Abstract
Fungicides are widely used in conventional agriculture to control fungal diseases, but may also affect non-target microorganisms such as arbuscular mycorrhizal (AM) fungi. These root symbionts develop extended mycelial networks within the soil via mechanisms such as anastomosis that indistinctly concerns intact and damaged hyphae, the latter being named hyphal healing mechanism (HHM). The HHM differs between Glomeraceae and Gigasporaceae. However, the effects of fungicides on this mechanism in unknown. Here, the impact of azoxystrobin, pencycuron, flutolanil, and fenpropimorph at 0.02 and 2 mg L–1 were tested in vitro on the HHM of Gigaspora sp. MUCL 52331 and Rhizophagus irregularis MUCL 41833, and repair events visualized carefully under a dissecting bright-field light microscope. Azoxystrobin was the more detrimental for both AM fungi at 2 mg L–1, while fenpropimorph impacted only R. irregularis (stimulating at low and inhibiting at high concentration). Conversely, flutolanil and pencycuron did not impact any of the two AM fungi. The mechanisms involved remains to be elucidated, but perturbation in the still-to-be firmly demonstrated spitzenkörper or in sterols content as well as a process of hormesis are possible avenues that deserve to be explored in view of a rationale management of chemicals to control fungal pathogens without harming the beneficial AM fungi.
Keywords: arbuscular mycorrhizal fungi, extraradical mycelium, hyphal healing mechanism, fungicides, growing hyphal tips
Introduction
The extraradical mycelium (ERM) of arbuscular mycorrhizal (AM) fungi is fundamental in plant nutrition and maintenance of biological fertility in agroecosystems (Pepe et al., 2018; de Novais et al., 2019a). Keeping the integrity of this belowground network is thus essential, not only for the survival of these fungi but also for their manifold benefits to plants. Mechanisms such as anastomosis and healing are pivotal for the spread and maintenance/survival of AM fungal colonies (de la Providencia et al., 2005). Both mechanisms drastically differ between genera (de la Providencia et al., 2005; Voets et al., 2006) and could be affected by agricultural practices such as plowing (Brito et al., 2012) or application of pesticides (de Novais et al., 2019b; Hage-Ahmed et al., 2019).
Anastomosis, that is the process of fusion between branches of the same or different hyphae to constitute a mycelial network (Kirk et al., 2008), has been abundantly reported in the fungal kingdom (Glass et al., 2000) and described in intact two-dimensional (Giovannetti et al., 2004) as well as three-dimensional (de la Providencia et al., 2005) ERM networks of AM fungi. It indistinctly concern intact and damaged hyphae, the latter being described as a hyphal healing mechanism (HHM) (de la Providencia et al., 2007). The HHM has been described in four successive events. First, it begins with the formation of a septum near or in the apical zone on both sides of the injured hyphae, which prevents massive cytoplasmic/protoplasmic leakage into the surrounding environment. Secondly, one or several growing hyphal tips (GHTs) emerge from both extremities of the cut hyphae either protruding through the septum or emerging behind it. Thirdly, the GHTs elongate and grow toward each other and in most cases enter into contact. Fourthly, fusion is observed between GHTs with re-establishment of cytoplasmic/protoplasmic flow (de la Providencia et al., 2005). Importantly, the HHM differ between Glomeraceae and Gigasporaceae. Indeed, the HHM in Glomeraceae is oriented toward the reconnection of the affected area by linking several hyphae in relatively small vicinity or by recolonization of roots and substrate, by contrast to Gigasporaceae in which the HHM is nearly always oriented toward the re-establishment of hyphal integrity (de la Providencia et al., 2007). This suggests that both fungi have developed different strategies to grow and survive under adverse conditions (de la Providencia et al., 2005).
Fungicides are widely used in conventional agriculture to control fungal diseases. Unfortunately, these molecules may also affect soil plant-beneficial microorganisms such as AM fungi (Jin et al., 2013; Buysens et al., 2015). Their impact on these belowground microorganisms have been investigated under field (e.g., Schalamuk et al., 2014; Rivera-Becerril et al., 2017), greenhouse (e.g., Jin et al., 2013; Channabasava et al., 2015; Rabab and Reda, 2019) and growth chamber (de Novais et al., 2019a) conditions as well as in vitro on root organs (e.g., Campagnac et al., 2008; Zocco et al., 2008) or whole plants (e.g., Zocco et al., 2011).
In vitro cultivation systems offer several advantages, among which, the absence of any confounding effects caused by unwanted contaminants or environmental factors (e.g., soil physico-chemical parameters) and the easy non-destructive observations of growth and development of fungi. Spore germination (Chiocchio et al., 2000; Zocco et al., 2008; Buysens et al., 2015), root colonization (Campagnac et al., 2008, 2009; Calonne et al., 2010, 2012), anastomosis formation (Cardenas-Flores et al., 2011; de Novais et al., 2019a,b), sterol biosynthesis pathway (Campagnac et al., 2010; Calonne et al., 2012) and transport of nutrients (e.g., phosphorus) from fungus to plant (Zocco et al., 2011) have been investigated in vitro in presence of different types of fungicides. Results differed significantly with fungicide and dose of application. However, no study has reported the effects of fungicides on the HHM and thus on the ability of hyphae to maintain integrity following physical disturbance.
Azoxystrobin, pencycuron, flutolanil and fenpropimorph are amongst the most frequently used fungicides to control soil fungal diseases. Azoxystrobin is a systemic fungicide that belongs to the class of methoxyacrylates, which are derived from the naturally occurring strobilurins. It is the most widely sold fungicide worldwide (Zhang Q. et al., 2019), used against several fungal diseases of many edible crops and ornamental plants. It exhibits its fungicidal activity by binding to the quinol oxidation (Qo) site of cytochrome b to inhibit mitochondrial respiration in fungal species from the Ascomycota, Basidiomycota, and Deuteromycota and fungal-like species from the Oomycota (Bartlett et al., 2002; Feng et al., 2020). Pencycuron is a phenylurea fungicide of contact, which is highly specific to Rhizoctonia solani, inhibiting mycelial growth by blocking cell division and destroying the cytoskeleton of the microtubules during mitosis (Young, 2012). Flutolanil is a systemic phenyl benzamide fungicide, used against diseases caused by Basidiomycota in crop plants (Zhao et al., 2019). It mainly inhibits the hyphal growth and infection formation, and strongly reduce the mycelial O2 consumption of R. solani as well as the activity of succinate dehydrogenase complex (Complex II) in mitochondria (Mol et al., 2019). Finally, fenpropimorph is a morpholine of broad-spectrum considered as a sterol biosynthesis inhibitor (SBI). It specifically inhibits at low concentrations the sterol Δ8→Δ7-sterol isomerase, and additionally, when it is used at higher concentrations, inhibits Δ14-sterol reductase (Marcireau et al., 1990). This fungicide is mainly applied in cereals to control Blumeria (powdery mildew) and Puccinia (cereal rust) species (Stenzel and Vors, 2019).
A number of studies under strict in vitro culture conditions have investigated the effects of these fungicides on AM fungi. Azoxystrobin and its formulation Amistar did not impact spore germination and root colonization of potato associated with R. irregularis MUCL 41833 at threshold concentration (IC50 ≤ 0.1 mg L–1 a.i.) for the control of R. solani, while at 10 times this threshold, spores production and mycelium development were significantly affected (Buysens et al., 2015). In the same study, at threshold value for the control of R. solani, pencycuron and its formulation Monceren, did not affect spore germination and intra- or ERM development (Buysens et al., 2015). Finally, flutolanil and its formulation Monarch at threshold value for the control of R. solani did not affect spores germination or ERM development but decreased root colonization and arbuscules formation. In other studies, using root organ cultures (ROC) (Campagnac et al., 2008, 2009, 2010; Zocco et al., 2008; Oger et al., 2009) or whole plants (Zocco et al., 2011), the effects of fenpropimorph on the AM fungal symbiosis was demonstrated. Fenpropimorph presented a high toxicity with drastic sterols modifications in the host roots which was mirrored by a drastic reduction of root growth, root colonization and decrease of phosphorus transport, alkaline phosphatase and succinate dehydrogenase activities of the ERM (Zocco et al., 2011). All these studies suggested that fungicides may have undesirable effects on AM fungi, but none considered their effects on HHM.
The objective of this study was to investigate under in vitro culture conditions the impact of two different concentrations (0.02 and 2 mg L–1) of azoxystrobin, pencycuron, flutolanil, and fenpropimorph on the HHM of two AM fungi (Gigaspora sp. MUCL 52331 and R. irregularis MUCL 41833) belonging to phylogenetically distant families and thus having different life history strategies.
Materials and Methods
Biological Material
The AM fungi Gigaspora sp. (Gerdemann and Trappe) MUCL 52331 and Rhizophagus irregularis (Błaszk., Wubet, Renker, and Buscot) C. Walker and A. Schüßler as [“irregulare”] MUCL 41833 were supplied by the Glomeromycota in vitro collection (GINCO –1). Both strains were maintained in association with Ri T-DNA transformed chicory (Cichorium intybus L.) roots on 135 mm diam. Petri plates containing 100 ml Modified Strullu–Romand (MSR) medium (Declerck et al., 1998).
Fungicide Medium Preparation
The active ingredients (a.i.) of four fungicides that disrupt respiration (azoxystrobin and flutolanil), cytoskeleton and motor proteins (pencycuron) and sterol biosynthesis in membranes (fenpropimorph) (Fungicide Resistance Action Committee, 2020) were supplied by Sigma-Aldrich, Inc., [Darmstadt, Germany]. Each fungicide was dissolved in a solution of acetone (5 ml L–1 of MSR medium) and added to a bottle containing 50 ml sterilized (121°C for 15 min) MSR medium. The a.i. were added at a concentration of 0.02 and 2 mg L–1 MSR medium (Zocco et al., 2008). The bottles contained a stirring magnet to avoid solidification and obtain a homogeneous concentration of the different a.i. One bottle containing MSR medium without fungicides but added with acetone (MSRacetone) and another with MSR medium alone (MSRcontrol) were used as controls.
Experimental Design
The Petri plates were placed in a slope (∼10°) during filling of the MSR medium so that medium was thinner at one side of the Petri plate at solidification. One chicory root piece was placed in the thicker side of the Petri plate and associated with one of the AM fungi. For Gigaspora sp., a 3 cm × 3 cm piece of gel containing mycelium and three spores from a 6-month-old ROC was placed close to the root, while for R. irregularis, a 2.5 cm × 2.5 cm sliced piece of gel containing mycelium and approximately 500 spores from a 2-month-old ROC was used. The Petri plates were incubated in an inverted position at 27°C in a growth chamber under dark conditions. After 12 and 6 weeks of growth for Gigaspora sp. and R. irregularis, respectively, Petri plates showing adequate ERM development were selected. Between 6 and 10 hyphae growing on the surface of the MSR medium in the thinner part of the Petri plates and showing dense cytoplasmic/protoplasmic flow under a dissecting bright-field light microscope (Olympus SZ2-CTV, Japan) at 40× magnification were selected in each Petri plate. The selected hyphae were chosen at a reasonable distance from each other to avoid as much as possible interferences (i.e., hyphae contact from different cuts). Under horizontal laminar hood, hyphae were cut with a sterilized scalpel under the dissecting bright-field light microscope at 6.7× to 40× magnification. Immediately after cutting, 20 μl of MSR medium without fungicides (MSRcontrol) or containing one of the two concentrations of fungicides or containing only acetone (MSRacetone) was immediately added at the place of injury. The HHM was analyzed in injured hyphae belonging to two AM fungal colonies (i.e., two Petri plates) per strain (Gigaspora sp. and R. irregularis) and for each control treatment (MSRtreatment, MSRAcetone) and fungicide concentration (0.02 and 2 mg L–1). The fungicide treatments were labeled as follows: azoxystrobin0.02 mgL–1, azoxystrobin2 mgL–1, flutolanil0.02 mgL–1, flutolanil2 mgL–1, pencycuron0.02 mgL–1, pencycuron2 mgL–1, fenpropimorph0.02 mgL–1, and fenpropimorph2 mgL–1. The cut hyphae were observed cautiously at regular intervals under the dissecting bright-field light microscope at 100× or 200× magnification, first frequently during the first 60 min, then each hour until 16 h and finally after 24, 30, 36, and 48 h. The hyphae were visualized carefully under a dissecting bright-field light microscope (Olympus BH2–RFCA, Japan) at 40× or 100× magnification (Figures 1A,B).
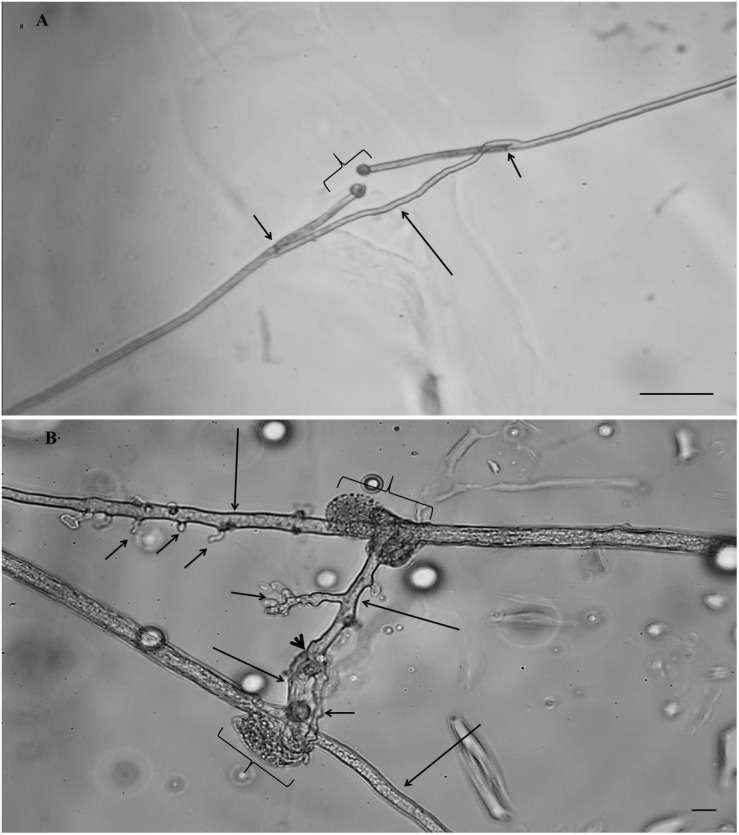
FIGURE 1.
Hyphal healing mechanism (HHM) of AM fungi in the control treatment (A) Gigaspora sp. MUCL 52331, the plugs were formed at both extremities in front of the hyphal injury (braces), two growing hyphal tips (GHTs) emerged at both extremities behind the septum (short arrows) and fused (large arrow) with re-establishment of cytoplasmic/protoplasmic flow (scale bar 50 μm). (B) Rhizophagus irregularis MUCL 41833, the plugs were formed at both extremities in front of the hyphal injury (braces), two GHTs from each injury site emerged through the septum (large arrows) and one GHT of each side contacted and fused (arrowhead) with re-establishment of cytoplasmic/protoplasmic flow. Several hyphal branches (short arrows) developed from the GHTs (scale bar 10 μm).
Data Collection
Following hyphae cutting, four events in the HHM (de la Providencia et al., 2005) were monitored: (1) septum formation near or in the apical zone on both sides of the cut hyphae (result not shown); (2) emission of GHTs through the septum or behind it and production of new branches on the GHTs; (3) elongation, orientation, and contact of the GHTs, and (4) fusion and re-establishment of cytoplasmic/protoplasmic flow. Additionally, the number of GHTs and total number of hyphal branches coming from the GHTs were counted at 48 h. The observed numbers are reported in Figures 2, 3. The percentage of observed events within each treatment and control group are reported in Table 1 and (Supplementary Table 2 for the MSRcontrol group).
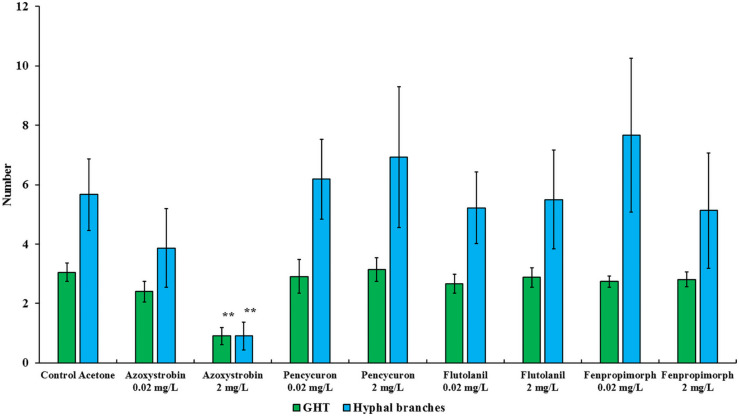
FIGURE 2.
Number of growing hyphal tips (GHTs) and their hyphal branches produced in the hyphal healing mechanism (HHM) of Gigaspora sp. MUCL 52331 in absence (control acetone) or in presence of increasing concentrations of azoxystrobin, pencycuron, flutolanil and fenpropimorph (0.02 and 2 mg L–1). Data were obtained 48 h after hyphal physical injury and addition of MSR medium containing or not the fungicide at the place of hyphal injury. Kruskal-Wallis followed by a Dunn multiple comparison post hoc test was performed to validate significant difference between fungicides treatments and their respective control acetone: *, **, and *** indicate P < 0.05; 0.01 and 0.001, respectively. P-values below 0.05 are significant.
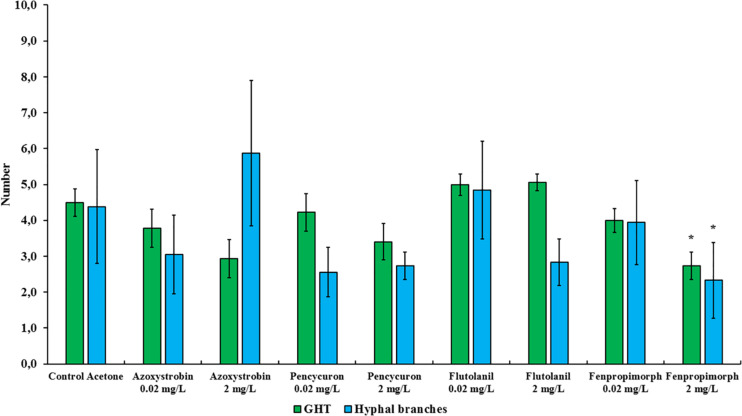
FIGURE 3.
Number of growing hyphal tips (GHTs) and their hyphal branches produced in the hyphal healing mechanism (HHM) of Rhizophagus irregularis MUCL 41833 in absence (control acetone) or in presence of increasing concentrations of azoxystrobin, pencycuron, flutolanil and fenpropimorph (0.02 and 2 mg L–1). Data were obtained 48 h after hyphal physical injury and addition of MSR medium containing or not the fungicide at the place of hyphal injury. Kruskal-Wallis followed by a Dunn multiple comparison post hoc test was performed to validate significant difference between fungicides treatments and their respective control acetone: *, **, and *** indicate P < 0.05; 0.01 and 0.001, respectively. P-values below 0.05 are significant.
TABLE 1.
Descriptive data percentages of hyphal branches production, growing hyphal tips (GHTs) emission, contact and fusion of the hyphal healing mechanism (HHM) of Gigaspora sp. MUCL 52331 and Rhizophagus irregularis MUCL 41833 in absence (control acetone) or in presence of increasing concentrations of azoxystrobin, pencycuron, flutolanil and fenpropimorph (0.02 and 2 mg L–1).
| Gigaspora sp. MUCL 52331 | Rhizophagus irregularis MUCL 41833 | |||||
| Control acetone | 0.02 mg L–1 | 2 mg L–1 | Control acetone | 0.02 mg L–1 | 2 mg L–1 | |
| Azoxystrobin | ||||||
| Hyphal branches (%) | 94.4 | 86.7 | 35 | 73.7 | 66.7 | 50 |
| GHT emission (%) | 94.4 | 86.7 | 30 | 100 | 88.9 | 87.5 |
| GHT contact (%) | 88.9 | 86.7 | 15 | 73.7 | 66.7 | 37.5 |
| GHT fusion (%) | 88.9 | 73.3 | 10 | 68.4 | 55.5 | 31.2 |
| Number of injured hyphae (n) | 18 | 15 | 20 | 19 | 18 | 16 |
| Pencycuron | ||||||
| Hyphal branches (%) | 81.2 | 81.8 | 64.3 | 94.4 | 88.9 | 86.7 |
| GHT emission (%) | 87.5 | 81.8 | 92.8 | 94.7 | 100 | 100 |
| GHT contact (%) | 81.2 | 81.8 | 85.7 | 89.5 | 83.3 | 73.3 |
| GHT fusion (%) | 81.2 | 81.8 | 71.4 | 73.7 | 72.2 | 60 |
| Number of injured hyphae (n) | 16 | 12 | 14 | 19 | 18 | 15 |
| Flutolanil | ||||||
| Hyphal branches (%) | 72.2 | 83.3 | 81.2 | 94.7 | 100 | 100 |
| GHT emission (%) | 94.4 | 94.4 | 93.7 | 100 | 100 | 100 |
| GHT contact (%) | 83.3 | 77.7 | 93.7 | 89.5 | 90 | 88.9 |
| GHT fusion (%) | 83.3 | 72.2 | 75 | 73.7 | 75 | 77.8 |
| Number of injured hyphae (n) | 18 | 18 | 16 | 19 | 20 | 18 |
| Fenpropimorph | ||||||
| Hyphal branches (%) | 87.5 | 86.7 | 56.2 | 85 | 86.7 | 70.6 |
| GHT emission (%) | 100 | 100 | 93.7 | 90 | 100 | 88.2 |
| GHT contact (%) | 100 | 93.3 | 87.5 | 85 | 80 | 35.3 |
| GHT fusion (%) | 87.5 | 73.3 | 75 | 70 | 73.3 | 29.4 |
| Number of injured hyphae (n) | 16 | 15 | 16 | 19 | 18 | 15 |
Statistical Analysis
To evaluate the impact of the fungicides on emission, contact and fusion of GHTs, each hyphal injury from all treatments, was considered. A Cox proportional hazard model for right and interval censored (time-to-event) data was fitted using R software version 4.0.0. Specifically, the MIICD.coxph function of the MIICD R package was used (Delord, 2015). The non-observed events of the HHM after 48 h were considered as right censored. More information on the time-to-event statistical modeling framework and more precisely on the Cox model can be found in Cox (1972) and George et al. (2014). The hazard ratio’s (hr) reported in Table 2 and Supplementary Table 2 and defined as exp (β), where the β’s are the coefficient estimates obtained from the fit of the Cox model, are interpreted as follows: an hazard ratio greater (respectively, smaller) than one means that the event is more (respectively, less) likely [i.e., the event occurs more (respectively, less) often or is sooner (respectively, later) observed] within the given treatment group compared to the control. In our analysis, MSRacetone is defined as the control group. For a seek of simplicity, Table 1 only reports the estimates (obtained from the Cox model) for the treatment groups. The ones corresponding to MSRwater are reported in Supplementary Table 1 since no significant difference was highlighted by the analysis between MSRacetone and MSRwater.
TABLE 2.
Results of Cox proportional hazards regression analysis on growing hyphal tips (GHTs) emission, contact and fusion events of the hyphal healing mechanism (HHM) for Gigaspora sp. MUCL 52331 and Rhizophagus irregularis MUCL 41833 in presence of increasing concentrations of azoxystrobin, pencycuron, flutolanil and fenpropimorph (0.02 and 2 mg L–1).
| Gigaspora sp. MUCL 52331 | Rhizophagus irregularis MUCL 41833 | ||||||
| Fungicide concentration (mg L–1) | β | Hazard ratio | P-value | β | Hazard ratio | P-value | |
| Azoxystrobin | |||||||
| GHT emission | 0.02 | −0.392 | 0.676 | 0.296 | −0.457 | 0.633 | 0.187 |
| 2 | −2.009 | 0.134 | <0.001 | −0.685 | 0.504 | 0.056 | |
| GHT contact | 0.02 | −0.424 | 0.655 | 0.259 | −0.200 | 0.818 | 0.613 |
| 2 | −2.444 | 0.064 | <0.001 | −1.147 | 0.317 | 0.020 | |
| GHT fusion | 0.02 | −0.568 | 0.567 | 0.140 | −0.345 | 0.708 | 0.416 |
| 2 | −3.129 | 0.044 | <0.001 | −1.224 | 0.294 | 0.021 | |
| Pencycuron | |||||||
| GHT emission | 0.02 | 0.083 | 1.086 | 0.847 | 0.484 | 1.623 | 0.164 |
| 2 | 0.124 | 1.133 | 0.748 | 0.520 | 1.682 | 0.154 | |
| GHT contact | 0.02 | 0.243 | 1.275 | 0.578 | 0.114 | 1.120 | 0.749 |
| 2 | −0.050 | 0.951 | 0.901 | −0.135 | 0.873 | 0.728 | |
| GHT fusion | 0.02 | −0.092 | 0.912 | 0.840 | 0.258 | 1.295 | 0.506 |
| 2 | −0.311 | 0.733 | 0.464 | −0.151 | 0.860 | 0.725 | |
| Flutolanil | |||||||
| GHT emission | 0.02 | 0.628 | 1.873 | 0.073 | NA | NA | NA |
| 2 | 0.403 | 1.496 | 0.267 | NA | NA | NA | |
| GHT contact | 0.02 | 0.178 | 1.195 | 0.633 | 0.075 | 1.078 | 0.825 |
| 2 | 0.539 | 1.714 | 0.144 | 0.002 | 1.002 | 0.995 | |
| GHT fusion | 0.02 | 0.049 | 1.050 | 0.899 | 0.139 | 1.149 | 0.709 |
| 2 | −0.222 | 0.801 | 0.569 | 0.120 | 1.127 | 0.752 | |
| Fenpropimorph | |||||||
| GHT emission | 0.02 | 0.139 | 1.149 | 0.711 | 1.275 | 3.580 | <0.001 |
| 2 | −0.415 | 0.661 | 0.282 | −0.345 | 0.708 | 0.334 | |
| GHT contact | 0.02 | −0.475 | 0.622 | 0.201 | 0.805 | 2.238 | 0.033 |
| 2 | −0.414 | 0.661 | 0.263 | −1.294 | 0.274 | 0.007 | |
| GHT fusion | 0.02 | −0.538 | 0.584 | 0.185 | 0.638 | 1.893 | 0.109 |
| 2 | −0.247 | 0.781 | 0.533 | −1.224 | 0.294 | 0.019 | |
Values were considered significant at P < 0.05 compared to the acetone control treatment. β = Regression coefficient. Hazard ratio = Exp (β). NA, Not applicable.
In addition, the number of GHTs and hyphal branches produced were subjected to Kruskal–Wallis non-parametric test followed by a post hoc Dunn multiple comparison test to validate significant difference between fungicides treatments and their respective control acetone using the software IBM SPSS statistic 26 software. For all analyses, statistical significance was established at a 95% confidence level (i.e., when the p-value is less than 0.05).
Results
Hyphal Healing Mechanism in Gigaspora sp. and R. irregularis in the MSRacetone and MSRcontrol Treatments
Whatever the AM fungus, a loss of cytoplasmic/protoplasmic content was observed at both extremities of the cut hyphae (Figures 1A,B). A septum was formed and a change in color inside the hyphae (dark brown to brown) was noticed within the first 180 min. The mechanism of HHM slightly differed between both AM fungi, especially in the number of GHTs produced. With Gigaspora sp., a low number of GHTs (with rare hyphal branches) was noticed emerging behind both extremities of the injured hyphae (Figure 1A). In most of the cases the GHTs grew toward each other, reconnected and re-established cytoplasmic/protoplasmic flow. In contrast, R. irregularis produced a high number of GHTs at both extremities growing through the septum and often bearing several hyphal branches. Hyphal re-growth appeared disorganized with a lower percentage of reconnections as compared with Gigaspora sp. Following healing, cytoplasmic/protoplasmic flow was re-established. Whatever the parameter (emission, contact and fusion of GHTs and number of GHTs and hyphal branches produced), no significant difference was highlighted between the MSRacetone and MSRcontrol treatments (see data in supporting information), thus the impact of fungicides on the HHM was compared solely to the MSRacetone control treatment.
Impact of Fungicides on the Hyphal Healing Mechanism in Gigaspora sp. MUCL 52331
The impact of azoxystrobin, pencycuron, flutolanil and fenpropimorph at 0.02 and 2 mg L–1 was evaluated on the HHM in Gigaspora sp. MUCL 52331 (Tables 1, 2). A significant effect of the azoxystrobin2 mgL–1 treatment was noticed for GHTs emission (hr = 0.134, P < 0.001), contact (hr = 0.064, P < 0.001) and fusion (hr = 0.044, P < 0.001) (Table 2). All events were less often (or later in time) observed in presence of azoxystrobin2 mgL–1 as compared to the MSRacetone treatment. The number of GHTs (P = 0.010) and hyphal branches (P = 0.004) was significantly lower in the azoxystrobin2 mgL–1 treatment as compared to the MSRacetone treatment (Figure 2). Within a period of approximately 360 min following injury in the azoxystrobin2 mgL–1 treatment, the hyphae that did not produce GHTs showed retraction of cytoplasm in the opposite direction to the tip followed by formation of numerous septa inside the hyphae (result not shown). Although the percentage of all HHM observed events was lower in the azoxystrobin0.2 mgL–1 compared to the ones in the MSRacetone treatment, no significant effect was revealed from the Cox Model (Table 2). The emission, contact and fusion of GHTs (Tables 1, 2) as well as number of GHTs and hyphal branches were not affected at both concentrations of pencycuron, flutolanil and fenpropimorph (Figure 2) as compared to the MSRacetone treatment.
Impact of Fungicides on Hyphal Healing Mechanism in Rhizophagus irregularis MUCL 41833
The impact of azoxystrobin, pencycuron, flutolanil and fenpropimorph at 0.02 and 2 mg L–1 was evaluated on the HHM of R. irregularis MUCL 41833 (Tables 1, 2). The results obtained from the Cox model revealed that the azoxystrobin2 mgL–1 treatment had a significant impact on GHTs contact and fusion as the hazard ratio’s were significantly less than 1 (hr = 0.317, P = 0.020, and hr = 0.294, P = 0.021, respectively) meaning that both events were less frequently observed within the azoxystrobin2 mgL–1 treatment as compared to the MSRacetone one, while no significant effect of the azoxystrobin2 mgL–1 on GHTs emission was found. Although a decreased trend in the percentage of observed GHTs emission, contact, and fusion events was noted in the azoxystrobin0.02 mgL–1 treatment, no significant difference was reported by our analysis (Tables 1, 2). The number of GHTs and hyphal branches did not differ at both concentrations of azoxystrobin compared to the MSRacetone treatment (Figure 3). After the formation of GHTs, the HHM process was inhibited in the azoxystrobin2 mgL–1 treatment. At the end of the evaluation, a limited growth of GHTs was observed and no septa was noticed in the injured hyphae of the azoxystrobin2 mgL–1 treatment with the exception of a septum in the area of the lesion.
The GHTs emission, contact, and fusion events were not significantly affected by both concentrations of pencycuron and flutolanil as compared to the MSRacetone treatment (Tables 1, 2). In the case of flutolanil, the Cox proportional hazard model was not performed in the GHTs emission event because GHTs formation was observed in all the injuries (Table 1). Moreover, the number of GHTs and hyphal branches did not differ at both concentrations treatments (pencycuron and flutolanil) as compared to the MSRacetone treatment (Figure 3).
Finally, the results of the Cox model showed that the fenpropimorph2 mgL–1 treatment reduced significantly the percentage of observed GHTs contact (hr = 0.274, P = 0.007) and fusion (hr = 0.294, P = 0.019) (Table 2). In presence of fenpropimorph2 mgL–1, the number of GHTs (P = 0.015) and the number of hyphal branches (P = 0.039) was significantly lower as compared to the MSRacetone treatment (Figure 3). After the formation of GHTs, the HHM process was inhibited in the fenpropimorph2 mgL–1 treatment. At the end of the evaluation, a limited growth of GHTs was observed and no septa was noticed in the injured hyphae of fenpropimorph2 mgL–1 treatment with the exception of a septum in the area of the lesion. On the other hand, in the fenpropimorph0.02 mgL–1 treatment, GHTs emission (hr = 3.580, P < 0.001) and contact (hr = 2.238, P = 0.033) were stimulated significantly, showing this event more frequently than in the MSRacetone treatment (Table 2).
Discussion
Seed dressing or foliar application of chemical fungicides are considered essential agricultural practices to control fungal diseases in crops intended for market. They have different modes of action and can target a broad range or a specific group of fungi, but in numerous cases, they also affect non-target fungi. It is important to understand the effects of fungicides on these non-target organisms, because some of them (e.g., AM fungi) play key roles in plant growth and health and thus help to optimize strategies of fungicide applications. Here, the effects of fungicides with different modes of action were investigated on the HHM, a strategy allowing fungi to preserve their mycelial networks integrity, of two AM fungi (Gigaspora sp. MUCL 52331 and R. irregularis MUCL 41833) with contrasting life history strategies.
In the absence of fungicides, similar results to those obtained in the studies of de la Providencia et al. (2005) and de la Providencia et al. (2007) on Glomeraceae and Gigasporaceae grown in vitro were obtained, demonstrating that the approach considered in the present study was adequate for studying the HHM in presence of fungicides. These authors suggested that in Gigasporaceae, the HHM (i.e., GHTs contact and subsequent cytoplasmic/protoplasmic flow re-establishment) is the most probable way to maintain the viability of hyphae in adverse conditions. In contrast, in Glomeraceae, the HHM might increase the ability of the fungus to colonize the roots due to the proliferation of new branches at the apex of the injured hyphae as well as the reconnection of the affected area by networking several hyphae in a relatively small vicinity.
In presence of fungicides, differences were noticed between active ingredients (a.i.) and concentrations. Azoxystrobin was detrimental to both fungi at the concentration of 2 mg L–1 but not at 0.02 mg L–1. Pencycuron and flutolanil at both concentrations did not impact the HHM of the two fungi, while fenpropimorph had contrasting effects on both fungi. With R. irregularis, the HHM was stimulated at 0.02 mg L–1 and inhibited at 2 mg L–1 fenpropimorph, while no effect was observed at both concentrations in Gigaspora sp.
Azoxystrobin at 2 mg L–1 was the most threatening a.i. impacting all the HHM events in Gigaspora sp. and the contact and fusion of GHTs in R. irregularis, while at 0.02 mg L–1 no effects were noticed on any of the fungi. At the highest concentration of 2 mg L–1, an evident retraction of cytoplasm in the opposite direction to the tips and subsequent formation of numerous walls inside the hyphae was observed when GHTs were not produced [70%] or did not enter into contact [85%] in Gigaspora sp. In R. irregularis, the GHTs [87.5%] were produced but only a few contacts [37.5%] and fusions [31.2%] were observed and the hyphae that did not form GHTs presented a main septum in the lesion area and did not produce numerous walls as in Gigaspora sp. These results complement the observations made by Buysens et al. (2015) on the effect of azoxystrobin on AM fungi. These authors demonstrated under similar in vitro culture conditions that azoxystrobin at 0.1 mg L–1 (as formulation Amistar) affected spore production and hyphal length of R. irregularis MUCL 41833 associated with potato, while root colonization was reduced at 1 (a.i., or formulation Amistar) and 10 mg L–1 a.i. Greenhouse studies further demonstrated that soil drench with azoxystrobin inhibited root colonization of Glomeraceae members in sugarcane (Vuyyuru et al., 2018). Moreover, root colonization and enzymatic activity of Funneliformis coronatum associated with maize plants was also inhibited, demonstrating the fungicidal activity of this strobilurin, probably on the respiratory electron transfer within mitochondria, where succinate dehydrogenase is part of complex II (Diedhiou et al., 2004). Interestingly, foliar application of azoxystrobin did not impact AM fungal root colonization (Diedhiou et al., 2004; Hernández-Dorrego and Mestre-Parés, 2010; Campos et al., 2015). A similar trend was observed with kresoxim-methyl, another strobilurin fungicide (Diedhiou et al., 2004). Both strobilurin fungicides present low uptake into leaves and absence of phloem mobility (Bartlett et al., 2002) probably explaining the absence of effects on AM fungi in the roots. Interestingly, Buysens et al. (2015) evaluated that 0.75 mg L–1 azoxystrobin applied in vitro could be considered close to the recommended dosage of Amistar applied against R. solani in potato crop production (1500 g a.i., ha–1) (Service Public Fédéral, 2015). Therefore, the concentration of 2 mg L–1 of a.i., used at the place of hyphal injury was probably above the recommended field dosage, while 0.02 mg L–1 was below. From these results, it is speculative to assert that field recommended dosage may impact the fungus significantly, because several aspects (e.g., biodegradation, soil type and soil particles size) might affect fungicide exposure to AM fungi (O’Connor et al., 2002; Rivera-Becerril et al., 2017). However, our results complement those obtained by Buysens et al. (2015), suggesting that the direct application in the culture medium of azoxystrobin at concentrations equal or above 0.1 mg L–1 (a.i., or formulated product) affect the development of AM fungi, therefore not excluding an impact on its development under field conditions following soil application.
The strong negative impact of azoxystrobin at 2 mg L–1 on the HHM (in particular contact and fusion of GHTs) of both AM fungi could possibly been attributed to the perturbation of apical vesicular bodies in the hyphal tip called Spitzenkörper. This structure consists in many small vesicles, ribosomes and cytoskeletal elements present in GHTs (Steinberg, 2007) playing key roles in hyphal growth, orientation, branching and morphogenesis (Hickey et al., 2002; Harris et al., 2005). This structure has not formally been described in AM fungi. Although, in Gigasporaceae, a cluster of spherical lipid bodies was observed behind the apex of growing germ tubes (Bentivenga et al., 2013) as well as “spitzenkörper-like” structures at the hyphal apex of Gigaspora margarita (Ramos et al., 2008a,b). In Glomeraceae their presence has never been proved but only suggested (de la Providencia et al., 2005). A perturbation of these structures by chemicals could explain the lack of recognition between GHTs in R. irregularis and Gigaspora sp. The effects of chemical molecules with fungicidal effects on the Spitzenkörper remains poorly investigated. Carbendazim, an inhibitor of ß-tubulin assembly in mitosis, was reported to inhibit completely the Spitzenkörper on living hyphal tip cells in Fusarium acuminatum (Ascomycota) (Howard and Aist, 1977, 1980). Instead, in Sclerotium rolfsii (Basidiomycota), the Spitzenkörper position was displaced within the apical hyphal zone changing the growth direction when hyphae were treated with cyproconazole, an inhibitor of ergosterol synthesis, causing alteration of the microtubule cytoskeleton and abnormal wall deposition (Roberson et al., 1989). These studies demonstrate that chemicals may impact growth and orientation of hyphae, hence affecting other processes such as anastomosis between branches of intact hyphae or contact and fusion of GHTs in injured hyphae. Although this has to be demonstrated for those fungi and extended to AM fungi with molecules such as azoxystrobin.
Pencycuron at 0.02 and 2 mg L–1 did not impact the HHM of both AM fungal strains. Buysens et al. (2015) also demonstrated that pencycuron at 0.01 mg L–1 a.i., did not affect the ERM development or root colonization. To the contrary, at the concentration of 0.5 mg L–1 a.i., or formulation and 5 mg L–1 a.i., root colonization of R. irregularis was reduced. One, 10, 100 mg L–1 a.i., or formulation further impacted spore germination (Buysens et al., 2015). These authors calculated that a concentration of 0.25 mg L–1 used in vitro was equivalent to the recommended dose of Monceren applied in the field to control R. solani in potato crop production (500 g ha–1 a.i.). Our results suggested that the direct application of pencycuron at 0.02 and 2 mg L–1 had no noticeable effects on the HHM, while higher concentrations above the recommended dose calculated in vitro may affect other processes (e.g., spore germination, root colonization) of AM fungal life cycle. Pencycuron is the only fungicide of the phenylurea group, which also includes herbicides for weed control in agricultural and non-agricultural systems (Navarro et al., 2012) and is known to inhibit cell division of Rhizoctonia sp. The absence of effect of pencycuron on the HHM at concentrations up to 2 mg L–1 may suggest that the enzymes involved in cell division in AM fungi are less sensitive than those in Rhizoctonia sp. A study under greenhouse conditions nonetheless demonstrated that the cytokinin-like growth regulator thidiazuron (phenylurea compounds) applied on leaves at 20 mg L–1 (91 μM) decreased root colonization of R. intraradices associated with the perennial grass Miscanthus giganteus (Schmidt et al., 2017), suggesting an indirect effect on AM colonization by a decrease in active cytokinines in the plant shoot. A modification in plant metabolism by the application of pencycuron on the leaves may thus impact AM fungal colonization more severely than a direct contact with the molecule.
Flutolanil at both concentrations did not affect the HHM of Gigaspora sp. and R. irregularis. This corroborates partially the study of Buysens et al. (2015). These authors noticed that flutolanil at 0.1 mg L–1 a.i., did not affect ERM development, while spore germination was reduced at 10 and 100 mg L–1 a.i., or formulation Monarch and root colonization decreased at 1 mg L–1 a.i., or formulation Monarch and 10 mg L–1 a.i. They suggested that flutolanil was more detrimental to the intraradical structures (hyphae and arbuscules) than ERM structures because it is an inhibitor of the succinate dehydrogenase and has a high systemic activity. Systemic fungicides are thought to be more detrimental to AM fungi because of their accumulation in or on the roots (Diedhiou et al., 2004; Burrows and Ahmed, 2007; Jin et al., 2013). The absence of impact of flutolanil at 0.02 and 2 mg L–1 on the HHM of AM fungi suggests that the enzymatic metabolisms of the respiration pathway in AM fungi are probably less sensitive to flutolanil than those of Basidiomycota plant pathogens. In addition, 0.02 and 2 mg L–1 concentrations could be considered lower or higher, respectively, than the recommended dose reported by Buysens et al. (2015). These authors hypothesized a value of 0.09 mg L–1 in vitro considering the recommended dose of Monarch applied to control R. solani in potato crop production (184 g ha–1 a.i.). Our results thus suggest a tolerance of AM fungi to the direct application of flutolanil even at a concentration above the recommended dose estimated in vitro.
Fenpropimorph at the highest concentration impacted the HHM in R. irregularis, while the opposite was noticed at the lowest concentration. Curiously, no effect was observed on Gigaspora sp. It is not excluded that these differences may be related to the sterol composition of both fungi. Indeed, a variation in the composition and content of sterols has been observed in the spores of AM fungal species in the Glomeraceae, Gigasporaceae, and Acaulosporaceae families (Grandmougin-Ferjani et al., 1999), not excluding the possibility of differences in the GHTs.
Sterols accumulation at the fungal growing tips supports hyphal growth facilitating apical endocytosis and organizing the cytoskeleton (Steinberg, 2007). Differences in sterol composition in GHTs may lead to differences in sensitivity to SBIs fungicides as fenpropimorph. In R. irregularis, GHT production was not affected by the high concentration of fenpropimorph, but reduced contacts and thus fusions were observed, suggesting perturbation of the GHTs recognition as with azoxystrobin. A common attraction (positive tropism) between GHTs was observed with Gigasporaceae members and suggested in Glomeraceae (de la Providencia et al., 2005). These authors hypothesized that the growth of both GHTs toward each other was due to elicitation of diffusible substance and that this mechanism was led by one GHT in a sequence of a signaling-response.
The stimulatory effect observed on HHM of R. irregularis at low concentration (0.02 mg L–1) and opposite observed at high concentration (2 mg L–1) of fenpropimorph could be related to a phenomenon named hormesis. The hormetic effect is a biphasic dose response characterized by temporal stimulation at low doses and inhibition at high doses of a stressor agent, suggesting an adaptive response that can be either directly induced or be the result of overcompensation after the alteration of homeostasis in a biological process (Calabrese and Baldwin, 2002). The hormetic effect of fungicides on mycelial growth and virulence have been previously reported in Oomycota, Ascomycota and Basidiomycota plant pathogens (Garzon and Flores, 2013; Lu et al., 2018; Zhang R. et al., 2019). To the best of our knowledge, hormesis has never been addressed in Glomeromycota and would deserve attention owing to the importance of these organisms for crops (Hage-Ahmed et al., 2019).
A strong impact of fenpropimorph at concentrations equal or above 0.2 mg L–1 under in vitro culture conditions was observed by Campagnac et al. (2008) and Zocco et al. (2008) on ERM development of R. irregularis (hyphal growth, spore production and mycelium architecture). This impact resulted in a decreased capacity of the fungus to transport P to the host plant and a drastic decrease of alkaline phosphatase and succinate dehydrogenase activities measured in the ERM (Zocco et al., 2011). With the same AM fungus, an induction of oxidative stress was observed in presence of fenpropimorph at 10 mg L–1, suggesting the perturbation of other biological processes such as response to biotic and abiotic environmental stimuli (González-Guerrero et al., 2010).
In conclusion, this work reports for the first time the effects of fungicides with different modes of action on the HHM in two phylogenically distant AM fungi (R. irregularis MUCL 41833 and Gigaspora sp. MUCL 52331). Azoxystrobin (a broad-spectrum fungicide) was the more detrimental on both fungi at concentration slightly above the recommended field dosage. Conversely, the contact fungicide pencycuron (Rhizoctonia-specific) and flutolanil (Basidiomycota-specific) did not impact any of the two AM fungi, while fenpropimorph only impacted R. irregularis (stimulating at low and inhibiting at high concentrations). The mechanisms behind the observed results remains to be elucidated, but perturbation in the still-to-be firmly demonstrated spitzenkörper or sterols content as well as a process of hormesis are avenues to be explored. This study broadens our knowledge on the impact of fungicides on AM fungi and opens new avenues for the rationale management of chemical inputs to control pathogenic fungi while limiting their impact on AM fungi. It also increases our knowledge of the different strategies for AM fungal colony to survive under adverse conditions in agricultural soils.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
VHR-M: data collection, analysis and interpretation, drafting the work, commentaries corrections, final approval, and agreement with all aspects of the work. MC-S: data collection, analysis and interpretation, draft work commentaries and corrections, final approval, and agreement with all aspects of the work. VB: analysis and interpretation of the data, draft correction and final approval and agreement with all aspects of the work. MG-R: contribution to the development of the experiment, draft correction and final approval, and agreement with all aspects of the work. SD: Substantial contributions to the conception and design of the experiments, interpretation of the data, draft corrections final approval, and agreement with all aspects of the work. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research has benefited from the statistical consult with Statistical Methodology and Computing Service, technological platform at UCLouvain – SMCS/LIDAM, UCLouvain.
Funding. This work was supported by the National Council of Science and Technology (CONACyT), Mexico, grant number 211447 to VHR-M.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2021.642094/full#supplementary-material
References
- Bartlett D. W., Clough J. M., Godwin J. R., Hall A. A., Hamer M., Parr-Dobrzanski B. (2002). The strobilurin fungicides. Pest Manag. Sci. 58 649–662. 10.1002/ps.520 [DOI] [PubMed] [Google Scholar]
- Bentivenga S. P., Kumar T. K., Kumar L., Roberson R. W., McLaughlin D. J. (2013). Cellular organization in germ tube tips of Gigaspora and its phylogenetic implications. Mycologia 105 1087–1099. 10.3852/12-291 [DOI] [PubMed] [Google Scholar]
- Brito I., Goss M. J., de Carvalho M., Chatagnier O., van Tuinen D. (2012). Impact of tillage system on arbuscular mycorrhizal fungal communities in the soil under Mediterranean conditions. Soil Till. Res. 121 63–67. 10.1016/j.still.2012.01.012 [DOI] [Google Scholar]
- Burrows R. L., Ahmed I. (2007). Fungicide seed treatments minimally affect arbuscular-mycorrhizal fungal (AMF) colonization of selected vegetable crops. J. Biol. Sci. 7 417–420. 10.3923/jbs.2007.417.420 [DOI] [Google Scholar]
- Buysens C., Dupre de Boulois H., Declerck S. (2015). Rhizoctonia solani impact the nontarget arbuscular mycorrhizal fungus Rhizophagus irregularis? Mycorrhiza 25 277–288. 10.1007/s00572-014-0610-7 [DOI] [PubMed] [Google Scholar]
- Calabrese E. J., Baldwin L. A. (2002). Defining hormesis. Hum. Exp. Toxicol. 21 91–97. 10.1191/0960327102ht217oa [DOI] [PubMed] [Google Scholar]
- Calonne M., Fontaine J., Debiane D., Laruelle F., Grandmougin-Ferjani A., Sahraoui A. L. (2010). “Propiconazole toxicity on the nontarget organism, the arbuscular mycorrhizal fungus, Glomus irregulare,” in Fungicides, ed. Carisse O. (Rijeka: IntechOpen; ), 325–346. [Google Scholar]
- Calonne M., Sahraoui A. L., Campagnac E., Debiane D., Laruelle F., Grandmougin-Ferjani A., et al. (2012). Propiconazole inhibits the sterol 14α-demethylase in Glomus irregulare like in phytopathogenic fungi. Chemosphere 87 376–383. 10.1016/jchemosphere.2011.12.027 [DOI] [PubMed] [Google Scholar]
- Campagnac E., Fontaine J., Sahraoui A. L., Laruelle F., Durand R., Grandmougin-Ferjani A. (2008). Differential effects of fenpropimorph and fenhexamid, two sterol biosynthesis inhibitor fungicides, on arbuscular mycorrhizal development and sterol metabolism in carrot roots. Phytochemistry 69 2912–2919. 10.1016/j.phytochem.2008.09.009 [DOI] [PubMed] [Google Scholar]
- Campagnac E., Fontaine J., Sahraoui A. L., Laruelle F., Durand R., Grandmougin-Ferjani A. (2009). Fenpropimorph slows down the sterol pathway and the development of the arbuscular mycorrhizal fungus Glomus intraradices. Mycorrhiza 19 365–374. 10.1007/s00572-009-0238-1 [DOI] [PubMed] [Google Scholar]
- Campagnac E., Sahraoui A. L., Debiane D., Fontaine J., Laruelle F., Garcon G., et al. (2010). Arbuscular mycorrhiza partially protect chicory roots against oxidative stress induced by two fungicides, fenpropimorph and fenhexamid. Mycorrhiza 20 167–178. 10.1007/s00572-009-0267-9 [DOI] [PubMed] [Google Scholar]
- Campos A. A. B., Scotton J. C., Costa W. L. F., Giassi V., Pinto D. F. P., Homma S. K. (2015). Seleção de fungicidas visando à preservação de fungos micorrízicos arbusculares nativos no cultivo do feijoeiro. Rev. Bras. Eng. Agríc. Ambient. 19 898–902. 10.1590/1807-1929/agriambi.v19n9p898-902 [DOI] [Google Scholar]
- Cardenas-Flores A., Cranenbrouck S., Draye X., Guillet A., Govaerts B., Declerck S. (2011). The sterol biosynthesis inhibitor molecule fenhexamid impacts the vegetative compatibility of Glomus clarum. Mycorrhiza 21 443–449. 10.1007/s00572-011-0385-z [DOI] [PubMed] [Google Scholar]
- Channabasava A., Lakshman H. C., Jorquera M. A. (2015). Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L). J. Soil Sci. Plant Nutr. 15 35–45. 10.4067/S0718-95162015005000004 27315006 [DOI] [Google Scholar]
- Chiocchio V., Venedikian N., Martinez A. E., Ana Menendez A. M., Ocampo J. A., Godeas A. (2000). Effect of the fungicide benomyl on spore germination and hyphal length of the arbuscular mycorrhizal fungus Glomus mosseae. Internat. Microbiol. 3 173–175. [PubMed] [Google Scholar]
- Cox D. (1972). Regression models and life-tables. J. R. Stat. Soc. Series B Stat. Methodol. 34 187–220. [Google Scholar]
- de la Providencia I. E., Fernández F., Declerck S. (2007). Hyphal healing mechanism in the arbuscular mycorrhizal fungi Scutellospora reticulata and Glomus clarum differs in response to severe physical stress. FEMS Microbiol. Lett. 268 120–125. 10.1111/j.1574-6968.2006.00572.x [DOI] [PubMed] [Google Scholar]
- de la Providencia I. E., de Souza F. A., Fernandez F., Séjalon-Delmas N., Declerck S. (2005). Arbuscular mycorrhizal fungi exhibit distinct pattern of anastomoses formation and hyphal healing mechanism between different phylogenic groups. New Phytol. 165 261–271. 10.1111/j.1469-8137.2004.01236.x [DOI] [PubMed] [Google Scholar]
- de Novais C. B., Avio L., Giovannetti M., Faria S. M., Siqueira J. O., Sbrana C. (2019b). Interconnectedness, length and viability of arbuscular mycorrhizal mycelium as affected by selected herbicides and fungicides. Appl. Soil Ecol. 143 144–152. 10.1016/j.apsoil.2019.06.013 [DOI] [Google Scholar]
- de Novais C. B., Giovannetti M., De Faria S. M., Sbrana C. (2019a). Two herbicides, two fungicides and spore-associated bacteria affect Funneliformis mosseae extraradical mycelium structural traits and viability. Mycorrhiza 29 341–349. 10.1007/s00572-019-00901-6 [DOI] [PubMed] [Google Scholar]
- Declerck S., Strullu D. G., Plenchette C. (1998). Monoxenic culture of the intraradical forms of Glomus sp. isolated from a tropical ecosystem: a proposed methodology for germplasm collection. Mycologia 90 579–585. 10.1080/00275514.1998.12026946 [DOI] [Google Scholar]
- Delord M. (2015). MIICD: Data Augmentation and Multiple Imputation for Interval Censored Data, an R library. R package version 2.1. Available online at: http://CRAN.R-project.org/package=MIICD (accessed May 14, 2020). [Google Scholar]
- Diedhiou P. M., Oerke E. C., Dehne H. W. (2004). Effects of the strobilurin fungicides azoxystrobin and kresoximmethyl on arbuscular mycorrhiza. Z. Pflanzenkr. Pflanzenschutz 111 545–556. [Google Scholar]
- Feng Y., Huang Y., Zhan H., Bhatt P., Chen S. (2020). An overview of strobilurin fungicide degradation: current status and future perspective. Front. Microbiol. 11:389. 10.3389/fmicb.2020.00389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fungicide Resistance Action Committee (2020). FRAC Code List© 2020: Fungicides Sorted by Mode of Action. Available online at: https://www.frac.info/docs/default-source/publications/frac-code-list/frac-code-list-2020-finalb16c2b2c512362eb9a1eff00004acf5d.pdf?sfvrsn=54f499a_2 (accessed June 22, 2020). [Google Scholar]
- Garzon C. D., Flores F. J. (2013). “Hormesis: biphasic dose-responses to fungicides in plant pathogens and their potential threat to agriculture,” in Fungicides – Showcases of Integrated Plant Disease Management from Around the World, ed. Nita M. (Rijeka: IntechOpen; ), 311–328. [Google Scholar]
- George B., Seals S., Aban I. (2014). Survival analysis and regression models. J. Nucl. Cardiol. 21 686–694. 10.1007/s12350-014-9908-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti M., Sbrana C., Avio L., Strani P. (2004). Patterns of below-ground plant interconnections established by means of arbuscular mycorrhizal networks. New Phytol. 164 175–181. 10.1111/j.1469-8137.2004.01145.x [DOI] [PubMed] [Google Scholar]
- Glass N. L., Jacobson D. J., Shiu K. T. (2000). The genetics of hyphal fusion and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Genet. 34 165–186. 10.1146/annurev.genet.34.1.165 [DOI] [PubMed] [Google Scholar]
- González-Guerrero M., Oger E., Benabdellah K., Azcón-Aguilar C., Lanfranco L., Ferrol N. (2010). Characterization of a CuZn superoxide dismutase gene in the arbuscular mycorrhizal fungus Glomus intraradices. Curr. Genet. 56 265–274. 10.1007/s00294-010-0298-y [DOI] [PubMed] [Google Scholar]
- Grandmougin-Ferjani A., Dalpé Y., Hartmann M. A., Laruelle F., Sancholle M. (1999). Sterol distribution in arbuscular mycorrhizal fungi. Phytochemistry 50 1027–1031. 10.1016/S0031-9422(98)00636-0 [DOI] [Google Scholar]
- Hage-Ahmed K., Rosner K., Steinkellner S. (2019). Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 75 583–590. 10.1002/ps.5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. D., Read N. D., Roberson R. W., Shaw B., Seiler S., Plamann M., et al. (2005). Polarisome meets spitzenkorper: microscopy, genetics, and genomics converge. Eukaryot. Cell 4 225–229. 10.1128/EC.4.2.225-229.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Dorrego A., Mestre-Parés J. (2010). Evaluation of some fungicides on mycorrhizal symbiosis between two glomus species from commercial inocula and Allium porrum L. seedlings. Span. J. Agric. Res. 8 43–50. 10.5424/sjar/201008S1-1222 [DOI] [Google Scholar]
- Hickey P. C., Jacobson D., Read N. D., Glass N. L. (2002). Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet. Biol. 37 109–119. 10.1016/s1087-1845(02)00035-x [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. (1977). Effects of MBC on hyphal tip organization, growth, and mitosis of Fusarium acuminatum, and their antagonism by D2O. Protoplasma 92 195–210. 10.1007/BF01279458 [DOI] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. (1980). Cytoplasmic microtubules and fungal morphogenesis: ultrastructural effects of methyl benzimidazole-2-yl-carbamate determined by freeze-substitution of hyphal tip cells. J. Cell Biol. 87 55–64. 10.1083/jcb.87.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Germida J. J., Walley F. L. (2013). Suppressive effects of seed-applied fungicides on arbuscular mycorrhizal fungi (AMF) differ with fungicide mode of action and AMF species. Appl. Soil Ecol. 72 22–30. 10.1016/j.apsoil.2013.05.013 [DOI] [Google Scholar]
- Kirk P. M., Cannon P. F., Minter D. W., Stalpers J. A. (2008). Ainsworth and Bisby’s Dictionary of the Fungi. Wallingford: CABI Publishing. [Google Scholar]
- Lu X. M., He S., Ma H. J., Li J. H., Zhu F. X. (2018). Hormetic effects of flusilazole preconditioning on mycelial growth and virulence of Sclerotinia sclerotiorum. Plant Dis. 102 1165–1170. 10.1094/PDIS-10-17-1638-RE [DOI] [PubMed] [Google Scholar]
- Marcireau C., Guilloton M., Karst F. (1990). In vivo effects of fenpropimorph on the yeast Saccharomyces cerevisiae and determination of the molecular basis of the antifungal property. Antimicrob. Agents Chemother. 34 989–993. 10.1128/AAC.34.6.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol G. P. S., Aruldhas D., Joe I. H., Balachandran S., Anuf A. R., George J. (2019). Structural activity (monomer and dimer), spectroscopic analysis, chemical reactivity, fungicidal activity and molecular dynamics simulation of phenyl benzamide fungicides: a combined experimental and theoretical approach. J. Mol. Struct. 1193 24–44. 10.1016/j.molstruc.2019.05.022 [DOI] [Google Scholar]
- Navarro S., Hernández-Bastida J., Cazaña G., Pérez-Lucas G., Fenoll J. (2012). Assessment of the leaching potential of 12 substituted phenylurea herbicides in two agricultural soils under laboratory conditions. J. Agric. Food Chem. 60 5279–5286. 10.1021/jf301094c [DOI] [PubMed] [Google Scholar]
- O’Connor P., Smith S., Smith F. (2002). Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol. 154 209–218. 10.1046/j.1469-8137.2002.00364.x [DOI] [Google Scholar]
- Oger E., Ghignone S., Campagnac E., Fontaine J., Grandmougin-Ferjani A., Lanfranco L. (2009). Functional characterization of a C-4 sterol methyl oxidase from the endomycorrhizal fungus Glomus intraradices. Fungal Genet. Biol. 46 486–495. 10.1016/j.fgb.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Pepe A., Giovannetti M., Sbrana C. (2018). Lifespan and functionality of mycorrhizal fungal mycelium are uncoupled from host plant lifespan. Sci. Rep. 8:10235. 10.1038/s41598-018-28354-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabab A. M., Reda E. A. (2019). Impact of ridomil, bavistin and agrothoate on arbuscular mycorrhizal fungal colonization, biochemical changes and potassium content of cucumber plants. Ecotoxicology 28 487–498. 10.1007/s10646-019-02042-0 [DOI] [PubMed] [Google Scholar]
- Ramos A. C., Façanha A. R., Feijó J. A. (2008a). Proton (H+) flux signature for the presymbiotic development of the arbuscular mycorrhizal fungi. New Phytol. 178 177–188. 10.1111/j.1469-8137.2007.02344.x [DOI] [PubMed] [Google Scholar]
- Ramos A. C., Façanha A. R., Feijó J. A. (2008b). “Ion dynamics during the polarized growth of arbuscular mycorrhizal fungi: from presymbiosis to symbiosis,” in Mycorrhiza, ed. Varma A. (Berlin: Springer-Verlang; ), 241–260. [Google Scholar]
- Rivera-Becerril F., Tuinen D. V., Chatagnier O., Rouard N., Béguet J., Kuszala C., et al. (2017). Impact of a pesticide cocktail (fenhexamid, folpel, deltamethrin) on the abundance of Glomeromycota in two agricultural soils. Sci. Total Environ. 577 84–93. 10.1016/j.scitotenv.2016.10.098 [DOI] [PubMed] [Google Scholar]
- Roberson R. W., Fuller M. S., Grabski C. (1989). Effects of the demethylase inhibitor, Cyproconazole, on hyphaI tip cells of Sclerotium rotfsii I. A light microscope study. Pest Biochem. Physiol. 34 130–142. 10.1016/0048-3575(89)90151-X [DOI] [Google Scholar]
- Schalamuk S., Velazquez S., Simón M. R., Cabello M. (2014). Effect of Septoria leaf blotch and its control with commercial fungicides, on arbuscular-mycorrhizal-fungal colonization, spore numbers, and morphotype diversity. J. Plant Prot. Res. 54 9–14. 10.2478/jppr-2014-0002 [DOI] [Google Scholar]
- Schmidt C. S., Mrnka L., Frantík T., Motyka V., Dobrev P. I., Vosátka M. (2017). Combined effects of fungal inoculants and the cytokinin-like growth regulator thidiazuron on growth, phytohormone contents and endophytic root fungi in Miscanthus × giganteus. Plant Physiol. Biochem. 120 120–131. 10.1016/j.plaphy.2017.09.016 [DOI] [PubMed] [Google Scholar]
- Service Public Fédéral (2015). Santé Publique, Sécurité de la Chaîne Alimentaire et Environnement. Service Produits Phytopharmaceutiques et Engrais. Available online at: http://search.fytoweb.be/Downloads/Aktes/Erkenning/10311P-B.pdf (accessed July 14, 2020). [Google Scholar]
- Steinberg G. (2007). Tracks for traffic: microtubules in the plant pathogen Ustilago maydis. New Phytol. 174 721–733. 10.1111/j.1469-8137.2007.02072.x [DOI] [PubMed] [Google Scholar]
- Stenzel K., Vors J. P. (2019). “Sterol biosynthesis inhibitors,” in Modern Crop Protection Compounds, eds Jeschke P., Witschel M., Krämer W., Schirmer U. (Weinheim: Wiley-VCH; ), 797–844. [Google Scholar]
- Voets L., de la Providencia I. E., Declerck S. (2006). Glomeraceae and Gigasporaceae differ in their ability to form hyphal networks. New Phytol. 172 185–188. 10.1111/j.1469-8137.2006.01873.x [DOI] [PubMed] [Google Scholar]
- Vuyyuru M., Sandhu H. S., McCray J. M., Raid R. N. (2018). Effects of soil-applied fungicides on sugarcane root and shoot growth, rhizosphere microbial communities, and nutrient uptake. Agronomy 8:223. 10.3390/agronomy8100223 [DOI] [Google Scholar]
- Young D. H. (2012). “Fungicides acting on mitosis and cell division,” in Modern Crop Protection Compounds, eds Krämer W., Schirmer U., Jeschke P., Witschel M. (Weinheim: Wiley-VCH; ), 739–748. [Google Scholar]
- Zhang Q., Zhu D., Ding J., Zheng F., Zhou S., Lu T., et al. (2019). The fungicide azoxystrobin perturbs the gut microbiota community and enriches antibiotic resistance genes in Enchytraeus crypticus. Environ. Int. 131:104965. 10.1016/j.envint.2019.104965 [DOI] [PubMed] [Google Scholar]
- Zhang R., Zhang Y., Xu Q., Li J., Zhu F. (2019). Hormetic effects of mixtures of dimethachlone and prochloraz on Sclerotinia sclerotiorum. Plant Dis. 103 546–554. 10.1094/PDIS-06-18-1071-RE [DOI] [PubMed] [Google Scholar]
- Zhao C., Zhang X., Hua H., Han C., Wu X. (2019). Sensitivity of Rhizoctonia spp. to flutolanil and characterization of the point mutation in succinate dehydrogenase conferring fungicide resistance. Eur. J. Plant Pathol. 155 13–23. 10.1007/s10658-019-01739-6 [DOI] [Google Scholar]
- Zocco D., Fontaine J., Lozanova E., Renard L., Bivort C., Durand R., et al. (2008). Effects of two sterol biosynthesis inhibitor fungicides (fenpropimorph and fenhexamid) on the development of an arbuscular mycorrhizal fungus. Mycol. Res. 112 592–601. 10.1016/j.mycres.2007.11.010 [DOI] [PubMed] [Google Scholar]
- Zocco D., van Aarle I. M., Oger E., Lanfranco L., Declerck S. (2011). Fenpropimorph and fenhexamid impact phosphorus translocation by arbuscular mycorrhizal fungi. Mycorrhiza 21 363–374. 10.1007/s00572-010-0344-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.