Sir,
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In general, diabetes, hypertension, cardiac disease, chronic lung disease, cerebrovascular disease, chronic kidney disease, immunosuppression and cancer are associated with a higher risk of mortality in COVID-19 patients.1 Williamson et al.2 reported that hepatic disease is one of the factors associated with COVID-19-related death. Remdesivir is one of the approved drugs effective against COVID-19 in Japan. Remdesivir particularly improves the survival outcomes of patients under oxygen support compared with those of patients receiving placebo.3 However, clinical trials on remdesivir treatment excluded patients with hepatic impairment.3–6 Currently, little is known about the experience of remdesivir administration in COVID-19 patients with severe hepatic cirrhosis. Herein, we present the case of a COVID-19 patient with severe hepatic Child–Pugh class C cirrhosis, who underwent remdesivir treatment.
A man in his 70s was admitted for fever, cough, fatigue, anorexia, accumulation of ascites and suspected peritonitis. He was an ex-smoker and long-term heavy drinker who was diagnosed with alcohol-related cirrhosis because of ascites as well as right-lobe atrophy and left-lobe hypertrophy on abdominal CT. He had undergone a pylorectomy 2 years before admission with a past medical history significant for type 2 diabetes, hypertension and angina pectoris. In total, 4700 mL of ascites was aspirated and was found to be non-infectious. On day 3 of his illness, he developed a prolonged fever and chills. Chest CT revealed ground-glass opacity in both lungs. He was treated with ceftriaxone and azithromycin with a presumptive diagnosis of pneumonia. Simultaneously, we tested for SARS-CoV-2 antigen and the test was positive. On day 4 of his illness, his vital signs were as follows: temperature, 38.4°C; respiratory rate, 24 breaths per min; and oxygen saturation, 86%. Oxygen therapy was started with a non-rebreather mask (3 L/min) and he was diagnosed with moderate COVID-19 according to WHO criteria.1
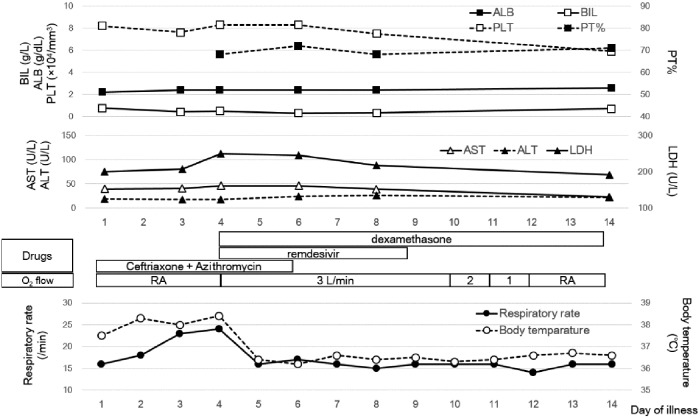
As we were concerned about the worsening of his respiratory condition, remdesivir (200 mg on the first day and 100 mg from the second day) and dexamethasone (6.6 mg/day) were initiated. However, his Child–Pugh score7 was class C (total score: 10) and each criterion was as follows: encephalopathy, none; ascites, moderate; bilirubin, 0.49 mg/mL; albumin, 2.4 mg/mL; and prothrombin time, 68%. Remdesivir was administered after obtaining informed consent from the patient and after explaining that there were insufficient data on its efficacy and safety in patients with chronic hepatic disease. Figure 1 shows the time course of the patient’s respiratory function, clinical features and hepatic laboratory test results. On day 1 of remdesivir and dexamethasone administration, his fever resolved and his respiratory rate normalized. Remdesivir was administered for 5 days (days 4 to 8) and dexamethasone for 10 days. On day 12, his oxygen saturation and respiratory rate normalized; oxygen mask use was therefore stopped. Almost no change was found in his liver function; no adverse events occurred from day 4, the first day of remdesivir administration, to day 17. He was discharged home on day 20 of illness. On day 39 after the end of remdesivir administration, his liver function did not worsen.
Figure 1.
Time course of respiratory function, clinical features and hepatic laboratory tests. BIL, bilirubin; ALB, albumin; PLT, platelet; PT, prothrombin time; LDH, lactate dehydrogenase; RA, room air.
Patients with cirrhosis are at increased risk of infection and associated complications due to cirrhosis-associated immune dysfunction, which is particularly important for patients with decompensated cirrhosis.8 Particularly, patients with Child–Pugh class C cirrhosis seem to experience a fatal course of COVID-19.8 There are few reports on remdesivir treatment for COVID-19 patients with cirrhosis and a recent review did not mention any details on this topic.9 Besides, this is the first report (to the best of our knowledge) of remdesivir administration in a COVID-19 patient with severe hepatic Child–Pugh class C cirrhosis. Remdesivir was hydrolysed and it yielded GS-441524 quickly, which was eliminated mainly through renal excretion. Therefore, the pharmacokinetics of remdesivir may not be very different between patients with hepatic cirrhosis and ordinary adults. This patient was able to complete a 5 day remdesivir treatment and experienced no adverse effects of note. However, a report of pharmacovigilance analysis suggested a higher risk of liver impairment with remdesivir treatment than with other drugs, regardless of the presence of COVID-19 with liver abnormalities.10 Additional studies are needed to determine whether or not remdesivir administration is effective and safe for COVID-19 patients with severe hepatic cirrhosis and whether dose adjustment is needed. Our report provides evidence of successful treatment with remdesivir of a COVID-19 patient with Child–Pugh class C cirrhosis. Continuous follow-up regarding the long-term prognosis of remdesivir treatment is needed.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.
References
- 1. WHO. Clinical Management of COVID-19: Interim Guidance . https://apps.who.int/iris/handle/10665/332196.
- 2. Williamson EJ, Walker AJ, Bhaskaran K et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beigel JH, Tomashek KM, Dodd LE et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med 2020; 383: 1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Zhang D, Du G et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Spinner CD, Gottlieb RL, Criner GJ et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA 2020; 324: 1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ISRCTN registry. WHO Public Health Emergency SOLIDARITY Trial of Treatments for SARS-COV-2 Infection in Hospitalized Patients. https://www.isrctn.com/ISRCTN83971151.
- 7. Albers I, Hartmann H, Bircher J et al. Superiority of the Child-Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol 1989; 24: 269–76. [DOI] [PubMed] [Google Scholar]
- 8. Qi X, Liu Y, Wang J et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2020; 70: 433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanafy AS, Abd-Elsalam S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: a hepatology perspective. World J Gastroenterol 2020; 26: 7272–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montastruc F, Thuriot S, Durrieu G. Hepatic disorders with the use of remdesivir for coronavirus 2019. Clin Gastroenterol Hepatol 2020; 18: 2835–6. [DOI] [PMC free article] [PubMed] [Google Scholar]