Abstract
Aims
In order to determine acute cardiac involvement in patients with COVID-19, we quantitatively evaluated tissue characteristics and mechanics by non-invasive cardiac magnetic resonance (CMR) in a cohort of patients within the first 10 days of the onset of COVID symptoms.
Methods and results
Twenty-five patients with reverse transcription polymerase chain reaction confirmed COVID-19 and at least one marker of cardiac involvement [cardiac symptoms, abnormal electrocardiograph (ECG), or abnormal cardiac biomarkers] and 25 healthy age- and gender-matched control subjects were recruited to the study. Patients were divided into those with elevated (n = 8) or normal TnI (n = 17). There were significant differences in global longitudinal strain among patients who were positive and negative for hs-TnI, and controls [−12.3 (−13.3, −11.5)%, −13.1 (−14.2, −9.8)%, and −15.7 (−18.3, −12.7)%, P = 0.004]. Native myocardial T1 relaxation times in patients with positive and negative hs-TnI manifestation (1169.8 ± 12.9 and 1113.2 ± 31.2 ms) were significantly higher than the normal (1065 ± 57 ms) subjects, respectively (P < 0.001). The extracellular volume (ECV) of patients who were positive and negative for hs-TnI was higher than that of the normal controls [32 (31, 33)%, 29 (27, 30)%, and 26 (24, 27.5)%, P < 0.001]. In our study, quantitative T2 mapping in patients who were positive and negative for hs-TnI [51 (47.9, 52.8) and 48 (47, 49.4) ms] was significantly higher than the normal [42 (41, 45.2) ms] subjects (P < 0.001).
Conclusion
In patients with early-stage COVID-19, myocardial oedema, and functional abnormalities are a frequent finding, while irreversible regional injury such as necrosis may be infrequent.
Keywords: magnetic resonance imaging, coronavirus, inflammation, diagnosis
Introduction
COVID-19 is a global pandemic that is continuing to affect many patients and thus requires the medical community to have a thorough understanding of its associated morbidity and mortality, especially its underlying pathophysiology. Previous studies have shown that patients infected with COVID-19 during the acute phase could manifest with myocardial injury, which can be associated with a higher risk of in-hospital mortality.1,2 Troponin is used in clinical practice as the key marker to detect myocardial injury and elevated troponin may trigger further investigation. Endomyocardial biopsy, the reference standard for the diagnosis of myocarditis based on histopathology, immunohistology, and molecular techniques to identify viral genomes, is not typically conducted in myocarditis patients because of possible sampling errors and the inherent procedural risk.3 Because myocardial injury can be reversible in COVID-19 patients following prompt treatment, a reliable tool for early diagnosis of myocardial injury is warranted.4 Cardiovascular magnetic resonance (CMR) can non-invasively identify myocardial oedema qualitatively and quantitatively, with or without necrosis according to the ‘2018 updated Lake Louise Criteria’, and provide specific markers for acute myocardial inflammation, such as localization and severity.5 Novel approaches for tissue characterization such as T1 mapping, T2 mapping, extracellular volume (ECV) mapping, and the combination of imaging criteria with multiple biomarkers further increases the accuracy of CMR.6 These methods can detect diffuse myocardial oedema and have shown the potential to improve diagnostic sensitivity and specificity in myocarditis.7 In addition, strain analysis based on cine MR images has proven effective in the evaluation of both regional and global myocardial dysfunction in patients with acute myocarditis.8,9 Puntmann et al.10 found that cardiac involvement could be revealed by CMR in 78 patients (78%) who recovered from COVID-19, and 60 patients (60%) manifested with ongoing myocardial inflammation. In contrast to this previous study, we sought to investigate patients during the acute presentation with COVID infection. Furthermore, the clinical value of novel mapping techniques and strain quantification for assessing myocardial inflammation has not been fully explored. The present study recruited patients with confirmed COVID-19 and suspected myocardial injury, and analysed the comprehensive parameters of CMR in order to evaluate the feasibility of the quantitative assessment of myocardial injury in the early stages of the disease by multiparametric CMR.
Methods
Fifty subjects were referred to CMR between 15 March 2020, and 15 April 2020, at Shanghai Public Health Clinical Center of Fudan University and Renji Hospital of Shanghai Jiao Tong University. Twenty-five patients with laboratory-confirmed COVID-19 and suspected myocardial involvement based on cardiac symptoms, abnormal Electrocardiograph (ECG), or abnormal cardiac biomarkers were recruited, and 25 age-matched and gender-matched healthy subjects without any known underlying cardiac diseases were recruited in our study as the control group. Our study complied with the Declaration of Helsinki and was approved by the institutional research ethics committee at the Shanghai Public Health Clinical Center and Renji Hospital. COVID-19 infection was confirmed in the Shanghai Public Health Clinical Center according to the interim guidance of the World Health Organization.11 All these patients were confirmed with a positive COVID-19 nucleic acid antibody in the Centre for Disease Control. In order to prevent the spread of the virus, COVID-19 patients were transported in MRI-compatible beds, were assigned a dedicated room, and also underwent CMR examination within the same dedicated room (Supplementary data online, Figure S1). All subjects gave written informed consent. All patients in our study with COVID-19 manifested with either cardiac symptoms (palpitation), abnormal ECG, or any variety of abnormal cardiac biomarkers [including increased creatinine kinase, creatinine kinase-myocardial band (CK-MB), high-sensitivity troponin I (hs-TnI), lactate dehydrogenase (LDH), and amino-terminal pro-brain natriuretic peptide (pro-BNP)].
Cases of pneumonia due to other causes such as common bacterial and viral pathogens were excluded. Exclusion criteria for all subjects were any contraindications to CMR (such as severe claustrophobia, defibrillators, and implanted pacemakers or other metallic implanted devices). Previously ischaemic and non-ischaemic cardiomyopathy patients were not included in this study in order to isolate acute myocardial injury.
Cardiac magnetic resonance
All CMR scans were performed on two 3.0 T MRI scanners of the same model (uMR780, United Imaging Healthcare, Shanghai, China) within 10 days after a confirmed diagnosis. CMR-exams were performed before publication of the recent Society for Cardiovascular Magnetic Resonance (SCMR) recommendations for CMR-protocols in COVID,12 but used a very similar protocol. Steady-state free precession cine imaging, T2 mapping, pre- and post-contrast T1 mapping,13 T2-weighted Short-tau Triple Inversion Recovery (T2-STIR), first-pass perfusion, and late gadolinium enhancement (LGE) were acquired. Details of the sequences are described in Supplementary data online, Table S1. The T1 and T2 values are the average of all myocardium from all three slices.
Image analysis
Two radiologists with 10 years (L-.M.W.) and 7 years (B.-H.C.) of clinical experience analysed the CMR images together and were blind to whether the subject was diagnosed with COVID-19 or healthy control. Patients were evaluated based on the updated lake Louise criteria.5 Left ventricular function and cardiac mechanics were analysed offline with dedicated commercial software Cvi42 version 5.11.3 (Circle Cardiovascular Imaging Inc., Calgary, Canada). The analysis of cardiac mechanics primarily included parameters from the tissue tracking software package, including peak global longitudinal strain (pGLS, %). T1 and T2 relaxation times were extracted from the T1 and T2 mapping by Cvi42. ECV was calculated using following equation 1 from native T1 and post-contrast T1 mapping images with Cvi42.
Native T1 refers to T1 mapping before the administration of contrast. Epicardial and endocardial borders were delineated to exclude pericardial effusion, epicardial fat, papillary muscles, and blood pooling from the analysis. Global T1, T2, and ECV were measured, respectively.
Statistical analysis
Statistical analysis was performed using MedCalc Version 11.4.2.0 (MedCalc Software, Ostend, Belgium) and SPSS Statistics Version 26.0 (IBM SPSS Inc., Chicago, IL, USA). The normality of variable distribution was tested by the Kolmogorov–Smirnov method and qualitative inspection of Q–Q plots. Continuous data distributed normally were presented as mean±SD when compared with independent t-test between two groups and one-way ANOVA test among three groups. Non-normally distributed data were presented as median (inter-quartile range) and compared with the Kruskal–Wallis test. Categorical and binary variables were expressed as n (%). The χ2 and Fisher’s Exact tests were used to compare the differences between two groups. A two-tailed P value of <0.05 was considered statistically significant.
Results
Population characteristics
A total of 50 subjects were recruited for our study. As shown in Figure 1, 25 of 120 patients were included due to cardiac involvement. Hs-TnI in 8 (32%) of 25 patients, CK in 3 (12%), LDH in 4 (16%), and Pro-BNP in 1 (4%) were all higher than the normal reference ranges, and the overall distribution was 0.03 (0.02–0.06) ng/mL, 72 (63.5–120) U/L, 186 (176–209.5) mmol/L, and 25.11 (16.2–71.7) mmol/L, respectively. In total, 56% (19/25) of patients had an abnormal ECG indicated by ST-segment elevation, and 36% (9/25) of patients had sinus arrhythmia. Less common ECG manifestations were T-wave depression and inversion (6/25), widened p-wave/short PR interval (5/25), tendency towards right deviation (5/25), complete right bundle branch block (2/25), and abnormal Q waves (1/25). Three (12%) patients exhibited palpitation.
Figure 1.
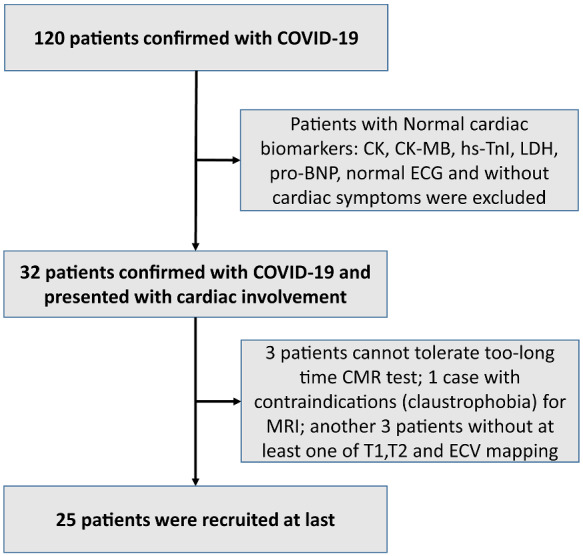
Flowchart of patient recruitment. Twenty-five patients with COVID-19 who presented with suspected cardiac involvement were recruited.
The baseline characteristics in patients with COVID-19 and the reference ranges of quantitative parameters are given in Table 1. Eight (32%) patients presented with positive hs-TnI. The average ages of the positive and negative hs-TnI patients were 20.50 (19–27) and 27 (18–42.5), respectively. Fifteen (60%) of them were male. Most patients presented with symptoms of fever (14, 56%) and cough (13, 52%); the peak temperature was 37.4 ± 0.9°C. The time duration between the onset of symptoms to the CMR scan was between 3 and 8 days.
Table 1.
Baseline characteristics
| Characteristics | Reference range | Total COVID-19 (n = 25) | hs-TnI (+, n = 8) | hs-TnI (−, n = 17) | P |
|---|---|---|---|---|---|
| Age (years) | – | 23 (18–33.5) | 20.5 (19–27) | 27 (18–42.5) | 0.56 |
| Male | – | 15 (60%) | 5 (62.5%) | 10 (58.8%) | 0.60 |
| Fever | – | 14 (56%) | 3 (37.5%) | 11 (67.7%) | 0.39 |
| PT (°C) | – | 37.4 ± 0.9 | 37.2 ± 0.9 | 37.5 ± 1 | 0.59 |
| Cough | – | 13 (52%) | 4 (50%) | 9 (52.9%) | 0.61 |
| Palpitation | 3 (12%) | 2 (25%) | 1 (5.9%) | 0.23 | |
| CMRTime days | 6.7 ± 5.7 | 5.5 ± 1.7 | 6.1 ± 3.1 | 0.64 | |
| Inpatient days | – | 19 (15–24) | 22.5 (16–25.8) | 17 (14.5–23) | 0.24 |
| Smoking history | – | 2 (8%) | 1 (12.5%) | 1 (5.9%) | 0.55 |
| History of alcohol | – | 2 (8%) | 1 (12.5%) | 1 (5.9%) | 0.55 |
| Comorbidity | – | 4 (16%) | 1 (12.5%) | 3 (17.6%) | 0.62 |
| CK (U/L) | 29–168 | 72 (63.5–120) | 67.5 (63–115.3) | 79 (65.5–123.5) | 0.48 |
| CK-MB (ng/mL) | 0–24 | 12 ± 2.3 | 12.2 ± 2 | 11.9 ± 2.5 | 0.72 |
| LDH (mmol/L) | 120–250 | 186 (176–209.5) | 181 (173.3–202.5) | 187 (177–239.5) | 0.24 |
| Pro-BNP (pg/mL) | 0–250 | 25.1 (16.2–71.7) | 27.1 (17.6–86.3) | 23 (14.6–62.8) | 0.55 |
| WBC (/L) | 3.5–9.5 | 4.8 (3.9–6.7) | 4.9 (4.6–5.9) | 4.8 (3.6–7.4) | 0.81 |
| Neutrophil (/L) | 1.8–6.3 | 3 (2.1–3.1) | 2.7 (2.2–3.6) | 3.1 (1.8–4.7) | 0.66 |
| Leukocytes (/L) | 1.1–3.2 | 1.3 (1.1–1.7) | 1.7 (0.9–1.7) | 1.3 (1.1–1.7) | 0.51 |
| CT pneumonia | – | 17 (68%) | 4 (50%) | 13 (76.5%) | 0.36 |
| Abnormal ECG | – | 19 (56%) | 7 (87.5%) | 12 (70.6%) | 0.62 |
| PE | – | 7 (28%) | 5 (62.5%) | 2 (11.8%) | 0.020 |
| Clinical severity | |||||
| Mild | 18 (72%) | 3 (37.5%) | 15 (88.2%) | 0.020 | |
| Moderate | 7 (28%) | 5 (62.5%) | 2 (11.8%) |
Numbers are given as median (interquartile ranges) or mean ± standard deviation or as absolute frequency with percentages in parentheses. hs-TnI (−): 0–0.04 (pg/mL).
CMRTime, Time duration between onset of symptoms to CMR scan; CK, creatine kinase; CK-MB, Creatinine kinase-myocardial band; ECG, Electrocardiograph; hs-TnI, high-sensitivity troponin I; LDH, Lactate dehydrogenase; Pro-BNP, pro-B-type natriuretic peptide; PT, Peak temperature; Total, total patients with COVID-19; WBC, white blood cell.. P values represent the comparison between hs-TnI (+) and hs-TnI (−).
One patient had a decreased white blood cell (WBC) count than the normal range, while two were higher. The WBC (22/25, 88%) and neutrophil counts (20/25, 80%) were normal in most patients. The 8 of 25 patients had decreased leukocytes compared with the normal range. One patient had a comorbidity of thalassaemia, another had complications from hypertension (10 years), another diabetes mellitus (5 years), and lastly, one patient had hyperlipidaemia. Two patients previously infected with chronic hepatitis B and human immunodeficiency virus (HIV), respectively, were in the negative CMR group. Seventeen (68%) patients showed typical COVID-19 pneumonia on CT. In these seventeen patients, a total of sixty pulmonary lesions were observed on CT imaging, including pure ground-glass-opacity (GGO): 18/60 (30%), GGO with reticular and/or interlobular septal thickening: 16/60 (26.7%), GGO with consolidation: 14/60 (23.3%), or consolidation: 12/60 (20%). Furthermore, 18 (72%) patients had mild type COVID-19 and 7 (28%) moderate type COVID-19.
Left ventricular mechanics analysis
In the cardiac functional evaluation, we found that most of the parameters in patients with COVID-19 were lower than subjects in the healthy control group. As shown in Table 2, LVEF of patients positive or negative for hs-TnI were significantly lower than those of controls, respectively (52.4 ± 2.8%, 55.1 ± 6.1%, and 64.6 ± 4.6%, P < 0.01). In addition, five (20%) patients manifested with regional wall motion abnormalities in the regions with cardiac involvement.
Table 2.
The characteristics of CMR
| Normal (n = 25) | hs-TnI (+, n = 8) | hs-TnI (−, n = 17) | P | |
|---|---|---|---|---|
| LVEDV (mL) | 107.6 (98.7–128.3) | 116.5 (99.7–127.1) | 113.8 (100.5–129.8) | 0.96 |
| LVESV (mL) | 43.2 ± 15.5 | 58.7 ± 17a | 51.2 ± 9.6 | 0.020 |
| SV (mL) | 67.5 (65.2–79.8) | 58.8 (53.5–67.6)a | 61.3 (55.4–72.3)b | 0.030 |
| HR (/min) | 65 (63–71) | 73 (63.5–81) | 72 (69.5–73.5)b | 0.030 |
| CO (L/min) | 4.8 (4.4–5.5) | 4.66 (4.3–6) | 4.3 (4–5.3) | 0.26 |
| LVEF (%) | 64.6 ± 4.6 | 52.4 ± 2.8a | 55.1 ± 6.1b | <0.001 |
| MyoMass (g) | 87.1 (74.6–114.9) | 91.6 (79.9–103.7) | 80.2 (74.3–104.2) | 0.77 |
| iLVEDV (mL/m2) | 69.8 (64.1–70.8) | 66.9 (60.9–74.8) | 66.2 (61–72.4) | 0.72 |
| iLVESV (mL/m2) | 25.4 ± 6.3 | 30.3 ± 5.2a | 29.9 ± 5.2b | 0.020 |
| iSV (mL/m2) | 42.9 (40.3–51.4) | 35.9 (33.3–46.1)a | 36.8 (34.4–38.3)b | <0.001 |
| CI (L/min/m2) | 2.9 (2.7–3.2) | 2.7 (2.4–3.5) | 2.6 (2.4–2.7)b | 0.002 |
| pGLS (%) | −15.7 (−18.3– −12.7) | −12.3 (−13.3– −11.5)a | −13.1 (−14.2–−9.8)b | 0.004 |
| Native T1 | 1065 ± 57 | 1169.8 ± 12.9a | 1113.2 ± 31.2b,c | <0.001 |
| ECV | 26 (24–27.5) | 32 (31–33)a | 29 (27–30)b,c | <0.001 |
| T2 mapping | 42 (41–45.2) | 51 (47.9–52.8)a | 48 (47–49.4)b | <0.001 |
Numbers are given as median (interquartile ranges) or mean ± standard deviation or as absolute frequency with percentages in parentheses.
CI, cardiac index; CO, cardiac output; HR, heart rate; i, index; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricle ejection fraction; LVESV, left ventricular end-systolic volume; MyoMass, Myocardial Mass; Normal, control group; pGLS, peak global longitudinal strain; SV, stroke volume.
P value <0.05 for comparing COVID-19 patients with positive hs-TnI and controls.
P value <0.05 for comparing COVID-19 patients with negative hs-TnI and controls.
P value <0.05 for comparing COVID-19 patients with positive and negative hs-TnI.
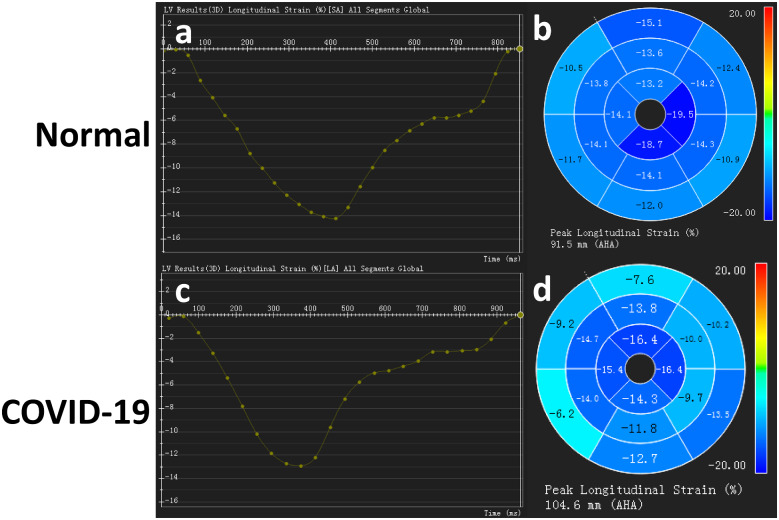
There were significant differences in cardiac mechanics represented by strain analysis (GLS, global longitudinal strain) among patients with positive and negative hs-TnI compared with controls [−12.3 (−13.3, −11.5)%, −13.1 (−14.2, −9.8)%, and −15.7 (−18.3, −12.7)%, P = 0.004]. GLS significantly decreased in patients with confirmed COVID-19 compared with the healthy control subjects. Tissue tracking presented significant changes in global cardiac mechanics in patients with COVID-19. Figure 2 compares GLS between a COVID-19 patient with a positive hs-TnI and a healthy participant. In total, 7 (28%) patients displayed a small amount of pericardial effusion: 5 (62.5%) in the hs-TnI positive group and 2 (11.8%) in the hs-TnI negative group.
Figure 2.
GLS between a patient confirmed with COVID-19 with positive hs-TnI and a normal participant. The upper row Figures (A and B) of strain analysis represent a normal participant with normal left ventricular function and cardiac mechanics. The lower rows (C and D) show a patient confirmed with COVID-19 who had a positive hs-TnI and displayed decreased GLS.
Myocardial tissue characteristics
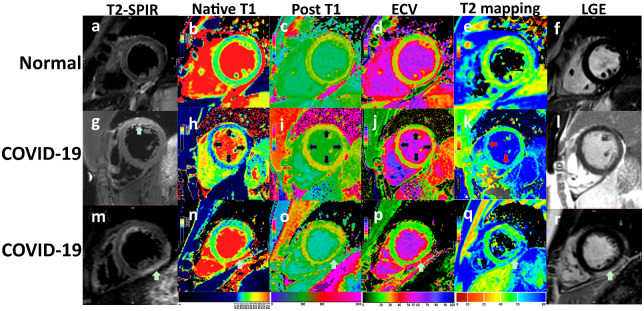
Among the COVID-19 patients, 14/25 (56%) displayed hyperintensity in T2-STIR, and 1/25 (4%) patients displayed positive LGE. T2-STIR hyperintensity was mainly located in the transmural myocardium (10/14), followed by the sub-epicardium (2/14) and mid-wall (2/14). A decreased perfusion was not found in any patients in our study after subjectively analysing the first-pass perfusion imaging. One patient displayed hyperintensity both on T2-STIR and LGE in the sub-epicardium of the left ventricular inferior and inferior-lateral wall of the basal and middle part (Figure 3). Differences between myocardial tissue characteristics are shown in Table 2. Native myocardial T1 relaxation times in patients with positive and negative hs-TnI manifestations (1169.8 ± 12.9 and 1113.2 ± 31.2 ms) were significantly higher than the normal (1065 ± 57 ms) subjects, respectively (P < 0.001). The ECV of patients with positive and negative hs-TnI was higher than that of the normal controls [32 (31–33)%, 29 (27–30)% vs. 26 (24–27.5)%, P < 0.001]. In our study, quantitative T2 mapping in patients with both a positive and negative hs-TnI [51 (47.9–52.8) and 48 (47–49.4) ms] was significantly higher than the normal [42 (41–45.2) ms] subjects (P < 0.001).
Figure 3.
Three cases of CMR measurements included T2-STIR, Native T1 mapping, Post-contrast T1 (post T1), ECV, T2 mapping, and PSIR. The upper row (A–F) represents a healthy participant. The figures in the second row are of a patient (G–L) confirmed with COVID-19 and had a slightly increased TnI (0.08 ng/mL). The anterior wall displayed transmural hyperintensity. T1, T2, and ECV also displayed diffuse hyperintensity. The global T1, T2, and ECV were higher than the normal group, although the differences of PSIR were not significant. The third patient (M–R) with COVID-19 (ECG: T-wave depression, short PR interval, TnI: 0.14 ng/mL, LDH: 302 mmol/L) displayed typical T2-STIR and LGE hyperintensity in the sub-epicardium of the left ventricular inferior and inferior-lateral wall of the basal and middle parts.
Discussion
This study investigated patients with recent COVID-19 infection and found evidence of cardiac involvement in patients with both normal and elevated cardiac biomarkers. CMR demonstrated abnormal myocardial mechanics and tissue characteristics with elevated markers of myocardial inflammation/oedema with parametric mapping, while myocardial infiltration/necrosis on LGE CMR was rare.
COVID-19 was caused by a novel enveloped RNA beta-coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).14 Angiotensin-converting enzyme 2 (ACE2) is a human cell receptor that binds strongly to the S (spike) protein of COVID-19, and the myocardium expresses high amounts of ACE2.15 ACE2 is the critical receptor for severe COVID-19 infections. Studies have found that COVID-19 may infect human capillary vessels directly. Vascular endothelial growth factor A (VEGF-A)-mediated increases in lung vessel permeability may be antagonized by ACE2 during acute lung injury.16 The binding of the SARS-CoV spike protein to ACE2 causes ACE2 down-regulation, which in turn leads to the increase of angiotensin II, increased vascular permeability, and ultimately, oedema formation.17 In pericytes of adult human hearts, highly expressed ACE2 indicates an intrinsic susceptibility of the human heart to COVID-19. The injury of pericytes may lead to capillary endothelial cell dysfunction, which induces microvascular dysfunction and leakage.18 The human recombinant soluble ACE2 may inhibit the growth of COVID-19 at very early stages.19 Myocardial injury (as expressed by elevated TnI, ECG abnormalities and arrhythmias, cardiac arrest, viral myocarditis and heart failure with increased NT-proBNP and decreased systolic function on CMR) after SARS-CoV-2 infection is reflective of severe COVID-19.1,2,17 The inflammatory cytokine storm contributes to the cause of multiple organ failure, which is a notable cause of death in COVID-19 patients.20 In consideration of the ACE2-related pathogenesis of COVID-19, SARS-CoV-2 comes in direct contact with myocytes and can result in infectious myocarditis as well as apoptosis-induced cardiac injury.
Several case reports and smaller studies of recovered COVID-19 patients have evaluated cardiac involvement by CMR.2,10,21–29 One study recruited 26 patients with CMR performed 35–60 days after the onset of cardiac symptoms following COVID-19 infection.21 In that study, 58% of patients had abnormal CMR findings, including regional oedema and LGE. The largest study to date by Puntmann et al.10 recruited 100 patients recovered from COVID-19 illness, and reported abnormal CMR findings in 78 patients (78%), with 73% showing raised native myocardial T1 and 60% raised myocardial T2. hsTnI was raised in 5% of their patients at the time of CMR and in 15% during the acute illness. In comparison, in our study, 88% (22/25) patients had abnormal native T1 values and 96% (24/25) patients had abnormal T2 values, indicating global myocardial inflammation. hsTnI was elevated in 8 of our 25 patients (32%). Notably, in our study patients were scanned during the acute phase of their illness (3–8 days after diagnosis), while Puntmann’s study scanned patients after recovery (64–92 days after diagnosis). Together, these two studies suggest that CMR markers of myocardial inflammation may be raised early and may persist beyond the acute phase of infection. Puntmann’s study did not include ECV evaluation or myocardial mechanics analysis, but in three patients with severe CMR abnormalities endomyocardial biopsy was undertaken, which revealed active lymphocytic inflammation. The evidence from current studies gives indications of cardiac involvement in COVID-19 infection but is insufficient to elucidate any specific cardiac mechanics and tissue characteristics of myocardial injury in the early stages of the disease.
In our study, 14 patients had positive T2-STIR, and 1 patient had LGE positive findings. Most of the T2-STIR hyperintensity was mainly distributed in the transmural myocardium, while only one patient displayed hyperintensity on T2-STIR consistent with LGE in the sub-epicardium of the inferior left ventricular and inferior-lateral wall in the basal and middle parts. Currently, another study had reported that one patient displayed diffuse transmural oedema.27 Diffuse T-lymphocyte inflammatory infiltrates and interstitial oedema was found. Only limited foci of myocardial necrosis without fibrosis were observed at a 7-day history. Although no SARS-CoV-2 genes were identified, it was characterized as acute virus-negative lymphocytic myocarditis associated with COVID-19.24 We hypothesize the reasons for this phenomenon are as follows: in the absence of LGE in most cases in the early stages of the disease, oedema may reflect reversible myocardial injury. CMR, combined with other cardiac biomarkers, may be more sensitive to detecting myocardial injury in patients with COVID-19.
Absolute ECV values, T1, and T2 relaxation times, when used as the quantitative CMR approaches, could be beneficial on account of being independent of the MRI signal intensities for a reference tissue. Studies have found that ECV values, T1, and T2 relaxation times can improve the diagnostic performance of CMR significantly compared with the former conventional Lake-Louise criteria.30–32
Recently it has been found that the stage of clinically defined myocarditis could be better characterized by native T1-based algorithms compared with the traditional T2WI and LGE images.32 Native T1 relaxation times can also be affected by fibrosis tissue, which could interfere with this technique as the approach to evaluate acute myocardial inflammation.33 Along with current research, our study implies that the increased native myocardial T1 relaxation times represent both acute myocardial inflammation and chronic injury in patients with myocarditis. ECV can be used as a non-specific marker for an increased extracellular space, while T2 is specifically sensitive to water-bound protons. Troponin is used in clinical practice as the key marker to detect myocardial injury and elevated troponin may trigger further investigation. In our study, there were significant differences among patients with a positive hs-TnI, negative TnI, and controls. The significant differences between patients with cardiac damage and control subjects imply noteworthy changes in myocardial tissue characterization.
Left ventricular function and mechanics analysis
A previous study reported a COVID-19 patient with increased myocardial enzymes (high-sensitivity troponin T and CK-MB) manifesting with diffuse hypokinesis of LV with an estimated LVEF of 40%.2 In our study, we found that LV function was reduced when compared with the normal subjects, especially in those that were positive for cardiac changes, as found on CMR. The higher LV mass may suggest an increased interstitial space due to inflammation.
Patients with COVID-19 who also displayed myocardial injury in the earlier phases of disease can undergo CMR examination in a dedicated room. According to our study, the alterations found on CMR in this acute stage reflect myocardial inflammation and myocardial injury. The decreased global longitudinal strain may indicate early dysfunction after cardiac involvement. Minor changes in cardiac function in patients with acute myocarditis can be distinguished from healthy subjects through quantitative GLS in echocardiography.34 We hypothesize the decrease in global longitudinal strain after myocardial injury may lead to significant changes in myocardial mechanics in the early stages.
Limitations
Our study has some limitations: First, the number of COVID-19 infections was limited. And, the evaluation of myocardial biomarkers in some patients with suspected myocardial injury is lower than that seen in typical ischaemic cardiomyopathy. Second, our study was performed by applying clinical justification for patients suspected of acute myocardial involvement without using endomyocardial biopsy. Besides, troponin status was not a pre-specified analysis and that this may have compounded Type I errors and the likelihood of a false positive result. Finally, all patients in this study were in the early stages of infection and lacked long-term follow-up. They will be re-evaluated by CMR at 3-month follow-ups.
Conclusion
In patients with early-stage COVID-19, myocardial oedema and functional abnormalities are a frequent finding, while irreversible regional injury such as necrosis may be infrequent.
Supplementary data
Supplementary data are available at European Journal - Cardiovascular Imaging online.
Data availability
Part of our clinical data collected for this study will be made available to others after being publicated. After the approval of a proposal from readers and accepting a signed data access agreement, you can send a E-mail to us. And the use of data requires the consent of all authors (contact: Bing-Hua Chen, E-mail: chenbinghua0311@163.com).
Funding
This work was financially supported by the National Natural Science Foundation of China (NO. 81873886) and the National Natural Science Foundation of China (NO. 81873887), Shanghai Municipal Commission of Health and Family Planning Excellent Young Talent Program (NO. 2017YQ031), Shanghai Shenkang Hospital Development Center Clinical Research and Cultivation Project (NO. SHDC12018X21); Shanghai Science and Technology Innovation Action Plan, Technology Standard Project (NO. 19DZ2203800); Shanghai Jiao Tong University School of Medicine Double Hundred Outstanding Person Project (NO. 20191904); and Shanghai Jiao Tong University Medical Cross-Project (NO. YG2017QN44).
Conflict of Interest: We declare that we have no financial relationships with United Imaging Healthcare of China. And we declare no competing interests with all authors.
Supplementary Material
References
- 1. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc 1989;64:1235–45. [DOI] [PubMed] [Google Scholar]
- 4. Villa-Forte A, Mandell BF. Cardiovascular disorders and rheumatic disease. Rev Esp Cardiol 2011;64:809–17. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 2018;72:3158–76. [DOI] [PubMed] [Google Scholar]
- 6. Lurz P, Luecke C, Eitel I, Fohrenbach F, Frank C, Grothoff M et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-trial. J Am Coll Cardiol 2016;67:1800–11. [DOI] [PubMed] [Google Scholar]
- 7. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53:1475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doerner J, Bunck AC, Michels G, Maintz D, Baeßler B. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol 2018;104:120–8. [DOI] [PubMed] [Google Scholar]
- 9. Luetkens JA, Schlesinger-Irsch U, Kuetting DL, Dabir D, Homsi R, Doerner J et al. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 2017;27:4661–71. [DOI] [PubMed] [Google Scholar]
- 10. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance. World Health Organization. https://apps.who.int/iris/handle/10665/331446 (13 March 2020, date last accessed).
- 12. Kelle S, Bucciarelli-Ducci C, Judd RM, Kwong RY, Simonetti O, Plein S et al. Society for cardiovascular magnetic resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J Cardiovasc Magn Reson 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M et al. Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging 2017;18:744–51. [DOI] [PubMed] [Google Scholar]
- 14. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367:1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu X, Lin Q, Qin X, Ruan Z, Zhou J, Lin Z et al. ACE2 antagonizes VEGFa to reduce vascular permeability during acute lung injury. Cell Physiol Biochem 2016;38:1055–62. [DOI] [PubMed] [Google Scholar]
- 17. Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation 2020;141:1648–55. [DOI] [PubMed] [Google Scholar]
- 18. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 2020;116:1097–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Monteil V, Kwon H, Prado P, Hagelkruys A, Wimmer RA, Stahl M et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020;181:905–13.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sardari A Tabarsi P Borhany H Mohiaddin R Houshmand G. Myocarditis detected after COVID-19 recovery. European Heart Journal- Cardiovascular Imaging 2021;22:131–2. 10.1093/ehjci/jeaa166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C et al. Cardiac involvement in recovered COVID-19 patients identified by magnetic resonance imaging. JACC: Cardiovasc Imaging 2020;13:2330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paul J-F Charles P Richaud C Caussin C Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging 2020;21:776. 10.1093/ehjci/jeaa107. PMC: 32338706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 2020;41:1861–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luetkens JA, Isaak A, Zimmer S, Nattermann J, Sprinkart AM, Boesecke C et al. Diffuse myocardial inflammation in COVID-19 associated myocarditis detected by multiparametric cardiac magnetic resonance imaging. Circ Cardiovasc Imaging 2020;13:e010897. [DOI] [PubMed] [Google Scholar]
- 26. Trogen B Gonzalez F J Shust G F. COVID-19-Associated Myocarditis in an Adolescent. Pediatric Infectious Disease Journal 2020;39:e204–5. 10.1097/INF.0000000000002788 [DOI] [PubMed] [Google Scholar]
- 27. Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J 2020;41:1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C et al. Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology 2020;297:E283–E288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coyle J Igbinomwanhia E Sanchez-Nadales A Danciu S Chu C Shah N. A Recovered Case of COVID-19 Myocarditis and ARDS Treated With Corticosteroids, Tocilizumab, and Experimental AT-001. JACC: Case Reports 2020;2:1331–6. 10.1016/j.jaccas.2020.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 2014;7:667–75. [DOI] [PubMed] [Google Scholar]
- 31. Thavendiranathan P, Walls M, Giri S, Verhaert D, Rajagopalan S, Moore S et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging 2012;5:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hinojar R, Foote L, Arroyo Ucar E, Jackson T, Jabbour A, Yu CY et al. Native T1 in discrimination of acute and convalescent stages in patients with clinical diagnosis of myocarditis: a proposed diagnostic algorithm using CMR. JACC Cardiovasc Imaging 2015;8:37–46. [DOI] [PubMed] [Google Scholar]
- 33. Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart 2013;99:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luetkens JA, Homsi R, Sprinkart AM, Doerner J, Dabir D, Kuetting DL et al. Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 2016;17:154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Part of our clinical data collected for this study will be made available to others after being publicated. After the approval of a proposal from readers and accepting a signed data access agreement, you can send a E-mail to us. And the use of data requires the consent of all authors (contact: Bing-Hua Chen, E-mail: chenbinghua0311@163.com).