Abstract
Background
Intracerebral hemorrhage (ICH) recurrence risk is known to be higher in patients with cerebral amyloid angiopathy (CAA) as compared to other causes of ICH. Risk factors for ICH recurrence are not completely understood, and our goal was to study specific imaging microangiopathy markers.
Methods
Retrospective case-control study of patients with non-traumatic ICH admitted to a single center between 2014 and 2017 who underwent magnetic resonance imaging (MRI). Clinical characteristics of the index event and occurrence of death and ICH recurrence were collected from clinical records. MRI images were independently reviewed by 2 neuroradiologists. Groups of patients with CAA-related and CAA-unrelated ICH defined were compared. Presence of CAA was defined according to the Boston modified criteria. Survival analysis with Kaplan-Meier curves and Cox-regression analyses was performed to analyze ICH recurrence-free survival.
Results
Among 448 consecutive patients with non-traumatic ICH admitted during the study period, 104 were included in the study, mean age 64 years (±13.5), median follow-up of 27 months (interquartile range, IQR 16–43), corresponding to 272 person-years of total follow-up. CAA-related ICH patients presented higher burden of lobar microbleeds (p < 0.001), higher burden of enlarged perivascular spaces (EPVS) in centrum semiovale (p < 0.001) and more frequently presented cortical superficial siderosis (cSS; p < 0.001). ICH recurrence in patients with CAA was 12.7 per 100 person-years, and no recurrence was observed in patients without CAA. Variables associated with ICH recurrence in the whole population were age (hazard ratio [HR] per 1-year increment = 1.05, 95% CI 1.00–1.11, p = 0.046), presence of disseminated cSS (HR 3.32, 95% CI 1.09–10.15, p = 0.035) and burden of EPVS in the centrum semiovale (HR per 1-point increment = 1.80, 95% CI 1.04–3.12, p = 0.035).
Conclusions
This study confirms a higher ICH recurrence risk in patients with CAA-related ICH and suggests that age, disseminated cSS, and burden of EPVS in the centrum semiovale are associated with ICH recurrence.
Keywords: Intracerebral hemorrhage, Recurrence, Cerebral amyloid angiopathy
Introduction
Arteriolosclerotic microangiopathy and cerebral amyloid angiopathy (CAA) are among the most frequent causes of non-traumatic intracerebral hemorrhage (ICH) [1]. It is well known that CAA is associated with a high hemorrhage recurrence risk (up to 10% per year) [2], and that this risk is significantly higher when compared to patients with ICH related to other causes [3, 4]. The distribution of cerebral microbleeds is thought to reflect the etiology of the underlying microangiopathy, which translates into different hemorrhage recurrence risks, but the role of other in vivo biomarkers of recurrence has also been highlighted in more recent studies [5, 6]. Characterization of new imaging biomarkers of ICH recurrence may not only provide better prognostic information in patients with ICH at index event but may also help us to better understand the pathophysiology of the underlying cause. Our goal was to study ICH recurrence in patients with CAA-related and CAA-unrelated ICH, and to analyze factors associated with ICH recurrence irrespective of etiology, specifically magnetic resonance imaging (MRI) markers such as burden of enlarged perivascular spaces (EPVS) and white matter hyperintensities.
Methods
This was a retrospective case-control study of adult patients with non-traumatic ICH admitted to a single university hospital between 2014 and 2017. Records were retrieved according to ICD-9 and ICD-10 primary or secondary diagnosis of ICH. Patients with other diagnoses and underlying structural lesions such as vascular malformations and cerebral tumors were excluded after review of individual patient records. All patients who underwent brain MRI (including T1-, T2-, and T2*-weighted images and T2-FLAIR) were included in the study. All patients underwent 1.5-tesla MRI, and the MRI acquisition parameters were constant throughout the study period. Patients who did not undergo all of the aforementioned sequences, whose images were not available or whose images were of low quality for analysis were excluded. Demographic and clinical variables for the index event, and occurrence of death and non-traumatic ICH recurrence were collected from the digital clinical records. Patients were followed in our Neurology outpatient clinic as part of the routine clinical care, and information concerning death and ICH recurrence was collected from the digital clinical records (registration of death, subsequent in-hospital admission records, outpatient clinic records).
MRI images were independently reviewed by 2 senior neuroradiologists for the classification of the following: microbleed anatomical rating scale [7]; presence of cortical superficial siderosis (cSS; focal = restricted to 3 sulci, disseminated = involving more than 3 sulci) [8]; EPVS score (score 0 = no EPVS, score 1 = <10 EPVS, score 2 = 11–20 EPVS, score 3 = 21–40 EPVS, score 4 = >40 EPVS) [9]; and age-related white matter changes (ARWMC; rating for periventricular white matter changes: score 0 = no lesions, score 1 = focal lesions, score 2 = beginning confluence of lesions, score 3 = diffuse involvement of the entire region; rating for deep white matter changes: score 0 = no lesions, score 1 = 1 focal lesion (≥5 mm), score 2 = >1 focal lesion, score 3 = confluent lesions) [10]. Interobserver agreement was calculated using interclass correlation coefficient (ICC) for single measures with 95% CI. Disagreements were reviewed by the 2 raters and a consensus was reached. Patients were classified as CAA-related ICH if criteria for possible or probable CAA according to the modified Boston criteria [8] were fulfilled. Within the group with CAA-unrelated ICH, patients with mixed-location hemorrhage/microbleeds were identified [11].
Baseline variables of the patients with CAA-related ICH and CAA-unrelated ICH were compared using χ2 test, independent sample t test and Mann-Whitney test as appropriate. Kaplan-Meier curves for recurrence of ICH were constructed and Mantel-Cox test was used for group comparison. Univariable survival analyses for recurrence of ICH in the whole study population were performed using Cox regression. Variables flagged in the univariable analysis as significant were used to construct a multivariable Cox regression model. Survival time for Cox regression analyses was the time between hospital admission for index ICH and the occurrence of ICH recurrence, or loss to follow-up or death. Statistical significance was set as an alpha value of 0.05. Anonymized data is available on request.
Results
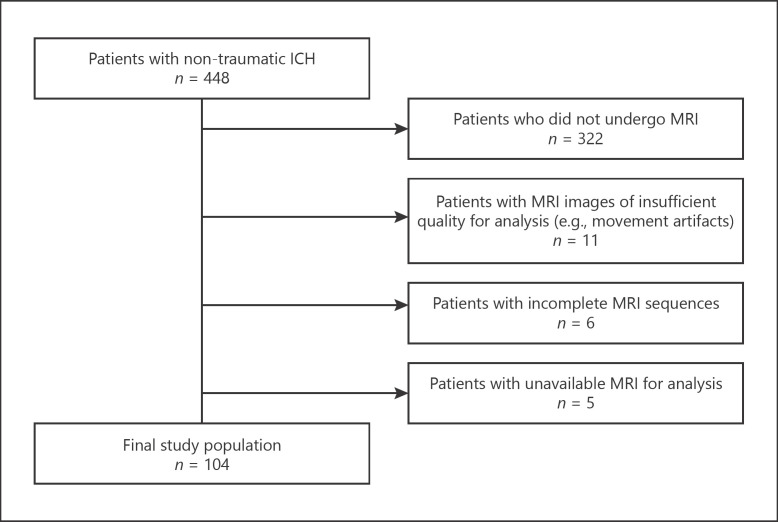
Among 448 consecutive patients with non-traumatic ICH admitted during the study period, 126 patients underwent brain MRI. From these, 22 patients were excluded because of missing MRI sequences, insufficient quality, or unavailability of images for analysis (Fig. 1). Patients who were excluded from the study were older, more frequently had antiplatelet therapy at baseline, deep ICH and intraventricular rupture, and presented a significantly higher 30-day mortality (online suppl. Table 1, see www.karger.com/doi/10.1159/000513503). The final study population consisted of 104 patients, with a mean age of 64 years (±13.5), 62 (59.6%) of male sex, and a median follow-up time of 27 months (interquartile range 16–43) corresponding to 272 person-years. Overall ICH recurrence during follow-up was 12.5% (4.8 per 100 person-years). Interobserver agreement for MRI variables was good to excellent (lowest ICC = 0.84 for deep ARWMC; higher ICC = 0.99 for counting of lobar microbleeds).
Fig. 1.
Patient selection flowchart.
Baseline characteristics of the groups with CAA-unrelated ICH (n = 56) and CAA-related ICH (n = 48) are presented in Table 1. Among patients with CAA-related ICH, the majority had probable CAA (n = 41), and only 7 patients had possible CAA according to the modified Boston criteria. Patients with CAA-related ICH were older (mean age 70.9 vs. 59.6 years, p = 0.007), presented lower systolic blood pressure (144 vs. 155 mm Hg, p = 0.029), lower diastolic blood pressure (75 vs. 87 mm Hg, p = 0.006), and lower blood glucose (109 vs. 202 mg/dL, p = 0.040) on admission. As expected, patients with CAA-related ICH more frequently presented lobar hemorrhages at the index event (93.8 vs. 19.6%, p < 0.001). Three patients with CAA-related ICH presented a cerebellar hemorrhage which was, in the 3 patients, superficial according to Pasi et al. [12]. Concerning MRI findings, patients with CAA-related ICH presented higher burden of lobar microbleeds (median number of lobar microbleeds 5 vs. 0, p < 0.001), higher burden of EPVS in centrum semiovale (median EPVS score 2.5 vs. 1, p < 0.001) and more frequently presented cSS (58.3 vs. 7.1%, p < 0.001). Among patients with CAA-unrelated ICH, we found 25 patients (44.6%) with mixed-location hemorrhage/microbleeds.
Table 1.
Baseline characteristics of patients according to the presence of CAA-unrelated and CAA-related ICH
| CAA-unrelated ICH (n = 56) | CAA-related ICH (n = 48) | p value | |
|---|---|---|---|
| Age, years | 59.6±13.9 | 70.9±10.2 | 0.007 |
| Male sex | 38 (68.9) | 24 (50.0) | 0.064 |
| Hypertension | 40 (71.4) | 36 (75.0) | 0.825 |
| Dyslipidemia | 27 (48.2) | 28 (58.3) | 0.303 |
| Diabetes | 10 (17.9) | 11 (22.9) | 0.522 |
| Antiplatelet therapy | 7 (12.5) | 9 (18.8) | 0.378 |
| Anticoagulation therapy | 9 (16.1) | 10 (20.8) | 0.531 |
| Admission Glasgow Coma Scale | 15 (13–15) | 14 (14–15) | 0.974 |
| Admission systolic blood pressure, mm Hg | 155 (133–174) | 144 (132–153) | 0.029 |
| Admission diastolic blood pressure, mm Hg | 87 (69–101) | 75 (67–83) | 0.006 |
| Admission glucose, mg/dL | 120 (109–149) | 109 (103–131) | 0.040 |
| Admission INR | 1.0 (1.0–1.2) | 1.1 (1.0–1.2) | 0.880 |
| Admission platelet count, /µL | 211 (153–254) | 201 (167–230) | 0.719 |
| ICH location | |||
| Lobar | 11 (19.6) | 45 (93.8) | <0.001 |
| Deep | 33 (58.9) | 0 | <0.001 |
| Infratentorial | 12 (21.4) | 3 (6.3) | 0.047 |
| Intraventricular rupture | 14 (25.0) | 14 (29.2) | 0.633 |
| Emergent neurosurgery | 6 (10.7) | 2 (4.2) | 0.282 |
| MRI findings | |||
| Age-related white matter changes score | |||
| Periventricular | 1 (1–2) | 2 (1–3) | 0.107 |
| Deep | 1 (1–2) | 2 (1–3) | 0.142 |
| Enlarged perivascular spaces score | |||
| Centrum semiovale | 1 (1–2) | 2.5 (1–3) | <0.001 |
| Basal ganglia | 2 (1–2) | 1 (1–2) | 0.119 |
| Number of microbleeds | |||
| Lobar | 0 (0–1) | 5 (1–9) | <0.001 |
| Basal ganglia | 0 (0–2) | − | <0.001 |
| Infratentorial | 0 (0–1) | 0 (0–1) | 0.930 |
| Cortical superficial siderosis | 4 (7.1) | 28 (58.3) | <0.001 |
| Focal (≤3 sulci) | 1 (1.8) | 13 (27.1) | <0.001 |
| Disseminated (>3 sulci) | 3 (5.4) | 15 (31.3) | 0.001 |
| 30-day mortality | 2 (3.6) | 1 (2.1) | 1.000 |
| Follow-up, months | 27 (21–43) | 24 (14–43) | 0.396 |
| ICH recurrence | 0 | 13 (27.1) | <0.001 |
Data is presented as n (%), mean ± SD, and median (interquartile range). CAA, cerebral amyloid angiopathy; ICH, intracerebral hemorrhage; INR, international normalized ratio; MRI, magnetic resonance imaging.
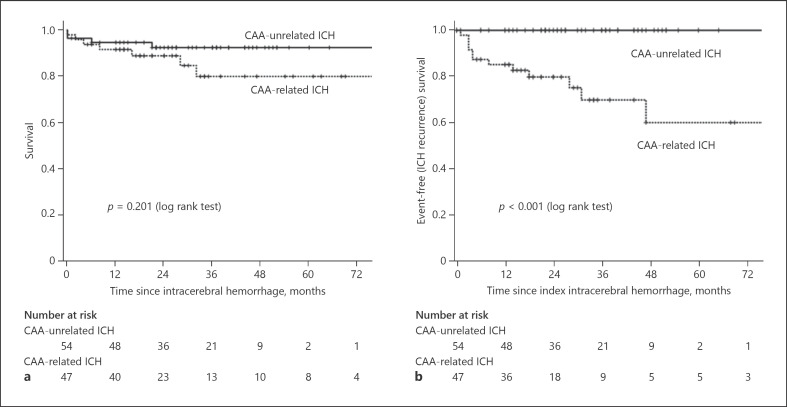
None of the patients with CAA-unrelated ICH experienced hemorrhage recurrence, while 13 patients with CAA-related ICH had at least a hemorrhage recurrence during the follow-up period (27.1%, 12.7 per 100 person-years). The Kaplan-Meier curves showed no difference concerning mortality between groups (p = 0.201) but showed significantly higher ICH recurrence in the group of patients with CAA-related ICH (p < 0.001; Fig. 2).
Fig. 2.
Kaplan-Meier curves for survival (a) and for ICH recurrence (b) according to the presence of CAA-related and CAA-unrelated ICH.
In the univariable survival analyses (Table 2), the only variables associated with ICH recurrence were age (hazard ratio [HR] per 1-year increment = 1.05, 95% CI 1.00–1.11, p = 0.046), presence of disseminated cSS (HR 3.32, 95% CI 1.09–10.15, p = 0.035), and burden of EPVS in the centrum semiovale (HR per 1-point increment = 1.80, 95% CI 1.04–3.12, p = 0.035). In the multivariable regression model, no variable was independently associated with ICH recurrence (Table 2).
Table 2.
Univariable and multivariable survival analyses using ICH recurrence during follow-up as endpoint in the Cox regression
| Univariable hazard ratio (95% CI) | p value | Multivariable hazard ratio (95% CI) | p value | |
|---|---|---|---|---|
| Age (per 1 year increase) | 1.05 (1.00–1.11) | 0.046 | 1.03 (0.97–1.09) | 0.318 |
| Male sex | 0.82 (0.28–2.45) | 0.722 | ||
| Hypertension | 0.69 (0.21–2.30) | 0.548 | ||
| Baseline systolic BP (per 10 mm Hg increment) | 0.92 (0.71–1.19) | 0.533 | ||
| Baseline diastolic BP (per 10 mm Hg increment) | 0.82 (0.56–1.22) | 0.331 | ||
| Diabetes | 1.48 (0.45–4.91) | 0.523 | ||
| Admission glucose (per 10 mg/dL increment) | 0.79 (0.62–1.01) | 0.057 | ||
| Dyslipidemia | 1.21 (0.40–3.62) | 0.736 | ||
| Diabetes | 1.48 (0.45–4.91) | 0.523 | ||
| Baseline antiplatelet therapy | 1.04 (0.22–4.86) | 0.958 | ||
| Baseline anticoagulation | 0.46 (0.06–3.60) | 0.461 | ||
| Baseline ICH volume (per 1 mL increment) | 1.02 (1.00–1.05) | 0.116 | ||
| Deep MB (per 1 MB increase) | 0.97 (0.88–1.07) | 0.511 | ||
| Lobar MB (per 1 MB increase) | 0.98 (0.94–1.03) | 0.472 | ||
| <5 lobar MB | 1.64 (0.49–5.44) | 0.422 | ||
| Focal cSS (≤3 sulci) | 1.67 (0.44–6.40) | 0.451 | ||
| Disseminated cSS (>3 sulci) | 3.32 (1.09–10.15) | 0.035 | 2.11 (0.62–7.16) | 0.230 |
| Periventricular ARWMC | 1.37 (0.67–2.81) | 0.387 | ||
| Deep ARWMC | 1.63 (0.84–3.19) | 0.152 | ||
| EPVS centrum semiovale | 1.80 (1.04–3.12) | 0.035 | 1.42 (0.78–2.61) | 0.254 |
| EPVS basal ganglia | 0.53 (0.20–1.37) | 0.189 |
95% CI, 95% confidence interval; BP, blood pressure; ICH, intracerebral hemorrhage; MB, microbleed; cSS, cortical superficial siderosis; ARWMC, age-related white matter hyperintensities; EPVS, enlarged perivascular spaces.
Discussion
This study confirms that ICH recurrence in patients with CAA-related ICH is significantly higher than in patients with CAA-unrelated ICH and found that increasing age, presence of disseminated cSS, and higher burden of EPVS in the centrum semiovale were associated with ICH recurrence in the whole study population.
The incidence of ICH recurrence in patients with CAA-related ICH is similar to larger cohort studies which reported incidences between 8 and 10% per year [13, 14, 15]. However, we did not find any ICH recurrence in the group with CAA-unrelated ICH. This may be explained by the small group size, considering that, according to the incidence of ICH recurrence reported in a recent meta-analysis [3], we would expect to find only 1 patient with recurrence in our group of CAA-unrelated ICH.
Even though an increasing burden of microbleeds has been associated with ICH recurrence both in CAA-related and CAA-unrelated ICH [3], more recent cohorts of CAA patients did not find this association and highlighted the importance of cSS as a more robust predictor for ICH recurrence [5, 6, 15]. In a meta-analysis of studies which included patients with CAA with and without ICH, even though cSS was found to be associated with occurrence of future ICH, disseminated cSS conferred a higher independent future ICH risk when compared to focal cSS [16]. Our results concerning the association of disseminated cSS with ICH recurrence are in line with these recent findings.
Few studies have addressed the role of other MRI microangiopathy markers as predictors of ICH recurrence [17]. The finding that a higher burden of EPVS in centrum semiovale is associated with ICH recurrence was only found in another larger study and may be a clue to better understanding the physiopathology of ICH in CAA. In a large single center cohort of CAA-related ICH survivors, Boulouis et al. [6] found that the total burden of microangiopathy (measured by the CAA-SVD-Score) was associated with a higher ICH recurrence, and that this association was mainly driven by the presence of cSS and higher burden of EPVS in the centrum semiovale. Perivascular spaces have been speculated to be involved in the pathogenesis of CAA, namely through dysfunction of amyloid-β clearance pathways [18]. It has been found that a higher burden of juxtacortical EPVS in patients with CAA was associated with increased severity of cortical accumulation of amyloid-β in vessel walls in the same regions [19], which could explain the association we found between EPVS in centrum semiovale and ICH recurrence risk. However, 2 other studies did not find such association between the severity of EPVS and recurrence risk in CAA-related ICH patients [14, 20], which warrants larger multicentric cohorts and meta-analyses to clarify this potential association.
The prevalence of hypertension and diabetes was similar in both groups of patients, which is probably related to the fact that the group with CAA-related ICH was significantly older, and raises the question of a possible contribution of arteriosclerotic microangiopathic changes for ICH also in this group of patients. However, it is interesting to note that systolic and diastolic blood pressures and glucose at admission were higher in patients with CAA-unrelated ICH, which suggests their contribution to the acute event, rather than a mere physiological reaction after ICH [21]. Inadequate blood pressure control after the acute phase of ICH is also known to be associated with ICH recurrence not only in patients with deep ICH, but also in patients with lobar ICH [22]. Unfortunately, we could not explore the contribution of blood pressure control to ICH recurrence because no systematic evaluation of blood pressure was carried out during follow-up in the routine clinical practice.
The main limitations of our study are related to its retrospective nature, its small sample size, possible recurrence detection bias in patients who die outside the hospital or who are admitted for ICH in other hospitals, low number of ICH recurrences, absence of ICH recurrence in the group of CAA-unrelated ICH, and exclusion of ICH patients who did not undergo MRI, which is reflected in the low mortality of included patients and represents a selection bias. We did not analyze recurrence predictors only in the subgroup of patients with CAA-related ICH because of the small sample size, and this could represent a methodological limitation because recurrences were only observed in this group. Our results concerning ICH recurrence predictors are derived from our whole study population of ICH patients irrespective of etiology, and even though none of the analyzed clinical or imaging marker is specific for CAA, and is also seen in patients with other ICH etiologies, our results must be interpreted with caution. The strengths of the study lie in the complete baseline clinical characterization, relatively long mean follow-up period in the Neurology outpatient clinic, as well as good interobserver agreement for MRI microangiopathy markers.
In conclusion, ICH recurrence is more frequent in patients with CAA-related ICH when compared with patients with CAA-unrelated ICH, and the most important predictors for ICH recurrence are the presence of cSS and higher burden of EPVS in the centrum semiovale.
Statement of Ethics
The study was approved by the Ethics for Health Commission of Hospital de Braga.
Conflict of Interest Statement
Authors report no disclosures.
Funding Sources
There are no funding sources to declare.
Author Contributions
J.P.: first manuscript draft writing; study design; data collection; statistical analysis. J.M.A., M.Q.-N., and J.S.-F.: final manuscript review; data collection. A.S.C.: final manuscript review; data collection; statistical analysis. F.S. and A.F.: final manuscript review; data processing and analysis. C.F.: final manuscript review; study design; important intellectual contribution. T.G.O.: final manuscript review; data collection; important intellectual contribution.
Supplementary Material
Supplementary data
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010 Jul;9((7)):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Charidimou A, Boulouis G, Gurol ME, Ayata C, Bacskai BJ, Frosch MP, et al. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017 Jul;140((7)):1829–50. doi: 10.1093/brain/awx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidimou A, Imaizumi T, Moulin S, Biffi A, Samarasekera N, Yakushiji Y, et al. Brain hemorrhage recurrence, small vessel disease type, and cerebral microbleeds: A meta-analysis. Neurology. 2017 Aug;89((8)):820–9. doi: 10.1212/WNL.0000000000004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinho J, Costa AS, Araújo JM, Amorim JM, Ferreira C. Intracerebral hemorrhage outcome: A comprehensive update. J Neurol Sci. 2019 Mar;398:54–66. doi: 10.1016/j.jns.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Charidimou A, Boulouis G, Roongpiboonsopit D, Xiong L, Pasi M, Schwab KM, et al. Cortical superficial siderosis and recurrent intracerebral hemorrhage risk in cerebral amyloid angiopathy: large prospective cohort and preliminary meta-analysis. Int J Stroke. 2019 Oct;14((7)):723–33. doi: 10.1177/1747493019830065. [DOI] [PubMed] [Google Scholar]
- 6.Boulouis G, Charidimou A, Pasi M, Roongpiboonsopit D, Xiong L, Auriel E, et al. Hemorrhage recurrence risk factors in cerebral amyloid angiopathy: comparative analysis of the overall small vessel disease severity score versus individual neuroimaging markers. J Neurol Sci. 2017 Sep;380:64–7. doi: 10.1016/j.jns.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009 Nov;73((21)):1759–66. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 8.Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010 Apr;74((17)):1346–50. doi: 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doubal FN, MacLullich AM, Ferguson KJ, Dennis MS, Wardlaw JM. Enlarged perivascular spaces on MRI are a feature of cerebral small vessel disease. Stroke. 2010 Mar;41((3)):450–4. doi: 10.1161/STROKEAHA.109.564914. [DOI] [PubMed] [Google Scholar]
- 10.Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. European Task Force on Age-Related White Matter Changes A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001 Jun;32((6)):1318–22. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 11.Pasi M, Charidimou A, Boulouis G, Auriel E, Ayres A, Schwab KM, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018 Jan;90((2)):e119–26. doi: 10.1212/WNL.0000000000004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasi M, Marini S, Morotti A, Boulouis G, Xiong L, Charidimou A, et al. Cerebellar hematoma location: implications for the underlying microangiopathy. Stroke. 2018 Jan;49((1)):207–10. doi: 10.1161/STROKEAHA.117.019286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biffi A, Halpin A, Towfighi A, Gilson A, Busl K, Rost N, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010 Aug;75((8)):693–8. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollenweber FA, Opherk C, Zedde M, Catak C, Malik R, Duering M, et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology. 2019 Feb;92((8)):e792–801. doi: 10.1212/WNL.0000000000006956. [DOI] [PubMed] [Google Scholar]
- 15.Raposo N, Charidimou A, Roongpiboonsopit D, Onyekaba M, Gurol ME, Rosand J, et al. Convexity subarachnoid hemorrhage in lobar intracerebral hemorrhage: A prognostic marker. Neurology. 2020;94:e968–e977. doi: 10.1212/WNL.0000000000009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charidimou A, Boulouis G, Greenberg SM, Viswanathan A. Cortical superficial siderosis and bleeding risk in cerebral amyloid angiopathy: A meta-analysis. Neurology. 2019 Dec;93((24)):e2192–202. doi: 10.1212/WNL.0000000000008590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charidimou A, Boulouis G. Intracerebral haemorrhage recurrence in cerebral amyloid angiopathy: time to look beyond microbleeds? J Neurol Sci. 2016 Aug;367:213–4. doi: 10.1016/j.jns.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, et al. colleagues from the Fondation Leducq Transatlantic Network of Excellence on the Role of the Perivascular Space in Cerebral Small Vessel Disease Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol. 2020 Mar;16((3)):137–53. doi: 10.1038/s41582-020-0312-z. [DOI] [PubMed] [Google Scholar]
- 19.van Veluw SJ, Biessels GJ, Bouvy WH, Spliet WG, Zwanenburg JJ, Luijten PR, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016 Mar;36((3)):576–80. doi: 10.1177/0271678X15620434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo HW, Jo KI, Yeon JY, Kim JS, Hong SC. Clinical features of high-degree centrum semiovale-perivascular spaces in cerebral amyloid angiopathy. J Neurol Sci. 2016 Aug;367:89–94. doi: 10.1016/j.jns.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Piran P, Lerario MP, Ong H, Gupta A, Murthy SB, et al. Differences in admission blood pressure among causes of intracerebral hemorrhage. Stroke. 2020 Feb;51((2)):644–7. doi: 10.1161/STROKEAHA.119.028009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biffi A, Anderson CD, Battey TW, Ayres AM, Greenberg SM, Viswanathan A, et al. Association between blood pressure control and risk of recurrent intracerebral hemorrhage. JAMA. 2015 Sep;314((9)):904–12. doi: 10.1001/jama.2015.10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data