Abstract
Background:
Platelet concentrates usage in the treatment of intrabony defects has been improved due to advancement of research. Many generation of platelet concentrates were used, but research regarding advanced platelet-rich fibrin (A-PRF) regarding periodontal treatment is scanty.
Aim:
The purpose of the study was to evaluate and compare PRF and A-PRF in the treatment of human periodontal infrabony defects (IBDs) both clinically and radiographically.
Materials and Methods:
Twenty-eight patients having IBDs were divided into Group A (PRF) and Group B (A-PRF). Clinical parameters such as plaque index, gingival index, probing pocket depth (PPD), and clinical attachment level (CAL) were recorded at baseline and 3 and 6 months and radiographic examination at baseline and 6 months were also recorded to evaluate defect fill, resolution, and change in the alveolar crest height. Then, all the data were tabulated in a Microsoft Excel sheet and subjected to statistical analysis. Mean and standard deviations of the clinical and radiographic parameters were calculated, and unpaired t-test was performed to assess intergroup comparison at different time intervals.
Results:
Intragroup comparison showed statistically significant improvement in PPD and CAL at 3 and 6 months while statistically significant improvement was observed in mean defect fill and resolution in Group B.
Conclusion:
Individually, both the materials have shown promising results. However, statistically, PRF group (Group A) showed better treatment outcome in terms of bone fill and A-PRF group (Group B) in terms of soft tissue healing.
Keywords: Advanced platelet-rich fibrin, infrabony defect, periodontal regeneration, platelet-rich fibrin
Introduction
Periodontitis is one of the widespread diseases affecting humankind in various forms and severities.[1] The goal of periodontal therapy is the regeneration of lost attachment apparatus, facilitating periodontal maintenance.[2] Several regenerative procedures such as bone grafts,[3] guided tissue regeneration,[4] root conditioning,[5] and growth factors[6] have been tried; however, till date, no graft material is considered as the gold standard.[7]
Platelet-rich fibrin (PRF) was developed in France by Choukroun et al. (2001).[8] It is a second-generation platelet concentrate, used to accelerate hard and soft tissue healing.[9] Advanced PRF (A-PRF) is a third-generation product composed of platelets and white blood cells. The protective membranes that are produced release key proteins, accelerate soft and hard tissue healing which further gains the potential for periodontal regeneration.[10] To the best of our knowledge, no study reported the clinical use of A-PRF for the treatment of periodontal infrabony defects (IBDs). Thus, the purpose of present study was to evaluate and compare the clinical and radiographic outcome of PRF and A-PRF in the treatment of human IBDs.
Materials and Methods
This study is a prospective, single-blinded, randomized clinical trial. A total of 30 patients were selected from the Outpatient Department of Periodontics and Implantology, Institute of Dental Sciences, Bareilly. They were informed about the study and informed consent was obtained and required ethical approval was obtained. Finally, 28 patients were selected and randomly allocated to either - Group A: open flap debridement (OFD) +PRF or Group B: OFD + A-PRF based on coin-toin method. The following clinical parameters were recorded at baseline and 3 and 6 months postoperatively: (1) plaque index (PI), (2) gingival index (GI), (3) probing pocket depth (PPD) [Figure 1a-d], and (4) clinical attachment level (CAL) and the intraoral periapical radiographs using long cone paralleling technique. The radiographic assessment of the percentage of bone defect fill and resolution and the change in the level of alveolar crest (AC) was done by Digimizer Image Analysis Software, MedCalc Software Ltd, Ostend, Belgium.[11]
Figure 1.
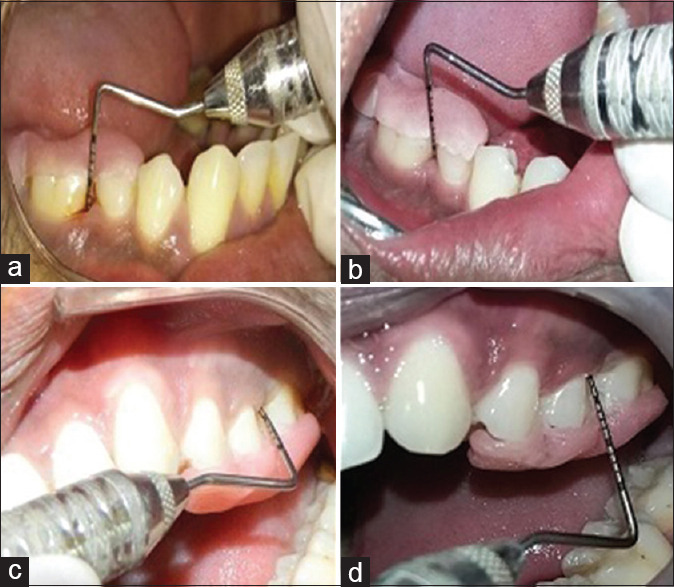
(a and b) Depicts pre-operative and post-operative probing pocket depths of group a; (c and d) depicts pre-operative and post-operative probing pocket depths of group b
Inclusion criteria
Patients having moderate-to-advanced chronic periodontitis with PPD ≥5 mm, CAL ≥3 mm following phase I therapy, radiographic evidence of vertical bone loss (≥3 mm), good general health, and no systemic disease.
Exclusion criteria
Patients showing unacceptable oral hygiene after phase I therapy, pregnant women, lactating mothers, smokers, occlusal disharmony, and parafunctional habits.
Clinical measurements were recorded using HU-Friedy UNC 15 probe and standardized using customized acrylic stents with grooves, prepared on the study models. The selected subjects underwent phase I therapy. Detailed instructions regarding self-performed plaque control measures were given. Intraexaminer calibration was achieved when 10 patient's measurements were assessed 24 h apart before the initiation of the study. Calibration was taken into account only when the values at baseline and 24 h were similar to ±1 mm at 90% level.[12]
Presurgical procedure
After completion of patient recruitment, phase I therapy was performed which includes scaling and root planing with hand and ultrasonic instrumentation. Then, the patient was advocated for oral hygiene instructions and kept on 0.2% chlorhexidine gluconate mouthwash (Rexidine™) for a period of 2 weeks. Then, the patient was maintained for re-evaluation. Before performing the flap surgery, the patient underwent routine blood investigations such as Clotting Time (CT), Bleeding Time (BT), Haemoglobin Percentage (HB%), Hepatitis B surface Antigen (HBsAG), Hepatitis C Virus (HCV) so that underlying abnormalities can be ruled out and surgical procedures can be performed in a routine way.
Preparation of platelet-rich fibrin and advanced platelet-rich fibrin clots
On the day of surgery, just 20 min before performing surgery, 10 ml of the blood was drawn from antecubital vein and transferred to sterile glass tubes (Borosilicate glass, New Delhi, India) and subjected to centrifugation (Remi R-8C, New Delhi, India) based on Choukroun et al.,[8,13] 2001 (2800 rpm for 12 min) protocol and Ghanaati et al.,[10] 2014 (1500 rpm for 14 min) protocol. In both forms of PRF clots, there will be a formation of three layers – top layer buffy coat, central fibrin clot, and bottom red blood cell layer. PRF and A-PRF clots were carefully retrieved using sterile tweezers and placed on sterile gauze, and excess plasma was removed and formed into PRF and A-PRF membranes.
Surgical procedure
After a re-evaluation period of 6–8 weeks, the surgical site was anesthetized with 2% lignocaine hydrochloride with adrenaline (1:80,000). Crevicular incisions were given on the facial and lingual/palatal sides reaching the tip of the interdental papilla using BP knife with blade no. 12/15.[14] Full thickness flap was reflected using periosteal elevator, preserving the interdental papilla. After flap reflection and exposure of osseous defect, a thorough debridement and root planing was done using curettes.
In Group A, freshly prepared PRF membrane, and in Group B, A-PRF membrane was placed After the defect was filled, the mucoperiosteal flaps were repositioned and secured using 3-0 silk sutures (Mersilk-Ethicon silk, Johnson and Johnson Ltd., India) by interrupted sutures[15,16] [Figures 1 and 2].
Figure 2.
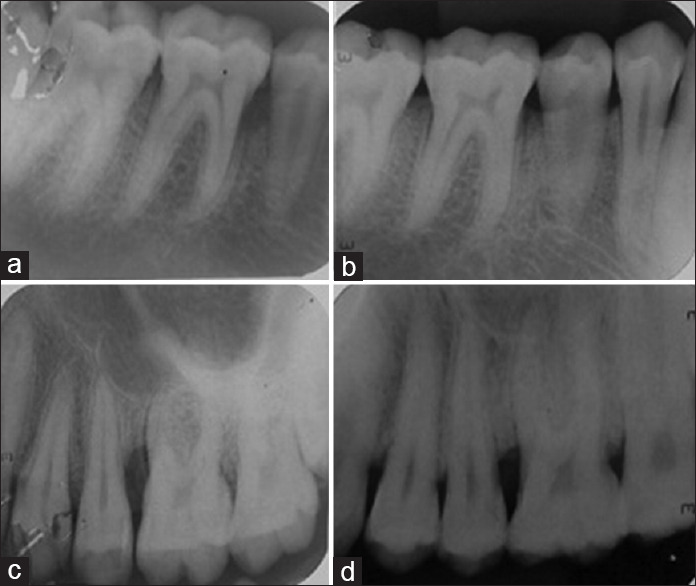
(a and b) Depicts pre-operative and post-operative intraoral periapical radiographs (iopa) of group a; (c and d) depicts pre-operative and post-operative intraoral periapical radiographs (iopa) of group b
The surgical area was covered with non-eugenol periodontal dressing (Coe-Pack™). Following medications were prescribed: capsule amoxicillin 500 mg t.i.d. and tablet ketorolac tromethamine 10 mg b.i.d. for 5 days with 0.2% chlorhexidine mouthwash (Rexidine™) b.i.d for 14 days. The patients were asked to refrain from eating hard food and brushing in the operated area. Sutures were removed after 10 days and the patients followed up after 3 and 6 months. Oral hygiene instructions were reinforced and scaling was done if necessary. All the surgeries were performed by a single experienced periodontal surgeon (HU), and radiographic measurements were recorded by another experienced periodontist (HB).
Radiographic parameters
All the radiographs [Figure 2a-d] were scanned using Epson L210 (Digital ICE Technologies) and measurements were carried out in Digimizer image analysis software, MedCalc Software Ltd, Ostend, Belgium.[11]
Statistical analysis
The clinical and radiographic results were averaged (mean ± standard deviation) at each time interval. The difference between each pair of measurements was calculated (baseline to 6 months). The paired t-test was applied to assess the statistical significance between time intervals within each group for all parameters.
Results
Clinical parameters
Regarding PI, Group A showed a mean reduction of 0.47 ± 0.29 at 3 months and 0.50 ± 0.42 at 6 months. However, Group B showed a mean reduction of 0.50 ± 0.22 at 3 months and 0.62 ± 0.22 at 6 months. Mean PI reduction was statistically significant at 3 and 6 months in both the groups compared to baseline but nonsignificant at 6 months compared with 3 months and between both groups for all time intervals [Table 1 and Graph 1].
Table 1.
Depicts the Mean-Standard deviations and mean difference of clinical parameters of both groups at different time intervals
| Clinical parameters | Difference between Groups A and B | P | |
|---|---|---|---|
| PI | Baseline | (−0.12)±(−0.20) | 0.6073 |
| 3 months | (−0.09)±(−0.09) | 0.6337 | |
| 6 months | 0.00±0.00 | 0.1643 | |
| GI | Baseline | (−0.05)±(−0.07) | 0.3506 |
| 3 months | 0.22±0.32 | 0.1387 | |
| 6 months | 0.12±0.36 | 0.323 | |
| PPD | Baseline | 0.54±0.36 | 0.123 |
| 3 months | 0.82±0.17 | 0.0012** | |
| 6 months | 0.62±0.11 | 0.00607** | |
| CAL | Baseline | 0.80±(−0.32) | 0.335 |
| 3 months | 0.75±(−0.34) | 0.031* | |
| 6 months | 0.67±0.35 | 0.0415* | |
*significant (P<0.05), **Highly Significant (P<0.01). PI: Plaque index; GI: Gingival index; PPD: Probing pocket depth; CAL: Clinical attachment level
Graph 1.
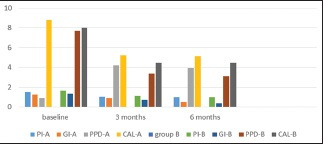
Various clinical parameters at different time periods in both the groups
Regarding GI, Group A showed mean reduction of 0.34 ± 0.20 at 3 months and 0.77 ± −0.10 at 6 months. However, in Group B, the mean reduction of 0.61 ± 0.19 at 3 months and 0.94 ± 0.33 at 6 months was observed; reduction was statistically significant at 3 and 6 months compared to baseline and nonsignificant for all time intervals in both groups [Table 1 and Graph 1].
For PPD, in Group A, a mean reduction of 3.73 ± 0.45 mm at 3 months and 4.00 ± 0.43 mm at 6 months was observed. However, in Group B, a mean reduction of 3.86 ± 0.26 mm at 3 months and 4.38 ± 0.18 mm at 6 months was observed. PPD reduction was highly statistically significant both intra- and inter-group after 3 and 6 months compared to baseline [Table 1 and Graph 1].
For CAL, in Group A, mean gain in CAL was 3.60 ± 0.35 mm at 3 and 3.67 ± 0.29 mm at 6 months, while in Group B, gain of 3.54 ± 0.33 mm at 3 months and 3.54 ± 0.26 mm at 6 months was observed. Both groups showed statistically significant gain after 3 and 6 months, compared to baseline, with greater gain in Group B [Table 1 and Graph 1], but values were statistically insignificant at 6 months, compared to 3 months (P = 1.00).
For radiographic evaluation, a mean defect fill of 1.99 ± 1.17 mm in Group A while 1.15 ± 1.08 mm in Group B after 6 months was highly statistically significant compared to baseline. Intergroup comparison showed greater gain in Cemento Enamel Junction (CEJ) to the Base of the Defect (BD) in Group A from baseline to 6 months [Table 2 and Graph 2].
Table 2.
Depicts the Mean-Standard deviations and mean difference of radiographic parameters for both the groups at different time periods
| Radiographic parameters | Difference between Groups A and B | Significance (P) | |
|---|---|---|---|
| Defect fill CEJ to BD | Baseline | 2.00±1.16 | 0.000009*** |
| 6 months | 0.00±0.00 | ||
| Difference | 0.84±0.09 | ||
| Reduction of AC height | Baseline | 1.51±0.75 | 0.000012*** |
| 6 months | 0.36±0.71 | ||
| Difference | 1.15±0.04 | ||
| Reduction of CEJ to AC | Baseline | 0.50±0.36 | |
| 6 months | 0.62±0.11 | 0.7834 | |
| Difference | (−0.25)±0.71 | ||
***very Highly Significant (P<0.001). CEJ: Cementoenamel junction; BD: Base of defect; AC: Alveolar crest
Graph 2.
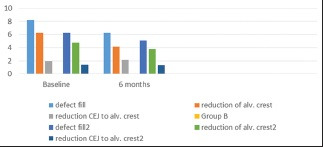
Various radiographic parameters at different time periods in both the groups
While coming to the mean defect resolution, a mean defect resolution of 2.14 ± 0.79 mm in Group A and 0.99 ± 0.75 mm in Group B at 6 months was highly significant compared to baseline. Intergroup comparison showed greater gain in AC to BD in Group A from baseline to 6 months [Table 2 and Graph 2].
Regarding change in AC height, the mean gain in AC height was −0.16 ± 0.66 mm in Group A and 0.99 ± −0.05 mm in Group B at 6 months. Intergroup comparison revealed statistically insignificant differences postoperatively (P = 0.2343) [Table 2 and Graph 2].
Discussion
PRF is a second-generation platelet concentrate, widely used to accelerate healing.[2] The fibrin clot protects the grafted biomaterials and facilitates cellular migration, necessary for the vascularization, and survival of the graft.
A-PRF is a relatively new concept. It is a third-generation platelet concentrate composed primarily of platelets and leukocytes. Growth factors released from A-PRF are Platelet Derived Growth Factor-AA (PDGF-AA), Platelet Derived Growth Factor-AB (PDGF-AB), Platelet Derived Growth Factor-BB (PDGF-BB), Transforming Growth Factor-β (TGF-β), Vascular Endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), Insulin Like Growth Factor (IGF). Proteins released from A-PRF contain more living progenitor cells, platelets, and neutrophilic granulocytes than PRF, thus influencing bone and soft tissue regeneration.[10]
The present study conducted to compare the efficacy of OFD with either PRF or A-PRF in the treatment of IBDs in moderate-to-severe periodontitis patients revealed that using either PRF or A-PRF alone, even with OFD, improves the clinical and radiographic outcome. The patients recruited had varied oral hygiene statuses, which were brought down to minimal PI scores following scaling and root planing, and were maintained for the two groups at 3 and 6 months also. Parameters, including CAL and PPD measurements, and the presence of Bleeding on Probing (BOP) were used to assess and monitor periodontal status.[17]
In the present study, A-PRF group was considered as test group and PRF group was considered as control. As many studies were performed regarding the use of PRF, it is used as standard control in the present study.
The results suggested improved PI from baseline to 6 months, but statistically insignificant differences between both the groups were observed, in accordance with Chacko etal.[18] Similarly, highly significant difference regarding mean reduction in GI was observed, which contributed to the maintenance of optimum oral hygiene by the patient and the frequent performance of oral prophylaxis.
The better results of Group B (A-PRF group) in the terms of PPD and CAL denotes better soft tissue healing response of A-PRF [Figure 1a and b]. The reason may be because decrease in the rotation per minute while increasing the centrifugation time in the A-PRF group gave an enhanced presence of neutrophilic granulocytes in the distal part of the clot, contributing to monocyte differentiation into macrophages. Thus, A-PRF might influence bone and soft tissue regeneration, especially through the presence of monocytes/macrophages and their growth factors. Radiographic parameters for the changes in the level of alveolar bone were done as per the method explained by Meador et al., 1985.[19]
According to Dohan Ehrenfest, et al.,[20] Leukocyte-Platelet Rich Fibrin (L-PRF) seems to be clinically better than A-PRF in terms of density of fibrin clot and intense release of growth factors. Pradeep et al.[21] evaluated the clinical and radiographic effectiveness of PRF and PRP in the treatment of IBD in patients with chronic periodontitis and showed PPD reduction, CAL gain, and bone fill in IBDs treated with PRF alone.
Intragroup comparisons in the present study showed highly significant difference in mean defect fill and defect resolution, compared to baseline, whereas highly significant results were in favor of Group A on intergroup comparisons [Figure 2a and b]. This result explained better osseous healing with PRF as compared to A-PRF. Hence, the present study observed enhanced soft tissue regeneration with A-PRF and osseous healing better with PRF, in accordance with Fujioka-Kobayashi et al.[22]
Very few studies were performed regarding the usage of A-PRF in the IBDs. Hence, more number of intercomparisons could not be performed with other study results, apart from described below. While coming to PRF, previous studies such as Thorat et al.[23] and Sharma et al.[24] reported reduction in PPD and CAL gain followed by increased radiographic bone fill in the treatment of IBDs when compared to OFD at different time intervals. The present study results share a similar pattern to their studies regarding PRF group (for hard tissue healing).
Even Pradeep et al.[21] revealed that the usage of a biomaterial such as a platelet concentrate always helps in better soft and hard tissue healing than OFD alone. Thus, the present study results follow a similar pattern.
The present study results were also in accordance with a previous study conducted by Suwondo et al.,[25] where they have obtained a decrease in PPD and gain in CAL in their 3 months of follow-up. However, hard tissue parameters improvements in the present study are in favor of PRF group than A-PRF group, which was not in accordance with the study conducted by Suwondo et al.[25] These variations regarding hard tissue healing might be due to weaker A-PRF structure than PRF when formed into membrane. Moreover, A-PRF membrane starts resolving within 3 days whereas PRF has a resorption time of 7–11 days.[13] Results from histological analysis reveal that A-PRF membrane does not have bone morphogenetic protein-2 (BMP-2) which improves osteoblast differentiation whereas it was reported in PRF.[26] Apart from this, interactions between VEGF and BMP-2 help in osteogenic effects and activation of BMP-2.[27]
In the present study, PPD reduction and CAL gain recorded at different time intervals shared similar pattern to a recent study conducted by Lei et al.;[14] however, coming to radiographic parameters, L-PRF had a better hard tissue healing than A-PRF which is opposite to that of Lei et al.[14] These variations in their results might be due to strict selection criteria, variations in defect morphology, and defect depths. Moreover, in their study, A-PRF results were compared with concentrated growth factors and concluded that both the materials were autologous cost-effective and easily procurable. Thus both A-PRF and concentrated growth factors can be used in the treatment of periodontal regeneration.
As the soft tissue and hard tissue healing occurs in different phases of wound healing, we can conclude that A-PRF secretes more growth factors compared to L-PRF which promote fibroblast proliferation, leading to better soft tissue healing, whereas PRF owing to its better organization and denser fibrin network might support the osseous healing better. One of the important criteria to measure the periodontal regeneration is the histological examination which was not attempted due to ethical concern. Hence, clinical and radiographical evaluation was done to evaluate the bone regeneration in IBDs. However, a long-term, multicenter, randomized controlled clinical trial is needed to determine the clinical and radiographic effects of A-PRF on bone regeneration.
Conclusion
Both the groups showed the potential of enhanced periodontal healing. However, statistically PRF was found to be better in terms of defect fill and defect resolution and A-PRF in terms of soft tissue healing. Thus, a long-term, multicenter, randomized controlled clinical trial is needed to substantiate the fact.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflict of interest.
References
- 1.Polson AM. Periodontal Regeneration: Current Status and Directions. Chicago: Quintessence Pub Co; 1994. pp. 11–20. [Google Scholar]
- 2.Grover V, Kapoor A, Malhotra R, Uppal RS. Evaluation of the efficacy of a bioactive synthetic graft material in the treatment of intrabony periodontal defects. J Indian Soc Periodontol. 2013;17:104–10. doi: 10.4103/0972-124X.107484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santana R, de Mattos C, Van Dyke T. Efficacy of combined regenerative treatments in human mandibular class ii furcation defects. J Periodontol. 2009;80:1756–64. doi: 10.1902/jop.2009.080605. [DOI] [PubMed] [Google Scholar]
- 4.Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: Combinations of barrier membranes and grafting materials-biological foundation and preclinical evidence: A systematic review. J Clin Periodontol. 2008;35:106–16. doi: 10.1111/j.1600-051X.2008.01263.x. [DOI] [PubMed] [Google Scholar]
- 5.Cortellini P, Tonetti MS. Focus on intrabony defects: Guided tissue regeneration. Periodontol 2000. 2000;22:104–32. doi: 10.1034/j.1600-0757.2000.2220108.x. [DOI] [PubMed] [Google Scholar]
- 6.Labahn R, Fahrenbach WH, Clark SM, Lie T, Adams DF. Root dentin morphology after different modes of citric acid and tetracycline hydrochloride conditioning. J Periodontol. 1992;63:303–9. doi: 10.1902/jop.1992.63.4.303. [DOI] [PubMed] [Google Scholar]
- 7.Lynch S, Williams R, Poison A, Howell T, Reddy M, Zappa U, Antoniades H. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16:545–8. doi: 10.1111/j.1600-051x.1989.tb02334.x. [DOI] [PubMed] [Google Scholar]
- 8.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:e55–62. [Google Scholar]
- 9.Anitua E, Andia I, Ardanza B, Nurden P, Nurden A. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 10.Ghanaati S, Booms P, Orlowska A, Kubesch A, Lorenz J, Rutkowski J, et al. Advanced platelet-rich fibrin: A new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40:679–89. doi: 10.1563/aaid-joi-D-14-00138. [DOI] [PubMed] [Google Scholar]
- 11.Babrawala I, Venkatesh P, Varadhan K. A novel approach using 15% natural chitosan gel in the management of intrabony defects: A pilot study. Chin J Dent Res. 2016;19:231–7. doi: 10.3290/j.cjdr.a37148. [DOI] [PubMed] [Google Scholar]
- 12.Pradeep AR, Shetty SK, Garg G, Pai S. Clinical effectiveness of autologous platelet-rich plasma and Peptide-enhanced bone graft in the treatment of intrabony defects. J Periodontol. 2009;80:62–71. doi: 10.1902/jop.2009.080214. [DOI] [PubMed] [Google Scholar]
- 13.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Lei L, Yu Y, Han J, Shi D, Sun W, Zhang D, et al. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficiency as adjunctive to the GTR procedure in periodontal intrabony defects. J Periodontol. 2020;91:462–72. doi: 10.1002/JPER.19-0290. [DOI] [PubMed] [Google Scholar]
- 15.Santosh Kumar BB, Aruna DR, Gowda VS, Galagali SR, Prashanthy R, Navaneetha H. Clinical and radiographical evaluation of a bioresorbable collagen membrane of fish origin in the treatment of periodontal intrabony defects: A preliminary study. J Indian Soc Periodontol. 2013;17:624–30. doi: 10.4103/0972-124X.119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khurana JV, Mali AM, Mali RS, Chaudhari AU. Comparative evaluation of healing after periodontal flap surgery using isoamyl 2-cyanoacrylate (bioadhesive material) and silk sutures: A split-mouth clinical study. J Indian Soc Periodontol. 2016;20:417–22. doi: 10.4103/0972-124X.194267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cobb CM, Williams KB, Gerkovitch MM. Is the prevalence of periodontitis in the USA in decline? Periodontol 2000. 2009;50:13–24. doi: 10.1111/j.1600-0757.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 18.Chacko N, Abraham S, Rao H, Sridhar N, Moon N, Barde D. A clinical and radiographic evaluation of periodontal regenerative potential of PerioGlas: A synthetic resorbable material in treating periodontal infrabony defects. J Int Oral Health. 2014;6:20–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Meador HL, Lane JJ, Suddick RP. The long-term effectiveness of periodontal therapy in a clinical practice. J Periodontol. 1985;56:253–8. doi: 10.1902/jop.1985.56.5.253. [DOI] [PubMed] [Google Scholar]
- 20.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–67. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Pradeep A, Rao N, Agarwal E, Bajaj P, Kumari M, Naik S. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: A randomized controlled clinical trial. J Periodontol. 2012;83:1499–507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 22.Fujioka-Kobayashi M, Miron R, Hernandez M, Kandalam U, Zhang Y, Choukroun J. Optimized platelet-rich fibrin with the low-speed concept: Growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88:112–21. doi: 10.1902/jop.2016.160443. [DOI] [PubMed] [Google Scholar]
- 23.Thorat M, Pradeep AR, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: A controlled clinical trial. J Clin Periodontol. 2011;38:925–32. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma A, Pradeep A. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: A randomized controlled clinical trial. J Periodontol. 2011;82:1705–12. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 25.Suwondo CI, Herawati D, Sudibyo S. Effect of advanced platelet-rich fibrin applications on periodontal regeneration in infrabony pocket treatment. Majalah Kedokteran Gigi Indonesia. 2018;4:154–60. [Google Scholar]
- 26.Titirinli K, Tekin U, Atıl F, Önder ME, Şenguven B, Ozgul O, Kocyigit ID. Evaluation of advanced platelet rich fibrin (A-PRF) on bone healing. Is it better than old version? A histological animal study. J Biomater Tissue Eng. 2017;7:478–83. [Google Scholar]
- 27.Yun YR, Jang JH, Jeon E, Kang W, Lee S, Won JE, et al. Administration of growth factors for bone regeneration. Regen Med. 2012;7:369–85. doi: 10.2217/rme.12.1. [DOI] [PubMed] [Google Scholar]


