Abstract
Background
Age and disease prevalence are the 2 biggest risk factors for Coronavirus disease 2019 (COVID-19) symptom severity and death. We therefore hypothesized that increased biological age, beyond chronological age, may be driving disease-related trends in COVID-19 severity.
Methods
Using the UK Biobank England data, we tested whether a biological age estimate (PhenoAge) measured more than a decade prior to the COVID-19 pandemic was predictive of 2 COVID-19 severity outcomes (inpatient test positivity and COVID-19-related mortality with inpatient test-confirmed COVID-19). Logistic regression models were used with adjustment for age at the pandemic, sex, ethnicity, baseline assessment centers, and preexisting diseases/conditions.
Results
Six hundred and thirteen participants tested positive at inpatient settings between March 16 and April 27, 2020, 154 of whom succumbed to COVID-19. PhenoAge was associated with increased risks of inpatient test positivity and COVID-19-related mortality (ORMortality = 1.63 per 5 years, 95% CI: 1.43–1.86, p = 4.7 × 10−13) adjusting for demographics including age at the pandemic. Further adjustment for preexisting diseases/conditions at baseline (ORM = 1.50, 95% CI: 1.30–1.73 per 5 years, p = 3.1 × 10−8) and at the early pandemic (ORM = 1.21, 95% CI: 1.04–1.40 per 5 years, p = .011) decreased the association.
Conclusions
PhenoAge measured in 2006–2010 was associated with COVID-19 severity outcomes more than 10 years later. These associations were partly accounted for by prevalent chronic diseases proximate to COVID-19 infection. Overall, our results suggest that aging biomarkers, like PhenoAge may capture long-term vulnerability to diseases like COVID-19, even before the accumulation of age-related comorbid conditions.
Keywords: Biological age, Biomarkers, Phenotypic age, UK Biobank
Coronavirus disease 2019 (COVID-19) represents one of the biggest threats to public health in nearly 100 years. While efforts are being undertaken to distribute vaccines and antibody tests for COVID-19, in the interim, there is a critical need for assessing risk stratification and to explore the use of geroscience-guided interventions seeking to improve outcomes by targeting biological aging. Accurately identifying those most at risk of severe complications or death will facilitate treatment decisions and inform guidelines regarding shelter-in-place and social distancing policies. As such, a major priority is in developing biomarkers that prognostically inform on severity of COVID-19 disease progression (1).
The risk of fatality and/or severe complications due to COVID-19 infection is strongly age-dependent. On March 27, 2020, the U.S. Center of Disease Control projected that persons aged 85 and older have predicted mortality rates of 10.4%–27.3%, compared to 4.3%–10.5% for individuals aged 75–84 years, 2.7%–4.9% for individuals 65–74 years, 1.4%–2.6% for those 55–64 years, and <1% for those 20–54 years of age (2). All-in-all, those aged 85 and older have a mortality risk that is 100-fold higher than for those under the age of 50, and currently 8 out of 10 COVID-19 deaths in the United States are among adults aged 65 or older (3). In addition to age, the U.S. Center of Disease Control reports that morbidity prevalence—particularly cardiovascular disease, diabetes mellitus, chronic kidney disease, and chronic lung disease (4)—exacerbate risk of death or symptomatic complications. Similar COVID-19 comorbidity associations were reported in other countries, for example, United Kingdom (5), China (6), and Italy (7).
Previous studies have predicted COVID-19 outcomes (pneumonia and mortality) using hospital inpatient data including demographics, signs and symptoms, clinical biomarkers, and imaging features. The performance in terms of C-statistic/index or area under the receiver operating characteristic (ROC) curve (AUC) was generally over 90% but subject to bias and overfitting (8). One study predicted hospital admission related to upper respiratory infections (pneumonia, influenza, acute bronchitis, etc.), proxy events of COVID-19, using over 500 diagnostic features from thousands of general population samples (9). The resulting AUCs were 70%–80% but may not be generalizable to COVID-19 (10). A recent study used 98 variables (demographics, recent vital signs, COVID-19 testing status and symptoms, as well as preexisting medical diagnoses) and 12 502 patients (9931 training and 2531 testing samples) with COVID-19-related visits to Massachusetts General Hospital emergency department and/or respiratory illness clinics (initiated for the pandemic) from March 7 to May 2, 2020, to predict an ordinal severity outcome within 7 days: (i) no event, (ii) hospitalized, (iii) ICU care and/or mechanical ventilation, or (iv) death, where the training results (AUC 0.76 for hospitalization, 0.79 for critical illness: ii, iii, or iv, and 0.93 for death vs others for each) were used to derive an acuity score for outpatient screening (11).
In recent years, we have developed and widely validated several biomarkers of aging (12–14) that strongly predict morbidity and mortality risk, in both short-term (1 year) and long-term (25+ years) follow-ups (12,14,15). Based on these observed trends, we hypothesize that biological aging, above and beyond chronological age, is a robust determinant of symptom severity following COVID-19 infection. We aimed to assess the risk and predictive performance of accelerated aging for COVID-19 severe infection using a biological age measure, named phenotypic age (PhenoAge). PhenoAge was previously trained using 42 biomarkers as inputs into a supervised machine learning model to predict all-cause mortality (12,15). We applied this measure to biomarker data from 2006 to 2010 of participants from a large community cohort, UK Biobank (UKB) (16,17). Combined with information on disease diagnoses updated to March 2020, we tested whether accelerated aging was predictive of COVID-19 severity based on mortality data and COVID-19 test results linked from the UK National Health Service (18).
Method
UK Biobank Data
UKB (16,17) is a volunteer community cohort, recruiting over 500 000 participants between the ages of 40 and 70 during 2006–2010. We restricted analyses to participants attending baseline assessment centers in England, excluding those who died before March 16, 2020 (first test date in the UKB COVID-19 test results). At recruitment (baseline), biological samples of participants were collected for biomarker assays. The disease status was confirmed based on self-reported doctor diagnoses at baseline or hospital admission records updated to March 2020. Also, the mortality data were used based on death certificates to October 2020. These phenotypic data are linked to the UK national COVID-19 test results, currently from March 16 to December 21, covering the first peak of COVID-19 incidence.
COVID-19 Severity Outcomes
Early testing in the United Kingdom was largely restricted to hospital inpatients with clinical signs of infection (18). We used 2 COVID-19 severity outcomes: inpatient test positivity between March 16 and April 27, 2020, and COVID-19-related mortality (death certificate ICD code: U071 or U072) with inpatient test-confirmed COVID-19 between March 16 and April 27, 2020. To ensure that test positivity is a proxy for COVID-19 severity, we restricted positives to inpatient positives between March 16 and April 27, 2020, when only symptomatic individuals were tested (19). As of April 27, 2020, hospitals under the UK National Health Service direction have tested all nonelective patients admitted overnight, including asymptomatic patients (19). The death data including causes up to October 2020, were used to determine death status for inpatient positives between March 16 and April 27, 2020. For the test positivity outcome, we compared inpatient test positives between March 16 and April 27 to untested samples and samples tested negative, excluding positive cases at noninpatient settings between March 16 and April 27, and positive cases regardless of origins from April 27 to December 21, 2020, as the severity of these people are uncertain. We included untested samples as they were enriched for milder or asymptomatic COVID-19 responses. For the mortality outcome, we compared COVID-19-related deaths with inpatient test-confirmed COVID-19 between March 16 and April 27, 2020 to untested samples, samples tested negative, and inpatient positives (March 16 to April 27, 2020) who survived, excluding those as for inpatient test positivity and deaths unrelated to COVID-19, based on ICD codes on death certificates.
PhenoAge
PhenoAge was developed based on mortality scores from the Gompertz proportional hazard model on chronological age and 9 biomarkers, which were selected from 42 biomarkers by Cox penalized regression model for best predicting all-cause mortality as a surrogate of biological aging in the National Health and Nutrition Examination Survey III (NHANES III) (12). Other methods can be used to derive biological age estimates from the set of biomarkers applied here. PhenoAge is more interpretable than the biomarkers given that it is expressed in units of years, on the same scale as chronological age. Therefore, it can be directly contrasted against chronological age to access accelerated aging. PhenoAge is proportional to 10-year mortality risk (12), assuming generalizability of the NHANES III training results to the UKB.
Biomarkers in UKB were measured at baseline (2006–2010) for all participants (measurement details in the UK Biobank Biomarker Panel (20) and UK Biobank Haematology Data Companion Document (21)). To correct distribution skewness, we set the top and bottom 1% of values to the 99th and 1st percentiles. The formula of PhenoAge is given by
where
and age denotes the chronological age.
Statistical Methods
We tested associations of (i) age at the pandemic (set as March 16, 2020, the first COVID-19 test date in the data, a shift of age at baseline), (ii) PhenoAge at baseline (10+ years prior), and (iii) preexisting diseases or conditions with the 2 COVID-19 severity outcomes, using the logistic models below.
M1: age at the pandemic
M2: age at the pandemic + PhenoAge acceleration (PhenoAgeAccel) at baseline
M3: age at the pandemic + preexisting diseases or conditions at baseline
M4: age at the pandemic + preexisting diseases or conditions at baseline + PhenoAgeAccel at baseline
We also modeled age at the pandemic and preexisting diseases/conditions to March 2020 (M5) and PhenoAgeAccel additionally (M6),
M5: age at the pandemic + preexisting diseases or conditions to March 2020
M6: age at the pandemic + preexisting diseases or conditions to March 2020 + PhenoAgeAccel at baseline
where PhenoAgeAccel was estimated by the residual of PhenoAge adjusted for chronological age at baseline in a linear regression model. As such, PhenoAgeAccel represents how much older (or younger) an individual’s PhenoAge is relative to what is expected based on his/her chronological age. A value of 5 suggests a participant is 5 years older than expected (faster ager), while a value of −5 suggests he/she is 5 years younger than expected (slow ager). Thus, we hypothesize that higher PhenoAgeAccel (faster biological aging) will be positively associated with COVID-19 severity.
There is no implication that PhenoAge is a better predictor than the biomarkers in PhenoAge especially when the outcome is not all-cause mortality. For sensitivity analysis, we replaced PhenoAgeAccel in M6 with the biomarkers in PhenoAge to determine relative contributions of the biomarkers to associations with the 2 COVID-19 severity outcomes.
where each biomarker was z-transformed to be in the same scale for the effect comparison with other biomarkers. We acknowledge that this model may be biased to overfitting in comparison to M6 since unlike PhenoAge for which the weighting of biomarkers was derived from an independent sample, these weights will be derived within the UKB sample for the outcome of interest.
The above models (M1–M7) were also adjusted for sex, self-reported ethnicity, and baseline UKB assessment centers in England to account for geographic differences in the prevalence of COVID-19. The preexisting diseases/conditions were mostly selected as those included in Atkins et al (5) (ICD-10 codes in Supplementary Table S1): dementia, type 2 diabetes, history of pneumonia, depression, atrial fibrillation, hypertension, chronic obstructive pulmonary disease (COPD), chronic kidney disease, rheumatoid arthritis, coronary artery disease, history of delirium, stroke, asthma, previous falls/fragile fractures, osteoarthritis, and liver disease.
We estimated the proportions of mediated effects of included diseases (to March 2020) and comorbidities (number of diagnosed diseases) for the association between accelerated aging (1 if PhenoAgeAccel > 0 and 0 if PhenoAge ≤ 0) and inpatient test positivity or COVID-19-related mortality, using participants free of the considered diseases at baseline, a logistic regression model for a binary-dependent variable, and a Poisson regression model for a count-dependent variable (comorbidities), adjusting for age at the pandemic, sex, ethnicity, and assessment centers.
We also evaluated M1–M7 for predictive power by AUC using 10-fold cross-validation. Specificity, positive and negative predictive values were reported when the sensitivity was controlled at a desired level by manipulating the predicted probability threshold to predict who will be severely infected. All the statistical analyses were performed in R version 3.4.1. The mediation analysis was conducted using the “mediation” R package (22).
Results
445 875 participants attended baseline assessment centers in England, United Kingdom. Participants who died before the pandemic (set as March 16, 2020, n = 25 321) were excluded. 47 572 in England were tested between March 16 and December 21, 2020, including 30 887 inpatient and 16 685 noninpatient samples. 6254 noninpatient positives (236 between March 16 and April 27; 6018 from April 27 to December 21, 2020) and 1504 inpatient positives from April 27 to December 21, 2020 were further excluded as well as participants with any missing data of demographics, comorbidities, or PhenoAge, leaving a total of 339 285 samples.
Among the included samples, 186 125 (54.6%) were female. 94.3% of participants self-identified as White (n = 319 786), 1.7% identified as Black (n = 5905), and 4.0% identified as Other, which included Mixed, Asian, and Chinese (n = 13 594). The mean attained age on March 16, 2020 (age at the pandemic) was 67.9 (SD = 8.1), where 217 313 (64.1%) were 65 years and older. The mean chronological age (56.3 ± 8.1) was 2.5 years older than the mean PhenoAge (53.9 ± 9.4), both at baseline/recruitment of UKB. PhenoAge and chronological age at baseline were highly correlated with Pearson correlation coefficient r = 0.857 (95% CI: 0.856–0.858). A summary of the PhenoAge biomarkers at baseline is provided in Table 1.
Table 1.
Characteristics of the Included Samples (n = 339 285): Participants Attending Baseline Assessment Centers in England and Alive Before the Pandemic (set as March 16, 2020)
| Included Samples | Frequency (%) or Mean ± SD |
|---|---|
| Sex (=female) | 186 125 (54.6%) |
| Ethnicity | |
| White | 319 786 (94.3%) |
| Black | 5905 (1.70%) |
| Others (incl. Asian, Chinese, and Mixed) | 13 594 (4.0%) |
| Age at baseline (years) | 56.3 ± 8.1 |
| Attained age on March 16, 2020 (years) | 67.9 ± 8.1 |
| COVID-19 severity groups | |
| Inpatient positives between March 16 and April 27, 2020 | 613 (0.18%) |
| COVID-19-related deaths to October 2020 | 154 (0.05%) |
| Other-cause deaths to October 2020 | 23 (0.01%) |
| Survivors to October 2020 | 436 (0.13%) |
| Tested negative | 32 098 (9.46%) |
| Untested | 306 574 (90.36%) |
| PhenoAge biomarkers | |
| Albumin (g/L) | 45.28 ± 2.54 |
| Alkaline phosphatase (U/L) | 82.73 ± 22.37 |
| Creatinine (μmol/L) | 71.95 ± 14.13 |
| log C-reactive protein (CRP) (mg/dL) | 0.30 ± 1.04 |
| Glucose (mmol/L) | 5.09 ± 0.93 |
| Lymphocyte percentage (%) | 29.03 ± 7.14 |
| Mean corpuscular volume (fL) | 91.04 ± 4.22 |
| Red blood cell distribution width (RDW) (%) | 13.46 ± 0.85 |
| White blood cell count (1000 cells/μL) | 6.83 ± 1.69 |
Six hundred and thirteen participants tested positive at inpatient settings between March 16 and April 27. Of these, 154 (25.1%) died from COVID-19. Three individuals were excluded for the COVID-19-related mortality outcome, with the specimen sample taken date slightly later than the date of death (≤3 days). For the rest of COVID-19-related deaths, the median duration from the first inpatient positive result to death was 7 days (first quartile: 4, third quartile: 11.75, range: 0–113 days). In the same testing period (March 16 to April 27, 2020), 436 inpatient positives survived to October 2020, and 23 died from causes unrelated to COVID-19.
The mean duration between baseline blood draw and the onset of the pandemic, set as March 16, 2020, was 11.5 years with the SD 0.8 years. Boxplots and violin plots for PhenoAge acceleration by status of demographic, COVID-19 severity, or disease status at the pandemic (Supplementary Figures S1–S4) showed upward trends for severe cases of COVID-19 and those with preexisting diseases/conditions particularly type 2 diabetes and chronic kidney disease, compared to their counterparts.
Based on logistic regression model results, men were more likely to test positive at an inpatient setting for COVID-19 and die with test-confirmed COVID-19 than women. Similarly, participants with self-reported Black ethnicity were more likely to test positive at an inpatient setting than those with self-reported White ethnicity, regardless of models (Tables 2–4). Increased age at the pandemic was associated with increased risks of inpatient test positivity and COVID-19-related mortality but age at the pandemic was not associated with inpatient test positivity (p > .05) after adjusting for concurrent disease prevalence (M5 and M6).
Table 2.
Models for COVID-19 Inpatient Test Positivity and COVID-19-Related Mortality With Inpatient Test-Confirmed COVID-19: M1 and M2
| M1: Age at the Pandemic (March 16, 2020) | M2: Age at the Pandemic (March 16, 2020) + PhenoAgeAccel at Baseline | |||
|---|---|---|---|---|
| Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead (n = 151) vs Positive Alive, Untested or Negative (n = 339 108) | Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead (n = 151) vs Positive Alive, Untested or Negative (n = 339 108) | |
| Sex* (=male) |
1.56 (1.33, 1.83)
p = 4.6e-08 |
2.07 (1.48, 2.89)
p = 1.8e-05 |
1.41 (1.20, 1.66)
p = 2.9e-05 |
1.70 (1.21, 2.38)
p = 2.1e-03 |
| Ethnicity† | ||||
| Black |
3.09 (2.06, 4.63)
p = 4.7e-08 |
3.44 (1.48, 8.00)
p = 4.2e-03 |
2.81 (1.87, 4.22)
p = 6.1e-07 |
2.96 (1.26, 6.92)
p = .012 |
| Other (incl. Asian, Chinese, and Mixed) |
2.09 (1.52, 2.88)
p = 5.5e-06 |
1.78 (0.85, 3.72) p = .12 |
2.01 (1.46, 2.77)
p = 1.7e-05 |
1.65 (0.79, 3.46) p = .18 |
| Age at the pandemic (per 5 years) |
1.12 (1.07, 1.18)
p = 1.1e-05 |
1.95 (1.69, 2.25)
p = 1.9e-20 |
1.12 (1.06, 1.17)
p = 2.3e-05 |
1.92 (1.67, 2.21)
p = 1.2e-19 |
| PhenoAgeAccel (per 5 years) |
1.37 (1.27, 1.47)
p = 1.1e-17 |
1.63 (1.43, 1.86)
p = 4.7e-13 |
Notes: In bold if p < .05; assessment center results skipped.
*Reference: female.
†Reference: White.
Table 3.
Models for COVID-19 Inpatient Test Positivity and COVID-19-Related Mortality With Inpatient Test-Confirmed COVID-19: M3 and M4
| M3: Age at the Pandemic (March 16, 2020) + Preexisting Diseases or Conditions at Baseline | M4: Age at the Pandemic (March 16, 2020) + Preexisting Disease or Conditions at Baseline + PhenoAgeAccel at Baseline | |||
|---|---|---|---|---|
| Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead (n = 151) vs Positive Alive, Untested or Negative (n = 339 108) | Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead (n = 151) vs Positive Alive, Untested or Negative (n = 339 108) | |
| Sex* (=male) |
1. 50 (1.28, 1.77)
p = 1.0e-06 |
1.96 (1.39, 2.75)
p = 1.1e-04 |
1.41 (1.19, 1.66)
p = 4.5e-05 |
1.69 (1.20, 2.38)
p = 2.9e-03 |
| Ethnicity† | ||||
| Black |
2.89 (1.93, 4.35)
p = 3.1e-7 |
3.11 (1.32, 7.29)
p = 9.2e-03 |
2.77 (1.84, 4.16)
p = 1.0e-06 |
2.92 (1.24, 6.88)
p = .014 |
| Other (incl. Asian, Chinese, and Mixed) |
1.94 (1.41, 2.68)
p = 5.4e-5 |
1.66 (0.79, 3.50) p = .18 |
1.94 (1.40, 2.67)
p = 5.8e-05 |
1.68 (0.80, 3.53) p = .17 |
| Age at the pandemic (per 5 years) |
1.07 (1.01, 1.13)
p = .019 |
1.81 (1.57, 2.10)
p = 1.6e-15 |
1.07 (1.02, 1.13)
p = 9.8e-03 |
1.82 (1.58, 2.11)
p = 6.1e-16 |
| PhenoAgeAccel (per 5 years) |
1.25 (1.16, 1.35)
p = 6.1e-09 |
1.50 (1.30, 1.73)
p = 3.1e-08 |
||
| Dementia (n1 = 0; n2 = 0) | ||||
| Type 2 diabetes (n1 = 41; n2 = 11) |
2.0 (1.43, 2.81)
p = 5.6e-05 |
1.54 (0.81, 2.93) p = .19 |
1.59 (1.12, 2.25)
p = 9.1e-03 |
1.02 (0.53, 1.98) p = .95 |
| History of pneumonia (n1 = 15; n2 = 3) | 1.07 (0.64, 1.8) p = .80 |
0.71 (0.22, 2.27) p = .57 |
1.04 (0.62, 1.76) p = .87 |
0.69 (0.22, 2.19) p = .53 |
| Depression (n1 = 64; n2 = 17) |
1.77 (1.36, 2.30)
p = 2.3e-05 |
1.98 (1.15, 3.40)
p = .014 |
1.71 (1.31, 2.23)
p = 6.9e-05 |
1.89 (1.10, 3.25)
p = .022 |
| Atrial fibrillation (n1 = 21; n2 = 8) |
1.68 (1.07, 2.64)
p = .024 |
1.77 (0.85, 3.69) p = .13 |
1.58 (1.01, 2.49)
p = .045 |
1.56 (0.74, 3.26) p = .24 |
| Hypertension (n1 = 250; n2 = 85) |
1.41 (1.18, 1.67)
p = 1.1e-04 |
1.91 (1.36, 2.67)
p = 1.7e-04 |
1.33 (1.12, 1.59)
p = 1.3e-03 |
1.73 (1.23, 2.43)
p = 1.5e-03 |
| COPD (n1 = 28; n2 = 13) |
1.88 (1.26, 2.80)
p = 1.8e-03 |
3.61 (1.98, 6.59)
p = 3.0e-05 |
1.74 (1.17, 2.59)
p = 6.6e-03 |
3.08 (1.68, 5.65)
p = 2.8e-04 |
| Chronic kidney disease (n1 = 4; n2 = 1) | 2.38 (0.87, 6.48) p = .091 |
1.99 (0.27, 14.56) p = .50 |
1.71 (0.62, 4.72) p = .30 |
|
| Liver disease (n1 = 11; n2 = 3) |
2.86 (1.55, 5.27)
p = 7.5e-04 |
3.37 (1.05, 10.75)
p = .04 |
2.61 (1.42, 4.82)
p = 2.1e-03 |
2.72 (0.85, 8.73)
p = .092 |
| Rheumatoid arthritis (n1 = 8; n2 = 4) | 0.93 (0.46, 1.88) p = .84 |
1.70 (0.62, 4.61) p = .30 |
0.80 (0.40, 1.62) p = .54 |
1.26 (0.46, 3.46) p = .65 |
| Coronary artery disease (n1 = 60; n2 = 19) | 1.18 (0.89, 1.58) p = .25 |
0.96 (0.58, 1.61) p = .88 |
1.14 (0.86, 1.53) p = .37 |
0.91 (0.54, 1.52) p = .72 |
| Delirium (n1 = 0; n2 = 0) | ||||
| Stroke (n1 = 5; n2 = 2) | 2.20 (0.90, 5.39) p = .083 |
2.57 (0.62, 10.59) p = .19 |
2.16 (0.88, 5.27) p = .092 |
2.47 (0.60, 10.19) p = .21 |
| Asthma (n1 = 84; n2 = 13) | 1.05 (0.83, 1.33) p = .69 |
0.52 (0.28, 0.96)
p = .035 |
1.02 (0.80, 1.29) p = .89 |
0.49 (0.26, 0.90)
p = .022 |
| Previous falls/fragile fractures (n1 = 26; n2 = 6) |
1.73 (1.16, 2.57)
p = 6.8e-03 |
1.20 (0.49, 2.94) p = .69 |
1.69 (1.14, 2.52)
p = 9.2e-03 |
1.14 (0.46, 2.80) p = .77 |
| Osteoarthritis (n1 = 69; n2 = 24) | 1.09 (0.84, 1.41) p = .51 |
1.19 (0.75, 1.87) p = .46 |
1.07 (0.83, 1.38) p = .62 |
1.15 (0.73, 1.80) p = .56 |
Notes: In bold if p < .05; assessment center results skipped; n1: number of inpatient positives between March 16 and April 27, 2020; n2: number of COVID-19-related deaths following an inpatient positive result between March 16 and April 27, 2020.
*Reference: female.
†Reference: White.
Table 4.
Models for COVID-19 Inpatient Test Positivity and COVID-19-Related Mortality With Inpatient Test-Confirmed COVID-19: M5 and M6
| M5: Age at the Pandemic (March 16, 2020) + Preexisting Diseases or Conditions to March 2020 | M6: Age at the Pandemic (March 16, 2020) + Preexisting Diseases or Conditions to March 2020 + PhenoAgeAccel at Baseline | |||
|---|---|---|---|---|
| Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead (n = 151) vs Positive Alive, Untested or Negative (n = 339 108) | Positive (n = 613) vs Untested or Negative (n = 338 672) | Positive Dead vs Positive Alive, Untested or Negative (n = 338 108) | |
| Sex* (=male) |
1.41 (1.19, 1.66)
p = 6e-05 |
1.76 (1.24, 2.49)
p = 1.6e-03 |
1.37 (1.16, 1.62)
p = 2.0e-04 |
1.66 (1.17, 2.37)
p = 4.6e-03 |
| Ethnicity† | ||||
| Black |
2.59 (1.71, 3.90)
p = 6.1e-06 |
2.39 (1.00, 5.68)
p = .049 |
2.56 (1.69, 3.86)
p = 8.1e-06 |
2.34 (0.98, 5.59) p = .055 |
| Other (incl. Asian, Chinese, and Mixed) |
1.81 (1.30, 2.50)
p = 3.8e-04 |
1.38 (0.65, 2.94) p = .40 |
1.82 (1.31, 2.52)
p = 3.3e-04 |
1.42 (0.67, 3.02) p = .37 |
| Age at the pandemic (per 5 years) | 0.95 (0.90, 1.00) p = .057 |
1.46 (1.26, 1.69)
p = 3.9e-07 |
0.95 (0.90, 1.01) p = .09 |
1.48 (1.28, 1.71)
p = 1.7e-07 |
| PhenoAgeAccel (per 5 years) |
1.10 (1.02, 1.19)
p = .016 |
1.21 (1.04, 1.40)
p = .011 |
||
| Dementia (n1 = 60; n2 = 31) |
5.56 (4.00, 7.74)
p = 2.5e-24 |
7.20 (4.37, 11.86)
p = 9.0e-15 |
5.62 (4.04, 7.82)
p = 1.2e-24 |
7.35 (4.46, 12.11)
p = 4.7e-15 |
| Type 2 diabetes (n1 = 131; n2 = 52) |
1.65 (1.32, 2.05)
p = 9.9e-06 |
2.04 (1.39, 2.99)
p = 2.6e-04 |
1.54 (1.23, 1.93)
p = 2.1e-04 |
1.80 (1.21, 2.67)
p = 3.7e-03 |
| History of pneumonia (n1 = 112; n2 = 41) |
2.27 (1.79, 2.89)
p = 1.4e-11 |
2.28 (1.48, 3.50)
p = 1.7e-04 |
2.24 (1.76, 2.84)
p = 3.9e-11 |
2.20 (1.43, 3.39)
p = 3.3e-04 |
| Depression (n1 = 104; n2 = 31) |
1.38 (1.10, 1.73)
p = 5.3e-03 |
1.50 (0.97, 2.31) p = .07 |
1.37 (1.09, 1.72)
p = 6.1e-03 |
1.50 (0.97, 2.32) p = .068 |
| Atrial fibrillation (n1 = 99; n2 = 36) |
1.47 (1.15, 1.88)
p = 2.3e-03 |
1.29 (0.84, 1.97) p = .24 |
1.46 (1.14, 1.87)
p = 2.7e-03 |
1.27 (0.83, 1.95) p = .26 |
| Hypertension (n1 = 352; n2 = 118) |
1.47 (1.22, 1.77)
p = 6.0e-05 |
2.22 (1.46, 3.38)
p = 2e-04 |
1.44 (1.19, 1.74)
p = 1.3e-04 |
2.15 (1.41, 3.28)
p = 3.5e-04 |
| COPD (n1 = 71; n2 = 29) | 1.21 (0.92, 1.61) p = .18 |
1.69 (1.06, 2.69)
p = .028 |
1.18 (0.89, 1.57) p = .24 |
1.61 (1.01, 2.57)
p = .047 |
| Chronic kidney disease (n1 = 72; n2 = 29) |
1.66 (1.26, 2.19)
p = 3.6e-04 |
1.65 (1.04, 2.61)
p = .032 |
1.57 (1.18, 2.08)
p = 1.7e-03 |
1.48 (0.93, 2.36) p = .10 |
| Liver disease (n1 = 36; n2 = 6) | 1.27 (0.89, 1.81) p = .19 |
0.61 (0.26, 1.43) p = .26 |
1.25 (0.87, 1.78) p = .23 |
0.58 (0.24, 1.37) p = .21 |
| Rheumatoid arthritis (n1 = 26; n2 = 12) | 1.16 (0.77, 1.75) p = .47 |
1.60 (0.84, 3.07) p = .15 |
1.12 (0.74, 1.68) p = .60 |
1.46 (0.76, 2.82) p = .26 |
| Coronary artery disease (n1 = 133; n2 = 47) | 1.03 (0.83, 1.29) p = .79 |
0.98 (0.66, 1.44) p = .90 |
1.03 (0.82, 1.28) p = .83 |
0.96 (0.65, 1.43) p = .85 |
| Delirium (n1 = 40; n2 = 19) |
1.79 (1.21, 2.66)
p = 3.8e-03 |
1.85 (1.01, 3.38)
p = .045 |
1.79 (1.21, 2.66)
p = 3.9e-03 |
1.85 (1.01, 3.39)
p = .045 |
| Stroke (n1 = 30; n2 = 11) |
1.41 (0.95, 2.08)
p = .089 |
1.30 (0.68, 2.49) p = .43 |
1.39 (0.94, 2.06)
p = .10 |
1.26 (0.66, 2.43) p = .48 |
| Asthma (n1 = 117; n2 = 24) | 1.09 (0.88, 1.35) p = .42 |
0.71 (0.44, 1.13) p = .15 |
1.09 (0.88, 1.35) p = .45 |
0.70 (0.44, 1.13) p = .14 |
| Previous falls/fragile fractures (n1 = 140; n2 = 48) |
1.87 (1.50, 2.32)
p = 2.3e-08 |
1.68 (1.11, 2.54)
p = .014 |
1.85 (1.49, 2.31)
p = 3.5e-08 |
1.65 (1.09, 2.50)
p = .018 |
| Osteoarthritis (n1 = 80; n2 = 28) | 0.84 (0.65, 1.07) p = .16 |
0.89 (0.58, 1.38) p = .61 |
0.83 (0.65, 1.07) p = .15 |
0.89 (0.58, 1.37) p = .60 |
Notes: In bold if p < .05; assessment center results skipped; n1: number of inpatient positives between March 16 and April 27, 2020; n2: number of COVID-19-related deaths following an inpatient positive result between March 16 and April 27, 2020.
*Reference: female.
†Reference: White.
Independent of age at the pandemic and other demographics, increased PhenoAge was associated with an increased risk of inpatient test positivity (odds ratio [OR] = 1.37 per 5 years, 95% CI: 1.27–1.47, p = 1.1 × 10−17) and COVID-19-related mortality (OR = 1.63 per 5 years, 95% CI: 1.43–1.86, p = 4.7 × 10−13) (Table 2 M2). The associations above were modestly reduced with additional adjustment for prevalent diseases at baseline (inpatient test positivity: OR = 1.25 per 5 years, 95% CI: 1.16–1.35, p = 6.1 × 10−9 and COVID-19-related mortality: OR = 1.50 per 5 years, 95% CI: 1.30–1.73, p = 3.1 × 10−8) (Table 3 M4). At baseline, there were no participants diagnosed with dementia or having history of delirium. The association with type 2 diabetes was significantly reduced for inpatient test positivity (M3: OR = 2.0 to M4: OR = 1.59) but remained statistically significant (p < .05) when adjusting for differences in PhenoAgeAccel (Table 3 M3–M4). We investigated if the association attenuation with type 2 diabetes can be explained by specific PhenoAge biomarkers, known to be associated with type 2 diabetes, particularly glucose, creatinine, and C-reactive protein (CRP) (log-transformed). PhenoAgeAccel in M4 was adjusted for chronological age and one of the biomarkers additionally and the change in OR associated with type 2 diabetes from M3 to M4 was compared to that with PhenoAgeAccel adjusted for chronological age only. The association attenuation with type 2 diabetes is attributed to a biomarker if the association attenuation with type 2 diabetes was significantly reduced when PhenoAgeAccel is further adjusted for that biomarker before entering M4. We expected the biomarkers contributed to the attenuation differently depending on the shared association between type 2 diabetes and PhenoAge determined by the biomarker. We found that the association attenuation with type 2 diabetes was more attributed to glucose (M3: OR = 2.0 to M4 with PhenoAgeAccel further adjusted for glucose: OR = 1.93), followed by log(CRP) (M3: OR = 2.0 to M4 with PhenoAgeAccel further adjusted for log(CRP): OR = 1.69), and then creatinine (M3: OR = 2.0 to M4 with PhenoAgeAccel further adjusted for creatinine: OR = 1.59), compared to the change from M3 to M4 (M3: OR = 2.0 to M4: OR = 1.59). Overall, the findings above suggest that PhenoAgeAccel as a function of multiple biomarkers cannot be replaced by any single biomarker in PhenoAge for associations with COVID-19 severity. Of note, the disease associations from M3 and M4 could be biased by those who developed diseases after baseline, so the results are not as reliable as those from M5 and M6, for example, negative associations between asthma and COVID-19-related mortality from M3 and M4.
When disease diagnoses were updated to March 2020, the association between PhenoAgeAccel (PhenoAge adjusted for baseline chronological age) and either severity outcome was further reduced (Table 4 M6) but remained statistically significant at the 5% significance level. Additionally, the attenuated association with type 2 diabetes from M3 to M4 was not found from M5 to M6. Dementia, type 2 diabetes, history of pneumonia, hypertension, delirium, and previous falls or fragile fractures were associated with inpatient test positivity and COVID-19-related mortality in both M5 and M6. M6 and M7 shared similar associations with diseases and demographics but the association with sex significantly increased from M6 to M7 for COVID-19-related mortality. Interestingly, albumin (OR = 0.87 per SD increase, 95% CI: 0.80–0.94, p = 5.9 × 10−4) and log(CRP) (OR = 1.10 per SD increase, 95% CI: 1.01–1.20, p = .037) more than a decade ago remained significantly associated with inpatient test positivity but not with COVID-19-related mortality likely due to a lack of power given the ORs were similar for both outcomes (Supplementary Table S2).
174 558 participants free of the considered diseases were used for mediation analysis, including 216 inpatient positives where 165 survived and 39 died due to COVID-19. We found that 22% of the effect of accelerated aging (ie, PhenoAgeAccel > 0) on inpatient test positivity was mediated by comorbidities (number of diagnosed diseases through March 2020, 41 158 about 24% reported at least one incident disease diagnosis), which mediated 18% of the effect of accelerated aging on COVID-19-related mortality. The mediation effect varied with diseases, mostly by type 2 diabetes with 13% for inpatient test positivity and 8% for COVID-19-related mortality, followed by hypertension (9% and 8%) and pneumonia (8% and 8%) (Supplementary Tables S4 and S5). Of note, the disease mediation effects are not additive as they likely share common mechanisms. Given that the mediation analyses are exploratory due to limited inpatient positives and COVID-19-related deaths, more research needs to be conducted to confirm any causal links.
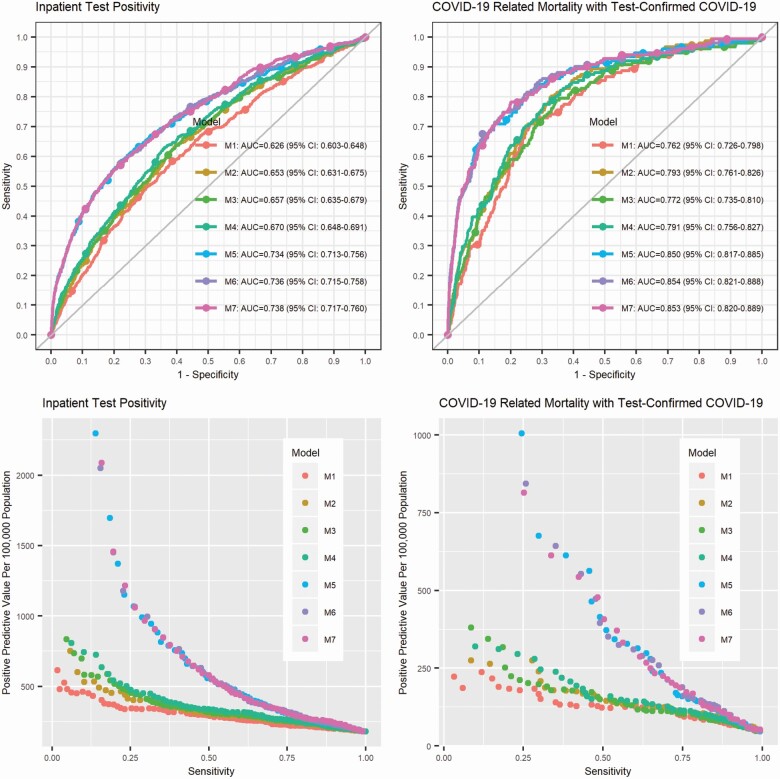
Using samples with complete data to train M1–M7 models, the AUC was higher for COVID-19 mortality than that for inpatient test positivity (Figure 1). Using baseline and up-to-date disease diagnosis data significantly improved AUC compared to using baseline data only. The AUC was similar for models using more or less baseline data, that is, M1–M4, and was not substantially increased by baseline PhenoAge, additional to demographics and baseline disease states.
Figure 1.
Receiver operating characteristic curves (ROCs), areas under the ROC curves (AUCs), and positive predictive value versus sensitivity for inpatient test positivity and COVID-19-related mortality with inpatient test-confirmed COVID-19.
As shown in Figure 1, the difference in positive predivtive value (PPV) among models for either severity outcome decreased when sensitivity increased. For inpatient test positivity, 278 per 100 000 identified cases are expected to be tested positive or hospitalized (PPV = 278/100 000) using M5 including recent disease states and the predicted probability threshold 0.00116 for 80% sensitivity, and the PPV increases to 442/100 000 when the sensitivity is set to 0.6, compared to the sample inpatient test positivity rate 181 per 100 000. Adding PhenoAgeAccel or biomarkers in PhenoAge at baseline to M5 did not significantly improve the predictive performance (M6, M7 vs M5) (Supplementary Table S3). For COVID-19-related mortality, 141 per 100 000 identified cases are expected to die with inpatient test-confirmed COVID-19 (PPV = 141/100 000) using M5 including recent disease states and the predicted probability threshold 0.00035 for 80% sensitivity, and the PPV increases to 314/100 000 when the sensitivity is set to 0.6, compared to the sample mortality rate 45 per 100 000. Adding PhenoAgeAccel or biomarkers in PhenoAge at baseline to M5 did not significantly improve the predictive performance (M6, M7 vs M5) (Supplementary Table S3).
Discussion
Higher PhenoAge was linked to COVID-19 severity with adjustment for chronological age. The association was modestly reduced by prevalent diseases at baseline and decreased when disease states were updated to the start of the pandemic. Our mediation analysis results suggested that only ~20% of the association between accelerated aging a decade or more prior and increased COVID-19 severity was mediated by incident diseases (acquired over that period of time).
The association between age-adjusted PhenoAge and COVID-19 severity, with involvement of disease pathology, may be explained by mechanisms underlying accelerated aging by PhenoAge. In our recent genome-wide association study on PhenoAge acceleration, we observed enrichment for biological processes involved in immune system, cell function, and carbohydrate homeostasis (23). A methylation clock (DNAmPhenoAge) trained using PhenoAge as a surrogate for biological age, instead of chronological age, has been shown to be associated with activation of pro-inflammatory, interferon, DNAm damage repair, transcriptional/translational signaling, and various markers of immunosenescence: a decline of naïve T cells and shortened leukocyte telomere length (12). Overall, this suggests that the accelerated biological aging profile captured by PhenoAge is largely characterized by accelerated inflammaging (chronic low-grade inflammation) (24), whose underlying mechanisms may exacerbate COVID-19 symptoms. This is also supported by the observation that albumin and CRP more than a decade ago were associated with increased risk of inpatient test positivity.
While we modeled PhenoAge and PhenoAge biomarkers alternatively using exactly the same data, they have different indications in the context of COVID-19 severity. As we have emphasized in the Method section that PhenoAge was pretrained using a U.S. cohort (NHANES III) to be a proxy of biological aging via the surrogate of all-cause mortality, it is expected to be a robust predictor for a wide range of age-related outcomes, hypothetically including COVID-19 severity, which is tested in this study. It should be noticed that PhenoAge is not a unique predictor for biological aging. Other sets of variables have been used to develop biological age predictors, eg, (14). These variables may or may not have direct connection to biological aging. Therefore, it is less meaningful to discuss the contribution of each biomarker to the association between PhenoAgeAccel and COVID-19 severity. However, we acknowledge the importance of research on prognostic biomarkers for COVID-19 severity. Compared to biological age predictors, we tend to have a better understanding of biomarkers, which would suggest underlying mechanisms and potential interventions.
The case fatality rate of COVID-19 increases with chronological age, PhenoAge, and accumulation of chronic comorbidities. There is therefore a potential that the effects of COVID-19 on mortality may be reduced by reversing/slowing the aging process. Drugs under development to slow the aging process may provide future avenues for reducing the risks associated viral infections, like COVID-19. This is inline with the geroscience framework and further supported by our previous work showing that people with younger biological ages are less prone to a plethora of diverse age-related diseases (25). By intervening to slow aging, therapeutics may in turn reduce pathological burden as well as minimize the risks of diseases like COVID-19, which are exacerbated by aging and pathology. To date, a number of aging therapeutics are being considered, which target key pathways and hallmarks of aging—reducing insulin/IGF-1 signaling (rapamycin), removing senescent cells (senolytics), and/or improving insulin sensitivity (metformin). Increasing autophagy and reducing age-related inflammation are also emerging as key mechanisms targeted by these drugs (26). Overall, our findings further substantiate the role of biological aging in the progression of COVID-19.
The predictive performance for COVID-19 severity outcomes was significantly improved by basic demographics and recent disease states, compared to the prevalence of test positivity and mortality in the cohort. Adding current PhenoAge may further improve the performance as the variation of biological age in relative to chronological age increases with age; therefore, current PhenoAge may be a more sensitive measure than PhenoAge at baseline for accelerated aging. However, models to predict rare events like COVID-19 severity outcomes inevitably have low positive predictive values. In other words, people with predicted probabilities greater than a threshold are at higher risk than others for severe COVID-19 but the absolute risk remains low. This model or other improved models with additional input could be a useful tool for risk evaluation, which in combination with educational intervention may encourage behavioral changes and greatly reduce the fatality rate of COVID-19.
There are limitations to this study, which warrant acknowledgement. First, we did not include cancers in the analysis as the status of cancer (eg, progressing vs remission) is not available, which is strongly associated with mortality related to COVID-19 (27). Additionally, some participants are not old enough to develop late-onset diseases. As disease cases may be misclassified as nondisease cases, the disease OR estimates were likely to be biased toward the null (28). Also, clinical severity data are not available, but we used the mortality data to derive the severity outcome, COVID-19-related mortality with inpatient test-confirmed COVID-19. Lastly, the UKB sample is known to be healthier than the general population (29); however, risk factor associations are usually generalizable (30).
In conclusion, PhenoAge more than a decade ago prior to the pandemic was associated with COVID-19 severity. Our analysis suggested that this acted partly through disease pathology. Accelerated aging by PhenoAge has previously reported to be largely characterized by inflammaging (24) and may represent susceptibility to innate immune overreaction, as in the case of cytokine storm. Overall, our findings have major public health implications for COVID-19 risk stratification. It provides additional justification for the design, testing, and ultimate clinical targeting of geroscience-guided therapies seeking to improve COVID-19 outcomes by targeting biological aging (25). Such approaches may be transformational by offering opportunities for use in both the current and future pandemics involving other infections by targeting the vulnerable host as opposed to the pathogen.
Supplementary Material
Funding
The authors are supported by grants funded by the National Institute on Aging, National Institute of Health: R00AG052604 for C.-L.K. and M.E.L.; R21AG060018 for C.-L.K., L.C.P., G.A.K., and D.M.; R33AG061456 for G.A.K. D.M. and L.C.P. are supported by the University of Exeter Medical School, and in part by the University of Connecticut School of Medicine. J.L.A. is funded by the Medical Research Council (MR/S009892/1) in the United Kingdom. J.A.H.M. is funded by the National Institute for Health Research (NIHR) in the United Kingdom (NIHR Doctoral Research Fellowship, DRF-2014-07-177).
Conflict of Interest
M.E.L. is named on patent applications for epigenetic clocks and holds licenses for the clocks she has developed. M.E.L. also serves as the Bioinformatics Advisor for Elysium Health.
Acknowledgments
UKB received an approval from the UK Biobank Research Ethics Committee (REC; REC reference 11/NW/0382). All the participants provided written informed consent to participate in the study and for their data to be used in future research. This research was conducted using the UKB resource, under the application 14631. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and social care.
Author Contributions
C.-L.K. and M.E.L. designed the study. J.L.A., L.C.P., and C.-L.K. processed the data. C.-L.K. performed the data analysis. All the authors contributed to the manuscript and approved the final version.
References
- 1. Yan L, Zhang H-T, Goncalves J, et al. An interpretable mortality prediction model for COVID-19 patients. Nat Mach Intell. 2020;2(5):283–288. doi: 10.1038/s42256-020-0180-7 [DOI] [Google Scholar]
- 2. Bialek S, Boundy E, Bowen V, et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19—United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1517–1521. doi: 10.15585/mmwr.mm6942e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wortham JM, Lee JT, Althomsons S, et al. Characteristics of persons who died with COVID-19—United States, February 12-May 18, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:923–929. doi: 10.15585/mmwr.mm6928e1 [DOI] [PubMed] [Google Scholar]
- 5. Kuo C-L, Pilling LC, Atkins JL, et al. ApoE e4e4 genotype and mortality with COVID-19 in UK Biobank. J Gerontol A Biol Sci Med Sci. 2020;75(9):1801–1803. doi: 10.1093/gerona/glaa169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gentile S, Strollo F, Ceriello A. COVID-19 infection in Italian people with diabetes: lessons learned for our future (an experience to be used). Diabetes Res Clin Pract. 2020;162:108137. doi: 10.1016/j.diabres.2020.108137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeCaprio D, Gartner J, McCall CJ, et al. Building a COVID-19 vulnerability index. J Med Artif Intell. 2020;3. doi: 10.21037/jmai-20-47 [DOI] [Google Scholar]
- 10. Tang X, Du RH, Wang R, et al. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195–205. doi: 10.1016/j.chest.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun H, Jain A, Leone MJ, et al. CoVA: an acuity score for outpatient screening that predicts Coronavirus disease 2019 prognosis. J Infect Dis. 2021;223(1):38–46. doi: 10.1093/infdis/jiaa663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10:573–591. doi: 10.18632/aging.101414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3 [DOI] [PubMed] [Google Scholar]
- 14. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15:e1002718. doi: 10.1371/journal.pmed.1002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong J, Rudkin JK, Allen N, et al. Dynamic linkage of COVID-19 test results between Public Health England’s second generation surveillance system and UK Biobank. Microb Genomics. 2020;6(7):1–9. doi: 10.1099/mgen.0.000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. COVID-19 Test Results Data. 2020. http://biobank.ndph.ox.ac.uk/showcase/exinfo.cgi?src=COVID19_tests. Accessed October 23, 2020.
- 20. UK Biobank Biomarker Panel. https://www.ukbiobank.ac.uk/media/oiudpjqa/bcm023_ukb_biomarker_panel_website_v1-0-aug-2015-edit-2018.pdf. Accessed March 29, 2021.
- 21. Sheard SM, Nicholls R, Froggatt J. UK Biobank Haematology Data Companion Document.2017. https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/haematology.pdf. Accessed October 23, 2021.
- 22. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. Mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1–38. doi: 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- 23. Kuo CL, Pilling LC, Liu Z, Atkins JL, Levine ME. Genetic associations for two biological age measures point to distinct aging phenotypes. medRxiv. 2020. doi: 10.1101/2020.07.10.20150797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 25. Sierra F. Geroscience and the Coronavirus pandemic: the whack-a-mole approach is not enough. J Am Geriatr Soc. 2020;68:951–952. doi: 10.1111/jgs.16489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19:513–532. doi: 10.1038/s41573-020-0067-7 [DOI] [PubMed] [Google Scholar]
- 27. Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146:195–203. doi: 10.1093/oxfordjournals.aje.a009251 [DOI] [PubMed] [Google Scholar]
- 29. Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.