Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes de COVID-19 disease use as a principal receptor the angiotensin-converting enzyme-2 (ACE2). It has been suggested that dipeptidyl peptidase-4 (DPP4) can be another possible receptor for this virus. The present study aimed to establish if the DPP4 levels and DPP4 polymorphisms are associated with COVID-19 disease and its severity.
Methods
The study included 107 COVID-19 patients and 263 matched-healthy controls. Fifty patients required invasive mechanical ventilation. The DPP4 was quantified in serum using the Bioplex system. Based on the previous results and the functional prediction analysis, we select for the study 5 DPP4 polymorphisms (rs12617336, rs12617656, rs1558957, rs3788979, and rs17574) and these were determined using the 5´exonuclease TaqMan assays.
Results
Low levels of DPP4 were observed in COVID-19 patients (46.5 [33.1–57.7] ng/mL) when compared to healthy controls (125.3 [100.3–157.3] ng/mL) (P < 0.0001). Also, patients that required mechanical ventilation showed lower DPP4 levels (42.8 [29.8–56.9] ng/mL) than those that did not need this procedure (49.2 [39.9–65.6] ng/mL) (P = 0.012). DPP4 levels correlated negatively with age, fibrinogen, and platelet levels, and positively with albumin, alanine aminotransferase, and percentage of neutrophils. The DPP4 rs3788979 polymorphism was associated with a high risk of COVID-19 disease and, the TT genotype carriers had the lowest DPP4 levels.
Conclusions
In summary, in the present study, an association of low levels of DPP4 with COVID-19 disease and severity was found. The association of the DPP4 rs3788979 polymorphism with COVID-19 is also reported.
Keywords: Angiotensin converting enzyme-2, COVID-19 disease, Dipeptidylpeptidase-4, Polymorphisms, Severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic produced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that has produced 114,853.685 confirmed cases globally with 2554.694 deaths as of March 4, 2021 [1]. SARS-CoV-2 belongs to the Coronaviridae family and can infect humans and several animal species [2]. This virus has a high genetic similarity with SARS-like coronavirus (96%) and SARS-CoV (79.5%) [3]. It has been demonstrated that the virus uses the angiotensin converting enzyme-2 (ACE2) receptor and transmembrane protease serine protease-2 (TMPRSS2) to enter the host cells [4]. Other proteases, such as cathepsins, furin, neutrophil elastase (ELANE), and probably TMPRSS11A [[5], [6], [7]] could be involved in this phenomenon. Another receptor for the corona-like viruses is the dipeptidyl peptidase 4 (DPP4), also known as CD26, which was reported to be the principal receptor for MERS-CoV cell entry [8]. MERS-CoV and SARS-CoV-2 possess structural similarities and are major causes of severe pneumonia in humans [9]. Indeed, using a modelled homo-trimer structure of COVID-19 spike glycoprotein that considered open (ligand-bound) and closed (ligand-free) conformation, Vankadari et al. established that the S1 domain of SARS-CoV-2 spike glycoprotein interacts human DPP4, which could be another receptor for the virus [10]. DPP4 is a protein that participates in various physiological processes and is present in two forms; it can be either anchored to the cell membrane or soluble, circulating in plasma [11]. Apart from its possible role in SARS-CoV-2 entry into the host cell, this protein has been significantly related to the presence of hypertension, obesity and insulin resistance [[12], [13], [14]]. Considering that DPP4 inhibitors increase half-life of glucagon-like peptide 1 (GLP-1), they have been used in the treatment of type 2 diabetes mellitus (T2DM) [15]. Considering that obesity and diabetes are important risk factors for SARS-CoV-2 infection and severity of COVID-19, the study of DPP4 protein in these patients can provide important information. A recent study in a very small group of individuals showed that patients hospitalized for COVID-19 have reduced levels of DPP4 compared to a control group [16]. This study also included a group of patients with sepsis, in whom no differences in DPP4 levels were found, suggesting that the decrease in this protein is conditioned by the SARS-CoV-2 infection. The DPP4-encoding gene is polymorphic; an association of the enzyme levels was found with not only some genotypes of these polymorphisms [17] but also some diseases, such as type 2 diabetes mellitus (T2DM) [17] and myocardial infarction [18]. Thus, the aim of the present study was to evaluate the DPP4 concentration in a group of patients with COVID-19 and establish if these levels are related with severity and some clinical findings. In addition, we evaluated the association of DPP4 polymorphisms with SARS-CoV-2 infection, COVID-19 severity, and DPP4 levels.
2. Methods
2.1. Subjects
We enrolled 107 consecutive patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection confirmed by RT-PCR test in at least one biological sample. The patients were attended in the intensive care unit of our Institute from May 11 to July 31, 2020. Fifty patients required invasive mechanical ventilation. Demographic, clinical, and laboratory parameters of COVID-19 patients were obtained. All patients or their relatives signed the institutional consent letter.
To evaluate if DPP4 serum concentrations in COVID-19 patients were different from healthy controls, we used a case control study design. For each COVID-19 patient, we matched 3 controls by age, sex, and body mass index. Of the 107 COVID-19 patients, 83 patients were individually matched with 3 healthy controls, while 7 patients were matched with only 2 controls each. Therefore, in the present study, we examined circulating DPP4 serum concentrations in 90 COVID-19 patients and 263 healthy controls (172 men and 91 women). The healthy control individuals were selected among the participants of the Genetics of Atherosclerotic Disease (GEA) Mexican study control group [19]. The GEA control group included individuals without personal or family history of premature coronary disease. Anthropometric, biochemical, clinical, and demographic characteristics, as well as cardiovascular risk factors were determined in all controls as previously described [[19], [20], [21]]. Criteria definitions and methods have been reported previously [[22], [23], [24], [25]]. Obesity [19,20], current smoking [20], hypertension [19,20], T2DM [19,20] definitions have already been published.
A serum DPP4 concentration ≤ p25 (89.8 ng/mL for women and 108.1 ng/mL for men) was considered low. This cut-off point was obtained from a GEA study subsample of non-obese non-hypertense volunteers (124 men and 183 women) with normal values of fasting lipids and glucose.
2.2. Sample handling
The samples were obtained in the intensive care unit following the institutional security protocols. Competent trained personnel carried out the handling and processing of the patients' blood samples, which were later transported to the laboratory for processing. Samples were centrifuged, and the serum was separated in a class II biological safety cabinet. Personnel handling the samples used personal protective equipment that included disposable gloves, a lab coat, and a surgical mask. Samples were collected and processed in a laboratory that adheres to the guidelines established in the Official Mexican Standards NOR-007-SSA3-2011, NOM-087-SEMARNAT-SSA1-2002, NOM-010-SSA2-2010, NOM-006-SSA2-2013, and NMX-EC-15189 IMNC-2015.
2.3. Quantification of DPP4 serum concentration
Bioplex system (R&D Systems, Minneapolis, USA) was used to quantified DPP4 serum concentration, according to manufacturer's instructions. A Bio-Plex Manager software was used for the data analyses. Results are expressed in ng/mL.
2.4. Genetic analysis
For the genomic DNA isolation from whole blood (containing EDTA) of the COVID-19 patients, we used QIAamp DNA Blood Mini kit (QIAGEN, Hilden, Germany). The genomic DNA from the control individuals had been previously extracted and the aliquots were stored at −70 °C. We used the SNP Function Prediction (http://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html), Human-transcriptome Database for Alternative Splicing (http://www.h-invitational.jp/h-dbas/), Splice Port: An Interactive Splice Site Analysis Tool (http://spliceport.cbcb.umd.edu/SplicingAnalyser.html), ESE finder (http://rulai.cshl.edu/cgi-bin/tools/ESE3/esefinder.cgi), HSF (http://www.umd.be/HSF/), and SNPs3D (http://www.snps3d.org/) bioinformatics tools to ascertain the possible functional effect of the DPP4 polymorphisms. Based on the previous results and the functional prediction analysis, we select for the study 5 DPP4 polymorphisms (rs12617336, rs12617656, rs1558957, rs3788979, and rs17574). Using 5′ exonuclease TaqMan genotyping assays, these polymorphisms were genotyped on an ABI Prism 7900HT Fast Real-Time PCR system (Applied Biosystems, Foster City, CA).
2.5. Statistical analysis
Data are expressed as frequencies, median (interquartile range) or mean ± standard deviation, as appropriate. For continuous variable comparisons, either Student's t-test or Mann–Whitney U test was used. The chi-squared test was employed for categorical variable comparisons. The frequencies of alleles and genotypes were determined by direct counting. We used the chi-squared test to determine the Hardy-Weinberg's equilibrium. Haplotype analysis and linkage disequilibrium were performed with Haploview software (version 4.1, Broad Institute of Massachusetts Institute of Technology and Harvard University, Cambridge, MA, USA). DPP4 serum concentration comparisons were evaluated by the Mann-Whitney U or Kruskal-Wallis test, as appropriate. Using logistic regression analysis adjusted for age, sex, body mass index, hypertension and type 2 diabetes mellitus, we appraised the independence and significance of the following associations (a) COVID-19 infection and DPP4 serum levels, (b) COVID-19 infection and DPP4 studied polymorphisms, and (c) DPP4 levels and the severity of COVID-19 infection (assessed by the need for mechanical invasive ventilation). The analysis of association of COVID-19 infection with each polymorphism under each inheritance model (additive, dominant, recessive, heterozygote, co-dominant1 and co-dominat2) was done individually. A P value <0.05 was considered significant. We used the SPSS software v15.0 (SPSS Chicago, IL) for all analyses.
3. Results
3.1. Clinical, biochemical, and demographic characteristics
One hundred-seven COVID-19 patients (68% male) were included in the study, with a median age of 55.76 years. Common symptoms were cough (62.5%), fever (61.7%), dyspnea (56.1%), fatigue (38.3%), and myalgia (38.3%). The most frequent comorbidities were hypertension in 45.8%, T2DM in 38.3%, and obesity in 34.6% (Table 1 ).
Table 1.
Characteristics of COVID-19 patients.
| All subjects (n = 107) | |
|---|---|
| Male (%) | 68 |
| Age (years) | 55.76 ± 13.59 |
| Body mass index (Kg/m2) | 29.08 ± 5.60 |
| Comorbidities | |
| Hypertension (%) | 45.8 |
| Type 2 diabetes mellitus (%) | 38.3 |
| Obesity (%) | 34.6 |
| Dyslipidemia (%) | 10.3 |
| Ischemic heart disease (%) | 8.4 |
| Tabaquism (%) | 7.5 |
| Valvular disease (%) | 4.7 |
| Chronic kidney disease (%) | 3.7 |
| Hyperthyroidism (%) | 3.7 |
| Gout (%) | 1.9 |
| Systemic lupus erythematosus (%) | 1.9 |
| Antiphospholipid syndrome (%) | 1.9 |
| Pulmonary hypertension (%) | 0.9 |
| Initial symptoms | |
| Cough (%) | 62.5 |
| Fever (%) | 61.7 |
| Dyspnea (%) | 56.1 |
| Fatigue (%) | 38.3 |
| Myalgia (%) | 38.3 |
| Arthralgia (%) | 33.6 |
| Headache (%) | 24.3 |
| Odynophagia (%) | 20.6 |
| Diarrhea (%) | 17.8 |
| Diaphoresis (%) | 12.1 |
| Shaking chills (%) | 11.2 |
| Emesis (%) | 9.3 |
| Chest pain (%) | 9.3 |
| Anosmia (%) | 7.5 |
| Dysgeusia (%) | 5.6 |
| Abdominal pain (%) | 5.6 |
| Hyporexia (%) | 4.7 |
| Rhinorrhea (%) | 1.9 |
Data are shown as mean ± standard deviation or percentage.
As mentioned above, of the 107 COVID-19 patients, 83 patients were individually matched with 3 healthy controls, and 7 patients with 2 controls each. Therefore, in the present study we examined the circulating DPP4 serum concentrations in 90 COVID-19 patients and 263 healthy controls; their clinical, demographic, and biochemical parameters are shown in Table 2 . When compared to controls, COVID-19 patients showed a high prevalence of hypertension, T2DM, as well as high levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase.
Table 2.
Demographic, clinical and biochemical parameters in COVID-19 patients and matched controls.
| All subjects (n = 353) | COVID-19 (n = 90) | Controls (n = 263) | Pa | |
|---|---|---|---|---|
| Male (%) | 65.4 | 65.5 | 65.4 | 0.543 |
| Age (years) | 53.9 ± 10.9 | 54.5 ± 11.6 | 53.7 ± 10.6 | 0.509 |
| Body mass index (Kg/m2) | 28 [26–31] | 28 [26–31] | 28 [26–31] | 0.340 |
| Type 2 diabetes mellitus (%) | 19.8 | 38.9 | 13.3 | <0.001 |
| Hypertension (%) | 17.8 | 44.4 | 8.7 | <0.001 |
| Creatinine (mg/dL) | 0.88 [0.71–1.06] | 0.85 [0.59–1.35] | 0.89 [0.74–1.04] | 0.006 |
| Alanine aminotranferase (U/L) | 27 [20–44] | 38 [21–62] | 25 [19–37] | <0.001 |
| Aspartate aminotranferase (U/L) | 26 [21–35] | 34 [22–55] | 25 [21−31] | <0.001 |
| Alkaline phosphatase (U/L) | 80 [66–99] | 88 [67–115] | 79 [66–94] | 0.010 |
Data are shown as mean ± standard deviation, median [interquartile range] or percentage.
Student's t-test, Mann Whitney's U test or Chi square test as appropriate.
3.2. DPP4 levels in COVID-19 patients and healthy controls
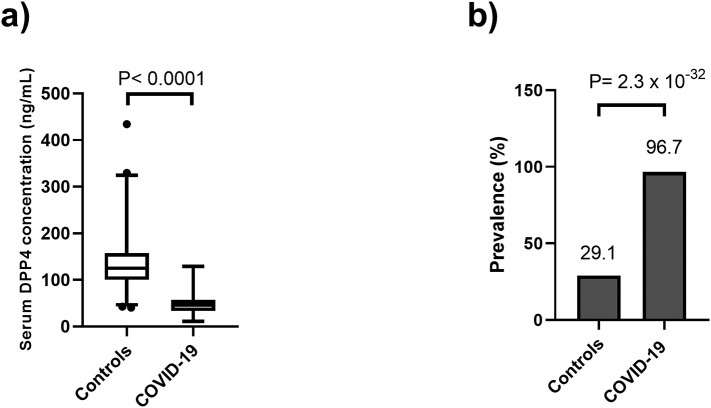
COVID-19 patients showed significantly lower serum DPP4 levels (46.5 [33.1–57.7] ng/mL) when compared to healthy controls (125.3 [100.3–157.3] ng/mL) (P < 0.0001) (Fig. 1a). In the same way, the prevalence of low levels of DPP4 was higher in COVID-19 patients than in controls (96.7 vs 29.1, P = 2.3 × 10−32) (Fig. 1b). We used logistic regression analyses adjusted for age, sex, body mass index, hypertension and type 2 diabetes mellitus to evaluate the independence and significance of the association between COVID-19 and DPP4 serum levels. We found that a low DPP4 concentration (<p25) was associated with a significantly higher risk of COVID-19 (OR [95%CI] = 70 [20−230], P = 1.69 × 10−11) (Data not shown).
Fig. 1.
DPP4 concentration and prevalence of low DPP4 in 90 COVID-19 patients matched with 263 healthy controls. a) serum DPP4 levels were significantly lower in COVID-19 patients (46.5 [33.1–57.7] ng/mL) compared with controls (125.3 [100.3–157.3] ng/mL, P < 0.0001). b) Prevalence of low DPP4 concentrations in COVID-19 patients and healthy controls (96.7 vs 29.1, P = 2.3 × 10−32).
3.3. DPP4 levels in COVID-19 patients with and without mechanical ventilation
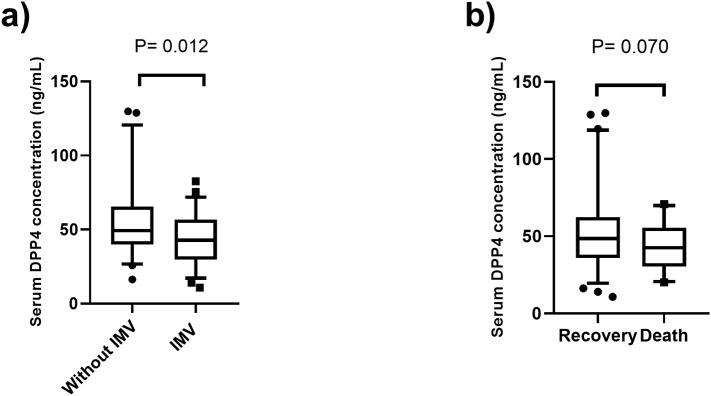
DPP4 levels were compared in patients who required invasive mechanical ventilation (IMV) and those who did not. The clinical and demographic characteristics of these groups are shown in Table 3 . In this analysis, we included 107 COVID-19 patients. Individuals who underwent IMV (82.0% male) had high levels of C-reactive protein, d-dimers, ferritin, and leucocytes when compared to non-seriously ill patients who did not require ventilation. The group of patients who received IMV showed low levels of DPP4 (42.8 [29.8–56.9] ng/mL) compared to individuals without ventilation (49.2 [39.9–65.6] ng/mL) (P = 0.012) (Fig. 2a). A marginal difference in DPP4 levels was observed between patients who recovered from the disease (48.9 [35.8–63.1] ng/mL) and those who died (42.5 [30.3–55.6] ng/mL) (P = 0.070) (Fig. 2b). The analysis of mortality was made in 102 patients, because 5 patients were transferred to another hospital and we did not know the outcome. Under different models adjusted for confounding variables (age, sex, BMI, T2DM, and hypertension), high DPP4 levels were associated with low risk of requiring IMV (Table 4 ).
Table 3.
Demographic, clinical and biochemical parameters of COVID-19 patients stratified by invasive mechanical ventilation.
| Invasive mechanical ventilation |
||||
|---|---|---|---|---|
| All patients (n = 107) | Yes (n = 50) | No (n = 57) | Pa | |
| Age (years) | 55.8 ± 13.6 | 58.4 ± 12.1 | 53.4 ± 14.4 | 0.055 |
| Male (%) | 55.1 | 82.0 | 56.1 | 0.004 |
| Body mass index (Kg/m2) | 27 [26–31] | 28 [26–31] | 27 [25–31] | 0.672 |
| Type 2 diabetes mellitus (%) | 38.3 | 52.9 | 31.5 | 0.397 |
| Hypertension (%) | 45.8 | 55.9 | 41.1 | 0.093 |
| Alanine aminotranferase (U/L) | 49 ± 41 | 46 ± 35 | 51 ± 45 | 0.546 |
| Aspartate aminotranferase (U/L) | 47 ± 40 | 53 ± 41 | 42 ± 38 | 0.166 |
| Alkaline phosphatase (U/L) | 97 ± 51 | 100 ± 46 | 94 ± 55 | 0.527 |
| Lactate deshidrogenase (U/L) | 275 [224–383] | 334 [260–436] | 243 [194–296] | 0.080 |
| Total bilirubin (mg/dL) | 0.60 [0.44–0.92] | 0.55 [0.38–1.02] | 0.69 [0.53–0.87] | 0.552 |
| Albumin (g/dL) | 2.81 [2.53–3.22] | 2.54 [2.36–2.79] | 3.18 [2.84–3.36] | <0.001 |
| C reactive protein (mg/L) | 97.6 [27.9–204.1] | 135.5 [55.1–231.3] | 73.9 [21.4–153.0] | 0.013 |
| D-Dimer (μg/mL) | 0.51 [0.24–0.90] | 0.75 [0.49–1.79] | 0.29 [0.17–0.64] | <0.001 |
| Fibrinogen (g/mL) | 5.01 ± 1.33 | 5.18 ± 1.30 | 4.85 ± 1.34 | 0.201 |
| Ferritin (g/mL) | 634 [355–998] | 771 [404–1142] | 556 [336–853] | 0.041 |
| Lymphocytes % | 11.8 [6.0–16.3] | 8.4 [3.3–13.3] | 14.6 [9.5–14.6] | <0.001 |
| Neutrophils % | 43.4 ± 40.5 | 39.1 ± 43.8 | 47.1 ± 37.3 | 0.313 |
| Leucocytes ×103/μL | 7.5 [5.5–11.4] | 9.4 [6.4–14.4] | 6.0 [4.7–8.8] | <0.001 |
| Platelets ×103/μL | 288.0 ± 125.6 | 291.1 ± 119.3 | 285.3 ± 131.8 | 0.813 |
| International normalized ratio | 1.21 ± 0.36 | 1.19 ± 0.22 | 1.22 ± 0.45 | 0.752 |
| Mortality (%)b | 27.5 | 47.9 | 9.3 | <0.001 |
Data are shown as mean ± standard deviation, median [interquartile range] or percentage.
Student's t-test, Mann Whitney's U test or Chi square test as appropriate.
This analysis was made in 102 patients.
Fig. 2.
DPP4 concentration in COVID-19 patients stratified: a) without invasive mechanical ventilation (IMV) (n = 57, 49.2 [39.9–65.6] ng/mL) and with IMV (n = 50, 42.8 [29.8–56.9] ng/mL) and b) those who recovered (n = 74, 48.9 [35.8–63.1] ng/mL) and who died (n = 28, 42.5, [30.3–55.6] ng/mL). The analysis of mortality was made in 102 patients, because 5 patients were transferred to another hospital and we did not know the outcome.
Table 4.
Association between serum DPP4 concentration and invasive mechanical ventilation in COVID-19 patients.
| Odds ratio [95% confidence interval] | p | |
|---|---|---|
| Unadjusted | 0.970 [0.949–0.992] | 0.008 |
| Model 1 | 0.971 [0.949–0.994] | 0.013 |
| Model 2 | 0.973 [0.951–0.996] | 0.022 |
| Model 3 | 0.974 [0.951–0.997] | 0.025 |
| Model 4 | 0.972 [0.949–0.996] | 0.021 |
| Model 5 | 0.974 [0.950–0.998] | 0.031 |
Model 1: adjusted for age > 60 years.
Model 2: Model 1 + adjusted for sex.
Model 3: Model 2 + adjusted for body mass index.
Model 4: Model 3 + adjusted for type 2 diabetes mellitus.
Model 5: Model 4 + hypertension.
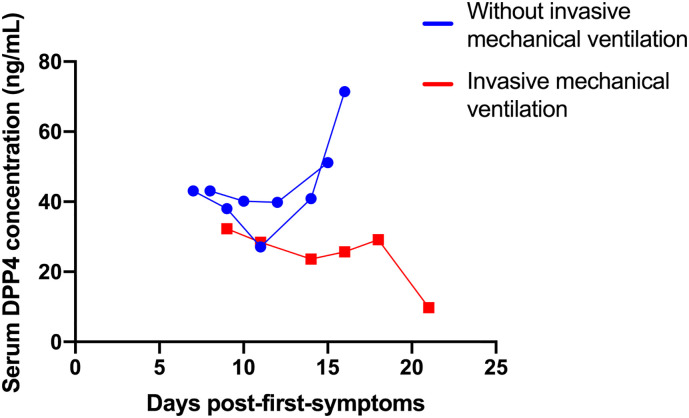
In three COVID-19 patients from the total of 107, DPP4 levels were measured several days after the diagnosis and their admission to the intensive care unit. As can be seen in Fig. 3 , in the two COVID-19 patients who recovered without IMV, serum DPP4 levels increased, whereas they decreased in one mechanically ventilated patient.
Fig. 3.
DPP4 concentrations in three COVID-19 patients measured several days after the diagnosis and their admission to the intensive care unit. Two COVID-19 patients who recovered without invasive mechanical ventilation and one mechanically ventilated patient are showed.
3.4. Correlation of DPP4 levels with clinical characteristics in COVID-19 patients
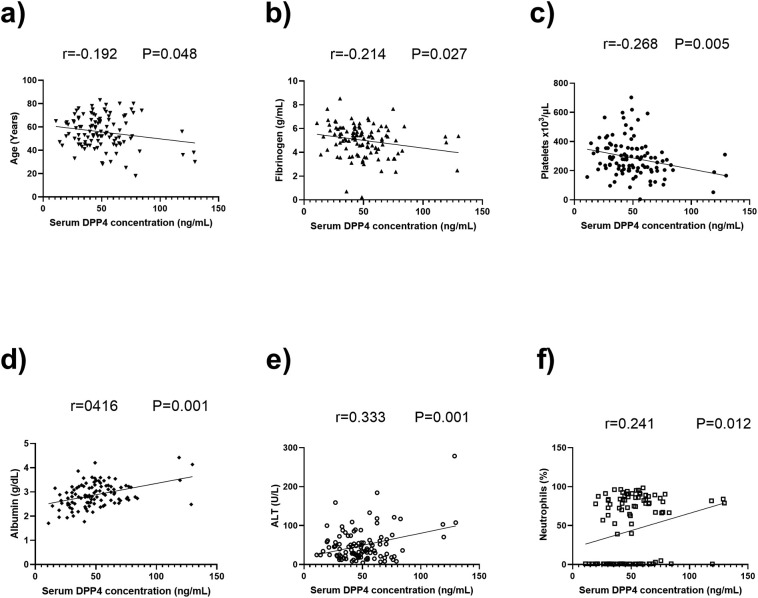
DPP4 levels correlated negatively with age (r = −0.192, P = 0.048), fibrinogen (r = −0.214, P = 0.027) and platelet levels (r = −0.268, P = 0.005), and positively with albumin (r = 0.416, P = 0.001), alanine aminotranferase (r = 0.333, P = 0.001), and percentage of neutrophils (r = 0.241, P = 0.012) (Fig. 4 ).
Fig. 4.
Pearson correlations between DPP4 levels and quantitative variables in COVID-19 patients: r and P values are shown.
3.5. Association of DPP4 polymorphisms with COVID-19
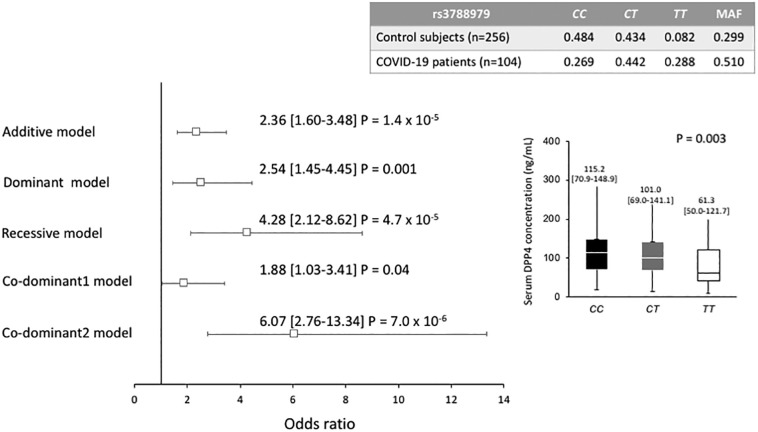
Of the 5 polymorphisms studied, rs3788979 showed a different distribution of its genotypes in COVID-19 patients and healthy controls. Under different models adjusted for age, sex, BMI, T2DM, and hypertension, rs3788979 was associated with a high risk of COVID-19 (OR = 2.36, 95% CI: 1.60–3.48, Padditive = 1.4 × 10−5; OR = 2.54, 95% CI: 1.45–4.45, Pdominant = 0.001; OR = 4.28, 95% CI: 2.12–8.62, Precessive = 4.7 × 10−5; OR = 1.88, 95%CI: 1.03–3.41, Pcodominant 1 = 0.04; OR = 6.07, 95% CI: 2.76–13.34, Pcodominant 2 = 7.0 × 10−6) (Fig. 4). On the other hand, in the total population (patients and controls), different DPP4 levels were observed in the rs3788979 genotypes; of note, the lowest levels were found in the TT genotype carriers (P = 0.003) (Fig. 5 ).
Fig. 5.
Association between DPP4 rs3788979 gene polymorphism and COVID-19 infection and DPP4 serum concentration stratified by rs3788979 genotypes.
Each model was adjusted for age, sex, body mass index, type 2 diabetes mellitus and hypertension.
MAF: Minor allele frequency.
4. Discussion
In a cohort of COVID-19 patients and controls, we found significantly lower levels of DPP4 in COVID-19 patients. Enzyme levels were also low in patients receiving IMV; by the same token, a low risk of requiring IMV was associated with a high DPP4 concentration. High enzyme levels correlated negatively with age, fibrinogen, and platelets, and positively with albumin, ALT, and percentage of neutrophils. The rs3788979 DPP4 polymorphism was associated with a high risk of COVID-19.
DPP4 is an enzyme that has been associated with the presence of hypertension, insulin resistance, and T2DM [13,14,17,26]. At the same time, this protein has been reported to be the receptor for corona-like viruses, such as MERS [27]. With a bioinformatics analysis, it has been recently established that the spike protein of the SARS-CoV-2 interacts with DPP4 [10], suggesting a possible role of this protein in the infection and severity of the disease. In a small number of COVID-19 patients, Schlicht et al. [16] reported low levels of DPP4 as compared to healthy controls; this result had been previously reported in MERS-CoV infected subjects [28]. DPP4 is a ubiquitous serine peptidase that is expressed in endothelial, bronchiolar epithelial, alveolar epithelial [29], and blood cells (particularly lymphocytes) [30]. This protein is found in two forms: one bound to the cell membrane and another one dissolved in plasma. Concerning the SARS infection, DPP4 could have several functions; one could be its role in the entry of the virus to the host cell. Another function could be the systemic effect that it may have, considering that it is produced by the adipose tissue as a proinflammatory adipokine with an important effect on the T cell activation, cell adhesion, and apoptosis [31]. Its relationship with the inflammatory process and insulin resistance is relevant since T2DM and obesity are major risk factors for a severe course of a COVID-19 disease [32]. Recently Solerte et al., showed that the DPP4 inhibitor Sitagliptin is beneficial in COVID-19 because this type of DPP4 inhibitor induces a compensatory up-regulation of DPP4 expression and release [33]. This result agrees with our findings. High circulating levels of DPP4 have been reported in individuals with obesity in several populations with an important correlation with insulin resistance [26,34]. However, the relationship of the plasma DPP4 activity with obesity and T2DM is contradictory; increased and decreased activity levels in patients with T2DM and obesity have been reported [[35], [36], [37], [38]]. Similarly, low levels of DPP4 have been reported in some conditions, such as rheumatoid arthritis [39], pregnancy [40], and hypoalphalipoproteinemia [personal communication]. These findings appear to be contrary to those expected. However, epigenetic modifications and changes at DNA level are part of the complex mechanisms that modulate the production of several biomolecules, including DPP4.
It is difficult to establish whether low DPP4 levels in patients are a cause or consequence of SARS-CoV-2 infection, however, the fact that uninfected control individuals have higher levels of DPP4 suggests that variation at low of this enzyme could be a consequence of the infection. In this context, low serum levels of DPP4 in COVID-19 patients could be due to several mechanisms; one would involve the enzyme as a receptor for the virus, which could modify the structure of the protein at the cell membrane, preventing its proteolytic cleavage. Hence, individuals infected with SARS would consequently have a lower DPP4 serum level, which is demonstrated in our study. Another mechanism affecting the soluble enzyme concentration could involve alterations at the intracellular level caused by the virus, which could prevent an adequate assembly of DPP4. This effect could decrease the amount of biomolecule in the cell membrane and, consequently, in the serum of the patients. As mentioned, DPP4 is expressed in several blood cells, but predominantly in T lymphocytes [30] and specifically in CD4 T cells [41]. Measurement of DPP4 in lymphocyte culture has suggested that these cells are an important source of soluble DPP4 [42]. As in other coronavirus infections (SARS and MERS), the individuals infected with SARS-CoV-2 present with a marked lymphopenia [[43], [44], [45]]; this disorder is more severe in those individuals who do not survive the disease, while in surviving patients, T lymphocyte levels are gradually restored [46]. Considering these facts, the decreased number of lymphocytes (the primary cell source of soluble DPP4) could explain why low levels of DPP4 in serum are present in COVID-19 patients. Another possible function of DPP4 as it relates to COVID-19 is that it could act as a ‘decoy receptor’ for the SARS-CoV-2. In this case, viral particles bound to circulating DPP4 could be partially or totally preventing from entering cells. Thus, high circulating DPP4 levels would be expected to reduce the spread of the virus within the host, while low levels would be related to a greater entry of the virus into the cells and consequently a greater degree of infection. We suggest that the latter is what would be happening in the patients analyzed in our study.
It has been suggested that the variability in SARS-CoV-2 infection prevalence and COVID-19 mortality in different populations depend largely on individual variations in genes that encode proteins relevant in viral pathogenicity mechanisms. Bioinformatics, case-control and genome-wide association (GWA) studies have shown polymorphisms located in several genes as possible susceptibility markers for COVID-19 infection and severity in several populations [[47], [48], [49], [50], [51]]. Located in the 2p24.3 region, the gene that encodes DPP4 is polymorphic and some of its polymorphisms have been associated not only with the risk of developing some diseases, such as myocardial infarction [18], rheumatoid arthritis [52], and T2DM [17], but also with variations in levels of DPP4 [17] and apolipoprotein B [53]. In our study, an association of the DPP4 rs3788979 polymorphism with risk of COVID-19 was established. We also found that the lowest levels of this enzyme were present in TT genotype carriers; this polymorphism was previously associated with risk of myocardial infarction in patients with atherosclerosis [18]. In agreement with our results, Aghili et al. reported that individuals with the rs3788979 CT/TT genotypes presented with low levels of DPP4.
Recently, a GWA study of 2244 patients with COVID-19 from the UK detected 3 polymorphisms associated with severe COVID-19, one of them located in the dipeptidyl peptidase 9 (DPP9) gene (rs2109069) [54]. DPP9 and DPP4 belong to the S9B subfamily of peptidases with high structural resemblance [55]. Indeed, DPP9 participates in the immune and inflammatory processes. This enzyme is expressed in leukocytes [56], activated lymphocytes [57], lymphocytes infiltrating inflamed lungs [58], and bronchi after induction of experimental asthma [59].
A strength of our study is that patients were matched for age and sex with the control group. An important limitation of this work is that we did not measure the activity of the soluble or the membrane-bound DPP4. This is important because the two DPP4 forms could have a different effect in the SARS-CoV-2 infection and then in the COVID-19 disease. Another source of uncertainty is that the DPP4 variation during the course of the disease was only tracked in three individuals, and yet there was agreement in their levels. In the two individuals who did not require IMV, the enzyme concentration increased, while it diminished in the one who did receive mechanical ventilation.
In summary, the presence and severity of COVID-19 is associated with lower levels of DPP4, which could be a possible marker for disease monitoring during its course. Individuals with rs3788979 TT genotype might produce low levels of DPP4 and, as a result, be more susceptible to infection and progression of the disease.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
This work was supported by funds of the Instituto Nacional de Cardiología Ignacio Chávez.
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int. Data last updated: 2021/03/04, 3:29pm CEST. 2021.
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–74. [DOI] [PMC free article] [PubMed]
- 3.Zhou P, Yang X, Wang X, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–3. [DOI] [PMC free article] [PubMed]
- 4.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Millet J., Whittaker G. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. U. S. A. 2014;111(42):15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram S, Dijkman R, Habjan M, Heurich A, Gierer S, Glowacka I, et al. TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium. J. Virol. 2013;87(11):6150–60. [DOI] [PMC free article] [PubMed]
- 7.Gierer S, Bertram S, Kaup F, Wrensch F, Heurich A, Krämer-Kühl A, et al. The spike protein of the emerging betacoronavirus EMC uses a novel coronavirus receptor for entry, can be activated by TMPRSS2, and is targeted by neutralizing antibodies. J. Virol. 2013;87(10):5502–11. [DOI] [PMC free article] [PubMed]
- 8.Seys L., Widagdo W., Verhamme F., Kleinjan A., Janssens W., Joos G., et al. DPP4, the Middle East respiratory syndrome coronavirus receptor, is upregulated in lungs of smokers and chronic obstructive pulmonary disease patients. Clin. Infect. Dis. 2018;66(1):45–53. doi: 10.1093/cid/cix741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Z., Xu Y., Bao L., Zhang L., Yu P., Qu Y., et al. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1):59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vankadari N, Wilce J. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect. 2020;9(1):601–4. [DOI] [PMC free article] [PubMed]
- 11.Gorrell M., Gysbers V., McCaughan G. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand. J. Immunol. 2001;54(3):249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Lamers D., Famulla S., Wronkowitz N., Hartwig S., Lehr S., Ouwens D.M., et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60(7):1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng T, Gao Y, Baskota A, Chen T, Ran X, Tian H. Increased plasma DPP4 activity is predictive of prediabetes and type 2 diabetes onset in Chinese over a four-year period: result from the China National Diabetes and Metabolic Disorders Study. J. Clin. Endocrinol. Metab. 2014;99(11):E2330–4. [DOI] [PubMed]
- 14.Zheng T., Chen T., Liu Y., Gao Y., Tian H. Increased plasma DPP4 activity predicts new-onset hypertension in Chinese over a 4-year period: possible associations with inflammation and oxidative stress. J. Hum. Hypertens. 2015;29(7):424–429. doi: 10.1038/jhh.2014.111. [DOI] [PubMed] [Google Scholar]
- 15.Penaforte-Saboia J.G., Couri C., Vasconcelos Albuquerque N., Lauanna Lima Silva V., Bitar da Cunha Olegario N., Oliveira Fernandes V., et al. Emerging roles of dipeptidyl peptidase-4 inhibitors in delaying the progression of type 1 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:565–573. doi: 10.2147/DMSO.S294742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlicht K, Rohmann N, Geisler C, Hollstein T, Knappe C, Hartmann K, et al. Circulating levels of soluble Dipeptidylpeptidase-4 are reduced in human subjects hospitalized for severe COVID-19 infection. Int. J. Obes. 2020;44(11):2335–8. [DOI] [PMC free article] [PubMed]
- 17.Ahmed RH, Huri HZ, Al-Hamodi Z, Salem SD, Al-Absi B, Muniandy S. Association of DPP4 gene polymorphisms with type 2 diabetes mellitus in Malaysian subjects. PLoS One. 2016;11(4):1–12. [DOI] [PMC free article] [PubMed]
- 18.Aghili N., Devaney J.M., Alderman L.O., Zukowska Z., Epstein S.E., Burnett M.S., et al. Polymorphisms in dipeptidyl peptidase IV gene are associated with the risk of myocardial infarction in patients with atherosclerosis. Neuropeptides. 2012;46(6):367–371. doi: 10.1016/j.npep.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Posadas-Sánchez R, Ocampo-Arcos WA, López-Uribe ÁR, Posadas-Romero C, Villarreal-Molina T, Alvarez-León E, et al. Hepatic lipase (LIPC) C-514T gene polymorphism is associated with cardiometabolic parameters and cardiovascular risk factors but not with fatty liver in Mexican population. Exp. Mol. Pathol. 2015;98(1):93–8. [DOI] [PubMed]
- 20.Villarreal-Molina T, Posadas-Romero C, Romero-Hidalgo S, Antúnez-Argüelles E, Bautista-Grande A, Vargas-Alarcón G, et al. The ABCA1 gene R230C variant is associated with decreased risk of premature coronary artery disease: the Genetics of Atherosclerotic Disease (GEA) study. PLoS One. 2012;7(11):1–9. [DOI] [PMC free article] [PubMed]
- 21.Medina-Urrutia A., Posadas-Romero C., Posadas-Sánchez R., Jorge-Galarza E., Villarreal-Molina T., González-Salazar M.D.C., et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc. Diabetol. 2015;14(1):20. doi: 10.1186/s12933-015-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 23.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am. J. Clin. Nutr. 1988;48(6):1351–61. [DOI] [PubMed]
- 24.Mautner G.C., Mautner S.L., Froehlich J., Feuerstein I.M., Proschan M.A., Roberts W.C., et al. Coronary artery calcification: assessment with electron beam CT and histomorphometric correlation. Radiology. 1994;192(3):619–623. doi: 10.1148/radiology.192.3.8058924. [DOI] [PubMed] [Google Scholar]
- 25.Posadas-Sánchez R., Pérez-Hernández N., Rodríguez-Pérez J.M., Coral-Vázquez R.M., Roque-Ramírez B., Llorente L., et al. Interleukin-27 polymorphisms are associated with premature coronary artery disease and metabolic parameters in the Mexican population: the genetics of atherosclerotic disease (GEA) Mexican study. Oncotarget. 2017;8(38):64459–64470. doi: 10.18632/oncotarget.16223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohmann N, Schlicht K, Corinna G, Hollstein T, Knappe C, Krause L, et al. Circulating sDPP-4 is increased in obesity and insulin resistance but is not related to systemic metabolic inflammation. J. Clin. Endocrinol. Metab. 2021;106:592–601. [DOI] [PubMed]
- 27.Wang N., Shi X., Jiang L., Zhang S., Wang D., Tong P., et al. Structure of MERS-CoV spike receptor-binding domain complexed with human receptor DPP4. Cell Res. 2013;23(8):986–993. doi: 10.1038/cr.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inn K., Kim Y., Aigerim A., Park U., Hwang E., Choi M., et al. Reduction of soluble dipeptidyl peptidase 4 levels in plasma of patients infected with Middle East respiratory syndrome coronavirus. Virology. 2018;518:324–327. doi: 10.1016/j.virol.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widagdo W., Raj V., Schipper D., Kolijn K., van Leenders G., Bosch B., et al. Differential expression of the Middle East respiratory syndrome coronavirus receptor in the upper respiratory tracts of humans and dromedary camels. J. Virol. 2016;90(9):4838–4842. doi: 10.1128/JVI.02994-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorrell M., Wickson J., McCaughan G. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell. Immunol. 1991;134(1):205–215. doi: 10.1016/0008-8749(91)90343-a. [DOI] [PubMed] [Google Scholar]
- 31.Boonacker E., Van Noorden C. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell Biol. 2003;82(2):53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 32.Mesas A., Cavero-Redondo I., Álvarez-Bueno C., Sarriá Cabrera M., Maffei de Andrade S., Sequí-Dominguez I., et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solerte S, D'Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43(12):2999–3006. [DOI] [PMC free article] [PubMed]
- 34.Ahmed R., Huri H., Muniandy S., Al-Hamodi Z., Al-Absi B., Alsalahi A., et al. Altered circulating concentrations of active glucagon-like peptide (GLP-1) and dipeptidyl peptidase 4 (DPP4) in obese subjects and their association with insulin resistance. Clin. Biochem. 2017;50(13–14):746–749. doi: 10.1016/j.clinbiochem.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Mannucci E., Pala L., Ciani S., Bardini G., Pezzatini A., Sposato I., et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48(6):1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 36.Ryskjaer J., Deacon C., Carr R., Krarup T., Madsbad S., Holst J., et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur. J. Endocrinol. 2006;155(3):485–493. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 37.McKillop A., Duffy N., Lindsay J., O’Harte F., Bell P., Flatt P. Decreased dipeptidyl peptidase-IV activity and glucagon-like peptide-1(7-36)amide degradation in type 2 diabetic subjects. Diabetes Res Clin Pr. 2008;79(1):79–85. doi: 10.1016/j.diabres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Meneilly G, Demuth H, McIntosh C, Pederson R. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV response to oral glucose. Diabet. Med. 2000;17(5):346–50. [DOI] [PubMed]
- 39.Gotoh H., Hagihara M., Nagatsu T., Iwata H., Miura T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin. Chem. 1989;35(6):1016–1018. [PubMed] [Google Scholar]
- 40.Krepela E, Kasafírek E, Vicar J, Kraml J. An assay of dipeptidyl peptidase IV activity in human serum and serum of pregnant women with glycyl-l-proline-1-naphthylamide and other glycyl-l-proline-arylamides as substrates. Physiol Bohemoslov. 1983;32(4):334–45. [PubMed]
- 41.Boonacker E, Wierenga E, Smits H, Van Noorden C. CD26/DPPIV signal transduction function, but not proteolytic activity, is directly related to its expression level on human Th1 and Th2 cell lines as detected with living cell cytochemistry. J. Histochem. Cytochem. 2002;50(9):1169–77. [DOI] [PubMed]
- 42.Uematsu T, Urade M, Yamaoka M. Decreased expression and release of dipeptidyl peptidase IV (CD26) in cultured peripheral blood T lymphocytes of oral cancer patients. J Oral Pathol Med. 1998;27(3):106–10. [DOI] [PubMed]
- 43.He Z., Zhao C., Dong Q., Zhuang H., Song S., Peng G., et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int. J. Infect. Dis. 2005;9(6):323–330. doi: 10.1016/j.ijid.2004.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cameron M., Bermejo-Martin J., Danesh A., Muller M., Kelvin D. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133(1):13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–9. [DOI] [PMC free article] [PubMed]
- 46.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vargas-Alarcón G., Posadas-Sánchez R., Ramírez-Bello J. Variability in genes related to SARS-CoV-2 entry into host cells (ACE2, TMPRSS2, TMPRSS11A, ELANE, and CTSL) and its potential use in association studies. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smatti M., Al-Sarraj Y., Albagha O., Yassine H. Host genetic variants potentially associated with SARS-CoV-2: a multi-population analysis. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.578523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elhabyan A., Elyaacoub S., Sanad E., Abukhadra A., Elhabyan A., Dinu V. The role of host genetics in susceptibility to severe viral infections in humans and insights into host genetics of severe COVID-19: a systematic review. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovsyannikova I, Haralambieva I, Crooke S, Poland G, Kennedy R. The role of host genetics in the immune response to SARS-CoV-2 and COVID-19 susceptibility and severity. Immunol. Rev. 2020;296(1):205–19. [DOI] [PMC free article] [PubMed]
- 51.Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020;383(16):1522–34. [DOI] [PMC free article] [PubMed]
- 52.Jiang L, Yin J, Ye L, Yang J, Hemani G, Liu A-J, et al. Novel risk loci for rheumatoid arthritis in Han Chinese and congruence with risk variants in Europeans. Arthritis Rheumatol. 2014;66(5):1121–32. [DOI] [PubMed]
- 53.Bailey S.D., Xie C., Paré G., Montpetit A., Mohan V., Yusuf S., et al. Variation at the DPP4 locus influences apolipoprotein B levels in south Asians and exhibits heterogeneity in Europeans related to BMI. Diabetologia. 2014;57(4):738–745. doi: 10.1007/s00125-013-3142-3. [DOI] [PubMed] [Google Scholar]
- 54.Pairo-Castineira E, Clohisey S, Klaric L, Bretherick A, Rawlik K, Parkinson N, et al. Genetic mechanisms of critical illness in Covid-19. Nature. 2021;591:92–98. [DOI] [PubMed]
- 55.Matteucci E., Giampietro O. Dipeptidyl peptidase-4 (CD26): knowing the function before inhibiting the enzyme. Curr. Med. Chem. 2009;16(23):2943–2951. doi: 10.2174/092986709788803114. [DOI] [PubMed] [Google Scholar]
- 56.Maes M., Dubois V., Brandt I., Lambeir A., Van der Veken P., Augustyns K., et al. Dipeptidyl peptidase 8/9-like activity in human leukocytes. J. Leukoc. Biol. 2007;81(5):1252–1257. doi: 10.1189/jlb.0906546. [DOI] [PubMed] [Google Scholar]
- 57.Chowdhury S., Chen Y., Yao T., Ajami K., Wang X., Popov Y., et al. Regulation of dipeptidyl peptidase 8 and 9 expression in activated lymphocytes and injured liver. World J. Gastroenterol. 2013;19(19):2883–2893. doi: 10.3748/wjg.v19.i19.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu D., Ajami K., Gall M., Park J., Lee C., Evans K., et al. The in vivo expression of dipeptidyl peptidases 8 and 9. J. Histochem. Cytochem. 2009;57(11):1025–1040. doi: 10.1369/jhc.2009.953760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schade J., Stephan M., Schmiedl A., Wagner L., Niestroj A., Demuth H., et al. Regulation of expression and function of dipeptidyl peptidase 4 (DP4), DP8/9, and DP10 in allergic responses of the lung in rats. J. Histochem. Cytochem. 2008;56(2):147–155. doi: 10.1369/jhc.7A7319.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]