Abstract
Background
Coronavirus disease 2019 (COVID-19) patients on haemodialysis (HD) have high mortality. We investigated the value of reverse transcription polymerase chain reaction (RT-PCR) and the dynamic changes of antibodies (enzyme-linked immunosorbent assay immunoglobulin M (IgM) + IgA and/or IgG) in a large HD cohort.
Methods
We conducted a prospective observational study in 10 Madrid HD centres. Infection rate, anti-SARS-CoV-2 antibody dynamics and the incidence of asymptomatic SARS-CoV-2 infection (defined by positive RT-PCR, IgM + IgA and/or IgG) were assessed.
Results
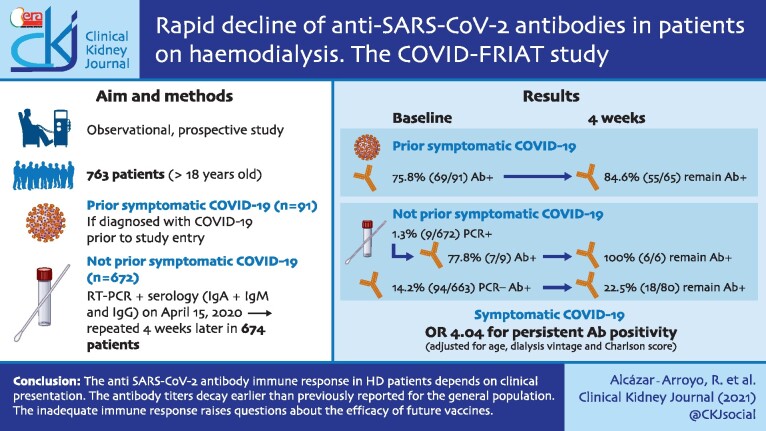
From 1 March to 15 April 2020, 136 of 808 (16.8%) HD patients were diagnosed with symptomatic COVID-19 by RT-PCR of nasopharyngeal swabs and 42/136 (31%) died. In the second fortnight of April, RT-PCR and anti-SARS-CoV-2 antibodies were assessed in 763 of the surviving patients. At this point, 69/91 (75.8%) symptomatic COVID-19 patients had anti-SARS-CoV-2 antibodies. Four weeks later, 15.4% (10/65) of initially antibody-positive patients had become negative. Among patients without prior symptomatic COVID-19, 9/672 (1.3%) were RT-PCR positive and 101/672 patients (15.0%) were antibody positive. Four weeks later, 62/86 (72.1%) of initially antibody-positive patients had become negative. Considering only IgG titres, serology remained positive after 4 weeks in 90% (54/60) of patients with symptomatic COVID-19 and in 52.5% (21/40) of asymptomatic patients. The probability of an adequate serologic response (defined as the development of anti-SARS-CoV-2 antibodies that persisted at 4 weeks) was higher in patients who had symptomatic COVID-19 than in asymptomatic SARS-CoV-2 infection {odds ratio [OR) 4.04 [95% confidence interval (CI) 2.04–7.99]} corrected for age, Charlson comorbidity index score and time on HD. Living in a nursing home [OR 5.9 (95% CI 2.3–15.1)] was the main risk factor for SARS-CoV-2 infection.
Conclusions
The anti-SARS-CoV-2 antibody immune response in HD patients depends on clinical presentation. The antibody titres decay earlier than previously reported for the general population. This inadequate immune response raises questions about the efficacy of future vaccines.
Keywords: antibodies, COVID-19, haemodialysis, SARS-CoV-2
Graphical Abstract
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic is a threat to global health and especially to vulnerable groups such as patients undergoing renal replacement therapy in haemodialysis (HD) centres. Patients on HD have a high COVID-19 mortality rate (24.9% in Spain) [1]. In addition, they are at greater risk for COVID-19 since they attend an HD facility two to six times a week, in most cases in shared healthcare transportation, spend >4 h in these units and may live in nursing homes. In 40% of HD patients, COVID-19 infection is asymptomatic, increasing the risk of nosocomial infection in HD units [2, 3]. Therefore strict protocol measures for the early detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in HD patients have been established. They include triage at the centre entrance and isolation and reverse transcription polymerase chain reaction (RT-PCR) testing in any patient with suspected infection [4]. The role of serology is unknown and the information available on the prevalence and dynamics of the antibody response to SARS-CoV-2 infection in HD patients is scarce [3, 5–7].
On 28 February 2020, the first case of COVID-19 was reported in Madrid, the Spanish region (6 663 394 inhabitants) most affected by COVID-19, with 348 954 cases diagnosed by RT-PCR and 12 225 deaths as of 23 November 2020. The first pandemic peak was on 30 March 2020, with a peak incidence (14-day cumulative COVID-19 cases) of 422 cases/100 000 inhabitants [8]. According to data from the Madrid Society of Nephrology as of 18 June 2020, 509 (14.7%) of the 3473 prevalent HD patients in Madrid had been infected by SARS-CoV-2 [9].
Without a specific vaccine or preventive treatments in a pandemic that involves a high percentage of asymptomatic patients, it is necessary to establish strategies that allow early detection of infected cases in dialysis units in order to organize healthcare while minimizing the risk of nosocomial infection. For this purpose, periodic serum antibodies and/or RT-PCR tests have been proposed for asymptomatic patients [6]. However, the usefulness of these strategies and the dynamics of anti-SARS-CoV-2 antibodies in HD patients remain unknown. Thus there are few data on the frequency of anti-SARS-CoV-2 antibody development and duration in HD patients. This information is critical to establish detection, isolation and follow-up plans.
The objectives of this study were to assess SARS-CoV-2 infection by RT-PCR and serology, the prevalence of asymptomatic SARS-CoV-2 infection and the dynamics of the antibody response to SARS-CoV-2 in a large representative sample of HD patients from Madrid hospitals and dialysis centres.
MATERIALS AND METHODS
Study population
This is an observational, open, prospective, multicentre study in all HD patients from 10 HD facilities, 8 managed by the Fundación Renal Íñigo Álvarez de Toledo (FRIAT) (4 hospitals and 4 outpatient dialysis facilities) and 2 hospitals in the Community of Madrid public health system. During the 6-week period after the onset of the pandemic, a specific triage strategy was used in every dialysis session to identify symptoms of COVID-19 or exposure to SARS-CoV-2 infected patients. A total of 136 patients were diagnosed as symptomatic COVID-19.
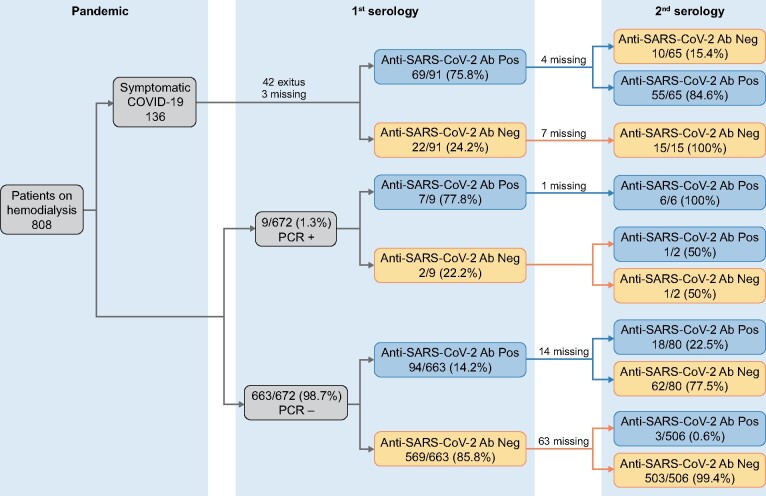
All HD patients >18 years of age on 15 April 2020 were invited to participate in the study that included a baseline SARS-CoV-2 RT-PCR and anti-SARS-CoV-2 serum antibody test and a second antibody test 4 weeks later. The only exclusion criterion was rejection to participate in the study. Patients were classified as having prior symptomatic COVID-19 diagnosed before 15 April or not. Those without a previous COVID-19 diagnosis and with a basal positive RT-PCR or serology [immunoglobulin G (IgG) and/or IgA + IgM] were classified as asymptomatic SARS-CoV-2 infection; others were defined as no evidence of previous or actual SARS-CoV-2 infection (Figure 1). For patients with prior COVID-19, only anti-SARS-CoV-2 antibodies were measured.
FIGURE 1:
Flow chart. Missing data are due to a change of dialysis facility (n = 38) or inadequate blood sample for analysis (n = 61).
At the time of the first serology, the accumulated incidence of COVID-19 infection in 14 days in the Community of Madrid system was 251 cases per 100 000 inhabitants, whereas at the time of the second assessment, 4 weeks later, the accumulated incidence had dropped to 28 cases per 100 000 inhabitants as a result of the strict confinement adopted by the national authorities. The peak accumulated incidence in the first COVID-19 pandemic wave in the Community of Madrid system was 422 cases per 100 000 inhabitants on 6 April 2020 [10].
The outcome of interest was an adequate serological response, defined as the development of anti-SARS-CoV-2 antibodies that persisted at 4 weeks. Data were obtained by two investigators from each centre.
The study was approved by the Research Ethics Committee of Puerta de Hierro Hospital in Madrid (CPMP/ICH/135/95) and was conducted in accordance with the Declaration of Helsinki and the European Union Clinical Trials Directive 2001/20/EC (EU CTD).
Laboratory measurements
Nasopharyngeal swabs were assessed for SARS-CoV-2 RNA by quantitative RT-PCR real-time amplification using specific oligonucleotides and a fluorescence-labelled probe that hybridized with a conserved target region of the ORF1ab and N genes (VIASURE SARS-CoV-2 RT-PCR Kit, CerTest Biotec, San Mateo de Gállego, Spain and TaqPath COVID-19 CE-IVD RT-PCR Kit, Thermo Fisher Scientific, Waltham, MA, USA).
Serum was tested for anti-SARS-CoV-2 IgA + IgM and for anti-SARS-CoV-2 IgG antibodies using an indirect enzyme-linked immunosorbent assay (ELISA; Vircell, Granada, Spain). The assay uses specific SARS-CoV-2 antigens from the Spike (S) glycoprotein and the nucleus capsid (N). Samples were diluted 1:20 in sample buffer and incubated at 37°C for 60 min in a 96‐well microtitre plate followed by protocol washing and incubation cycles, including controls and required reagents. Optical density (OD) was measured at 450 nm using a VirClia microplate reader (Vircell). The ELISA results are expressed as OD measurements using a microplate reader with a 450-nm filter and a 620-nm reference filter and interpreted according to the manufacturer’s protocol. The OD index results are the OD of the clinical sample:OD of the calibrator ratio, without units. The use of indexes allowed us compensate for interassay variability.
Anti-SARS‐CoV‐2 antibodies detected by this assay were shown to have neutralizing (potentially protective) properties in plaque reduction neutralizing tests [11]. The sensitivity and specificity reported by the manufacturer are 88% and 99%, respectively, for the combined IgM + IgA and 85% and 98%, respectively, for IgG, without specific data for HD patients. All serological samples were tested in the same reference laboratory.
Study variables
The past history of COVID-19 and outcomes before the start of the study were recorded for all prevalent dialysis patients in participating units as of 1 March 2020 to determine the baseline exposure to the virus and estimate the mortality rate. Demographic (age, sex, dialysis vintage and aetiology of kidney disease) and morbidity data [body mass index, hypertension, chronic obstructive pulmonary disease (COPD), diabetes, smoking, active neoplasms and treatment with renin–angiotensin–aldosterone system blockers] were collected. Risk factors for SARS-CoV-2 infection were also recorded (healthcare transportation, known exposure to an infected partner and living in nursing homes).
Statistical analysis
Data are shown as mean [standard deviation (SD)] or percentage, according to the type of variable analysed. We use the chi-squared test for association between qualitative variables and the Student’s t-test for quantitative variables. The 95% confidence interval (CI) for proportions was calculated using the Wald method and a P-value ˂0.05 was considered statistically significant. A logistic regression multivariate analysis was used to explore factors associated with an asymptomatic positive RT-PCR test or serologic response, using a change-in-estimate criterion; a 10% cut-off was considered to identify possible confounders. Missing data were excluded from the analysis. The statistical analysis was performed using Stata version 14.0 (StataCorp, College Station, TX, USA).
RESULTS
Incidence and mortality of symptomatic COVID-19 prior to study entry
As of 1 March 2020 there were 808 prevalent HD patients in the six hospitals and four outpatient dialysis centres. Through 15 April 2020 (baseline date), 136 (16.8%) patients had been diagnosed with COVID-19 [12]. This would be equivalent to an accumulated incidence of 5147 cases/100 000 patients, much higher than the maximum accumulated incidence recorded in Madrid (422/100 000 inhabitants) during the first pandemic wave. Infection rates were not homogeneous for different centres, but this was also the case for the general population living in the same healthcare areas. COVID-19 mortality among HD patients was 42/136 COVID-19 patients, corresponding to an estimated mortality for the Madrid HD population of 30.9% (95% CI 23.1–38.6) (Figure 1).
Detection of SARS-CoV-2 infection by RT-PCR and serology
Through 15 April 2020, 763 patients were included in the study, representing 99.6% of all HD patients >18 years of age in the participating centres. Patients were classified into two groups: symptomatic COVID-19 (n = 91) if diagnosed with COVID-19 prior to study entry or no COVID-19 (n = 672) (Figure 1). Symptomatic COVID-19 patients were older, had more risk factors for COVID-19 (including living with an infected partner, living in a nursing home, using shared ambulance transportation and previous hospital admissions) and more often received angiotensin-converting enzyme inhibitors (ACEIs) (Table 1).
Table 1.
Patients’ characteristics by COVID-19 status
| Characteristics | Previous COVID-19 |
No previous COVID-19 | All | P-value |
|---|---|---|---|---|
| n (%) | 91 (11.8) | 672 (88.2) | 763 | |
| Age (years), mean (SD) | 70.6 (14.1) | 64.9 (14.8) | 65.5 (14.9) | <0.001 |
| Male (%) | 53.9 | 66.8 | 65.3 | 0.02 |
| Dialysis vintage (months), median (IQR) | 32.4 (12.2–56.0) | 30.5 (14.3–72.2) | 31.0 (14.0–70.9) | 0.9 |
| Comorbidity | ||||
| Charlson comorbidity index, mean (SD) | 8.2 (2.6) | 7.5 (3.1) | 7.6 (3.0) | 0.04 |
| Overweight (%) | 32.1 | 33.5 | 33.3 | 0.8 |
| Obese (%) | 19.8 | 21.1 | 20.9 | 0.8 |
| Hypertension (%) | 81.6 | 78.8 | 79.1 | 0.6 |
| COPD (%) | 18.7 | 11.3 | 12.1 | 0.07 |
| Diabetes mellitus (%) | 43.4 | 37.2 | 37.9 | 0.3 |
| Smoker (%) | 14.5 | 17.1 | 16.8 | 0.6 |
| Neoplasm (%) | 7.9 | 6.1 | 6.3 | 0.5 |
| Risk factors for COVID-19 (%) | ||||
| Living with COVID-19 partner | 38 | 2.3 | 6.3 | <0.001 |
| Living in nursing home | 19.8 | 2.8 | 4.9 | <0.001 |
| Collective/public transport | 68.1 | 51.6 | 53.6 | 0.003 |
| Previous hospital admission | 15.9 | 8.6 | 9.6 | 0.03 |
| Treatment (%) | ||||
| ACEI | 26.3 | 16.6 | 17.7 | 0.03 |
| ARB | 10.4 | 17.8 | 17.0 | 0.1 |
| ACEI or ARB | 35.8 | 33.9 | 34.1 | 0.7 |
| MRA | 0 | 2.8 | 2.5 | 0.1 |
Overweight: body mass index 25–30 kg/m2; obese: body mass index >30 kg/m2. IQR, interquartile range; COPD, chronic obstructive pulmonary disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist.
Seroconversion after symptomatic COVID-19
In the 91 patients with symptomatic COVID-19, the mean time from COVID-19 diagnosis to baseline serology was 28.2 days (95% CI 5–53). IgM + IgA and/or IgG anti-SARS-CoV-2 antibodies were detected in 75.8% (69/91; 95% CI 67.0–84.6) of patients.
Non-COVID-19 patients
Among patients with no previous COVID-19, RT-PCR was positive in 9 of 672 (1.3%). Serum SARS-CoV-2 antibodies were detected in 7 of 9 RT-PCR-positive patients (77.8%) and in 94/663 RT-PCR-negative patients (14.2%). Overall, the incidence of detected asymptomatic SARS-CoV-2 infection was 15.3% (103/672; 95% CI 12.9–18.1). Taken as a whole, the proportion of asymptomatic infection was 53.1% (103/194) of all infected patients. The proportion of SARS-CoV-2 infection in the entire cohort was 25.4% (194/763; 91 symptomatic COVID-19 patients and 103 asymptomatic detected by RT-PCR or serology).
Antibody dynamics
After 4 weeks, serum anti-SARS-CoV-2 antibodies were again assessed in 674 patients (88.3% of the study population). At this time point, 55/65 [84.6% (95% CI 75.8–93.4)] of symptomatic COVID-19 patients with positive serology maintained anti-SARS-CoV-2 antibodies and 15.4% (10/65) became antibody negative. IgG antibodies remained positive in 54/60 [90% (95% CI 82.4–97.6)] of patients (Supplementary data, Figure S1 in Supplementary Appendix).
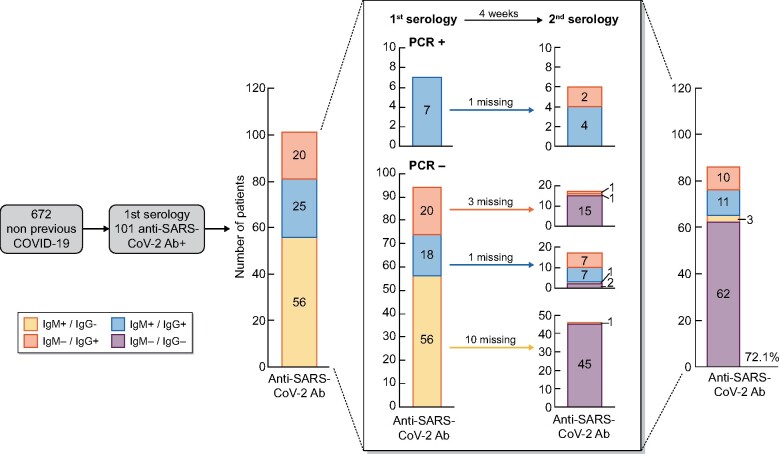
In patients with an asymptomatic SARS-CoV-2 infection, anti-SARS-CoV-2 antibodies persisted in all patients with a positive RT-PCR at baseline [6/6 (100%)] and in only 18/80 [22.5% (95% CI 13.3–31.7)] of those who were RT-PCR negative. Thus in 62/80 (77.5%) asymptomatic SARS-CoV-2 infection–RT-PCR negative patients, anti-SARS-CoV-2 antibodies disappeared 4 weeks later. Anti-SARS-CoV-2 IgG antibodies remained positive in 15/34 [44.1% (95% CI 27.4–60.8)] patients with baseline positivity and RT-PCR negative and in 52.5% (21/40) of all asymptomatic patients irrespective of RT-PCR results. However, only 9/63 [14.3% (95% CI 5.6–22.9)] of the baseline IgA+IgM–positive patients remained positive after 4 weeks and 18.8% (13/69) of all asymptomatic patients irrespective or RT-PCR results (Figure 2).
FIGURE 2:
Evolution and type of anti-SARS-CoV-2 antibodies in asymptomatic patients detected in the first serology and the evolution at 4 weeks.
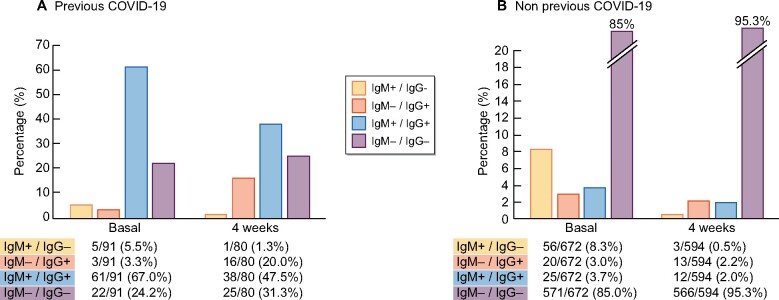
In the entire cohort (symptomatic COVID-19 and asymptomatic SARS-CoV-2 infection), only 52.3% (79/151) of patients with anti-SARS-CoV-2 antibodies remained positive after 4 weeks and 47.7% (72/151) of patients lost the antibodies previously detected. Overall the seroprevalence against SARS-CoV-2 in HD was 170/763 (22.3%) at baseline and 83/674 (12.3%) at 4 weeks (Figure 3).
FIGURE 3:
Seroprevalence of anti-SARS-CoV-2 antibodies in HD patients with (A) symptomatic COVID-19 and patients with (B) no previous diagnosis of COVID-19. Values represent the percentage of the whole cohort in each measure.
In both symptomatic and asymptomatic patients with baseline anti-SARS-CoV-2 antibodies, the initial IgG levels [OD index: 6.5 (SD 2.4) vs 5.1 (SD 3.1); P < 0.001] declined at 4 weeks [OD index: 5.0 (SD 2.6) vs 2.3 (SD 2.7); P < 0.001]. The decrease in IgG titres was lower in COVID-19 patients than in asymptomatic SARS-CoV-2-infected patients (12.0% versus 51.6%; P < 0.001).
We performed a logistic regression analysis to explore differences between patients with persistent anti-SARS-CoV-2 antibodies (adequate serologic response, n = 79) or with transient antibodies (inadequate serologic response, n = 94). The probability of an adequate serologic response was higher in patients who had symptomatic COVID-19 than in asymptomatic SARS-CoV-2 infection [odds ratio (OR) 4.04 (95% CI 2.04–7.99)] corrected for age, Charlson comorbidity index score and time on HD.
In patients with COVID-19, the mean time from diagnosis to baseline antibody assessment was 28.2 days (SD 12.2). This time was shorter in patients with inadequate serologic response than in those with persistent antibodies (20.3 versus 30.8 days; P < 0.001). Thus it is unlikely that antibodies were lost in patients in whom a longer time had elapsed since infection.
Patients with positive SARS-CoV-2 RT-PCR or serology had more risk factors for infection (living with an infected partner, living in a nursing home or public healthcare transportation) (Table 2). Since these risk factors may be combined (e.g. healthcare transportation is more frequent in nursing home patients), they may act as confounding factors. For this reason we performed a logistic regression by change-in-estimate criterion, selecting a final model including confounding factors; thus living in a nursing home [5.9 (95% CI 2.3–15.1)] and using public healthcare transportation [1.5 (95% CI 0.9–2.3)] were the main risk factors for SARS-CoV-2 infection (Table 3).
Table 2.
Characteristics of non-previous COVID-19 patients (n = 672) classified by basal RT-PCR and serologic screening into two groups: positive and negative SARS-CoV-2 infection
| Characteristics | Negative SARS-CoV-2 infectiona | Positive SARS-CoV-2 infectiona | P-value |
|---|---|---|---|
| n (%) | 569 | 103 | |
| Age (years), mean (SD) | 64.4 (14.7) | 67.6 (15.4) | 0.05 |
| Male (%) | 68.1 | 59.8 | 0.1 |
| Dialysis vintage (months), median (IQR) | 31.1 (14.9–73.8) | 26.4 (11.8–62.2) | 0.1 |
| Comorbidity | |||
| Charlson comorbidity index, mean (SD) | 7.4 (3.1) | 8.1 (3.1) | 0.05 |
| Overweightb (%) | 33.2 | 34.7 | 0.6 |
| Obeseb (%) | 21.7 | 17.8 | 0.4 |
| Hypertension (%) | 78.9 | 78.1 | 0.9 |
| COPD (%) | 10.7 | 15.1 | 0.2 |
| Diabetes mellitus (%) | 35.9 | 44.8 | 0.1 |
| Smoker (%) | 16.5 | 20.2 | 0.4 |
| Neoplasm (%) | 6.8 | 2.1 | 0.08 |
| Risk factors for COVID-19 (%) | |||
| Living with COVID-19 partner | 1.5 | 7.4 | <0.001 |
| Living in nursing home | 1.6 | 9.9 | <0.001 |
| Collective/public transport | 49.8 | 61.8 | 0.03 |
| Previous hospital admission | 8.6 | 8.5 | 0.9 |
| Treatment (%) | |||
| ACEI | 15.9 | 21.1 | 0.2 |
| ARB | 18.5 | 13.8 | 0.3 |
| ACEI or ARB | 33.9 | 33.7 | 0.9 |
| MRA | 2.8 | 3.2 | 0.8 |
IQR, interquartile range.
Negative SARS-CoV-2 infection includes patients with negative SARS-CoV-2 RT-PCR and anti-SARS-CoV-2 antibodies. Positive SARS-CoV-2 infection includes patients with positive SARS-CoV-2 RT-PCR and/or positive anti-SARS-CoV-2 antibodies. b Overweight: body mass index 25–30 kg/m2; obese: body mass index >30 kg/m2.
Table 3.
Logistic regression for risk of SARS-CoV-2 infection among HD patients
| Risk | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Collective/public transport | 1.45 | 0.93–2.27 | 0.10 | 1.48 | 0.95–2.31 | 0.08 |
| Living in a nursing home | 6.92 | 2.74–17.50 | <0.001 | 5.89 | 2.29–15.10 | <0.001 |
DISCUSSION
In this large prospective cohort of HD patients in Madrid at a time when the COVID-19 infection rate was declining, we reported a 25.4% (194/763) prevalence of present or past SARS-CoV-2 infection diagnosed by serology or RT-PCR, a high prevalence of asymptomatic patients and an alarming early washout of anti-SARS-CoV-2 antibodies (47.7% of the patients lost them within 4 weeks of the study), suggesting a suboptimal immune response in HD.
The severity of COVID-19 in HD was greater than that reported in the general population and 30.9% of those who had symptomatic COVID-19 infection died. Although COVID-19 mortality has evolved over the pandemic [13], official data from Spain’s Ministry of Health in the second half of April showed general mortality of 7.9% and 13.9% in those >70 years of age [14]. Mortality in other observational HD studies ranged from 16.2 to 41% [2, 15, 16]. Multiple comorbidities and frailty probably contribute to higher COVID-19 mortality in HD patients, although chronic kidney disease itself is a key independent risk factor [17].
The prevalence of asymptomatic SARS-CoV-2 infection in HD patients was high [103/194 (53.1% of the total infected)]—higher than previous general population reports, as it was 28.5% in the ENE-COVID population-based epidemiological study of SARS-CoV-2 seroprevalence in Spain [18] and similar to that described in HD in the UK (40.3%) [3] and in the Wuhan series (51%) [5]. The reasons for this high prevalence of asymptomatic COVID-19 on HD are unknown. COVID-19 symptoms can be unspecific and may be difficult to distinguish from usual symptoms of high-comorbidity patients. In fact, in asymptomatic patients, pulmonary infiltrates may be detected by high-sensitivity chest computed tomography (CT). In the Wuhan HD study of 46 patients with positive serology and negative RT-PCR (79% asymptomatic), chest CTs were normal in only 26%, compared with 55.9% of 623 patients without SARS-CoV-2 infection [5]. The most relevant clinical sign at routine screening at arrival in the dialysis facility for HD sessions was fever, but 34% of patients admitted for COVID-19 remain afebrile during the entire admission [19]. Asymptomatic patients are not detected in the daily HD screening for COVID-19 and therefore are a potential source of infection for other patients, reinforcing the need to maintain strict respiratory isolation measures in dialysis units and in transport to and from the units in all patients, independent of the presence of symptoms.
The overall seroprevalence of anti-SARS-CoV-2 antibodies was 22.3%, which was double the reported seroprevalence on the same dates in the general population in Madrid (11.5%) [18]. Prior HD reports found anti-SARS-CoV-2 prevalence in HD of 36.2% in 356 patients in the UK [3], 8.2% in 1014 patients in Wuhan [5] and 3.3% in 1542 patients in Honghu and Jingzhou, China [7].
The seroconversion rate in HD patients with symptomatic COVID-19 was only 75.8% (69/91) at baseline (i.e. on average, 28 days after the onset of symptoms) and decreases to 68.7% (55/80) 4 weeks later. These data contrast with those published for the general population. Thus, in a systematic review that includes 74 studies, at 4 weeks, 100% of the symptomatic COVID-19 patients had anti-SARS-CoV-2 IgG [20].
To our knowledge, this is the first multicentre prospective study that analyses anti-SARS-CoV-2 antibody dynamics in a large HD population. The HD population lost anti-SARS-CoV-2 antibodies rapidly, as seroprevalence declined from 22.3% to 12.3% in just 4 weeks. There were clear differences depending on whether COVID-19 was symptomatic or not. Anti-SARS-CoV-2 IgG antibodies remained positive at 4 weeks in 90% of symptomatic patients, while the figure was 52.5% for asymptomatic patients with baseline anti-SARS-CoV-2 IgG antibodies. Greater exposure to the virus (in quantity or duration) could induce a stronger immune response in symptomatic patients. These results expand the preliminary observation by Labriola et al. [21] in eight HD patients with SARS-CoV-2 infection and positive serology (six symptomatic and two asymptomatic), in whom anti-SARS-CoV-2 IgG was detectable for at least 3 months in all patients, but titres decreased progressively. Compared with the general population, the rate of antibody loss was higher in HD. Thus in the ENE-COVID-19 seroprevalence study in Spain [18], 14.4% of the general population lost anti-SARS-CoV-2 antibodies at 6 weeks. In asymptomatic patients, this figure rose to 24.0%. In this HD study, roughly half of patients who developed anti-SARS-CoV-2 antibodies lost them in 1 month (72/151). There is currently some debate about the dynamics of anti-SARS-CoV-2 antibodies [22–24]. Discrepancies may depend on different assays or the heterogeneity of the population studied, as more severe disease has been associated with higher and more stable neutralizing antibody titres than asymptomatic infection [25].
The impact of the present findings on long-term risk of reinfection should be studied. Confirmed COVID-19 reinfections of variable severity have been observed, including in an HD patient with previous asymptomatic infection [26]. In another published COVID-19 reinfection in a patient with preserved renal function, the disappearance of IgG antibodies before reinfection was documented [27]. It is still too early to know if these are anecdotal reports or if the risk of reinfection in patients who have lost the antibody titre is really significant or if there is a specific antibody titre that implies protection against reinfection. Indeed, as for other viral diseases, the absence of antibody detection may not imply the absence of protective immunity [28]. In fact, SARS-CoV-2 infection generates long-lasting memory B cells against several viral proteins, guaranteeing lasting B cell immunity even if antibody titres decay [29]. The risk of reinfection needs to be further explored due to its impact on future vaccination policies.
The lower SARS-CoV-2 seroconversion rate and faster anti-SARS-CoV2 antibody titre decay in HD patients may be related to impaired natural and adaptive immunity in chronic kidney disease that negatively impacts every step of the immune response, from antigen presentation by monocytes to T helper and cytotoxic cell responses and the development of long-lasting memory B cells [30]. Furthermore, dialysis worsens the chemotactic and phagocytic function of neutrophils [31]. These impaired responses lead to the suboptimal immune response to infections and the inadequate antibody production following vaccination observed in the dialysis population. The findings of this study suggest an inadequate immune response to SARS-CoV-2 in HD and the need to be alert to possible reinfections or inadequate response rates to vaccination programmes. Until these issues are clarified, the current policies of screening at admission and respiratory isolation in all HD facilities should be maintained.
The results of this study identify a need to redefine the role of anti-SARS-CoV-2 antibody testing in HD patients to reduce the risk of infection and to help manage dialysis station occupancy. At times of high community transmission, patient screening using both RT-PCR and serology is more efficient than RT-PCR alone, by detecting more asymptomatic potentially infective patients. RT-PCR + IgM measurement increased the sensitivity of detection of active infection to 98.6%, compared with only 51.9% if just a single RT-PCR test is done [32]. In addition, the rapid disappearance of antibodies in asymptomatic patients on HD suggests that periodic serology screening and systematic data collection may allow easy traceability of the antibody status in each patient, something similar to current practice for hepatitis B and C viruses. Knowledge of the SARS-CoV-2 status would be useful to confirm reinfections, distribute patients in dialysis units according to the risk of contagion and plan vaccination campaigns. The periodicity of serological screenings will depend on the epidemiological situation at each moment, being more frequent at times of high community transmission rates.
COVID-19 is more frequent in HD than in peritoneal dialysis patients or kidney transplant recipients [1]. Transmission within HD facilities may contribute to this difference, although universal protection measures minimize the risk of nosocomial infection [33]. We have further identified healthcare transport, living in a nursing home or living with a COVID-19 patient as risk factors associated with SARS-CoV-2 infection. Sharing healthcare transport with asymptomatic COVID-19 patients (later detected by RT-PCR screening) was associated with an almost 5-fold greater risk of COVID-19 infection [34]. Healthcare transport in Spain usually consists of collective ambulances lacking social distancing and, in the initial weeks of the pandemic, lacking face masks. Therefore strategies to promote private transport should be strengthened and, if healthcare transportation is used, the number of seats and the duration of the trip should be reduced.
This study has several limitations. The ELISA only assessed antibodies against protein S and the nucleocapsid of SARS-CoV-2 and may not reflect the dynamics of other antibodies. The high percentage of asymptomatic patients detected must be contextualized in the epidemic situation of Madrid, a European city with one of the highest community transmission rates, so the results cannot necessarily be extrapolated to other regions with a lower incidence of SARS-CoV-2 infection. As a strength, this is a prospective study in which almost all prevalent HD patients from the participating centres were included, the follow-up was well structured and the serological assays used the same method and a centralized reference laboratory.
In conclusion, HD patients have a high risk of infection by SARS-CoV-2, high mortality and a high percentage of asymptomatic infection. The rate of anti-SARS-CoV-2 development in HD patients was lower than in the general population and the antibody titre declined faster. This raises questions about the susceptibility of HD patients to reinfection and the response to the SARS-CoV-2 vaccine. These data support the European Renal Association–European Dialysis and Transplant Association call for dialysis patient-specific vaccine studies that explore the antibody response and the optimal vaccination schedule [17].
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared upon reasonable request to the corresponding author.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Joaquín Mendoza and Dr. José Rojas from Vircell. They gratefully acknowledge the cooperation of every health professional fighting against the COVID-19 pandemic.
FUNDING
The COVID-FRIAT project was funded by the Fundación Renal Íñigo Álvarez de Toledo.
AUTHORS’ CONTRIBUTIONS
R.A.-A., J.P. and B.M. designed the study. R.A.-A., J.P. and P.L.-S. performed the statistical analysis and drafted the document. All authors participated in data collection and interpretation. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no relevant financial interest in the scope of this study. The results presented in this article have not been presented previously in whole or part.
COVID-FRIAT STUDY GROUP
Alfredo Cordón and Alicia González Horna (Nephrology Department, Dialysis Center–Los Llanos, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain);
Ana Botella and Paula Manso del Real (Nephrology Department, Dialysis Center–Los Lauros, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain);
Jesús Hernández (Nephrology Department, Dialysis Center–Santa Engracia, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain);
Eva María García San Segundo (Nephrology Department, Dialysis Center–Santa Engracia, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain);
José Guerrero (Nephrology Department, Dialysis Center–Santa Engracia, Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain);
Mariano Acuña and Elena Guerrero Rodríguez (Nephrology Department, Dialysis Center–Los Llanos II. Fundación Renal Íñigo Álvarez de Toledo, Getafe, Spain);
Alberto Ortiz and Emilio Gonzalez Parra (Nephrology Department, Fundación Jiménez Díaz, Madrid, Spain);
Gema Fernández Juárez, Enrique Gruss and Almudena Ortigosa Barriola (Nephrology Department, University Hospital Fundación Alcorcón, Madrid, Spain);
Laura Rodríguez and Cristina Ledesma Torre (Nephrology Department, University Hospital de Villalba, Madrid, Spain);
Simona Alexandru and Dolores Piña Simón (Nephrology Department, University Hospital Rey Juan Carlos, Móstoles, Madrid, Spain);
Raquel Esteras (Nephrology Department, University Hospital Infanta Elena, Valdemoro, Madrid, Spain);
Adriana Iglesias González (Nephrology Department, University Hospital Infanta Elena, Valdemoro, Madrid, Spain);
Darío Janeiro Marín and Alicia Sánchez García (Nephrology Department, University Hospital Puerta de Hierro, Majadahonda, Spain);
David Hernán, Mónica Sánchez and Jesús Portillo (Fundación Renal Íñigo Álvarez de Toledo, Madrid, Spain.
Contributor Information
COVID-FRIAT study group:
Alfredo Cordón, Alicia González Horna, Ana Botella, Paula Manso del Real, Jesús Hernández, Eva María García San Segundo, José Guerrero, Mariano Acuña, Elena Guerrero Rodríguez, Alberto Ortiz, Emilio Gonzalez Parra, Gema Fernández Juárez, Enrique Gruss, Almudena Ortigosa Barriola, Laura Rodríguez, Cristina Ledesma Torre, Simona Alexandru, Dolores Piña Simón, Raquel Esteras, Adriana Iglesias González, Darío Janeiro Marín, Alicia Sánchez García, David Hernán, Mónica Sánchez, and Jesús Portillo
REFERENCES
- 1. Sánchez-Álvarez JE, Pérez-Fontán M, Jiménez Martín C. et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN). Nefrologia 2020; 40: 272–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albalate M, Arribas P, Torres E. et al. High prevalence of asymptomatic COVID-19 in haemodialysis: learning day by day in the first month of the COVID-19 pandemic. Nefrologia 2020; 40: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clarke C, Prendecki M, Dhutia A. et al. High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 2020; 31: 1969–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arenas MD, Villar J, González C. et al. Management of the SARS-CoV-2 (COVID-19) coronavirus epidemic in hemodialysis units. Nefrologia 2020; 40: 258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang H, Tian JB, Dong JW. et al. Serologic detection of SARS-CoV-2 infections in hemodialysis centers: a multicenter retrospective study in Wuhan, China. Am J Kidney Dis 2020; 76: 490–499.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stock da Cunha T, Gomá-Garcés E, Avello A. et al. The spectrum of clinical and serological features of COVID-19 in urban hemodialysis patients. J Clin Med 2020; 9: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X, Nie S, Sun J. et al. The cumulative rate of SARS-CoV-2 infection in Chinese hemodialysis patients. Kidney Int Rep 2020; 5: 1416–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centro de coordinación de Alertas y Emergencias Sanitarias. Ministerio de Sanidad y Consumo, Gobierno de España. Actualización número 68, Enfermedad por el coronavirus (COVID-19).https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_68_COVID-19.pdf (14 September 2020, date last accessed)
- 9. Tornero F. Incidencia de COVID-19 en la Comunidad de Madrid. Webinar SOMANE COVID-19. https://www.somane.org/modules.php?name=news& d_op=showNew&date=20200618&idnews=131 (15 September 2020, date last accessed)
- 10.Actualización diaria. Enfermedad por el coronavirus (COVID-19). Centro de Coordinación de Alertas y Emergencias Sanitarias. Ministerio de Sanidad. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/situacionActual.htm (15 September 2020, date last accessed)
- 11. Kohmer N, Westhaus S, Rúhl C. et al. Clinical performance of different SARS-CoV-2 IgG antibody tests. J Med Virol 2020; 92: 2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Clinical Management of COVID-19 Interim Guidance. March 27, 2020.https://www.who.int/publications/i/item/clinical-management-of-covid-19 (15 September 2020, date last accessed)
- 13. Ghayda RA, Lee KH, Han YJ. et al. Estimation of global case fatality rate of coronavirus disease 2019 (COVID-19) using meta-analyses: comparison between calendar date and days since the outbreak of the first confirmed case. Int J Infect Dis 2020; 100: 302–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Informe no 23. Situación de COVID-19 en España a 16 de abril de 2020. Equipo COVID-19. RENAVE. CNE. CNM. Instituto de Salud Carlos III. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%c2%ba%2023.%20Situaci%c3%b3n%20de%20COVID-19%20en%20Espa%c3%b1a%20a%2016%20de%20abril%20de%202020.pdf (15 September 2020, date last accessed)
- 15. Goicoechea M, Sánchez-Cámara LA, Macías N. et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int 2020; 98: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scarpioni R, Manini A, Valsania T. et al. COVID-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol 2020; 37: 2020-vol2. [PubMed] [Google Scholar]
- 17. Ortiz A, Cozzolino M, Duivenvoorden R. et al. Chronic kidney disease is the key risk factor for severe COVID-19: a call to action by the European Renal Association. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollán M, Pérez-Gómez B, Pastor-Barriusa R. et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Portolés J, Marques M, López-Sánchez P. et al. Chronic kidney disease and acute kidney injury in the COVID-19 Spanish outbreak. Nephrol Dial Transplant 2020; 35: 1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murchu E O, Byrne P, Walsh KA. et al. Immune response following infection with SARS-CoV-2 and other coronaviruses: a rapid review. Rev Med Virol 2021; 31: e2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Labriola L, Scohy A, Seghers F. et al. A longitudinal, 3-month serologic assessment of SARS-CoV-2 infections in a Belgian hemodialysis facility. Clin J Am Soc Nephrol 2021; doi: 10.2215/CJN.12490720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ibarrondo FJ, Fulcher JA, Goodman-Meza D. et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild COVID-19. N Engl J Med 2020; 383: 1085–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terpos E, Mentis A, Dimopoulos MA.. Loss of anti-SARS-CoV-2 antibodies in mild COVID-19. N Engl J Med 2020; 383: 1694–1698. [DOI] [PubMed] [Google Scholar]
- 24. Shu H, Wang S, Ruan S. et al. Dynamic changes of antibodies to SARS-CoV-2 in COVID-19 patients at early stage of outbreak. Virol Sin 2020; 35: 744–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long QX, Tang XJ, Shi QL. et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26: 1200–1204. [DOI] [PubMed] [Google Scholar]
- 26. Munoz Mendoza J, Alcaide ML.. COVID-19 in a patient with end-stage renal disease on chronic in-center hemodialysis after evidence of SARS-CoV-2 IgG antibodies. Reinfection or inaccuracy of antibody testing. IDCases 2020; 22: e00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. To KK-W, Hung IF-N, Chan K-H. et al. Serum antibody profile of a patient with COVID-19 reinfection. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baumgarth N, Nikolich-Žugich J, Lee FE. et al. Antibody responses to SARS-CoV-2: let’s stick to known knowns. J Immunol 2020; 205: 2342-2350 (doi: 10.4049/jimmunol.2000839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen-Contant P, Embong AK, Kanagaiah P. et al. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio 2020; 11: e01991-–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kato S, Chmielewski M, Honda H. et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3: 1526–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy S, Chitturi C, Yee J.. Vaccination in chronic kidney disease. Adv Chronic Kidney Dis 2019; 26: 72–78 [DOI] [PubMed] [Google Scholar]
- 32. Guo L, Ren L, Yang S. et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71: 778–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Sequera Ortiz P, Quiroga Gili B, de Arriba de la Fuente G. et al. Protocol against coronavirus diseases in patients on renal replacement therapy: dialysis and kidney transplant. Nefrologia 2020; 40: 213–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rincon A, Moreso F, López-Herradón A. et al. The keys to control a COVID-19 outbreak in a haemodialysis unit. Clin Kidney J 2020; 13: 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.