Abstract
Background
Several vaccines are now available under emergency use authorization in the United States and have demonstrated efficacy against symptomatic COVID-19. Vaccine impact on asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is largely unknown.
Methods
We conducted a retrospective cohort study of consecutive, asymptomatic adult patients (n = 39 156) within a large US healthcare system who underwent 48 333 preprocedural SARS-CoV-2 molecular screening tests between 17 December 2020 and 8 February 2021. The primary exposure of interest was vaccination with ≥1 dose of an mRNA COVID-19 vaccine. The primary outcome was relative risk (RR) of a positive SARS-CoV-2 molecular test among those asymptomatic persons who had received ≥1 dose of vaccine compared with persons who had not received vaccine during the same time period. RR was adjusted for age, sex, race/ethnicity, patient residence relative to the hospital (local vs nonlocal), healthcare system regions, and repeated screenings among patients using mixed-effects log-binomial regression.
Results
Positive molecular tests in asymptomatic individuals were reported in 42 (1.4%) of 3006 tests and 1436 (3.2%) of 45 327 tests performed on vaccinated and unvaccinated patients, respectively (RR, .44; 95% CI, .33–.60; P < .0001). Compared with unvaccinated patients, risk of asymptomatic SARS-CoV-2 infection was lower among those >10 days after the first dose (RR, .21; 95% CI, .12–.37; P < .0001) and >0 days after the second dose (RR, .20; 95% CI, .09–.44; P < .0001) in the adjusted analysis.
Conclusions
COVID-19 vaccination with an mRNA-based vaccine showed a significant association with reduced risk of asymptomatic SARS-CoV-2 infection as measured during preprocedural molecular screening. Results of this study demonstrate the impact of the vaccines on reduction in asymptomatic infections supplementing the randomized trial results on symptomatic patients.
Keywords: COVID-19, SARS-CoV-2, vaccination, asymptomatic
Among asymptomatic adults undergoing preprocedural SARS-CoV-2 molecular screening, risk of a positive test was lower among those >10 days after the first dose and >0 days after the second dose of an mRNA COVID-19 vaccine, compared with those who were unvaccinated.
Since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, more than 2 million lives have been lost and the global society has been disrupted in an unprecedented manner [1]. Despite significant efforts leveraging nonpharmacologic interventions such as use of face masks, physical distancing, community stay-at-home measures, quarantine, and isolation, spread has continued throughout much of the world. Ongoing infection and subsequent transmission from asymptomatic individuals is a significant contributing factor to the ongoing pandemic, with more than half of all transmission estimated to occur from individuals without symptoms [2]. Disrupting the rate of asymptomatic transmission is critical to bringing the pandemic to an end.
Through an unprecedented global effort at vaccine development, several vaccines have been licensed for use across the world. The data supporting the approval of these vaccines were based on reduction in symptomatic or severe COVID-19 disease. Published results from late-stage clinical trials show that the vaccine efficacy at preventing symptomatic COVID-19 disease ranged from 70.4% to 95% [3–5]. There is significant uncertainty, however, about the impact of COVID-19 vaccination on asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and transmission risk. The ability of vaccination to reduce asymptomatic or minimally symptomatic infection will be critical to ending the pandemic, given the relative contribution of asymptomatic infection to viral transmission. There are limited real-world data on the impact of vaccination on asymptomatic SARS-CoV-2 infection, which severely limits the development of postvaccination behavior recommendations and may contribute to vaccine hesitancy [6].
Within our healthcare system, patients are routinely evaluated for symptomatic and asymptomatic SARS-CoV-2 infection prior to surgery and medical procedures, which have potential to generate an aerosol. This approach has been in place since April 2020 in an effort to prevent patient harm from operative complications related to COVID-19 and decrease potential exposure and transmission to healthcare personnel [7]. Preoperative evaluation has included patient symptom questionnaires before and upon arrival at our medical facilities, combined with SARS-CoV-2 molecular testing performed just prior to selected medical and surgical procedures. In this study, we sought to evaluate the real-world impact of vaccination on preprocedural SARS-CoV-2 molecular test positivity among individuals without symptoms and to assess the impact of vaccination on asymptomatic/presymptomatic infection.
METHODS
Study Population
This was a retrospective cohort study that included all consecutive molecular screening tests performed in adult (≥18 years old) patients at Mayo Clinic campuses located in Rochester, Minnesota; Phoenix, Arizona; and the Mayo Clinic Health System (located in Minnesota and Wisconsin) who underwent preprocedural and presurgical SARS-CoV-2 molecular testing (henceforth referred to as preprocedural molecular screening) within 48–72 hours of their procedure. All patients undergoing testing between 17 December 2020 to 8 February 2021 were included; 17 December 2020 was chosen as this was the first date that vaccines were administered at these sites. In the Midwest region (Rochester, MN, campus and the Mayo Clinic Health System), criteria for preprocedural molecular screening were determined by a multidisciplinary COVID-19 Diagnostic Stewardship Committee, with ongoing review on a weekly basis [8]. The Arizona campus uses similar guidance to determine the need for preprocedural molecular screening. In general, all surgical procedures requiring general anesthesia and other select medical procedures (Supplementary Material) required preprocedural molecular screening. In addition to molecular screening, patients were asked whether they had fever or other COVID-19 symptoms that were new or not related to a pre-existing condition prior to their surgical procedure through a standardized phone or electronic questionnaire (Supplementary Material), as well as when arriving onsite for their procedure. Patients were not followed to assess for the development of subsequent symptoms. Patients tested due to symptoms or a known exposure were tested using an alternative ordering process and were excluded from this analysis. This study was deemed exempt by the Mayo Clinic Institutional Review Board.
Data Sources
For this study, all patient-level data from molecular screening tests (including test collection date/time and Mayo Clinic site), vaccinations (including vaccination manufacturer, date/time, dose, Mayo Clinic site), and patient demographic data (age, sex, race/ethnicity, state of residence) were captured in the electronic health record and compiled in an institutionally curated COVID-19 database housing distinct tables for molecular testing, serology testing, and inpatient COVID-19 data. This database represents the primary, centralized source of COVID-19 data at our institution and is easily accessible through Structured Querying Language (SQL) [9].
Exposure(s)
The primary exposure was vaccination with at least 1 dose of the BNT162b2 (Pfizer, Inc, New York, NY) or mRNA-1273 (Moderna, Inc, Cambridge, MA) SARS-CoV-2 vaccines prior to molecular screening. We assessed exposure as vaccinated (with any number of doses and at any time interval) prior to SARS-CoV-2 molecular screening versus unvaccinated prior to screening. We also conducted analyses categorizing patients by timing of vaccination (days from vaccination to screening) as well as by number of doses (0, 1, or 2) prior to screening. We further conducted a subgroup analysis for those receiving the Pfizer vaccine.
Outcome
The outcome was relative risk (RR) of a positive test at preprocedural molecular screening. The period prevalence, more commonly termed “percent positivity,” was also determined and aggregated by exposure categories. Molecular testing was performed through a combination of emergency use authorized methods depending on the Mayo Clinic location, including a SARS-CoV-2 laboratory-developed real-time polymerase chain reaction (PCR) [10], the APTIMA SARS-CoV-2 transcription-mediated amplification assay (Hologic, Marlborough, MA), and the Abbott RealTime SARS-CoV-2 real-time PCR method [11]. When available, we obtained the cycle threshold (Ct) or relative light unit (RLU) values for positive molecular test results. The real-time PCR Ct values are inversely proportional to the amount of viral RNA in the sample, while the RLU values are directly proportional to the concentration of target nucleic acid.
Statistical Analysis
Patient demographics including age, sex, race/ethnicity, county and state of residence, and whether the patient resided in the hospital’s Health Referral Region (HRR), a proxy for whether or not that patient was “local,” were gathered and compared between those who were vaccinated prior to molecular screening versus those who were unvaccinated at the time of screening using a chi-square test for sex and state of residence and a t test for age. We calculated the percent positivity of molecular screening comparing vaccinated and unvaccinated groups and compared these using log-binomial regression to estimate the unadjusted RR and 95% confidence interval (CI).
For patients vaccinated prior to molecular screening, we calculated “days-to-screening” by subtracting the date of molecular screening from the date of vaccination and plotted Kaplan-Meier survival analysis by number of doses received. Rarely, patients were vaccinated on the same day as their preprocedural molecular screening. For these patients (n = 151), we considered them to be “vaccinated” prior to screening and calculated the days-to-screening as zero. We categorized timing as follows: unvaccinated prior to screening, screening 0–10 days after the first dose, screening more than 10 days after the first dose, and screening more than 0 days after the second dose. Finally, we analyzed data based on the number of doses prior to screening. Patients were categorized as having received 0 doses (unvaccinated), 1 dose, or 2 doses. The percent positivity and exact 95% CIs for these 4 vaccination groups were calculated and analyzed as described above. We also conducted subgroup analysis by assessing the timing categories described above within patients who received only the BNT162b2 (Pfizer) vaccine. There were not a sufficient number of patients receiving the mRNA-1273 (Moderna) vaccine to perform a subgroup analysis for that group. All analyses were repeated with adjustment in mixed-effects models with random intercepts for each Mayo Clinic site (Rochester, Mayo Clinic Health System, and Arizona), a random residual to correct for intrapatient repeated measures, and fixed effects for age, sex, race/ethnicity, and patient residence relative to the hospital (local vs nonlocal).
RESULTS
There were 48 333 molecular screening tests performed among 39 156 unique patients during the study period. Mean (SD) age was 54.2 (19.7) years and 25 364 (52.5%) were female. There were 3006 (6.2%) screening tests performed on individuals who were vaccinated prior to their molecular screening (Table 1). Those who were vaccinated were significantly younger and more likely to be female compared with those without prior vaccination, reflecting the early focus on vaccinating healthcare workers. We observed differences in the race, state of residence, and residence within the local HRR. Among the vaccinated group, median (interquartile range) time from first dose of vaccine to their molecular screening was 16 days (7–27 days), with 707 (23.5%) screening tests in the vaccinated group having occurred among individuals who had received their second dose.
Table 1.
Study Population Characteristics by Vaccination Status Prior to Preprocedural Molecular COVID-19 Screening
| Screenings With at Least 1 Prior Vaccination (n = 3006, 6.2%) | Screenings With No Prior Vaccination (n = 45 327, 93.8%) | P a | |
|---|---|---|---|
| Mayo Clinic site, n (%) | <.001 | ||
| Arizona campus | 1467 (48.8) | 15 662 (34.6) | |
| Rochester campus | 1005 (33.4) | 15 450 (31.4) | |
| Mayo Clinic Health System | 534 (17.8) | 14 215 (31.4) | |
| Age, mean (SD), years | 46.9 (14.9) | 55.2(18.4) | <.001 |
| Sex, n (%) | <.001 | ||
| Male | 1057 (35.2) | 21 912 (48.3) | |
| Female | 1949 (64.8) | 23 415 (51.7) | |
| Race, n (%) | <.001 | ||
| White, non-Hispanic | 2401 (79.9) | 39 145 (86.4) | |
| African or African-American | 52 (1.7) | 1014 (2.2) | |
| Asian or Asian-American | 184 (6.1) | 1041 (2.3) | |
| Hispanic of any race | 180 (6.0) | 2238 (4.9) | |
| Other/unknown | 189 (6.3) | 1889 (4.2) | |
| Patient resides in local HRR, n (%) | 2537 (84.4) | 28 480 (62.8) | <.001 |
| State of residence, n (%) | <.001 | ||
| Minnesota | 1227 (40.8) | 18 170 (40.3) | |
| Wisconsin | 299 (10.0) | 5988 (13.3) | |
| Iowa | 20 (0.7) | 1867 (4.1) | |
| Arizona | 1405 (46.7) | 13 824 (30.6) | |
| Other | 55 (1.8) | 5478 (12.1) | |
| Timing (days to screening), n (%) | … | ||
| 0–10 days after first dose | 937 (31.2) | … | |
| >10 days after first dose, before second dose | 1362 (45.3) | … | |
| >0 days after second dose | 707 (23.5) | … | |
| Timing, median (IQR), days to screening | … | ||
| Days after first dose | 16 (7, 27) | … | |
| Days after second dose | 9 (5, 15) | … | |
| Number of doses, n (%) | |||
| 1 | 2299 (76.5) | … | |
| 2 | 707 (23.5) | … | |
| Manufacturer, n (%) | … | ||
| Pfizer | 2826 (94.0) | … | |
| Moderna | 178 (5.9) | … | |
| Missing/unknown/external | 2 (<0.1) | … |
Abbreviations: COVID-19, coronavirus disease 2019; HRR, Health Referral Region; IQR, interquartile range.
a t Test for continuous variables, chi-square for categorical variables, and Bonferroni-corrected for multiplicity.
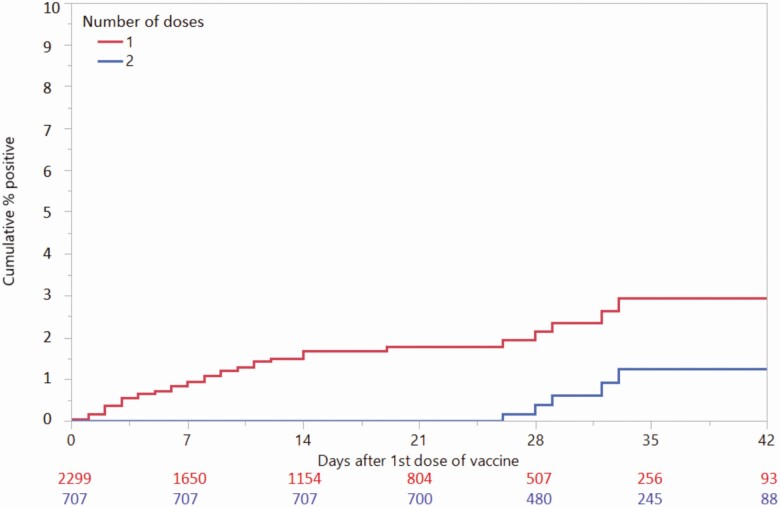
Among 45 327 screening tests performed on unvaccinated individuals without COVID-19 symptoms, 1436 (3.2%; 95% CI, 3.0–3.3%) were positive. Among 3006 screening tests performed on patients without COVID-19 symptoms who had received at least 1 dose of vaccine prior to molecular screening, 42 (1.4%; 95% CI, 1.0–1.8%) were positive. The cumulative percentages who were positive 6 weeks after the first dose of vaccine in those receiving 1 versus 2 doses were 2.9% and 1.3%, respectively (Figure 1).
Figure 1.
Survival analysis by time-to-preprocedural positive COVID-19 test after receiving first dose of vaccination, by total doses received. A person who received their first dose of vaccine on 1 January 2021, their second dose on 24 January 2021, and then had a positive molecular COVID-19 test on 26 January 2021 would appear on both the red (1-dose) and blue (2-dose) lines with the event on day 25 on the x-axis (event occurred 25 days after first dose). Abbreviation: COVID-19, coronavirus disease 2019.
In our primary analysis, the unadjusted RR for a positive test during asymptomatic preprocedural screening comparing vaccinated versus unvaccinated was .44 (95% CI, .33–.60; P < .0001) (Table 2). The RR for a positive test comparing screening more than 10 days after the first dose with unvaccinated was .28 (95% CI, .16–.49; P < .0001), and the RR for a positive test comparing screening more than 0 days after the second dose with unvaccinated was .27 (95% CI, .12–.60; P = .001). In the number of doses analysis, the RR for a positive test comparing 1 dose with unvaccinated was .49 (95% CI, .36–.69; P < .0001) and the RR for a positive test comparing 2 doses with unvaccinated was .27 (95% CI, .12–.60; P = .001).
Table 2.
Outcomes
| Unadjusted Molecular Test, Percent Positivity (95% Confidence Interval) | Unadjusted Relative Risk (95% Confidence Interval) | P | |
|---|---|---|---|
| Analysis 1 | |||
| Unvaccinated (reference) | 3.1 (3.0–3.3) | Reference | |
| At least 1 vaccination prior to screening | 1.4 (1.0–1.8) | .44 (.33–.60) | <.0001 |
| Analysis 2 | |||
| Unvaccinated (reference) | 3.1 (3.0–3.3) | Reference | |
| Screening 0–10 days after first dose | 2.6 (1.6–3.6) | .81 (.54–1.20) | .29 |
| Screening >10 days after first dose, before second dose | 0.9 (.4–1.4) | .28 (.16–0.49) | <.0001 |
| Screening >0 days after second dose | 0.9 (.3–1.8) | .27 (.12–0.60) | <.0001 |
| Analysis 3 | |||
| Unvaccinated (reference) | 3.1 (3.0–3.3) | Reference | |
| 1 dose prior to screening | 1.6 (1.1–2.1) | .49 (.36–.69) | <.0001 |
| 2 doses prior to screening | 0.9 (.2–1.5) | .27 (.12–.60) | .001 |
| Analysis 4—Pfizer only | |||
| Unvaccinated (reference) | 3.1 (3.0–3.3) | Reference | |
| Screening 0–10 days after first dose | 2.7 (1.6–3.8) | .86 (.58–1.30) | .48 |
| Screening >10 days after first dose, before second dose | 0.9 (0.4–1.4) | .27 (.15–.49) | <.0001 |
| Screening >0 days after second dose | 0.9 (0.2–1.5) | .27 (.12–.60) | .001 |
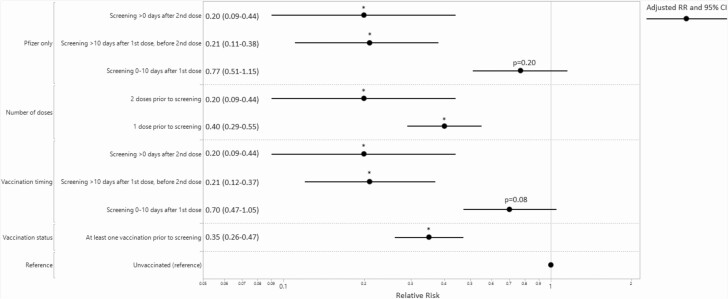
After adjustment for confounding variables and random effects, the adjusted RR (aRR) for a positive test during asymptomatic preprocedural screening comparing vaccinated versus unvaccinated was .35 (95% CI, .26–.47; P < .0001) (Figure 2). The aRR for a positive test comparing screening more than 10 days after the first dose with unvaccinated was .21 (95% CI, .12–.37; P < .0001), and the aRR for a positive test comparing screening more than 0 days after the second dose with unvaccinated was .20 (95% CI, .09–.44; P < .0001). Further analysis, including in the Pfizer-only subgroup analysis, remained significant after adjustment (Figure 2).
Figure 2.
Adjusted RR (with 95% CIs) comparing preprocedural COVID-19 molecular screening percent positive by vaccination status and timing. *Significant at P < .001. RR adjusted for age, sex, race/ethnicity, and patient residence in the hospital Health Referral Region (local vs nonlocal). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; RR, relative risk.
The molecular tests’ Ct or RLU values were available for 38 (90.5%) of 42 and 1116 (77.7%) of 1436 positive screening tests in the vaccinated and unvaccinated groups, respectively (Supplementary Material, Supplementary Tables 1 and 2). Multiple different testing methods were used in these patients, limiting the comparison between vaccinated and unvaccinated groups to only those tested using the same method. Interestingly, the Ct values of positive results from vaccinated individuals at our Arizona location were significantly lower (P < .01) than for unvaccinated individuals, but there were no other significant differences. Among positive tests in Arizona, there was a nonsignificant difference in the Ct value when analyzed by timing after vaccination (Supplementary Material, Supplementary Table 2).
DISCUSSION
In this real-world study, we observed that vaccination using an mRNA COVID-19 vaccine is associated with a reduced rate of asymptomatic SARS-CoV-2 infection among individuals tested during preprocedural molecular screening. We observed a significant decrease in asymptomatic infection, consistent in timing and magnitude with what has been observed in clinical trials evaluating the prevention of symptomatic infection after vaccination with mRNA vaccine [3, 4]. Among individuals who had received a single dose of vaccine more than 10 days prior to their preprocedural test, we observed a 72% reduction in the risk of a positive molecular screening test. When analysis was limited to those individuals who received 2 doses of vaccine, we observed a 73% reduction in the risk of a positive molecular screening test compared with those who were not vaccinated. After adjustment for multiple potential confounding factors, we observed an 80% reduction in the risk of a positive molecular screening test among tests performed in persons who had received 2 doses of vaccine, compared with those who were not vaccinated.
There are mixed data from published clinical trials regarding the efficacy of vaccination against asymptomatic infection. During the clinical trial to approve the mRNA-1273 vaccine (Moderna), SARS-CoV-2 PCR was performed in asymptomatic individuals 28 days from the first dose, just prior to the second dose [3]. That study observed a 62% reduction in the risk of asymptomatic infection in the vaccine group (14 of 14 550, 0.10%) compared with the placebo group (39 of 14 598, 0.27%). In our study, the majority (94%) of patients received the BNT162b2 mRNA vaccine (Pfizer) with a smaller number receiving the mRNA-1273 vaccine (5.9%). In the subgroup analysis of those more than 10 days after their first dose of the BNT162b2 mRNA vaccine, we observed a 79% reduction in the risk of a positive test, after adjustment for potential confounding factors. Our observation of a similar reduction in risk among individuals more than 10 days after the first vaccine dose who predominantly received the BNT162b2 vaccine suggests that both mRNA vaccines lead to early reduction in asymptomatic infection soon after the initial dose. Among those individuals receiving both doses of the BNT162b2 vaccine, we observed an 80% reduction in the risk of molecular test positivity, as compared with unvaccinated individuals, after adjustment for potential confounding factors. Compared with the clinical trial efficacy results reported for the BNT162b2 and mRNA-1273 vaccines [3, 5], this reduction in efficacy is not unexpected, given that 36% of post–second-dose tests were performed fewer than 7 days after their second dose of vaccine.
The impact of vaccine on asymptomatic SARS-CoV-2 infection is likely to be dependent on the efficacy of the specific vaccine. As an example, a single standard dose of the ChAdOx1 nCoV-19 vaccine (AstraZeneca) did not provide consistent protection against asymptomatic infection [12]. In an analysis performed from 22 to 90 days after the first vaccine dose, there was no protection against asymptomatic infection. However, among individuals who received 2 doses of vaccine 12 or more weeks apart, there was a 47% reduction in asymptomatic infection when measured 14 or more days after the second dose. Reduction in asymptomatic infection is also likely impacted by the timing of vaccine doses. Analyses similar to the one conducted in our study will be needed to better understand the real-world impact for other vaccines as they receive authorization in the United States.
There are several limitations to this study. First, there may have been unmeasured confounding factors that contributed to the lower rate of molecular test positivity within the group who received vaccination. Accordingly, one can only infer correlation between vaccination and reduced molecular test positivity, rather than causation. For most of the observation period, vaccine availability was limited to healthcare personnel and residents of long-term care facilities, due to the increased risk for COVID-19 exposure in these populations. However, one would not be surprised to find higher rates of test positivity in preprocedural screening of these groups; the fact that lower positivity rates were seen in the vaccinated cohort supports a significant mitigating effect of vaccination. We attempted to adjust for confounding factors through an adjusted analysis and observed that the strength of association between vaccine receipt and a decline in test positivity only strengthened. Nevertheless, it is possible that unmeasured confounders remain and contributed to our observations. This study was performed in a largely White, non-Hispanic population who were under the age of 65. A second limitation is that patients undergoing preprocedural molecular screening may have been symptomatic or in the presymptomatic phase of COVID-19 infection. We relied upon our existing clinical mechanisms to exclude tests ordered on symptomatic patients from this analysis. Some individuals may have not responded to the pre-visit phone call or electronic questionnaire or the personnel asking the questions may not have asked all of the questions. Patients may have not been truthful in their responses. We did not longitudinally follow these patients to assess for the development of subsequent symptoms. Therefore, our results may reflect a combination of asymptomatic and mildly symptomatic cases. We attempted to address this limitation by only including patients who underwent testing ordered via an order panel performed for preprocedural molecular testing, as well as through the existing preprocedural symptom questionnaire process to identify patients who were symptomatic. Patients who were identified as symptomatic on the questionnaire would have been tested through a separate process and excluded from this analysis. Finally, the likely enrichment of the vaccinated cohort with healthcare personnel and long-term-care residents could have impacted the rate with which vaccinated subjects reported mild symptoms at the time of preprocedural testing, in which case they would be tested under the symptomatic testing process and not analyzed in this study. The likelihood of false-positive molecular testing was not addressed in this study. While affecting both groups, a floor effect may have resulted in an underestimate in the reduction in asymptomatic test–positive persons in the vaccinated group as the unadjusted test positivity postvaccination falls within the range of the published molecular test false positivity rates.
In summary, previous receipt of an mRNA COVID-19 vaccine was associated with an 80% reduction of risk in asymptomatic COVID-19 in patients who have received 2 vaccines when compared with those who had not been vaccinated. These results are consistent with previously published data showing a reduction in asymptomatic infection following vaccination with an mRNA vaccine, even after 1 dose [3]. From a public health perspective, it will be important to determine if the current recommendations to maintain prevaccination behaviors for masking and social distancing will impact vaccine hesitancy. These data, together with further studies, will inform on the risk–benefit balance of current postvaccination guidelines.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by internal funding at the Mayo Clinic.
Potential conflicts of interest. M. B. reports personal fees from DiaSorin Molecular as an advisory board member, outside the submitted work. In the past 36 months, N. D. S. has received research support through Mayo Clinic from the Food and Drug Administration to establish the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (grant number U01FD005938); the Centers for Medicare and Medicaid Innovation under the Transforming Clinical Practice Initiative (TCPI); the Agency for Healthcare Research and Quality (grant numbers R01HS025164, R01HS025402, R03HS025517, K12HS026379); the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (grant numbers R56HL130496, R01HL131535, R01HL151662); the National Science Foundation; and the Patient Centered Outcomes Research Institute (PCORI) to develop a Clinical Data Research Network (LHSNet). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Coronavirus disease (COVID-19) dashboard. Geneva, Switzerland; World Health Organization. Available at: https://covid19.who.int/. Accessed 12 February 2021. [Google Scholar]
- 2. Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 2021; 4:e2035057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group . Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voysey M, Clemens SAC, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amit S, Alexsandra Beni S, Bibe A, et al. Post-vaccination COVID-19 among healthcare workers, Israel. Emerg Infect Dis 2021; 27. doi: 10.3201/eid2704.210016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Storino CB, Watson JC, Sanchez W, et al. Revamping outpatient care for patients without COVID-19. Mayo Clin Proc 2020; 95:S44–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah AS, Tande AJ, Challener DW, et al. Diagnostic stewardship: an essential element in a rapidly evolving COVID-19 pandemic. Mayo Clin Proc 2020; 95:S17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollock BD, Carter RE, Dowdy SC, et al. Deployment of an interdisciplinary predictive analytics task force to inform hospital operational decision-making during the COVID-19 pandemic. Mayo Clinic Proc 2020. doi: 10.1016/j.mayocp.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodino KG, Espy MJ, Buckwalter SP, et al. Evaluation of saline, phosphate-buffered saline, and minimum essential medium as potential alternatives to viral transport media for SARS-CoV-2 testing. J Clin Microbiol 2020; 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Challener DW, Shah A, O’Horo JC, Berbari E, Binnicker MJ, Tande AJ. Low utility of repeat real-time PCR testing for SARS-CoV-2 in clinical specimens. Mayo Clin Proc 2020; 95:1942–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voysey M, Costa Clemens SA, Madhi SA, et al. Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine. SSRN [Preprint]. February 1, 2021 [cited 2021 Feb 12]. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3777268. Accessed 12 February 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.