In the midst of a devastating pandemic with high transmissibility and case fatality, the mRNA COVID-19 vaccine trials represent hope of an end to the global burden of COVID-19 infection, hospital utilization, and death. The efficacy and safety of the vaccines have been demonstrated in adults across a range of demographics, with the exception of those who are pregnant and lactating. This systematic exclusion—common in clinical trials—represents a missed opportunity to protect a group at risk of adverse outcomes in the setting of COVID-19 infection. It has special implications on the healthcare workforce, a majority of whom are women and at high risk of COVID-19 infection.
The exclusion of pregnant and lactating women from COVID-19 vaccine trials (Text Box 1) reflects a historic pattern of ‘protection by exclusion’, representing an instance in which the estimated effect of a therapy on mother and child will rely on anecdotal and delayed reports from healthcare settings rather than the monitored setting of a clinical trial.2 This exclusion is not justified. For example, Pfizer and Moderna excluded pregnant and lactating women from their mRNA COVID-19 vaccine trials3,4 with no biological evidence to suggest that the vaccines are teratogenic, and no plausibility that they are transmitted in breast milk. The mRNA vaccines rapidly degenerate after injection in muscle cells, and aside from the post-vaccination immune response that can cause a fever, there is no reason to assume, a priori, that harm will come to pregnant and lactating women enrolled in the trials.4
Text Box 1.
COVID-19 Phase III trials that have excluded pregnant and lactating women
| Company | Vaccine | Trial registration number(s) | Ages included |
|---|---|---|---|
| Astra Zeneca (US) | AZD1222 | NCT04516746 | 18+ |
| Astra Zeneca (UK) | AZD1222 |
NCT04400838 (UK) NCT04536051 (Brazil) NCT04444674 (South Africa) |
5–12, 18+ |
| Janssen | Ad26.COV2.S | NCT04505722 | 18+ |
| Moderna | mRNA-1273 | NCT04470427 | 18+ |
| Pfizer | BNT162 | NCT04368728 | 12+ |
| Sinopharm | Sinopharm vaccine | NCT04510207 | 18+ |
| Sinovac | CoronaVac | NCT04456595 | 18+ |
Adapted from Doshi.1
The exclusion of pregnant and lactating women from the COVID-19 vaccine trials has implications on healthcare workers, who are among the highest risk groups for COVID-19 exposure and infection. As many as three out of four healthcare workers and four out of five nurses are women, and it is estimated that more than 300 000 health care workers in the USA alone are pregnant or immediately postpartum during vaccine implementing.5 The dual risk posed by COVID-19 to pregnant healthcare professionals—increased workplace exposure and increased risk of adverse outcomes if infected6—makes it particularly concerning that the trials failed to include pregnant or lactating individuals. Furthermore, when healthcare workers are infected, they become sources of nosocomial spread thereby posing a risk to patients and to other healthcare workers.
The recommendations for use of the COVID-19 Pfizer and Moderna vaccine in pregnant and lactating women now range from avoidance of the vaccine—as recommended by the World Health Organization and some regulatory agencies—to reliance on recipients to make choices guided by their values or their clinicians’ judgement.5,7–9 Some regulatory bodies recommend against pregnancy in the weeks following the vaccine. The rationale for the recommendations of vaccine avoidance in pregnancy, pregnancy avoidance in weeks following vaccination, and decision-making regarding vaccination safety by clinicians in absence of clinical trial data is unclear.
Pregnancy and lactation are two distinct biological states that are often conflated in the eligibility criteria of clinical trials. Drugs that may have evidence of teratogenicity in biological studies may not be secreted in breast milk, and drugs secreted in breast milk with harmful effects to baby may not be teratogenic. The grouping of pregnancy and lactation into a single condition that is systematically excluded from clinical trials is often ill-conceived.2 Indeed, the exclusions of one or both groups of patients from clinical trials is often without justification. For example, pregnant and lactating women were excluded from the COVID-19 hydroxychloroquine and azithromycin trials when these drugs have been used to treat pneumonia, malaria, and lupus in pregnant women and lactating women for several years.
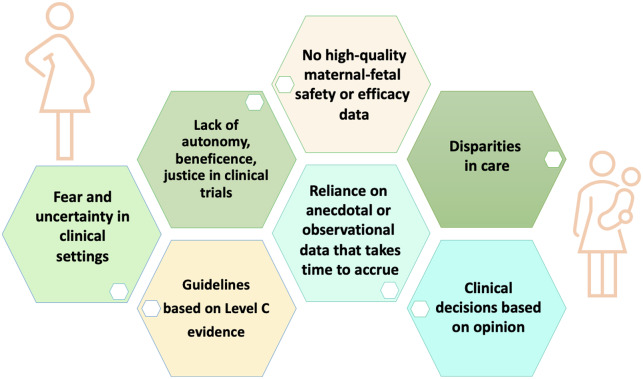
The systematic exclusion of pregnant and lactating women from clinical trials, while broadly accepted, does not uphold the ethical principles of justice, beneficence, and autonomy (Figure 1). Results from trials in non-pregnant populations cannot be safely extrapolated to pregnant women due to differences in physiology that influence the pharmacokinetics and pharmacodynamics of drugs. Recommendations for most therapies come with the stipulation that there is no safety or efficacy data to support their use in pregnant or lactating women.2 The treatment of many conditions during pregnancy or lactation relies on historic drugs for which there is only observational data to confirm safety. This applies to all cardiovascular conditions in pregnant and lactating women; the level of evidence to support treatment recommendations in these women in major guideline documents is almost exclusively ‘C’, based on small studies, observational data, or the consensus of experts.9 Pregnant and lactating women are thus deprived from the benefit of modern-day therapies that improve outcomes in other groups. Furthermore, trials that exclude pregnant and lactating women often also exclude those who can potentially become pregnant, and these criteria are independently associated with the under-enrolment of women in clinical trials.2,11
Figure 1.
The risks posed by systematic exclusion of pregnant and lactating women from clinical trials.
To ensure that safe vaccines and treatments are extended to all groups during the COVID-19 pandemic, it is important that exclusion criteria be considered carefully, keeping in mind those at risk of exposure, infection, and adverse outcomes. Exclusion criteria should be justified based on data from biological studies, trials in non-human primates, and in the case of drugs historically used for other indications, observational safety data. Clinical trialists and pharmaceutical companies can include maternal–fetal medicine specialists on their steering committees to obtain guidance and manage risk during the trial. As pregnancy and lactation are different biological conditions, they should not be combined into a single exclusion criterion in clinical trials.
The appropriateness of having regulators and clinicians make recommendations to pregnant and lactating women about a potentially life-saving vaccine in absence of high-quality evidence merit consideration. While the exclusion of pregnant and lactating women has become the norm in clinical trials across medical conditions, the stakes in doing so are perhaps the greatest during a pandemic that has taken an immeasurabletoll on healthcare professionals, the majority of whom are women. The categorical exclusion of pregnant and lactating women in COVID-19 trials to date represents a missed opportunity to inform the care of a demographic group that is often subjected to disparities in care.
Conflict of interest: none declared.
References
- 1. Doshi P, Will Covid-19 vaccines save lives? Current trials aren’t designed to tell us. BMJ 2020;371; m4037. doi: 10.1136/bmj.m4037. [DOI] [PubMed] [Google Scholar]
- 2. Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007;297:1233–1240. [DOI] [PubMed] [Google Scholar]
- 3. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott A, Flach B, Doria-Rose NA, Corbett KA, Morabito KM, O’Dell S, Schmidt SD, Swanson PA, Padilla M, Mascola JR, Neuzil KM, Bennett H, Sun W, Peters E, Makowski M, Albert J, Cross K, Buchanan W, Pikaart-Tautges R, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 2020;383:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Safety and efficacy of the BNT162b2 mRNA Covid-19. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oliver S. Clinical Considerations for Populations Included in Phase 1a. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/COVID-03-Oliver.pdf?fbclid=IwAR0yfyQbXDlGYP37QtVFrGhSZQrLCKRYppYJl7W-UD7VqcEUu6GSnrOaz4E (21 January 2021).
- 6. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qiu X, Yuan M, Coomar D, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, Mofenson L, Zamora J, Thangaratinam S, for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Center for Disease Control. Vaccination considerations for people who are pregnant or breastfeeding. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html#:∼:text=Routine 20testing 20and 20pregnancy,an 20mRNA 20COVID 2D19 20vaccine (21 January 2021).
- 8. The Society of Obstetricians and Gynaecologists of Canada Statement on COVID-19 Vaccination in Pregnancy. January 11, 2021. https://sogc.org/common/Uploaded20files/Covid20Information/SOGC_Statement_COVID19_Vaccination_in_Pregnancy.pdf (21 January 2020).
- 9. The Society of Obstetricians and Gynaecologists of Canada Statement on COVID-19 Vaccination in Pregnancy. https://sogc.org/common/Uploaded%20files/Covid%20Information/SOGC_Statement_COVID19_Vaccination_in_Pregnancy.pdf (21 January 2021).
- 10. Wall Street Journal. WHO recommends against Moderna, Pfizer vaccines for most pregnant women. https://www.wsj.com/articles/who-recommends-against-moderna-pfizer-vaccines-for-most-pregnant-women-11611775138 (27 January 2021).
- 11. Whitelaw S, Sullivan K, Eliya Y, Alruwayeh M, Thabane L, Yancy CW, Mehran R, Mamas MA, Van Spall HGC. Trial characteristics associated with under-enrolment of females in randomized controlled trials of heart failure with reduced ejection fraction: a systematic review. Eur J Heart Fail 2020; doi:10.1002/ejhf.2034. [DOI] [PubMed] [Google Scholar]