Abstract
Cannabis use is widespread among adolescents and has been associated with long-term negative outcomes on neurocognitive functions. However, the factors that contribute to the long-term detrimental effects of cannabis use remain poorly understood. Here, we studied how Reelin deficiency influences the behavior of mice exposed to cannabis during adolescence. Reelin is a gene implicated in the development of the brain and of psychiatric disorders. To this aim, heterozygous Reeler (HR) mice, that express reduced level of Reelin, were chronically injected during adolescence with high doses (10mg/kg) of Δ9-tetrahydrocannabinol (THC), a major psychoactive component of cannabis. Two weeks after the last injection of THC, mice were tested with multiple behavioral assays, including working memory, social interaction, locomotor activity, anxiety-like responses, stress reactivity, and pre-pulse inhibition. Compared to wild-type (WT), HR mice treated with THC showed impaired social behaviors, elevated disinhibitory phenotypes and increased reactivity to aversive situations, in a sex-specific manner. Overall, these findings show that Reelin deficiency influences behavioral abnormalities caused by heavy consumption of THC during adolescence and suggest that elucidating Reelin signaling will improve our understanding of neurobiological mechanisms underlying behavioral traits relevant to the development of psychiatric conditions.
1. Introduction
Heavy and frequent cannabis use by adolescents has been linked epidemiologically to increased risk of developing psychiatric conditions, including schizophrenia, psychosis, and substance use disorders (Volkow, 2016). Similarly, animal studies show that administration of cannabinoids (e.g. THC), during adolescence, perturbs a wide range of behaviors, including memory, social interaction, anxiety, and sensorimotor gating by targeting the endocannabinoid (eCB) system (Rubino et al., 2015). Despite the evidence of possible detrimental health outcomes associated with adolescent cannabis use, a recent survey in the US revealed a substantial increase in daily use of cannabis and a decreased perception of the risks associated with its regular use by adolescents (Johnston, 2018). Further, the increasing legalization of recreational cannabis use has led to calls to understand whether such policies put adolescents at higher risk of developing psychiatric disorders. All this emphasizes the need to better understand the neurobiological mechanisms associated with heavy consumption on cannabis during adolescence (Wilkinson et al., 2016).
During adolescence, the brain undergoes continuous remodeling of its structure, connectivity, and plasticity (Sturman and Moghaddam, 2011; Arain et al., 2013). In addition, substantial hormonal changes during adolescence influence not only reproductive functions, but also the emergence of sex differences in cognitive, social, and emotional behaviors (Schulz and Sisk, 2016). Thus, the adolescence is considered a critical period wherein brain development may be altered by the exposure to psychoactive drugs, which can lead to sex-specific behavioral abnormalities and increased risk for psychopathology in adulthood (Cousijn et al., 2018; Lisdahl et al., 2018).
However, the factors that contribute to long-term detrimental effects of cannabis exposure during adolescence remain poorly understood. The goal of this study is to examine the potential role of Reelin signaling in modulating the behavioral effects of cannabis on the adolescent brain.
Reelin is a protein of the extracellular matrix that is predominately expressed in neuronal cells and plays a key role in brain development and synaptic plasticity (D’Arcangelo et al., 1995). During embryonic stages, Reelin activates an extensive signaling cascade that is critical for the proper migration and cell positioning of cortical neurons (Sekine et al., 2014). During adolescence, Reelin signaling promotes the development of the synaptic excitation/inhibition (E/I) balance within the prefrontal cortex (Iafrati et al., 2014; Bouamrane et al., 2016). In the mature brain, Reelin is required for learning and memory by regulating the N-methyl-D-aspartate receptors (NMDA-R) function and the expression of neuronal activity-dependent genes (Weeber et al., 2002; Qiu et al., 2006; Niu et al., 2008; Rogers et al., 2011; Telese et al., 2015). In humans, Reelin deficiency has been linked to the development of psychiatric disorders (Ishii et al., 2016). Thus, the HR mice, that expressed lower level of Reelin, have been proposed as a valid animal model to study neurodevelopmental psychiatric disorders (Lossi et al., 2019). Whether there is a functional relationship between Reelin deficiency and the consequences of adolescent exposure to high levels of THC remains unknown.
To this aim, we examined the long-lasting behavioral outcomes of chronic adolescent exposure to high doses of THC (10mg/kg) in female and male HR mice. We compared HR mice to their WT littermate controls in a battery of behavioral tests exploring different facets of cognitive and emotional responsiveness, including working memory (Sannino et al., 2012), social interaction (Yang et al., 2011), anxiety-like responses (Bailey and Crawley, 2009), stress reactivity (Can et al., 2012), and pre-pulse inhibition (Geyer et al., 2002). This is the first study to investigate the relationship between Reelin deficiency and the effect of adolescent exposure to THC in mice.
2. Materials and Methods
2.1. Animals.
All experimental procedures were approved by the institutional animal care and use committee at University of California, San Diego. Mice were housed (3–4 per cage) under a 12h light/12h dark cycle and provided with food and water ad libitum. HR mice were bred in house using the B6C3Fe a/a-Relnrl/J line (The Jackson Laboratory, #000235) (D’Arcangelo et al., 1995).
2.2. Drug treatment protocol and experimental design for behavioral analysis.
THC was provided by the U.S. National Institute on Drug Abuse and was dissolved in a vehicle solution consisting of ethanol, tween, and 0.9% saline (1:1:18) on the day of administration. The “high” dose and chronic treatment protocol were selected to study the behavioral effects of heavy and chronic cannabis exposure. The dosage we used (10 mg/kg) was referred to as ‘high’ based on previous studies using low to high ranges of THC in mice (Trexler et al., 2018; Kasten et al., 2019). This dose was also selected to achieve physiologically relevant amounts of THC in mice based on plasma level concentrations observed in cannabis use in humans, as shown in Suppl. Fig. 1 and further explained in the discussion (Huestis et al., 1992; Abrams et al., 2007; Zuurman et al., 2008; Karschner et al., 2009; Nguyen et al., 2016). Vehicle or THC were administered daily to adolescent mice by intraperitoneal injections from post-natal day (PND) 28 to PND 48, which cover the adolescent period in mice (Laviola et al., 2003) (Fig. 1A). To examine the long-term effects of chronic adolescent exposure to THC, mice were tested two weeks after a drug abstinence period, starting at PND 63. We used 9 cohorts of mice to perform multiple behavioral assays. Cohorts 1 to 5 were subjected to locomotor activity, open field (OF), six-different objects (6-DOT), light-dark (LD), three-chamber social approach, and tail suspension (TS) tests. Cohorts 6 to 9 were subjected to locomotor activity, acoustic startle response (ASR) and pre-pulse inhibition (PPI) tests. Mice were tested between 10:00 am and 5:00 pm. Behavioral assays were conducted on separate days and all behavioral tests were performed once on each mouse. The number of mice used in each behavioral assay is reported in Table 1. To prevent bias due to olfactory cues, the behavioral apparatus was cleaned with diluted ethanol solution in between mice.
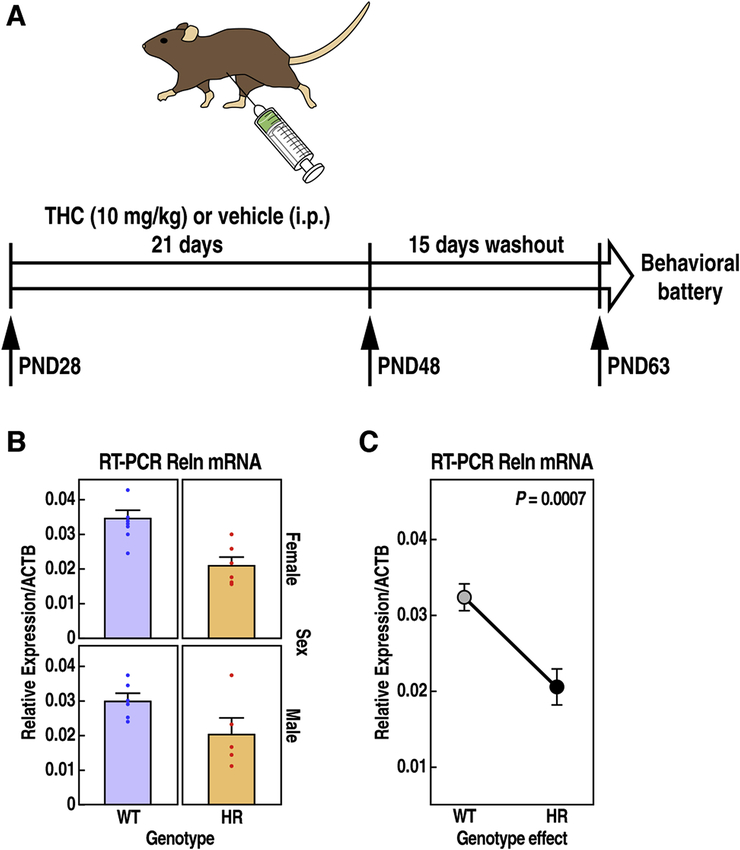
Figure 1: Experimental model.
(A) Schematic diagram illustrates the daily i.p. administration of THC (10 mg/Kg) during adolescence (PND28–48), and the day when behavioral assessment began (PDN63) after 15 days of washout. (B) Relative expression of Reelin mRNA transcript levels measured by RT-PCR and normalized to housekeeping gene ACTB in total RNA isolated from mouse brain tissues in WT and HR mice. (C) Reelin expression is reduced in HR mice compared to WT littermates (main genotype effect, P = 0.0007, LMM).
Table 1.
Number of mice used for behavioral analysis*
| BEHAVIORAL ASSAYS | MALE WT | FEMALE WT | MALE HET | FEMALE HET | ||||
|---|---|---|---|---|---|---|---|---|
| VEH | THC | VEH | THC | VEH | THC | VEH | THC | |
| Locomotor | 19 | 20 | 19 | 18 | 17 | 21 | 19 | 23 |
| Tail suspension | 24 | 24 | 20 | 18 | 18 | 22 | 20 | 22 |
| Open field | 17 | 15 | 10 | 12 | 10 | 11 | 16 | 14 |
| Light-dark | 18 | 16 | 13 | 12 | 9 | 11 | 15 | 13 |
| Social | 19 | 19 | 17 | 15 | 10 | 11 | 16 | 14 |
| 6-DOT | 12 | 13 | 9 | 9 | 10 | 11 | 16 | 14 |
| PPI | 23 | 24 | 24 | 23 | 20 | 22 | 25 | 26 |
| ASR | 23 | 24 | 25 | 23 | 20 | 22 | 25 | 26 |
The number of outliers for specific variables and removed from analysis of are reported in the method section
2.3. Six different objects test.
We used the 6-DOT to study short-term working memory in mice, as previously described (Sannino et al., 2012). First, mice were left free to explore an empty arena (60×40 ×35 cm) during a 10 min habituation trial. Afterwards, mice were exposed to six different objects for a total time of 10 min (familiarization trial). After an inter-trial interval of 1 minute, mice were exposed to identical copies of the familiar objects, but one object was substituted with a novel object (test trial). The exploration time across different trials was measured with Anymaze (Ugo Basile, Varese, Italy) and was used to calculate a discrimination index (DI) as follows: (time spent exploring novel object – average time spent exploring familiar objects) / Total time spent exploring novel + familiar objects. The time exploring the novel object and the DI were used as indexes of novelty-induced exploratory activity and working memory, respectively.
2.4. Three-chamber social approach test.
The three-chamber social approach test was used to measure social behaviors, as previously described (Yang et al., 2011). The apparatus comprised of three-chambered box with dividing walls with small openings to allow free exploration of the three chambers, such as one empty central chamber, one side chamber containing an empty small wire cage (novel object) and one side chamber containing a stranger mouse inside a small wire cage (novel mouse). The target mouse was first placed in the center chamber and allowed to explore the apparatus for 15 min. After introducing the novel mouse and the novel object in the side chambers, the mouse was allowed to explore for 10 min. The placement of the novel mouse or novel object in the left or right chambers was systematically alternated in between trials. The time spent in each compartment and the time spent actively sniffing the novel mouse or the novel object were manually scored. Longer time spent with or exploring the novel mouse versus the novel object was considered an index of sociability. Sociability index (SI) was calculated as [(time spent exploring or sniffing novel mouse - time spent exploring or sniffing novel object) / (total time spent exploring or sniffing novel mouse and novel object)].
2.5. Tail suspension test.
The TS test was used to measure motor responses under aversive conditions (Can et al., 2012). Mice were suspended by their tails with tape to a bar in a position that they could not escape or hold on to nearby surfaces. The test lasted for 6 minutes and the mobility time (s) was manually scored for each minute. We analyzed was the mobility time (s) as the sum of the final 5 minutes.
2.6. Open field test.
The OF test was used to measure anxiety-like behavior (Bailey and Crawley, 2009). Mice were placed randomly in one of the 4 corners of an open plexiglass arena (60×40×35 cm) for 5 minutes. The total time spent in and the latency to entry the center of the arena (s) were recorded and scored using Anymaze.
2.7. Light-dark test.
The LD test was used to measure anxiety-like behavior(Bailey and Crawley, 2009). The animals were tested for 10 min in a light–dark rectangular box (60×40×35 cm) in which the aversive light compartment (40×40×35 cm) was illuminated by a 100 lux light. The dark side (20×40×35 cm) had an opaque cover and ~ 0 lux of light. The two compartments were connected by an open doorway, which allowed the subjects to move freely between the two compartments. The test began by placing the animal in the dark compartment. The time spent in the light compartment (s), the latency to enter in the light chamber (s), and the total number of transitions, were measured using Anymaze.
2.8. Acoustic startle response and pre-pulse inhibition test.
The ASR and PPI tests were used to assess stress reactivity and sensorimotor gating functions. The tests were performed with a startle reflex measuring apparatus (SR-LAB; San Diego Instruments, San Diego, CA), as previously described (Toth et al., 2013). The system comprises a piezoelectric unit that transduces vibrations into signals when mice startle inside the plexigas cylinder. First, mice are placed in a plexiglas cylinder with background noise (65 decibel [db]) for 5 min (acclimation phase). Then, mice were subjected to a total of 179 trials, for a total of 25 min, including: (a) startle trials (40 milliseconds [ms] with 80, 90, 100, 110 and 120 db acoustic pulses), (b) prepulse+startle trials: 20 ms with acoustic prepulses of 3 (68), 6 (71) and 12 (77) db above background noise followed, 100ms later, by a 40 ms 120-db startling pulse. Startle amplitude was measured every 1ms over a 65ms period beginning at the onset of the startle stimulus. Average startle amplitude over the sampling period was taken as the dependent variable. Percent PPI at each pre-pulse intensity was calculated as 100 - [(startle response for prepulse/startle response for startle-alone trials) x 100].
2.9. Locomotor activity.
Locomotor activity was measured using the video tracking system Anymaze. Mice were placed in an empty open field (60×40×35 cm) for 20 minutes. The distance traveled (m) was recorded in 5 minutes intervals and used as an index of locomotor activity in a novel environment.
2.10. Body weight measurements.
Body weight (g) was measured through the course of the drug administration protocol and at PND63 when the behavioral assays began. The change in body weight was calculated as the difference between body weight at any given day and body weight at PND 28.
2.11. RNA Extraction, cDNA Synthesis and qPCR.
Brain tissue from PFC n = 8 female WT, n = 6 male WT, n = 6 female HR, n = 5 male HR mice was homogenized in TRIzol Reagent (#15596018, Thermo Fisher Scientific) and Zirconium beads (#Zr0B05-RNA, Next Advance) using the Bullet Blender homogenizer (BBX24B, Next Advance,). RNA was extracted on columns with the Direct-Zol RNA miniprep kit (#R2051, Zymo Research). To quantify Reelin expression levels, equal amounts of cDNA were synthesized using the Superscript VILO MasterMix (#11755–050, Thermo Fisher Scientific) and mixed with the qPCRBIO SyGreen Blue Mix (#17–507DB, PCR Biosystems) and 5 pmol of both forward (5’- GGACTAAGAATGCTTATTCC −3’) and reverse (5’- GGAAGTAGAATTCATCCATCAG −3’) Reelin primers. ACTB was amplified as an internal control (5’- ATGGAGGGGAATACAGCCC −3’) and reverse (5’- TTCTTTGCAGCTCCTTCGTT −3’).
2.12. Plasma THC analysis.
For determination of plasma THC levels, male (n = 8) and female (n = 8) mice were used for each group. For the acute administration group, blood samples were collected 30 minutes after a single injection of THC at PND 48. For the chronic administration group, mice were injected with THC once a day for 21 consecutive days (PND 28–48) and blood samples were collected 24 hours after the last injection. Blood samples (~250 μL) were collected in tubes containing EDTA, via syringe needle insertion in the heart ventricles following exposure to carbon monoxide. Plasma THC concentration was quantified using fast liquid chromatography/mass spectrometry (LC/MS) adapted from (Lacroix and Saussereau, 2012; Irimia et al., 2015; Nguyen et al., 2018). 50 μL of plasma were mixed with 50 μL of deuterated internal standard (100 ng/mL CBD-d3 and THC-d3 in acetonitrile; Cerilliant), and cannabinoids were extracted from samples using 300 μL acetonitrile and 800 μL of chloroform, dried and then reconstituted in 100 μL of a methanol/water (2:1) mixture. Separation was performed on an Agilent LC1100 using a Poroshell 120 EC-C18 column (4.0μm, 2.1mm x 100mm) using isocratic elution with water and methanol, both with 0.2 % formic acid (250 μL/min; 81% MeOH). THC was quantified using an Agilent 6140 single quadrupole MSD using electrospray ionization and selected ion monitoring [THC (m/z=315.2) and THC-d3 (m/z=318.2)]. A linear scaling correction factor was used to quantify THC in one subject with 40 μL of plasma. Calibration curves were conducted daily for each assay at a concentration range of 0–200 ng/mL and observed correlation coefficients were 0.999.
2.13. Statistical analysis.
To examine how factors (treatment, genotype, sex) affected mice behavior or THC level, we used linear mixed models (LMM) in JMP pro v. 15.0 (SAS Institute, Inc). LMM allow modeling of both fixed and random effects (which subsume repeated measures) (Quinn GP, 2002; Zuur AF, 2016). We incorporated the categorical predictor variables of interest as fixed effects and included all possible interactions. We included mouse cohorts and individual subjects as random effects to account for possible non-independence of the data. We ensured assumptions of approximate normality and variance homogeneity were met by inspecting plots of residuals versus predicted values, and by inspecting quantile-quantile plots with 95% confidence limit curves. When residual plots indicated that it was appropriate for repeated measures, we used a covariance structure that allowed variances to differ across the levels of the repeated variable (Garrett M. Fitzmaurice, 2011). We analyzed the untransformed data in all but few traits where a fourth root transformation mitigated variance heterogeneity (e.g. latency to light, latency to center, Vmax of acoustic startle, body weight change). When significant interactions were found, planned post-hoc pairwise comparisons were performed to identify differences among specific genotype and treatment groups. We report P-values that remained significant after controlling for multiple comparisons by holding the ‘false-discovery rate’ to 0.05 using the Benjamini-Hochberg method (Hochberg, 1995). Outliers were detected using the Huber M-estimation method (Huber, 1973) in JMP pro v. 15.0 and removed when appropriate (n = 3 in latency to light, n = 2 latency to center, n = 2 time spent sniffing, n = 1 sociability index, n = 1 in % PPI).
2.14. Factor analysis of behavioral assays.
Factor analysis is a data reduction method for understanding underlying relationships among variables (Bartholomew, 2008). We performed factor analysis using the variables from multiple behavioral assays, such as 6-DOT (DI, total exploration time in test trial [T3]), social interaction (time sniffing novel mouse, difference time sniffing novel mouse vs novel object), OF (time in center), LD (time in light), TS (mobility time). Factor analysis was computed in JMP 15.0 Pro and was conducted with varimax rotation with a factor-loading cutoff of 0.3 (Manly and Navarro Alberto; Stephens, 1996). The number of actors retained in our model was selected by inspecting the ‘elbow’ on the scree plot curve with factors retained if their eigenvalues were greater than 1 (Cattell, 1966). With these settings, a three-factor model was generated for our dataset. The loadings of the observed variables on the extracted factors are shown in Fig. 7. Factor scores for individual mice were extracted and used as variables for subsequent LMM analysis to identify their associations with treatment, sex, and genotype.
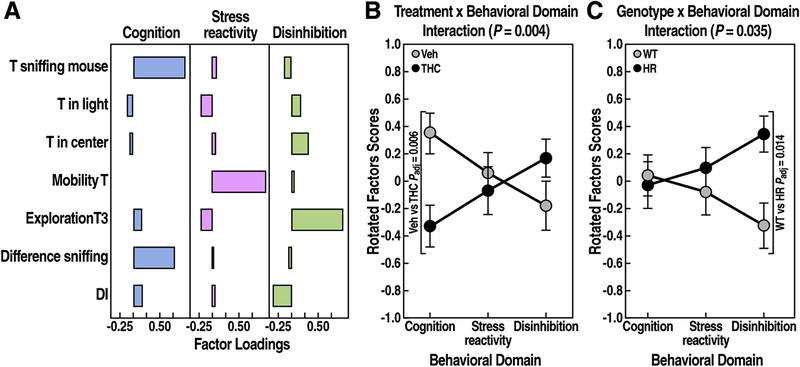
Figure 7: Factor analysis of behavioral tests.
(A) Factor loadings for each named behavioral domain are reported for several behavioral variables. LMM analysis for rotated factor scores revealed a (B) treatment x behavioral domain interaction (P = 0.004), and a (C) genotype x behavioral domain interaction (P = 0.035). FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
3. Results
3.1. Working memory was impaired after chronic adolescent exposure to THC, and in male HR mice
To study the long-term effects of chronic adolescent exposure to high doses of THC (10mg/kg), male and female mice were administered THC chronically during the adolescent period (PND 28–48) and were tested 2 weeks after the last injection of THC (Fig. 1A). Mice of both sexes showed similar level of THC in the plasma after acute or chronic injections (Fig. Suppl. 1A). To examine how Reelin signaling influences the behavioral effects of chronic adolescent exposure to THC, we used HR mice, which carry a null mutation in the Reelin gene (D’Arcangelo et al., 1995) and express ~40% reduced level of Reelin mRNA in brain tissues (Fig. 1B–C).
To assess the long-term effects of chronic adolescent exposure to THC on working memory in WT and HR mice, we used a modified novel object recognition test that uses 6 instead of 2 objects (6-DOT, Fig. 2A) (Sannino et al., 2012; Olivito et al., 2016). This test evaluates recognition memory under conditions of high loads of information processing, which is referred to as memory span and is considered a form of working memory. A discrimination index (DI) was calculated to determine the amount of time mice explored the novel object compared to the familiar objects. A preference for the novel object is considered as a sign that mice remember the familiar objects (Sannino et al., 2012). Higher rates of exploratory activity in response to the novel object is also interpreted as a sign of novelty seeking behavior (Flagel et al., 2014).
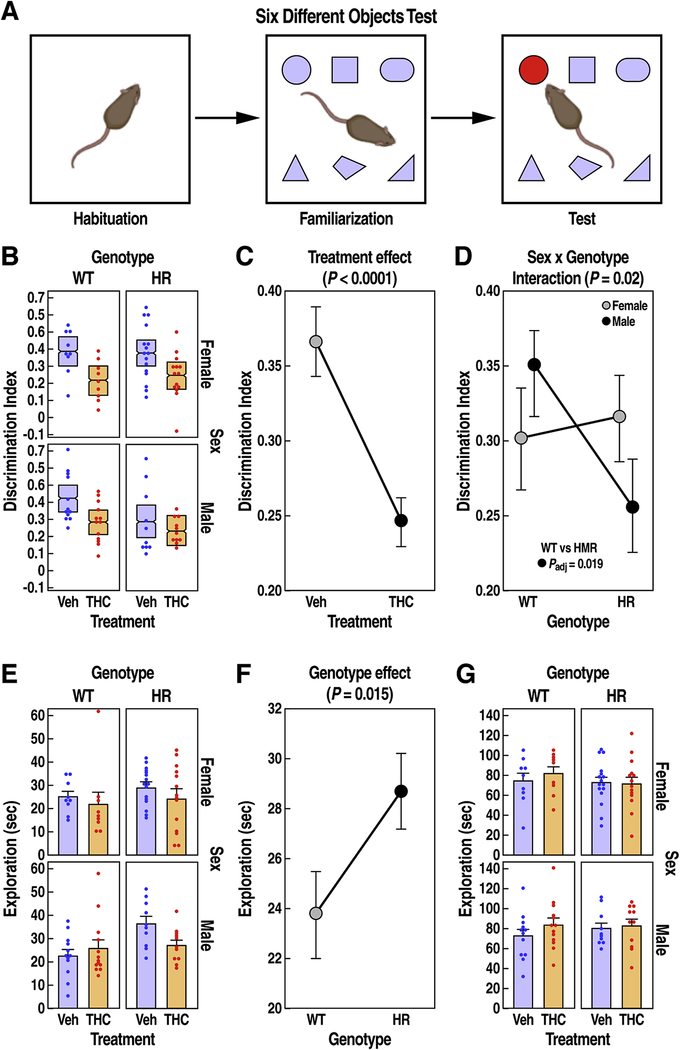
Figure 2: Six-different objects test in WT and HR mice after chronic adolescent exposure to THC.
(A) 6-DOT with habituation, familiarization, and test trials. (B) DI is shown as mean ± 95% confidence intervals. (C) THC reduced the DI (mean ± SEM) across all groups (main treatment effect, P < 0.0001, LMM). (D) Male HR mice showed a reduced DI (mean ± SEM) compared to male WT mice (sex x genotype interaction, P = 0.02, LMM). (E) Exploration time (s) in test trial is expressed as mean ± SEM. (F) HR mice spent longer time (mean ± SEM) exploring the objects in the test trial (main genotype effect, P = 0.015, LMM). (G) Exploration time (mean ± SEM) in familiarization trial did not change across groups. FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
Chronic THC exposure during adolescence decreased the DI (working memory) of mice across all groups by ~31% compared to the vehicle-treated control group (Fig. 2B–C, treatment effect, F1,86 = 16.4, P < 0.0001). HR mice also showed decreased DI, but the magnitude of the genotype effect varied among sexes (Fig. 2D, sex x genotype interaction, F1,86 = 5.7, P = 0.019). Compared to WT, female HR mice did not show impaired working memory, but male HR mice showed a decreased DI that was similar to the level of impairment caused by THC treatment in male WT mice (Fig. 2D, paired t test, t = 2.9, Padj = 0.019).
This assay also revealed that HR mice displayed a ~25% increase in novelty-induced exploratory activity compared to WT controls, while treatment had no detectable effect (Fig. 2E–F, genotype effect, F1,84 = 6.1, P = 0.015; treatment effect F1,81 = 2.3, P = 0.13). This effect was triggered by the novel object, as it was revealed during the test phase while the total exploratory activity toward objects did not vary among groups in the initial familiarization phase (Fig. 2G, main effects and interactions Ps > 0.3).
To further evaluate potential confounding effects of general locomotion or body weight having an impact on exploratory activity in this task, we analyzed changes in spontaneous locomotor activity in an empty arena or in body weight in WT and HR mice (Suppl. Fig. 1B–C). There was no effect of THC treatment, genotype, or sex on locomotor activity or body weight at the time of testing, suggesting that the deficits in working memory observed in WT and HR mice were not driven by changes in locomotion or body weight.
3.2. Social behaviors were impaired by chronic adolescent exposure to THC in male HR mice
To assess the long-term effects of chronic adolescent exposure to THC on social interaction behavior in WT and HR mice, we used the three-chamber social approach test in which a mouse is given the choice to spend time interacting with a novel mouse or a novel inanimate object, which are referred to as “exploration targets” in our analysis (Yang et al., 2011). We considered two aspects of social interaction: (1) time spent in the chamber with the novel mouse versus the novel object, and (2) time actively sniffing the novel mouse versus the novel object, which is a more specific measure of social interaction (Yang et al., 2011). Higher time spent in the chamber with or sniffing the novel mouse compared to the novel object was considered an index of “sociability”.
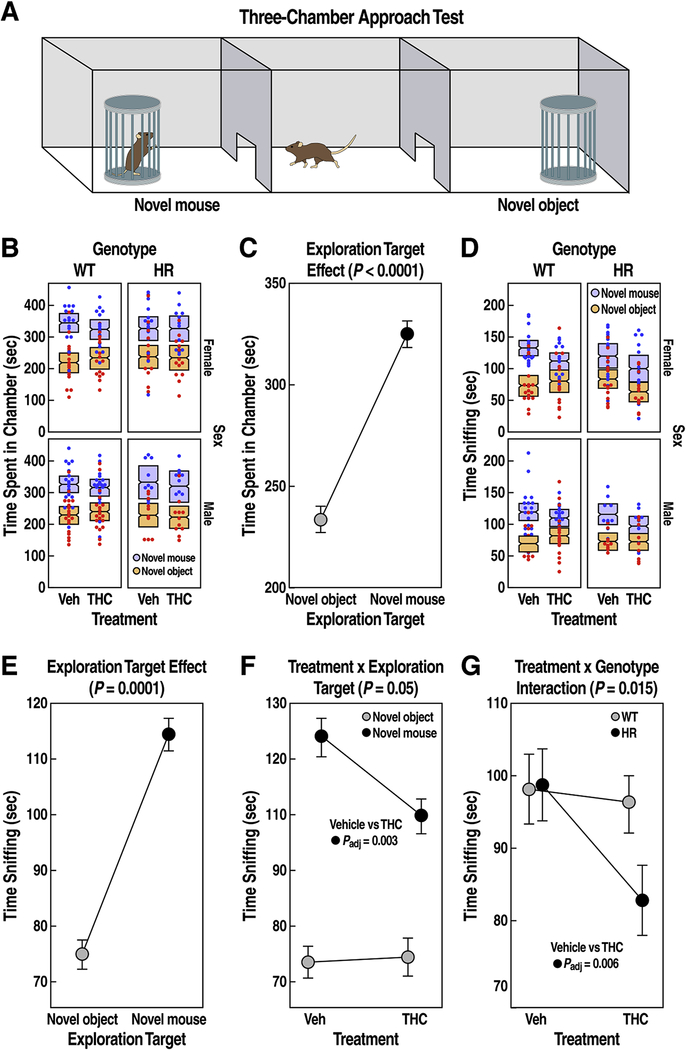
All mice spent longer time with novel mouse than the inanimate object (Fig. 3B–C, exploration target effect, F1,119 = 49.5, P < 0.0001), and sniffed the novel mouse more than the novel object (Fig. 3D–E, exploration target effect; F1,113 = 83.3, P < 0.0001), confirming that the behavioral assay can detect sociability across all groups. There were no significant effects of treatment, genotype and sex on time spent in the chamber (Fig. 3B, P > 0.1). In contrast, when analyzing time sniffing (Fig. 3D), the effect of chronic adolescent exposure to THC varied among the two exploration targets (Fig. 3F, treatment x exploration target interaction, F1,113 = 4.1, P = 0.046). Precisely, THC had no significant effect on time spent sniffing the novel inanimate object compared to vehicle (Fig. 3F, paired t test, t = 0.06, Padj = 1); however, THC caused a ~20% decrease in time spent sniffing the novel mouse compared to vehicle across all groups (Fig. 3F, t = 3.1, Padj = 0.003). The suppressive effect of THC on social interaction depended on the genotype (Fig. 3G, treatment x genotype interaction, F1,108 = 6.1, P = 0.015); a post-hoc analysis revealing that only HR mice treated with THC exhibited a significant reduction in overall time sniffing when compared to vehicle treated HR mice (paired t test, t = 3.2, Padj = 0.006). However, when a sociability index was calculated, we did not detect any effect of treatment or genotype (all effects and interaction Ps >0.1, Suppl. Fig. 2).
Figure 3: Social interaction behavior in WT and HR after chronic adolescent exposure to THC.
(A) Three-chamber interaction test diagram. Time (sec) spent in chamber (B) or sniffing (D) is expressed as mean ± 95% confidence intervals. (C) Mice spent more time (mean ± SEM) in the chamber or (D) more time sniffing (mean ± SEM) the novel mouse compared to the novel object (main exploration target effect, P < 0.0001, LMM). (E-F) THC reduced social investigation (post-hoc pairwise t test Padj = 0.003; treatment x exploration target interaction, P = 0.05, LMM). (G) HR exposed to THC during adolescence showed reduced social interaction behavior (post-hoc pairwise t test Padj = 0.006; treatment x genotype interaction, P = 0.015, LMM). FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
3.3. Chronic adolescent exposure to THC increased anxiety-like behavior in female HR mice
To evaluate how impaired Reelin signaling influences anxiety-like behavior in mice chronically exposed to THC during adolescence, we compared WT and HR mice in the LD (Fig. 4A) and OF (Fig. 4F) tests. These behavioral assays examine anxiety-like responses by which animals avoid illuminated or open areas (Crawley and Goodwin, 1980; Bailey and Crawley, 2009). Decreased anxiety (increased disinhibition) is represented by mice spending more time in the light or open compartments, and by having a shorter latency to enter the brightly lit or center chambers.
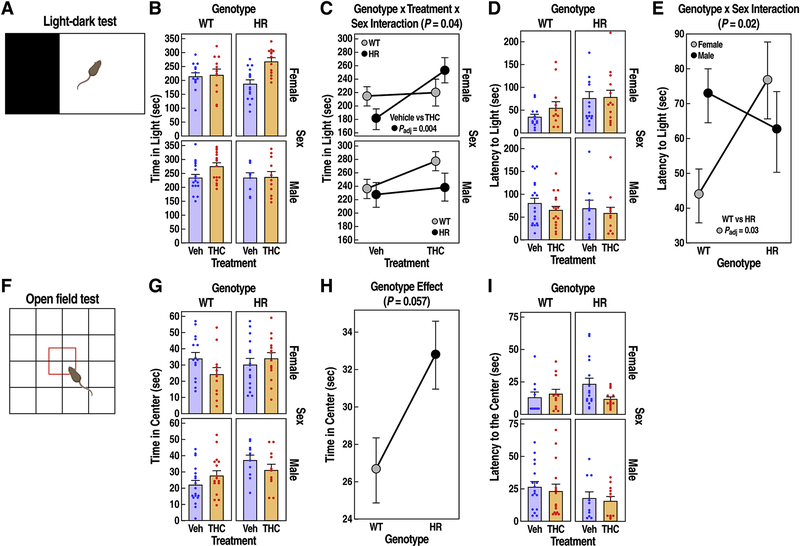
Figure 4: Anxiety-like responses in WT and HR following chronic adolescent exposure to THC.
(A) Light-dark test schematic. (B) Time (sec) spent in the light compartment is expressed as mean ± SEM. (C) Female HR treated with THC during adolescence showed reduced anxiety (treatment x genotype x sex interaction, P = 0.04, LMM). (D) Latency (sec) to enter the illuminated chamber is expressed as mean ± SEM. (E) Female HR mice showed increased latency (sec) to enter the light compared to WT controls (genotype x sex interaction, P = 0.02, LMM). (F) Open field test schematic. (G) Time (sec) spent in the center of the arena is expressed as mean ± SEM. (H) HR mice showed reduced anxiety (main genotype effect, P = 0.057, LMM). (I) Latency (sec) to enter the center compartment is expressed as mean ± SEM and did not change across groups. FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
In the LD test (Fig. 4B), THC increased the overall time spent in the light compartment (treatment effect, F1,102 = 8.5, P =0.0043), but the strength of these effects varied among genotypes and sexes (Fig. 4C, treatment x genotype x sex interaction, F1,97.5 = 4.4, P = 0.04). This effect was mainly driven by female HR mice treated with THC, which spent 43% longer time in the light compared to the vehicle-treated group (Fig. 4C, paired t test, t = 3.6, Padj = 0.004). A separate analysis revealed that the latency to enter the light chamber was also influenced by genotype and sex (Fig. 4D–E, sex x genotype interaction, F1,47 = 5, P = 0.0197). Female HR mice showed ~18% increase in latency compared to female WT, indicating an increased baseline anxiety-like responses (paired t test, t = 2.5, Padj = 0.03). However, the latency to enter the light compartment was not significantly influenced by THC (treatment effect, F1,97 = 0.02, P = 0.88).
In the open field test (Fig. 4G), HR mice spent ~18% more time in the center (Fig. 4H, genotype effect, F1,102 = 3.7, P = 0.057 marginally significant), suggesting a reduced anxiety-like behavior. Time spent in center also varied among sexes and treatment groups (treatment x genotype x sex interaction, F1,99 = 6.1, P = 0.016); however, despite this significant overall variability, a post-hoc analysis did not identify significant differences between specific pairs of groups. Latency to enter the open area did not vary among groups (Fig. 4I, all P > 0.2).
3.4. Male HR mice showed enhanced ability to strive against stress following chronic adolescent exposure to THC
To test the effect of adolescent exposure to THC on reactivity to aversive conditions in WT and HR mice, we performed the TS test (Fig. 5A), an assay commonly used to screen anti-depressive drugs and to measure behavioral despair in mice (Can et al., 2012). An increased mobility has been associated with enhanced ability to strive against stress and is broadly related to active coping, impulsive and aggressive behaviors (Strekalova et al., 2004; Brockhurst et al., 2015).
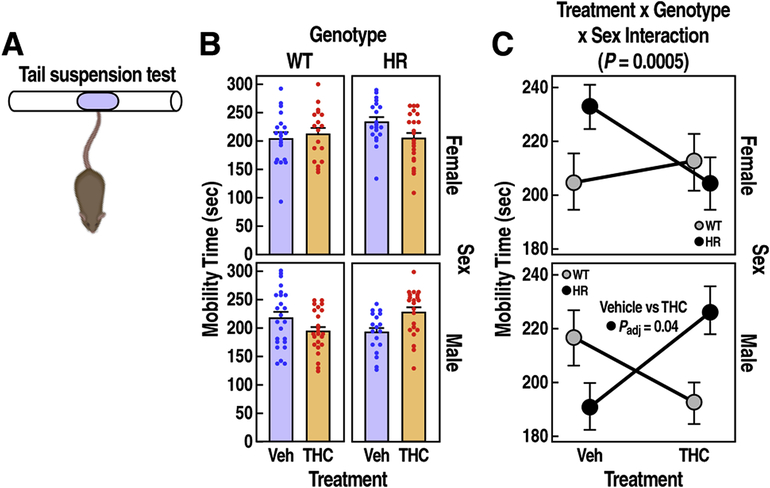
Figure 5: Reactivity to aversive conditions in WT and HR mice following chronic adolescent exposure to THC.
(A) Tail suspension test diagram. (B) Mobility time (sec) is expressed as mean ± SEM. (C) Male HR mice treated with THC during adolescence showed increased mobility (treatment x genotype x sex interaction, P = 0.0005, LMM). FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
We quantified total mobility time in a 6-minute tail suspension assay in WT and HR mice (Fig. 5B). THC treatment influenced total mobility time, but the effect varied among sexes and genotypes (Fig. 5C, treatment x sex x genotype interaction, F1,158 = 12.8, P = 0.0005). While THC did not significantly change the mobility time of either male or female WT mice compared to the vehicle controls (paired t test, t = 1.9, Padj = 0.07; t = 0.5, Padj = 0.6, respectively), it induced a 19% increase in mobility time for male HR mice (Fig. 5C, paired t test, t = 2.6, Padj = 0.04).
3.5. Reelin deficiency led to enhanced startle responses and pre-pulse inhibition
To evaluate psychotic-like behaviors in WT and HR mice following chronic adolescent exposure to THC, we quantified the ASR and the % PPI (Fig. 6A). The ASR is a reflexive reaction to a sudden acoustic stimulus (Swerdlow et al., 2001; Geyer et al., 2002). The % PPI is used as a measure of sensorimotor gating, which occurs when a weak pre-pulse stimulus suppresses the response to the subsequent startling stimulus. PPI is impaired in schizophrenia patients and serves as an animal model of schizophrenia (Cadenhead et al., 1993).
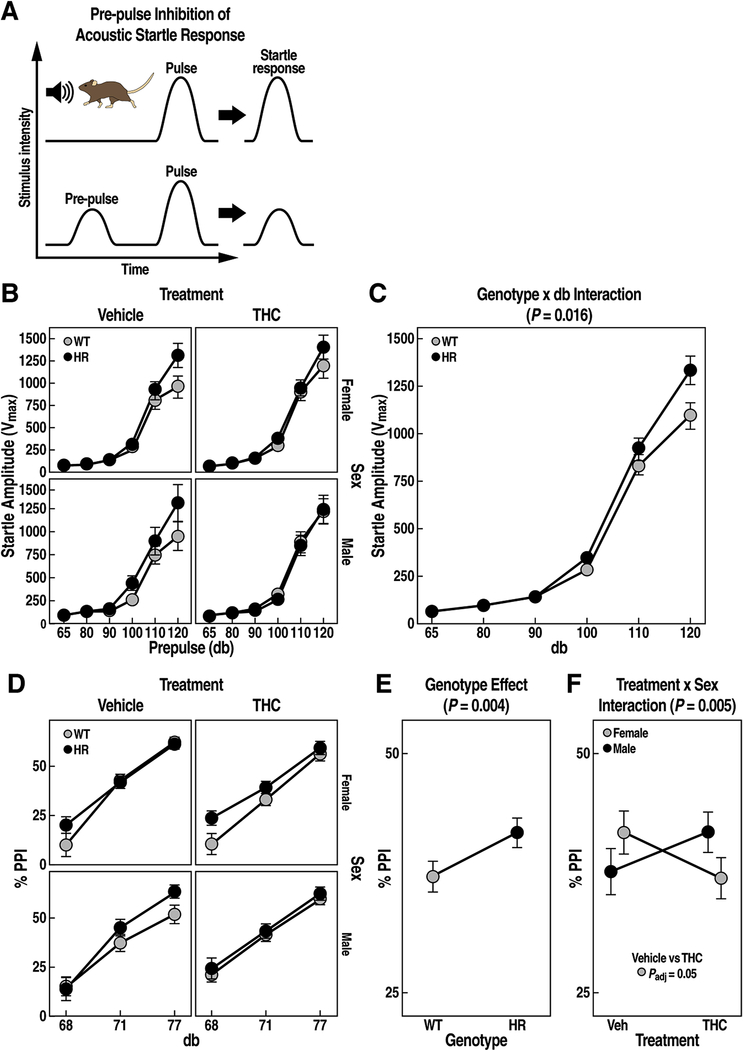
Figure 6: Acoustic startle reflex of pre-pulse inhibition in WT and HR mice following chronic adolescent exposure to THC.
(A) Pre-pulse inhibition of acoustic startle test diagram. (B) Startle amplitude is expressed as Vmax ± SEM. (C) HR mice show increased startle response (genotype x db interaction, P = 0.016, LMM). (D) Pre-pulse inhibition is expressed as % PPI ± SEM. (E) HM mice exhibited higher % PPI compared to WT controls (main genotype effect, P = 0.004, LMM). (F) Female treated with THC during adolescence showed reduced %PPI compared to vehicle (treatment x sex interaction, P = 0.005, LMM). FDR adjusted P < 0.05 for post-hoc pairwise t tests are reported.
We first examined the differences in ASR of WT and HR mice at various acoustic intensities (65, 80, 90, 100, 110, 120 db, Fig. 6B). THC did not influence ASR (treatment effect, F1,451 = 0.7, P = 0.4). Compared to WT, HR mice exhibited an overall ~3% higher startle response (genotype effect, F1,451 = 7.2, P = 0.008), which varied by the acoustic intensity of the pulse (Fig. 6C, genotype x db interaction, F5,408 = 2.8, P = 0.016). The increased startle tended to be stronger at 120db (Fig. 6B, paired t test, t = 2, Padj = 0.08 marginally significant).
When examining the % PPI at three pre-pulse intensities (Fig. 6D, 68, 71 and 77 db), HR mice showed an overall ~13% increase in % PPI compared to WT mice (Fig. 6E, genotype effect, F1,482 = 8.6, P = 0.004). Adolescent exposure to THC also had an effect on % PPI that varied among sexes (Fig. 6F, treatment x sex interaction, F1,482 = 8, P = 0.005). Female mice treated with THC tended to show ~12 % reduction in % PPI compared to vehicle-treated mice (Fig. 6F, paired t test, t = 2, Padj = 0.05).
3.6. THC and Reelin deficiency influenced behavioral domains that were associated with cognition and disinhibition
We performed a factor analysis to determine whether the multiple behavioral responses We performed a factor analysis to determine whether the multiple behavioral responses influenced by THC and Reelin deficiency.
The factor analysis identified three unique behavioral domains (factors), which explained 57% of total variance (Fig. 7A). The first factor explained 23.8% of the total variance and contained outcomes (positive loadings) from the social interaction and working memory tests (e.g., time sniffing novel mouse, discrimination index). As factor 1 largely reflected measures of cognitive functions, we named it “cognition”. The second factor explained 15.7% of the total variance and was named “stress reactivity” because the main outcome that contributed to this cluster was the mobility time of the TS test. The third factor (17% of the total variance) was named “disinhibition” as it contained behaviors that reflected increased exploratory or disinhibited behavior (e.g., time in the center of the open field, novel object exploration).
To determine whether these behavioral domains differed between treatments, genotypes, or sexes, we analyzed the rotator factor scores for each subject. We found that the behavioral domains were significantly influenced by treatment or genotype (Fig. 7B, treatment x behavioral domains interaction, F2,142 = 5.7, P = 0.004; Fig. 7C, genotype x behavioral domains interaction, F2,142 = 3.4, P = 0.035; respectively). Post-hoc analysis revealed that cognition was significantly decreased by THC (Fig. 7B, paired t test, t = 3.1, Padj = 0.006), and disinhibition was significantly increased by Reelin deficiency (Fig. 7C, paired t test, t = 2.8, Padj = 0.014). Neither treatment, genotype, nor sex significantly influenced the third behavioral domain, stress reactivity.
Overall, this exploratory data analysis is consistent with the results of the separate behavioral tests by indicating that adolescent exposure to THC detrimentally affected cognitive functions, and it revealed that reduced expression of Reelin led to disinhibitory behaviors, which were intensified by THC treatment in a sex-specific manner.
Discussion
The present study revealed for the first time that reduced levels of Reelin influences behavioral abnormalities caused by heavy consumption of THC during adolescence, in a sex-dependent manner. Here we discuss the potential implications and limitation of our findings.
Influence of THC and Reelin deficiency on cognitive functions
In line with previous reports in rodent models and clinical studies in humans, we found that chronic adolescent exposure to THC impaired working memory (Renard et al., 2014; Rubino and Parolaro, 2016; Hurd et al., 2019). Additionally, HR male mice showed lower working memory in comparison to the WT littermates and to a similar extent as did chronic adolescent exposure to THC. Although it is the first time that HR mice are tested in a task that measures memory span, such as the 6-DOT, this finding is consistent with studies demonstrating the crucial role of Reelin in mechanisms underlying learning and memory (Weeber et al., 2002; Rogers et al., 2013; Iafrati et al., 2014; Telese et al., 2015). Overall, these findings show that our experimental model reproduced previously published results; however, the lack of a genotype x treatment interaction in the 6-DOT suggests that the underlying mechanisms may be independent.
We found that social interaction was impaired by chronic adolescent exposure to THC with the strongest effects in HR mice. These observations are in line with evidence in human and preclinical studies showing social deficits induced by THC (Long et al., 2010). Only few studies have examined social behaviors of the HR mice and did not report prominent social deficits (Podhorna and Didriksen, 2004; Macri et al., 2010; Michetti et al., 2014). Thus, our study revealed for the first time that social interaction is reduced by THC in mice with Reelin deficiency. It will be important to examine if these behavioral changes are also associated with cellular and molecular changes in areas implicated in social behavior, including PFC, amygdala and striatum (Ko, 2017).
Consistently, the factor analysis revealed that variables from both working memory and social interaction tests contributed to a behavioral domain, named ‘cognition’, which was negatively influenced by adolescent exposure to THC. The negative effects of THC on cognitive behaviors are known to be mediated by CB1 receptors in rodents (Lichtman and Martin, 1996; Nava et al., 2001; Niyuhire et al., 2007; Wise et al., 2009; Puighermanal et al., 2013). Therefore, it is reasonable to infer that the behavioral effects induced by THC in our study are dependent on CB1 receptors; however, future research is necessary to confirm this.
Reelin deficiency is associated with disinhibitory phenotypes
We speculate that multiple behavioral responses exhibited by HR mice may reflect general behavioral disinhibition. In rodents, behavioral disinhibition is measured as a function of increased exploratory activity towards unfamiliar objects or environments, and has been associated with compulsive drug taking (Davis et al., 2008; Flagel et al., 2010; Belin et al., 2011). In humans, behavioral disinhibition reflects personality traits that encompasses impulsivity, risk-taking and novelty seeking phenotypes; these behavioral patterns are more pronounced in adolescence and have been linked to addiction susceptibility (Young et al., 2009). Here, we found that, compared to WT, both male and female HR mice explored the novel objects for longer time in the 6-DOT. Additionally, female HR mice treated with THC showed signs of reduced inhibitory control as they spent more time in the center chamber of the OF test or in the brightly lit compartment of the LD test. Consistently, variables from these tests contributed to the same behavioral domain (‘disinhibition’) revealed by the factor analysis, which was positively influenced by Reelin deficiency. These observations are also in line with a previous study showing decreased anxiety of HR mice in the elevated plus maze and increased motor impulsivity (Ognibene et al., 2007).
Collectively, these results suggest that Reelin deficiency leads to loss of inhibitory control, which may underlie behavioral traits linked to addiction vulnerability. Further supporting this hypothesis, in a recent genome-wide association study (GWAS), a variant of the Reelin gene was associated with higher likelihood to consume alcohol (P = 4 × 10−9, variant and risk allele rs756747-T) (Karlsson Linner et al., 2019). It will be important to experimentally test whether HR mice show increased drug taking behavior when exposed to different drugs of abuse.
Reelin deficiency leads to abnormal responses to aversive conditions
We observed abnormal behavior in HR mice in response to aversive conditions. First, male HR mice treated with THC showed prolonged mobility time in the TS test compared to vehicle group. This behavior has been linked to proactive coping in response to a threat (Strekalova et al., 2004; Brockhurst et al., 2015). Second, HR mice exhibited enhanced startle reactivity to acoustic stimuli compared to WT mice, which was not influenced by THC treatment. In a previous study, the startling response of HR mice did not change following a single acoustic pulse of 105db; however, in our study, we used five startling pulses from 80db to 120db that likely increased the sensitivity of the task. Elevated ASR has been observed in mental conditions associated with impaired emotional reactivity, such as posttraumatic stress disorders (Orr et al., 1995) and obsessive-compulsive disorders (Kumari et al., 2001). These observations led us speculate that reduced expression of Reelin may lead to altered emotional reactivity. This hypothesis deserves further examination of the HR mice using tasks specifically designed to assess impulsivity and aggression.
Reelin as a susceptibility factors for psychiatric disorders
The array of behavioral phenotypes exhibited by HR mice in our study, encompassing memory impairments, social deficits, poor inhibitory control, and altered stress responses, is reminiscent of the behaviors characterizing numerous psychiatric conditions, ranging from autism spectrum disorders (ASD) to schizophrenia and substance use disorders. Consistently, a role of Reelin in the development of these disorders is supported by several lines of evidence. In particular, whole exome sequencing studies identified de novo mutations in the Reelin gene in individuals with ASD (Neale et al., 2012; Iossifov et al., 2014; Wang et al., 2014). Further support for Reelin involvement in psychiatric disorders is provided by the observation of reduced expression of Reelin transcript or protein in postmortem brains of individuals affected by ASD (Fatemi et al., 2001), and schizophrenia (Guidotti et al., 2000; Ruzicka et al., 2007; Habl et al., 2012). These observations suggest that altered Reelin signaling may be a vulnerability factor for psychiatric disorders and that the HR mice represent a valuable animal model for translational research. However, we did not observe any PPI deficits in HR mice. In contrast, a previous study showed that a single in vivo injection of Reelin protein increases % PPI (Rogers et al., 2013). It is possible that, in our study, the elevated startle response observed in HR mice may act as a confounding factor for accurately assessing the effect of Reelin deficiency. It is also possible that impaired Reelin signaling contributes mainly to the cognitive, social and emotional deficits associated with schizophrenia, which are referred to as negative symptoms, as opposed to positive symptoms that include psychotic-like behaviors (Kirkpatrick et al., 2006).
Notably, THC reduced % PPI in female WT mice, suggesting that a history of drug exposure leads to psychotic-like behaviors. Conflicting results have been reported concerning the effects of adolescent chronic exposure to THC on psychotic-like behaviors in rodents (Rodriguez et al., 2017; Todd et al., 2017; Ibarra-Lecue et al., 2018). Differences in THC doses, mice genetic background and specific experimental conditions among different research groups may explain some of these conflicts.
Sex differences in behavioral responses associated with Reelin haploinsufficiency
Our study revealed numerous sex differences in the behavioral abnormalities associated with Reelin deficiency and/or adolescent exposure to THC. Because we did not observe sex differences in the plasma levels of THC following acute or chronic THC administration, we conclude that other factors are responsible for driving sex differences in the observed behavior. Male HR mice were more sensitive to working memory impairments, as well as to emotional reactivity in the TS test. In contrast, female mice, showed reduced anxiety-like behaviors (HR) or reduced % PPI (WT) in response to THC. Consistently, previous finding in rodents showed that chronic adolescent exposure to cannabinoids is associated with numerous behavioral sex differences; however, the knowledge of the underlying mechanisms remains limited (Craft et al., 2013). Given the critical role of Reelin brain development, it is possible that Reelin influences the development of the endocannabinoid system in a sex-specific manner, which could lead to sex differences in the behavioral effects of THC.
Limitations of the experimental model
Our study focused on the adolescent exposure to THC due to its relevance to human studies showing that cannabis is the most widely used illicit drug among adolescents. We explicitly note that the observed effects of adolescent exposure may also occur following chronic adult exposure, which remains to be investigated.
Our study used a single dosage of THC (10mg/kg) and did not examine overall dose response curves, which would permit better understanding of potential sex and genotype differences. However, this dosage can elevate plasma levels of THC in rodents similar to those detected in humans using cannabis; hence, it should adequately model heavy chronic THC exposure, which have been linked to detrimental health effects. Specifically, we show that 30 minutes after a single injection of THC (10 mg/kg) in mice the plasma concentrations of THC were similar to those reported in humans after smoking marijuana or vaping ethanolized (pure) THC (>100ng/ml) (Abrams et al, 2007; Zuurman et al, 2008, Huestis et al, 1992, Heustis et al, 2007). Moreover, we show that 24 hours after the last daily injection of chronic (21 days) THC treatment in mice the plasma levels of THC were similar to those detected in cannabis frequent users (> 6ng/ml) (Desrosiers et al., 2014; Lee et al., 2015).
In conclusion, our study indicates that Reelin deficiency contributes to behavioral alterations induced by THC exposure and suggests that elucidating Reelin signaling will improve our understanding of neurobiological mechanisms underlying behavioral traits relevant to the development of psychiatric conditions.
Supplementary Material
Supplementary Figure 1: THC plasma levels, locomotor activity and body weight. (A) THC plasma levels (mean ng/ml ±SEM) from female (F) and male (M) WT mice after one single injection of THC at PND 48 (acute) or 21 consecutive injections from PND 28 to PND 48 (chronic). (B) Twenty-minute locomotor activity is expressed as mean distance traveled (m) ± SEM for every 5-minute intervals. (C) Body weight (g) change is expressed as mean ± SEM for the THC administration course (day 1 to 14), and for day 63 when the behavioral testing started.
Supplementary Figure 2: Sociability index in WT and HR mice following chronic adolescent exposure to THC. (A) Sociability index calculated using time spent in the chamber with novel mouse versus novel object is shown as mean ± 95% confidence intervals. (B) Sociability index calculated using time sniffing novel mouse versus novel object is shown as mean ± 95% confidence intervals. Treatment, genotype and sex had no significant effect on both sociability indices.
Reelin deficiency influenced behavioral abnormalities caused by heavy consumption of THC during adolescence, in a sex-dependent manner
Social interaction was reduced by THC in HR mice
Decreased anxiety-like behavior was observed in female HR mice treated with THC
Enhanced stress reactivity was observed in male HR mice
Acknowledgments
This work was supported by the National Institute on Drug Abuse [DP1DA042232 to FT]. We thank R.F. Hechinger and S. Roige-Sanchez for helpful discussions and critical reading of the manuscript; A. Turner, H. Taylor, S. Nolan, and J. Hightower for technical assistance.
Footnotes
Declarations of interest: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL (2007) Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther 82:572–578. [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S (2013) Maturation of the adolescent brain. Neuropsychiatr Dis Treat 9:449–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN (2009) Anxiety-Related Behaviors in Mice. In: Methods of Behavior Analysis in Neuroscience (nd, Buccafusco JJ, eds). Boca Raton (FL). [Google Scholar]
- Bartholomew DJ (2008) Analysis of multivariate social science data, 2nd Edition. Boca Raton: CRC Press. [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouamrane L, Scheyer AF, Lassalle O, Iafrati J, Thomazeau A, Chavis P (2016) Reelin-Haploinsufficiency Disrupts the Developmental Trajectory of the E/I Balance in the Prefrontal Cortex. Front Cell Neurosci 10:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhurst J, Cheleuitte-Nieves C, Buckmaster CL, Schatzberg AF, Lyons DM (2015) Stress inoculation modeled in mice. Transl Psychiatry 5:e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Geyer MA, Braff DL (1993) Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry 150:1862–1867. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD (2012) The tail suspension test. J Vis Exp:e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB (1966) The Scree Test For The Number Of Factors. Multivariate Behav Res 1:245–276. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Luijten M, Feldstein Ewing SW (2018) Adolescent resilience to addiction: a social plasticity hypothesis. Lancet Child Adolesc Health 2:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Marusich JA, Wiley JL (2013) Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci 92:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK (1980) Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav 13:167–170. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723. [DOI] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB (2008) The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav 90:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers NA, Himes SK, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA (2014) Phase I and II cannabinoid disposition in blood and plasma of occasional and frequent smokers following controlled smoked cannabis. Clin Chem 60:631–643. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary JM, Halt AR, Realmuto GR (2001) Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord 31:529–535. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H (2014) Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology 76 Pt B:425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H (2010) An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology 35:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett M, Fitzmaurice NML, and Ware James H. (2011) Applied Longitudinal Analysis, 2nd Edition.
- Geyer MA, McIlwain KL, Paylor R (2002) Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry 7:1039–1053. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E (2000) Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry 57:1061–1069. [DOI] [PubMed] [Google Scholar]
- Habl G, Schmitt A, Zink M, von Wilmsdorff M, Yeganeh-Doost P, Jatzko A, Schneider-Axmann T, Bauer M, Falkai P (2012) Decreased reelin expression in the left prefrontal cortex (BA9) in chronic schizophrenia patients. Neuropsychobiology 66:57–62. [DOI] [PubMed] [Google Scholar]
- Hochberg Ba (1995) Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57:289–300. [Google Scholar]
- Huber PJ (1973) Robust Regression: Asymptotics, Conjecture, and Monte Carlo. Annals of Statistics 1:799–821. [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ (1992) Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 16:276–282. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, Melis M (2019) Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J Neurosci 39:8250–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iafrati J, Orejarena MJ, Lassalle O, Bouamrane L, Gonzalez-Campo C, Chavis P (2014) Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B-NMDARs and the mTOR pathway. Mol Psychiatry 19:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Lecue I, Mollinedo-Gajate I, Meana JJ, Callado LF, Diez-Alarcia R, Uriguen L (2018) Chronic cannabis promotes pro-hallucinogenic signaling of 5-HT2A receptors through Akt/mTOR pathway. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43:2028–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iossifov I et al. (2014) The contribution of de novo coding mutations to autism spectrum disorder. Nature 515:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kubo KI, Nakajima K (2016) Reelin and Neuropsychiatric Disorders. Front Cell Neurosci 10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, & Patrick ME (2018) Monitoring the Future national survey results on drug use, 1975–2017: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan [Google Scholar]
- Karlsson Linner R et al. (2019) Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet 51:245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, Huestis MA (2009) Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol 33:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Zhang Y, Boehm SL, 2nd (2019) Acute Cannabinoids Produce Robust Anxiety-Like and Locomotor Effects in Mice, but Long-Term Consequences Are Age- and Sex-Dependent. Front Behav Neurosci 13:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Fenton WS, Carpenter WT Jr., Marder SR (2006) The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull 32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J (2017) Neuroanatomical Substrates of Rodent Social Behavior: The Medial Prefrontal Cortex and Its Projection Patterns. Front Neural Circuits 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari V, Kaviani H, Raven PW, Gray JA, Checkley SA (2001) Enhanced startle reactions to acoustic stimuli in patients with obsessive-compulsive disorder. Am J Psychiatry 158:134–136. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31. [DOI] [PubMed] [Google Scholar]
- Lee D, Bergamaschi MM, Milman G, Barnes AJ, Queiroz RH, Vandrey R, Huestis MA (2015) Plasma Cannabinoid Pharmacokinetics After Controlled Smoking and Ad libitum Cannabis Smoking in Chronic Frequent Users. J Anal Toxicol 39:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR (1996) Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 126:125–131. [DOI] [PubMed] [Google Scholar]
- Lisdahl KM, Sher KJ, Conway KP, Gonzalez R, Feldstein Ewing SW, Nixon SJ, Tapert S, Bartsch H, Goldstein RZ, Heitzeg M (2018) Adolescent brain cognitive development (ABCD) study: Overview of substance use assessment methods. Dev Cogn Neurosci 32:80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T (2010) A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. The international journal of neuropsychopharmacology 13:861–876. [DOI] [PubMed] [Google Scholar]
- Lossi L, Castagna C, Granato A, Merighi A (2019) The Reeler Mouse: A Translational Model of Human Neurological Conditions, or Simply a Good Tool for Better Understanding Neurodevelopment? J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri S, Biamonte F, Romano E, Marino R, Keller F, Laviola G (2010) Perseverative responding and neuroanatomical alterations in adult heterozygous reeler mice are mitigated by neonatal estrogen administration. Psychoneuroendocrinology 35:1374–1387. [DOI] [PubMed] [Google Scholar]
- Manly BFJ, Navarro Alberto JA Multivariate statistical methods : a primer, Fourth edition. Edition.
- Michetti C, Romano E, Altabella L, Caruso A, Castelluccio P, Bedse G, Gaetani S, Canese R, Laviola G, Scattoni ML (2014) Mapping pathological phenotypes in reelin mutant mice. Front Pediatr 2:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F, Carta G, Colombo G, Gessa GL (2001) Effects of chronic Delta(9)-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology 41:392–399. [DOI] [PubMed] [Google Scholar]
- Neale BM et al. (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA (2016) Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology 109:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S, Yabut O, D’Arcangelo G (2008) The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J Neurosci 28:10339–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Martin BR, Lichtman AH (2007) Exposure to marijuana smoke impairs memory retrieval in mice. J Pharmacol Exp Ther 322:1067–1075. [DOI] [PubMed] [Google Scholar]
- Ognibene E, Adriani W, Granstrem O, Pieretti S, Laviola G (2007) Impulsivity-anxiety-related behavior and profiles of morphine-induced analgesia in heterozygous reeler mice. Brain Res 1131:173–180. [DOI] [PubMed] [Google Scholar]
- Olivito L, Saccone P, Perri V, Bachman JL, Fragapane P, Mele A, Huganir RL, De Leonibus E (2016) Phosphorylation of the AMPA receptor GluA1 subunit regulates memory load capacity. Brain Struct Funct 221:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK (1995) Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol 104:75–82. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Didriksen M (2004) The heterozygous reeler mouse: behavioural phenotype. Behav Brain Res 153:43–54. [DOI] [PubMed] [Google Scholar]
- Puighermanal E, Busquets-Garcia A, Gomis-Gonzalez M, Marsicano G, Maldonado R, Ozaita A (2013) Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Zhao LF, Korwek KM, Weeber EJ (2006) Differential reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J Neurosci 26:12943–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP KM (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Renard J, Krebs MO, Le Pen G, Jay TM (2014) Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci 8:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G, Neugebauer NM, Yao KL, Meltzer HY, Csernansky JG, Dong H (2017) Delta9-tetrahydrocannabinol (Delta9-THC) administration after neonatal exposure to phencyclidine potentiates schizophrenia-related behavioral phenotypes in mice. Pharmacology, biochemistry, and behavior 159:6–11. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Rusiana I, Trotter J, Zhao L, Donaldson E, Pak DT, Babus LW, Peters M, Banko JL, Chavis P, Rebeck GW, Hoe HS, Weeber EJ (2011) Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem 18:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Zhao L, Trotter JH, Rusiana I, Peters MM, Li Q, Donaldson E, Banko JL, Keenoy KE, Rebeck GW, Hoe HS, D’Arcangelo G, Weeber EJ (2013) Reelin supplementation recovers sensorimotor gating, synaptic plasticity and associative learning deficits in the heterozygous reeler mouse. J Psychopharmacol 27:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D (2016) The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biol Psychiatry 79:578–585. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, Sagheddu C, Ligresti A, Tonini R, Di Marzo V, Parolaro D (2015) Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 73:60–69. [DOI] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A (2007) Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry 12:385–397. [DOI] [PubMed] [Google Scholar]
- Sannino S, Russo F, Torromino G, Pendolino V, Calabresi P, De Leonibus E (2012) Role of the dorsal hippocampus in object memory load. Learn Mem 19:211–218. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL (2016) The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci Biobehav Rev 70:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine K, Kubo K, Nakajima K (2014) How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci Res 86:50–58. [DOI] [PubMed] [Google Scholar]
- Stephens D (1996) Hearing rehabilitation in a psychosocial framework. Scand Audiol Suppl 43:57–66. [PubMed] [Google Scholar]
- Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Moghaddam B (2011) The neurobiology of adolescence: changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev 35:1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156:194–215. [DOI] [PubMed] [Google Scholar]
- Telese F, Ma Q, Perez PM, Notani D, Oh S, Li W, Comoletti D, Ohgi KA, Taylor H, Rosenfeld MG (2015) LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 86:696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC (2017) Interactions between cannabidiol and Delta(9)-THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 27:132–145. [DOI] [PubMed] [Google Scholar]
- Toth M, Ziegler M, Sun P, Gresack J, Risbrough V (2013) Impaired conditioned fear response and startle reactivity in epinephrine-deficient mice. Behav Pharmacol 24:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler KR, Nass SR, Crowe MS, Gross JD, Jones MS, McKitrick AW, Siderovski DP, Kinsey SG (2018) Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug Alcohol Depend 191:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND (2016) Effects of Cannabis Use on Human Behavior-Reply. JAMA Psychiatry 73:996. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hong Y, Zou L, Zhong R, Zhu B, Shen N, Chen W, Lou J, Ke J, Zhang T, Wang W, Miao X (2014) Reelin gene variants and risk of autism spectrum disorders: an integrated meta-analysis. Am J Med Genet B Neuropsychiatr Genet 165B:192–200. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, Sweatt JD, Herz J (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem 277:39944–39952. [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Yarnell S, Radhakrishnan R, Ball SA, D’Souza DC (2016) Marijuana Legalization: Impact on Physicians and Public Health. Annu Rev Med 67:453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Thorpe AJ, Lichtman AH (2009) Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacology 34:2072–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN (2011) Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8:Unit 8 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK (2009) Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol 118:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur AF IE (2016) A protocol for conducting and presenting results of regression-type analyses. Methods in Ecology and Evolution 7:636–645. [Google Scholar]
- Zuurman L, Roy C, Schoemaker RC, Hazekamp A, den Hartigh J, Bender JC, Verpoorte R, Pinquier JL, Cohen AF, van Gerven JM (2008) Effect of intrapulmonary tetrahydrocannabinol administration in humans. J Psychopharmacol 22:707–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: THC plasma levels, locomotor activity and body weight. (A) THC plasma levels (mean ng/ml ±SEM) from female (F) and male (M) WT mice after one single injection of THC at PND 48 (acute) or 21 consecutive injections from PND 28 to PND 48 (chronic). (B) Twenty-minute locomotor activity is expressed as mean distance traveled (m) ± SEM for every 5-minute intervals. (C) Body weight (g) change is expressed as mean ± SEM for the THC administration course (day 1 to 14), and for day 63 when the behavioral testing started.
Supplementary Figure 2: Sociability index in WT and HR mice following chronic adolescent exposure to THC. (A) Sociability index calculated using time spent in the chamber with novel mouse versus novel object is shown as mean ± 95% confidence intervals. (B) Sociability index calculated using time sniffing novel mouse versus novel object is shown as mean ± 95% confidence intervals. Treatment, genotype and sex had no significant effect on both sociability indices.