of the following criteria: 1) respiratory distress (≥30 breaths/min), 2) resting oxygen saturation ≤93%, or 3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mm Hg (1 mm Hg = 0.133 kPa). Critical cases were defined as those with respiratory failure requiring mechanical ventilation or with other organ failure requiring intensive care unit admission. Patient survival was the primary clinical outcome assessed.
Statistical analysis
Continuous variables are expressed as medians and IQRs, and categorical variables are expressed as numbers and percentages (%). The baseline characteristics of the normal-nutrition group and risk-of-malnutrition group were compared using the Mann-Whitney test or chi-square test. Significant variables (P < 0.05) in the Mann-Whitney test or chi-square test were included in a multivariable logistic regression model to identify the independent factors of the risk of malnutrition. P values <0.05 were considered statistically significant. Data were analyzed using SPSS Statistics (version 23.0; IBM Corporation).
Results
Patient characteristics
From 29 January 2020 to 15 March 2020, a total of 803 patients with COVID-19 were consecutively admitted to our hospital; 760 of these patients met the inclusion criteria of this study (Figure 1). Among the 760 patients, the gender ratio was 1:1. The median age of patients was 60 y, and the median BMI (kg/m2) was 24.0. The median NRS score showed a risk of malnutrition. Fever was the most common symptom in patients with COVID-19. Other commonly reported symptoms were cough, fatigue, dyspnea, muscle soreness, headache, and diarrhea. The median serum albumin concentration was lower than that at the physiological level; 55.3% of patients had a serum albumin concentration <35 g/L. The median serum prealbumin concentration was within the physiological range; 25.5% of patients had a serum prealbumin concentration <150 mg/L. As the hospital was designated for patients with severe COVID-19, there were no mild COVID-19 cases in this study. Moderate COVID-19 cases represented 10% of all cases, 74.7% were severe cases, and 15.3% were critical cases. The median Barthel ADL score showed a mild impairment in ADL. The ADL score was 100 in 45.1% of patients, 61–99 in 37.6% of patients, 41–60 in 10.4% of patients, and ≤40 in 6.84% of patients. Of patients, 91.3% survived. Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of COVID-19 patients with normal nutrition and risk of malnutrition1
| All participants (n = 760) | Normal (n = 132) | Risk of malnutrition2 (n = 628) | P | |
|---|---|---|---|---|
| NRS score | 3.48 (2.22, 5.74) | 2 (2, 2) | 3 (3, 5) | <0.001 |
| Age, y | 60 (46, 74) | 49 (38,70) | 64 (56, 71) | <0.001 |
| <65, n (%) | 443 (58.3) | 120 (90.9) | 323 (51.4) | <0.001 |
| ≥65, n (%) | 317 (41.7) | 12 (9.1) | 305 | (48.6) |
| Gender, n (%) | ||||
| Male | 380 (50.0) | 71 (53.8) | 309 (49.2) | 0.338 |
| Female | 380 (50.0) | 61 (46.2) | 319 (50.8) | |
| Symptoms, n (%) | ||||
| Fever | 691 (90.9) | 101 (76.5) | 590 (93.9) | <0.001 |
| Cough | 539 (70.9) | 97 (73.5) | 442 (70.4) | 0.476 |
| Fatigue | 305 (40.1) | 49 (37.1) | 256 (40.8) | 0.438 |
| Dyspnea | 256 (33.7) | 48 (36.4) | 208 (33.1) | 0.474 |
| Muscle soreness | 243 (32.0) | 42 (31.8) | 201 (32.0) | 0.526 |
| Headache | 235 (31.0) | 37 (28.0) | 198 (31.5) | 0.429 |
| Diarrhea | 60 (7.89) | 11 (8.33) | 49 (7.80) | 0.837 |
| With comorbidities, n (%) | ||||
| Hypertension | 233 (30.7) | 47 (35.6) | 186 (29.6) | 0.175 |
| Diabetes mellitus | 145 (19.1) | 28 (21.2) | 117 (18.6) | 0.493 |
| Cerebrovascular disease | 134 (17.6) | 23 (17.4) | 111 (17.7) | 0.945 |
| Cardiovascular disease | 118 (15.5) | 20 (15.2) | 98 (15.6) | 0.896 |
| Chronic lung disease | 49 (6.45) | 10 (7.58) | 39 (6.21) | 0.561 |
| BMI, kg/m2 | 24.0 (20.6, 27.3) | 24.3 (22.1, 26.1) | 23.5 (21.7, 25.7) | 0.029 |
| <18.5, n (%) | 26 (3.42) | 2 (1.52) | 24 (3.82) | 0.057 |
| 18.5–24, n (%) | 396 (52.1) | 58 (43.9) | 338 (53.8) | |
| 24–28, n (%) | 267 (35.1) | 58 (43.9) | 209 (33.2) | |
| ≥28, n (%) | 71 (9.34) | 14 (10.6) | 57 (9.08) | |
| Albumin, g/L | 33.9 (27.8, 40.1) | 37.3 (35.1, 40.7) | 33.3 (29.1, 37) | <0.001 |
| <35, n (%) | 420 (55.3) | 30 (22.7) | 390 (62.1) | <0.001 |
| ≥35, n (%) | 340 (44.7) | 102 (77.3) | 238 (37.9) | |
| Prealbumin, mg/L | 223 (130, 316) | 270 (236, 328) | 215 (314, 281) | <0.001 |
| <150, n (%) | 194 (25.5) | 6 (4.55) | 188 (29.9) | <0.001 |
| ≥150, n (%) | 566 (74.5) | 126 (95.5) | 440 (70.1) | |
| Disease types, n (%) | ||||
| Moderate cases | 76 (10.0) | 42 (31.8) | 34 (5.41) | <0.001 |
| Severe cases | 568 (74.7) | 84 (63.6) | 484 (77.1) | |
| Critical cases | 116 (15.3) | 6 (4.55) | 110 (17.5) | |
| ADL score | 83.2 (60.2, 100) | 100 (95, 100) | 90 (70, 100) | <0.001 |
| 100, n (%) | 343 (45.1) | 98 (74.2) | 245 (39.0) | <0.001 |
| 61–99, n (%) | 286 (37.6) | 29 (22.0) | 257 (40.9) | |
| 41–60, n (%) | 79 (10.4) | 4 (3.03) | 75 (11.9) | |
| ≤40, n (%) | 52 (6.84) | 1 (0.76) | 51 (8.12) | |
| Clinical outcomes, n (%) | ||||
| Survived | 694 (91.3) | 132 (100) | 562 (89.5) | <0.001 |
| Died | 66 (8.68) | 0 (0.00) | 66 (10.5) | |
Values are medians (IQRs) unless otherwise indicated. ADL, activities of daily living; COVID-19, coronavirus disease 2019; NRS, Nutrition Risk Screening.
Risk of malnutrition was defined as NRS score ≥3.
Clinical variables associated with malnutrition risk in COVID-19 patients
Based on NRS scores, we classified study participants into 2 categories: normal nutrition (NRS score <3) and risk of malnutrition (NRS score ≥3). The percentage of patients at malnutrition risk was 82.6%. The median age in the risk-of-malnutrition group was significantly higher than that in the normal-nutrition group (P < 0.001). The median BMI in the risk-of-malnutrition group was significantly lower than that in the normal-nutrition group (P = 0.029). The median ADL score in the risk-of-malnutrition group was also lower than that in the normal-nutrition group (P < 0.001). However, there was no significant difference in gender composition between the 2 groups (P = 0.338). In addition, we found no significant differences in symptoms (cough, fatigue, dyspnea, muscle soreness, headache, and diarrhea) between the 2 groups; fever was the only symptom with a significantly different frequency between the groups. Furthermore, there were no significant differences in comorbidities (hypertension, diabetes mellitus, cerebrovascular disease, cardiovascular disease, and chronic lung disease) between the 2 groups (Table 1).
Patients in 2 subgroups of age <65 y and ≥65 y were noted with significant differences in malnutrition risk (P < 0.001). Malnutrition risk frequency were also found to be significantly different in the stratifications of albumin concentrations, prealbumin concentrations, and clinical outcomes (P < 0.001, respectively). The significant differences in malnutrition risk frequency were associated with disease severity (moderate cases, severe cases, and critical cases) and different functional performance in ADL (P < 0.001, respectively). However, the differences in malnutrition risk frequency in the different BMI groups were not significant (P = 0.057) (Table 1).
Independent factors associated with malnutrition risk in patients with COVID-19
Univariable analyses revealed that age, fever, BMI, albumin concentration, prealbumin concentration, disease severity, ADL score, and clinical outcomes were significantly associated with the risk of malnutrition ( Table 1). To identify independent factors associated with the risk of malnutrition in patients with COVID-19, we conducted multivariable logistic regression analyses. Age >65 y (P< 0.001), serum albumin concentrations <35 g/L (P < 0.001), serum prealbumin concentrations <150 mg/L (P = 0.042), critical disease type (P < 0.001), ADL score of 41–60 (P = 0.012), and ADL score ≤40 (P < 0.001) were significantly associated with the risk of malnutrition ( Table 2), whereas fever, BMI, and clinical outcomes did not show a significant link with the risk of malnutrition in patients with COVID-19.
Table 2.
Multivariable logistic regression models to identify the associated factors of risk of malnutrition in COVID-19 patients1
| B | SE | Wald | df | OR | 95% CI | P | |
|---|---|---|---|---|---|---|---|
| Age (y) | |||||||
| <65 | 1 | ||||||
| ≥65 | 1.69 | 0.329 | 26.2 | 1 | 5.40 | 2.83–10.3 | <0.001 |
| Albumin (g/L) | |||||||
| ≥35 | 1 | ||||||
| <35 | 1.28 | 0.246 | 27.1 | 1 | 3.61 | 2.23–5.84 | <0.001 |
| Prealbumin (mg/L) | |||||||
| ≥150 | 1 | ||||||
| <150 | 1.06 | 0.520 | 4.14 | 1 | 2.88 | 1.04–8.00 | 0.042 |
| Disease types | |||||||
| Moderate cases | 1 | ||||||
| Severe cases | 0.741 | 0.708 | 1.09 | 1 | 2.102 | 0.523–8.41 | 0.296 |
| Critical cases | 1.50 | 0.273 | 30.1 | 1 | 4.463 | 2.62–7.64 | <0.001 |
| ADL score | |||||||
| 100 | 1 | ||||||
| 61–99 | −20.6 | 8685 | 0.000 | 1 | 0.000 | 0.000 | 0.998 |
| 41–60 | 1.50 | 0.598 | 6.32 | 1 | 4.504 | 1.34–14.5 | 0.012 |
| ≤40 | 2.25 | 0.269 | 69.7 | 1 | 9.495 | 5.60–16.1 | <0.001 |
Adjusted for age, gender, BMI, albumin concentration, prealbumin concentration, disease type, ADL score, and clinical outcomes. ADL, activities of daily living; COVID-19, coronavirus disease 2019.
Severe cases vs. moderate cases.
Critical cases vs. moderate cases.
ADL score of 41–60 vs. ADL score of 100.
ADL score ≤40 vs. ADL score of 100.
The risk of malnutrition in older patients (≥65 y) was 5.40 times higher (95% CI: 2.83–10.3 times) than that in patients <65 y. Critically ill COVID-19 patients had a 4.46 times higher risk of malnutrition (95% CI: 2.62–7.64 times) than patients with moderate COVID-19. The risk of malnutrition in patients with an ADL score of 41–60 was 4.50 times higher (95% CI: 1.34–14.5 times) than that in patients with an ADL score of 100. Patients with an ADL score of ≤40 had a 9.49-times higher risk of malnutrition (95% CI: 5.60–16.1 times) than patients with an ADL score of 100 points. In addition, patients with albumin concentrations <35 g/L and prealbumin concentrations <150 mg/L had 3.61- and 2.88-times higher risk of malnutrition (95% CI: 2.23–5.84 and 1.04–8.00 times, respectively) than patients with albumin concentrations ≥35 g/L and prealbumin concentrations ≥150 mg/L (Table 2).
Discussion
In this study, we demonstrated that the risk of malnutrition was common in adult hospitalized patients with COVID-19. We also identified older age, low serum concentrations of albumin and prealbumin, critical cases, and poor ADL function as significant factors associated with the risk of malnutrition in adult patients with COVID-19. Therefore, nutritional support should be strengthened for patients with COVID-19, especially for elderly, critically ill patients and those with poor ADL function or low albumin and prealbumin concentrations. We believe that nutritional support may improve the clinical outcomes of patients with COVID-19.
Nutrition intake is vital for the survival and maintenance of immunological function. Poor nutrition impairs immune defense mechanisms and may increase susceptibility to infection (9). Furthermore, malnutrition could be a result of infection. Fever, the most common symptom of COVID-19, significantly increases energy consumption. In addition, reduced appetite in COVID-19 patients impacts nutrition intake. Diarrhea, another relatively common symptom of COVID-19, results in poor nutrient absorption. The psychological stress brought by the disease increases the risk of depression and anxiety, further impacting food intake (10). Thus, COVID-19 patients are inclined to be at risk of malnutrition.
Older age is a well-known risk factor for malnutrition, leading to significant morbidity and mortality in patients with chronic and acute diseases (5). Older individuals have worse outcomes and higher mortality upon SARS-CoV-2 infection (1., 2., 3.). In this study, we found that almost half of the patients in the risk-of-malnutrition group were aged over 65 y. Notably, 96.2% of older (≥65 y) COVID-19 patients had a risk of malnutrition. Multivariable logistic regression analyses revealed that age >65 y was an independent risk factor for malnutrition risk. The age-associated loss of skeletal muscle mass and function, oral and chewing problems, psychosocial issues, cognitive impairment, and low financial income may be factors contributing to the high prevalence of the risk of malnutrition in older patients with COVID-19.
Serum albumin and prealbumin are commonly used as nutritional status indicators. Their concentrations are affected by acute inflammation, malignancies, liver cirrhosis, and nephritis. In patients with inflammatory response, the synthesis of acute-phase proteins, such as C-reactive protein, ferritin, TNF-ɑ, and interleukins (11), requires the consumption of albumin. Decreased protein intake and increased protein consumption may lead to low serum albumin concentrations. A low albumin concentration has been recently identified as an independent prognostic factor in COVID-19 (12). In this study, we found that the median albumin concentrations in COVID-19 patients were lower than the physiological range and that low concentrations of albumin and prealbumin were independent factors associated with the risk of malnutrition. These findings suggest that maintaining a normal concentration of albumin and prealbumin may prevent malnutrition risk in patients with COVID-19.
We also found that the severity of the disease was associated with the nutritional status of COVID-19 patients. Critically ill COVID-19 patients often develop serious respiratory dysfunction and multiple organ failure, which may enhance energy consumption, increase protein breakdown, decrease muscle protein synthesis, and impair food intake. In this study, we found that critically ill COVID-19 patients were at a significantly higher malnutrition risk than those with moderate COVID-19. Consistently, early support of enteral nutrition in critically ill COVID-19 patients has been shown to significantly reduce mortality (13).
The Barthel index is a well-established indicator of daily activity performance, including the need for feeding assistance. Several studies have shown that nutritional status is associated with functional status and was able to predict functional decline (14, 15). In the present study, we found that patients in the risk-of-malnutrition group had a significantly worse ADL function than those in normal-nutrition group. We also found that moderate to severe impairment of daily activity performance was associated with the risk of malnutrition in COVID-19 patients.
Recently, the impact of obesity on the severity of COVID-19 has attracted considerable attention (16). To explore the relation between obesity and the risk of malnutrition, we classified patients as underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 24), overweight (24 ≤ BMI < 28), and obese (BMI ≥ 28). Notwithstanding the lower BMI in the risk-of-malnutrition group, there were no significant differences between the underweight, normal-weight, overweight, and obese groups. In addition, we found no association between comorbidities and the risk of malnutrition in COVID-19 patients.
There are some limitations to the present study. Importantly, it was a single-center study, and the hospital was designated for severe and critical COVID-19 cases, which may have led to selection bias. Moreover, we did not consider the association of dietary habits, mental condition, and social support with the nutritional status of patients. Furthermore, the effect of malnutrition as a risk factor for poor COVID-19 outcomes merits further investigation. Only 3.42% of our patients had a BMI < 18.5. The small number of undernourished subjects hindered the evaluation of malnutrition's significance in the clinical outcomes of COVID-19 patients. As the data are observational, all the observed relations are associations and causal statements cannot be made.
In conclusion, the risk of malnutrition was frequent in patients with COVID-19. Older age, critical cases, poor ADL function, and low albumin and prealbumin concentrations were independent factors associated with the risk of malnutrition in patients with COVID-19. Therefore, we recommend early nutritional support in COVID-19 patients to prevent further declines in the nutritional status and improve clinical outcomes.
Acknowledgments
We thank Dr. Yan Gan for the assistance in manuscript improvement and language editing. The authors’ responsibilities were as follows—PH: designed study; PH, AL, and JC: wrote the manuscript; PH and JC: performed the statistical analysis; QW, YM, YP, MZ, WZ, XC, WG, and JC: collected and analyzed the data; and all authors: read and approved the final manuscript.
Footnotes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang JL, Liu Y, Wei Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–500. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu KY, Shi HP. Explanation of expert recommendations on medical nutrition for patients with novel coronavirus pneumonia. Natl Med J China. 2020;100:724–728. doi: 10.3760/cma.j.cn112137-20200205-00196. [DOI] [PubMed] [Google Scholar]
- 5.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P, endorsed by the ESPEN Council ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O'Horo JC et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. Published online 2020 Apr 27. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed]
- 7.Li T, Zhang Y, Gong C, Wang J, Liu B, Shi L, Duan J. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020;74:871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Health Commission of China New coronavirus pneumonia prevention and control program. 6th ed. J Infect Contr China. 2020;2:192–195. [Google Scholar]
- 9.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system—working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen HC, Nguyen MH, Do BN, Tran CQ, Nguyen TTP, Pham KM, Pham LV, Tran KV, Duong TT, Tran TV, et al. People with suspected COVID-19 symptoms were more likely depressed and had lower health-related quality of life: the potential benefit of health literacy. J Clin Med. 2020;9:965. doi: 10.3390/jcm9040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016;46:239–248. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal E, Miller M, Yaxley A, Isenring E. Malnutrition in the elderly: a narrative review. Maturitas. 2013;76:296–302. doi: 10.1016/j.maturitas.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Li XY, Du B, Wang YS, Kang HYJ, Wang F, Sun B, Qiu HB, Tong ZH. The key points in treatment of the critical coronavirus disease 2019 patient. Chin J Tuberc Respir Dis. 2020;43:E026. doi: 10.3760/cma.j.cn112147-20200224-00159. [DOI] [PubMed] [Google Scholar]
- 14.Villafañe JH, Pirali C, Dughi S, Testa A, Manno S, Bishop MD, Negrini S. Association between malnutrition and Barthel index in a cohort of hospitalized older adults article information. J Phys Ther Sci. 2016;28:607–612. doi: 10.1589/jpts.28.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Reig M, Gómez-Arnedo L, Alfonso-Silguero SA, Juncos-Martínez G, Romero L, Abizanda P. Nutritional risk, nutritional status and incident disability in older adults: the FRADEA study. J Nutr Health Aging. 2014;18:270–276. doi: 10.1007/s12603-013-0388-x. [DOI] [PubMed] [Google Scholar]
- 16.Ryan DH, Ravussin E, Heymsfield S. COVID 19 and the patient with obesity—the editors speak out. Obesity (Silver Spring). 2020;28:847. doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]


