Abstract
Objective
To define the characteristics of solitary idiopathic choroiditis (SIC) in a consecutive series of patients and propose a nomenclature change to idiopathic scleroma.
Materials and Methods
Electronic patient records were retrospectively interrogated to identify all patients diagnosed with SIC between 2002 and 2019 in a tertiary referral ophthalmic hospital in the United Kingdom.
Results
Thirty-four eyes of 34 patients were found to have SIC. The mean age at diagnosis was 48 years (range 24–78) and 23 patients (68%) were female. All lesions were located posterior to the equator, most frequently in the inferotemporal quadrant (13 eyes, 38%). The lesions had a mean largest basal diameter of 1.2 ± 0.4 disc diameters (range 0.5–2) and their distance to the optic disc had a mean of 1.2 ± 0.9 disc diameters (range 0–3.3). All lesions were intrascleral on enhanced depth imaging optical coherence tomography, demonstrating a hypo-reflective zone within the sclera, with an underlying hyper-reflective zone in some cases. No lesion enlarged or developed features consistent with active inflammation after a median follow-up time of 0.9 years (range 0–16.8).
Discussion/Conclusion
Optical coherence tomography shows SIC to be an intrascleral lesion. Furthermore, we found no evidence of any inflammatory component. A nomenclature change to idiopathic scleroma is appropriate to prevent unnecessary investigation.
Keywords: Ocular oncology, Optical coherence tomography, Solitary idiopathic choroiditis
Introduction
Several authors have described a distinctive, yellow-white, post-equatorial tumor that is small and apparently benign. This lesion has been termed “unifocal helioid choroiditis” by Hong et al. [1] and “solitary idiopathic choroiditis” (SIC) by Shields et al. [2]; however, the etiology of this condition is unknown. Lesions with a similar appearance can be found in both inflammatory and neoplastic ocular conditions. Inflammatory lesions usually comprise choroidal granulomas caused by conditions such as sarcoidosis, tuberculosis, toxocariasis, and syphilis [3]. Neoplasms that mimic this condition include amelanotic choroidal nevus, amelanotic choroidal melanoma, choroidal osteoma, and choroidal metastasis. An early sclerochoroidal calcification may present in a similar fashion and has recently been described to be similarly confined to the sclera [4]. The recent development of optical coherence tomography with enhanced depth imaging (EDI-OCT) allows these lesions to be imaged with greater resolution and to a greater depth.
The aims of this study were to describe a series of cases presenting to Moorfields Eye Hospital with this tumor and to compare our findings with those previously reported.
Materials and Methods
The electronic patient records of Moorfields Eye Hospital were retrospectively interrogated and all patients diagnosed with SIC between 2002 and 2019 were reviewed. The following data were recorded: age at presentation, sex, visual acuity at first and final visit, ocular and medical co-morbidities, OCT, fundus auto-fluorescence photography, near-infrared photography, B-scan ultrasound, fluorescein angiography, and indocyanine green (ICG) angiography. Five cases including SIC in the differential diagnosis were excluded because we did not consider them to have this condition. The lesion diameters and distance to optic disc margin were measured as multiples of the vertical disc diameter in the same eye using the Optos widefield imaging software (Optos plc, Dunfermline, UK).
Results
The diagnosis of SIC was confirmed in 34 eyes of 34 patients (23 female, 11 male) with a mean age of 48 years (range 24–78). The lesion involved the left eye in 21 patients and the right eye in 13. The best corrected visual acuity at presentation had a median of 20/20 (range 20/15–20/40). Eight patients had ocular co-morbidities, which included eyelid melanoma (1), cataract (1), dry age-related macular degeneration (1), neovascular age-related macular degeneration (1), congenital hypertrophy of the retinal pigment epithelium (RPE) (1), choroidal nevus (1), central retinal vein occlusion (1), and retinal detachment (1). The central retinal vein occlusion and retinal detachment both affected the contralateral eye while the congenital hypertrophy of the RPE and choroidal nevus were ipsilateral. Systemic co-morbidities were found in two patients and included cutaneous nevi and cerebral astrocytoma, respectively. The referring diagnosis included: amelanotic nevus or melanoma (17), choroidal osteoma (6), choroidal nevus (3), scar (2), SIC (1), inflammatory lesion (1), and possible metastasis of cerebral astrocytoma (1). The remaining three patients had no diagnosis mentioned in the referral letter or no referral on file. Two patients reported symptoms, which included fluctuating vision and decreased vision with photopsia and floaters, respectively. These symptoms were considered to be unrelated to the SIC lesion. Twelve patients attended the service once only and were discharged for surveillance elsewhere. The remaining 22 patients had a median follow-up of 1.3 years (range 0.2–16.8).
Nine patients (26%) underwent investigations to determine the etiology of the lesion. These included angiotensin-converting enzyme (6), Venereal Disease Research Laboratory test for syphilis (6), QuantiFERON Gold (6), inflammatory markers (3), and chest X-ray (3). Other tests included investigations for Toxoplasma gondii, Toxocara canis, Bartonella henselae, and Borrelia burgdorferi serology, antinuclear antibodies, rheumatoid factor, antineutrophil cytoplasmic antibodies, antibodies to extractable nuclear antigens, smooth muscle antibodies, and double-stranded DNA antibodies. No systemic investigations revealed any cause for the SIC lesion. No patient received treatment for SIC. None of the lesions showed any evidence of activity at presentation or, to our knowledge, previously and subsequently. No patient experienced visual loss between their initial and final visit.
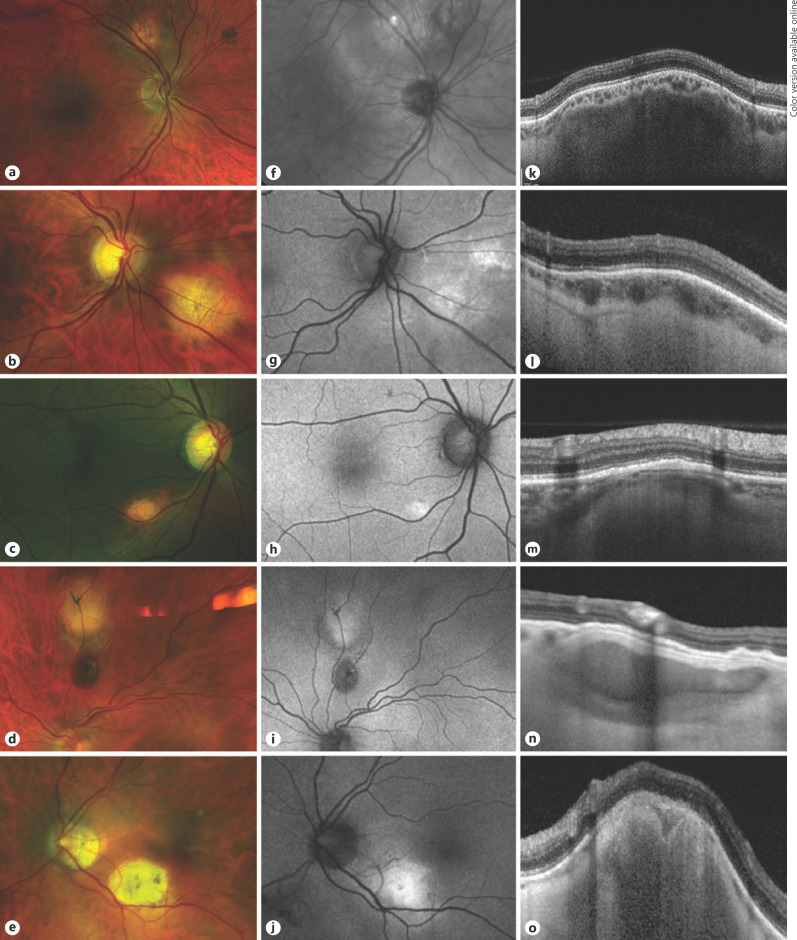
The characteristics of the lesions encountered are listed in Table 1. The lesions showed variation in color, position, and borders. All yellow-white lesions were visible on fundus auto-fluorescence and near-infrared imaging, whereas yellow or orange lesions showed varying degrees of visibility using these modalities. Most lesions (30, 88%) had poorly defined borders, whereas four (12%) had discrete margins with a punched-out appearance. Figure 1 illustrates the spectrum of ophthalmoscopic, auto-fluorescence, and OCT features.
Table 1.
Characteristics of SIC lesions and their features as delineated by varying image modalities
| Characteristic | |
|---|---|
| Size | |
| Largest basal diameter, DD | 1.2±0.4 (0.5–2) |
| Smallest basal diameter, DD | 1.0±0.4 (0.4–1.7) |
| Location | |
| Inferotemporal | 13 (38%) |
| Inferonasal | 11 (32%) |
| Superotemporal | 6 (18%) |
| Inferior midline | 3 (9%) |
| Superonasal | 1 (3%) |
| Post-equatorial | 34 (100%) |
| Distance from optic disc, DD | 1.2±0.9 (0–3.3) |
| Shape | |
| Circle | 19 (56%) |
| Oval | 5 (15%) |
| Poorly defined | 10 (29%) |
| Color | |
| Yellow | 19 (56%) |
| Yellow-white | 10 (29%) |
| Orange | 5 (15%) |
| Surface features | |
| Patchy retinal pigment epithelium | 15 (44%) |
| Pigment migration | 2 (6%) |
| OCT features | |
| Intralesional appearance | |
| Hypo-reflective area within sclera | 18 (53%) |
| Hyper-reflective zone within the | |
| hypo-reflective area | 16 (47%) |
| Shape of retinal elevation | |
| Single dome | 29 (85%) |
| Bilobed/sharp peak | 5 (15%) |
| Full choroidal compression | 29 (85%) |
| Choroidal vessels visible at apex | 24 (70%) |
| Associated retinal changes | |
| RPE thickening | 11 (32%) |
| RPE detachment | 9 (26%) |
| Outer retinal loss | 7 (21%) |
| Subretinal fluid | 4 (12%) |
| None | 5 (15%) |
| Auto-fluorescence | |
| Hyper-auto-fluorescent | 23 (67%) |
| Hypo-auto-fluorescent | 6 (18%) |
| Poorly visualised | 5 (15%) |
| Infrared | |
| Hyper-reflective | 22 (65%) |
| Hypo-reflective | 5 (15%) |
| Poorly visualised | 7 (20%) |
| B-scan ultrasound elevation, mm | 0.9±0.5 (0–1.7) |
Values are presented as n (%) or mean ± standard deviation (range). DD, disc diameters; OCT, optical coherence tomography; RPE, retinal pigment epithelium; SIC, solitary idiopathic choroiditis.
Fig. 1.
Spectrum of appearance of the solitary idiopathic choroiditis lesions in this series, imaged with color fundus photography (first column), auto-fluorescence (second column), and enhanced depth imaging optical coherence tomography (third column).
On OCT, SIC showed either (a) a hypo-reflective area under a band of hyper-reflective sclera (Fig. 1k) or (b) a further hyper-reflective mass beneath this (Fig. 1m). Although the whole lesion caused a dome-shaped elevation of the overlying retina, the scleral lesion itself tended to have a lumpy, irregular surface at the border between sclera and choroid (Fig. 1k). Lesions that did not fully indent the retina were generally small, with minimal or no retinal elevation (Fig. 1m, n).
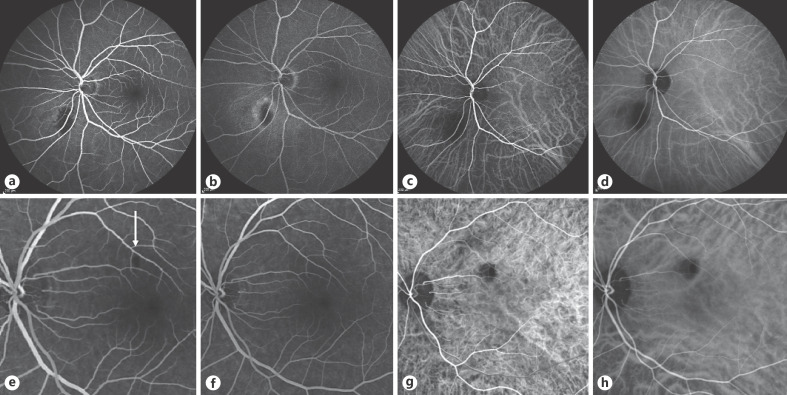
Five patients underwent fluorescein and ICG angiography. ICG angiography showed consistent hypo-fluorescence in all phases of the angiogram in those patients tested, whereas the fluorescein angiographic appearances depended on the degree of involvement of the overlying retina and RPE. One patient with RPE detachment and disruption of the ellipsoid zone showed hypo-fluorescence over the area of the ellipsoid abnormality with progressive hyper-fluorescence adjacent to this, consistent with RPE detachment (Fig. 2a–d). Another patient, who had no retinal abnormality overlying the SIC, showed only a small filling defect, which persisted during the arterial and venous phases and filled normally thereafter (Fig. 2e–h).
Fig. 2.
Early and late frames of the fluorescein angiogram and indocyanine green angiography of 2 patients. Patient 2 (second row) has a filling defect on the early fluorescein angiogram (arrow) and is filled in the later fluorescein frame.
Discussion
This study, completed in 2019, further characterises the multimodal imaging of SIC and expands the phenotypic spectrum of this disease. On EDI-OCT, all lesions known as SIC were confined entirely to the sclera. All lesions exhibited compression of the overlying choroid. Lesions with atrophy of the overlying choroid and RPE showed hyper-auto-fluorescence possibly arising from the exposed sclera, which can be observed in cases of thinned choroid, such as that seen in myopic degeneration [5, 6]. The hypo-fluorescence of some SIC lesions that was demonstrated by ICG is consistent with total compression of the choroidal blood vessels impeding blood flow through the choroid overlying the lesion. Although choroidal vessel lumina were visible at the apex of most lesions (23/34 eyes, 68%), the ICG findings suggest that these vessels were not being perfused.
All lesions in this series were post-equatorial. They were small with a mean largest basal diameter of 1.2 ± 0.4 disc diameters (range 0.5–2) and located close to the optic disc with a mean distance of 1.2 ± 0.9 disc diameters (range 0–3.3). The variation in appearance of the lesion on ophthalmoscopy and color photography was related to the degree of atrophy of the overlying RPE and retina, with all four punched-out lesions having loss of the RPE, ellipsoid zone, and outer nuclear layer on OCT. Fung et al. [7] describe an orange halo in 6/10 cases. Nine eyes (26%) in our series showed this halo, which corresponded to the lateral extent of the lesion visible clearly through the retina. The intralesional appearance on OCT consisted either of a hypo-reflective area under an initial hyper-reflective band of sclera (18, 53%) or a further dome-shaped zone of hyper-reflectivity posterior to this (16, 47%). Fung et al. [7] describe similar appearances, with a hyper-reflective band and shadowing in eight of their ten patients. This indicates that the lesion is not homogenous and that it exhibits areas of differing density within the sclera.
Our series demonstrates the benign nature of these lesions as none grew over time. Only two patients in this series reported symptoms, which we considered to be unrelated to the scleral lesion. There was no inflammation noted in any eye in this series. This is in contrast to the cohort of 60 cases reported by Shields et al. [2], in which most patients presented with symptoms, with about one-third of all lesions showing signs of inflammation. Notably, gamma-interferon testing was not available at the time of publication of this series [8]. As none of our cases showed any evidence of ocular inflammation, we can only surmise that the lesions in this series must have a different pathology. There remains a possibility that the lesions known as SIC in our series represent a group of different pathologies, given their spectrum of appearance, particularly on OCT (Fig. 1).
The strengths of this study include the large number of patients and their extensive multimodal imaging, which allowed further characterisation of this condition. Since our hospital provides a tertiary referral service, many patients were discharged from our care for surveillance at their local hospital so that the median follow-up time was short; however, it is likely that any patient developing symptoms or inflammation would have been returned to our care. Another limitation of our study is the lack of histology so that the precise etiology remains unknown.
There has been an evolution within the literature regarding the anatomy and nature of SIC. In 2002, Shields et al. [2] described this lesion as a choroidal inflammatory mass because it resembled a choroidal granuloma. In 2013, Fung et al. [7], using EDI-OCT, described these lesions as extending into the sclera from the choroid. In 2014, Shields et al. [9] described the lesion as either choroidal or scleral. We consider all lesions in our study to have been intrascleral, with varying degrees of compression of the overlying choroid.
Because of its scleral location and the absence of any signs of inflammation, we propose the term “idiopathic scleroma” for this lesion. Such nomenclature should discourage unnecessary systemic investigations for inflammatory disease and perhaps immunosuppressive therapy, which some might administer because of the term “solitary idiopathic choroiditis.” Others have recently reported similar findings in a series of 63 patients with unilateral findings and proposed the term “focal scleral nodule” for this lesion [10].
In conclusion, in view of the scleral location of these lesions and the lack of any inflammatory signs, we propose replacing the term “solitary idiopathic choroiditis” with “idiopathic scleroma.” We suggest that systemic investigations for inflammatory disease and immunosuppressive therapy are not required once the tumor has been localised to sclera by OCT and found to show no signs of inflammation.
Statement of Ethics
Ethics approval was granted for this study by the Audit Committee of Moorfields Eye Hospital Clinical Audit Department (Audit No. 502) which did not deem written consent necessary because this was a retrospective review of patients' charts to assess our practice without influencing treatment or investigations, which were performed solely in the course of routine care and not for the purposes of this study, which was conducted fully in accordance with the Declaration of Helsinki.
Conflict of Interest Statement
The authors declare no disclosures or conflicts of interest.
Funding Sources
This study did not receive any funding.
Author Contributions
B. Damato and W. Rahman conceived the manuscript. E. Duignan wrote the manuscript. E. Duignan, T. Moloney, and R. O'Day collected the relevant data. All authors revised successive drafts of the manuscript.
Acknowledgement
The authors wish to acknowledge our colleagues in the Medical Imaging Department, Moorfields Eye Hospital.
References
- 1.Hong PH, Jampol LM, Dodwell DG, Hrisomalos NF, Lyon AT. Unifocal helioid choroiditis. Arch Ophthalmol. 1997 Aug;115((8)):1007–13. doi: 10.1001/archopht.1997.01100160177006. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Shields CL, Demirci H, Hanovar S. Solitary idiopathic choroiditis: the Richard B. Weaver lecture. Arch Ophthalmol. 2002 Mar;120((3)):311–9. doi: 10.1001/archopht.120.3.311. [DOI] [PubMed] [Google Scholar]
- 3.Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 3rd ed. Philadelphia (Pa): Mosby; 2004. [Google Scholar]
- 4.Hasanreisoglu M, Saktanasate J, Shields PW, Shields CL. Classification of sclerochoroidal calcification based on enhanced depth imaging optical coherence tomography “mountain-like” features. Retina. 2015 Jul;35((7)):1407–14. doi: 10.1097/IAE.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 5.Sawa M, Ober MD, Freund KB, Spaide RF. Fundus autofluorescence in patients with pseudoxanthoma elasticum. Ophthalmology. 2006 May;113((5)):814–20.e2. doi: 10.1016/j.ophtha.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 6.Sparrow JR, Gregory-Roberts E, Yamamoto K, Blonska A, Ghosh SK, Ueda K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012 Mar;31((2)):121–35. doi: 10.1016/j.preteyeres.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung AT, Kaliki S, Shields CL, Mashayekhi A, Shields JA. Solitary idiopathic choroiditis: findings on enhanced depth imaging optical coherence tomography in 10 cases. Ophthalmology. 2013 Apr;120((4)):852–8. doi: 10.1016/j.ophtha.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro S, Andrews R, Sagoo M. Solitary idiopathic choroiditis. Case Rep Ophthalmol. 2014 Jan;5((1)):1–5. doi: 10.1159/000357470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shields CL, Pellegrini M, Ferenczy SR, Shields JA. Enhanced depth imaging optical coherence tomography of intraocular tumors: from placid to seasick to rock and rolling topography—the 2013 Francesco Orzalesi Lecture. Retina. 2014 Aug;34((8)):1495–512. doi: 10.1097/IAE.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 10.Fung AT, Waldstein SM, Gal-Or O, Pellegrini M, Preziosa C, Shields JA, et al. Focal Scleral Nodule: A New Name for Solitary Idiopathic Choroiditis and Unifocal Helioid Choroiditis. Ophthalmology. 2020 Nov;127((11)):1567–77. doi: 10.1016/j.ophtha.2020.04.018. [DOI] [PubMed] [Google Scholar]