Abstract
The SARS-Cov-2 virus caused a high morbidity and mortality rate disease, that is the COVID-19 pandemic. Despite the unprecedented research interest in this field, the lack of specific treatments leads to severe complications in a high number of cases. Current treatment includes antivirals, corticosteroids, immunoglobulins, antimalarials, interleukin-6 inhibitors, anti-GM-CSF, convalescent plasma, immunotherapy, antibiotics, circulation support, oxygen therapy, and circulation support. Due to the limited results, until specific treatments are available, other therapeutic approaches need to be considered. The endocannabinoid system is found in multiple systems within the human body, including the immune system. Its activation can lead to beneficial results such as decreased viral entry, decreased viral replication, and a decrease in pro-inflammatory cytokines such as IL-2, IL-4, IL-6, IL-12, TNF-α, or IFN-γ. Moreover, endocannabinoid system activation can lead to an increase in anti-inflammatory cytokines, mainly represented by IL-10. Overall, the cannabinoid system can potentially reduce pulmonary inflammation, increase the immunomodulatory effect, decrease PMN infiltration, reduce fibrosis, and decrease viral replication, as well as decrease the ‘cytokine storm’. Although the cannabinoid system has many mechanisms to provide certain benefits in the treatment of SARS-CoV-2 infected patients, research in this field is needed for a better understanding of the cannabinoid impact in this situation.
Keywords: COVID-19 treatment, SARS-CoV-2, cannabinoid, immune system, cytokine storm
1. Introduction
Viruses are submicroscopic agents which can invade the human body through respiratory, gastrointestinal, genitourinary systems, or skin tissue. Once inside the body, the viruses use the hosts’ cells to replicate their RNA or DNA. The immune system is usually efficient in treating most viral infections, but in some cases the virus is so aggresive, that the immune system alone cannot defend the body.
Such an aggressive virus is the coronavirus strain. The new coronavirus, SARS-CoV-2, causes an atypical respiratory disease, named Coronavirus disease 19 (COVID-19), and due to the worldwide spread and the high number of infected people, World Health Organization (WHO) declared a global pandemic (Zhang et al. 2020). So far, the infection has affected more than 72 million people worldwide, leading to more than 1.5 million deaths. This new virus primarily affects the respiratory tract, but other organs can also be affected (Lucaciu et al. 2020; Petrescu et al. 2020; Zhang et al. 2020). Several complications are associated with this disease, some of which can lead to death, even in the case of medical treatment. Recent research has demonstrated that the disease evolution severity largely depends on the presence of co-morbidities and the efficiency of the patients’ immune system (Xu et al. 2020; Zhang et al. 2020; Zhou et al. 2020). The treatment for the SARS-CoV-2 infection is complex. Besides other treatment strategies, combining anti-parasite drugs with immunomodulatory, antiviral, and common flu therapies has given some results (Guo et al. 2020; Zhang et al. 2020). Despite these treatments, the mortality rate can be up to 9% in some countries. This high mortality rate can be explained by the lack of targeted treatments. As a result, until more efficient targeted treatments are being tested and approved, other nonspecific treatments are being considered. Such an option is the activation of the cannabinoid system, which is found in multiple locations inside the human body, including the central nervous system, immune system, gastrointestinal or musculoskeletal system. Concerning the SARS-CoV-2 infection, the cannabinoid effects on the immune system have the potential to limit the abnormal function of the immune system and therefore decrease the overall mortality.
2. Pathophysiology of covid -19
2.1. Mechanism of SARS-CoV-2 invasion into host cells
SARS-CoV-2 belongs to the Coronavirinae family, presenting the largest genome among RNA viruses. This virus is an enveloped, positive-sense RNA virus, with spike-like projections on the surface (Jin et al. 2020). Viral replicase/transcriptase function is encoded in two-thirds of the genome, while the other third encodes viral structural proteins. The genome is packed into a helical nucleocapsid protected by a lipid bilayer. Based on their genomic structure, Coronaviruses are divided into four groups: α, β, γ, and δ. The first two types of coronaviruses infect only mammals (Rabi et al. 2020). SARS-CoV-2 is a β coronavirus. Four proteins are present in coronaviruses: nucleocapsid (N), envelop (E), membrane (M), and spike (S). The last-mentioned protein determines the host tropism, being the leading mediator of viral entry and it is formed out of transmembrane trimetric glycoprotein (Bosch et al. 2003). This protein has two subunits: S1 and S2, S1 is responsible for the process of binding to the host cell, and S2 for the fusion of the cell and virus membrane. Li et al. 2003 demonstrated that Angiotensin-converting enzyme 2 (ACE2) is a functional receptor for the SARS coronavirus. ACE2 is a type I integral membrane protein, a mono-carboxypeptidase that hydrolyzes angiotensin II. This protein is highly expressed on lung epithelial cells, in the heart, ileum, kidneys, and bladder (Zou et al. 2020).
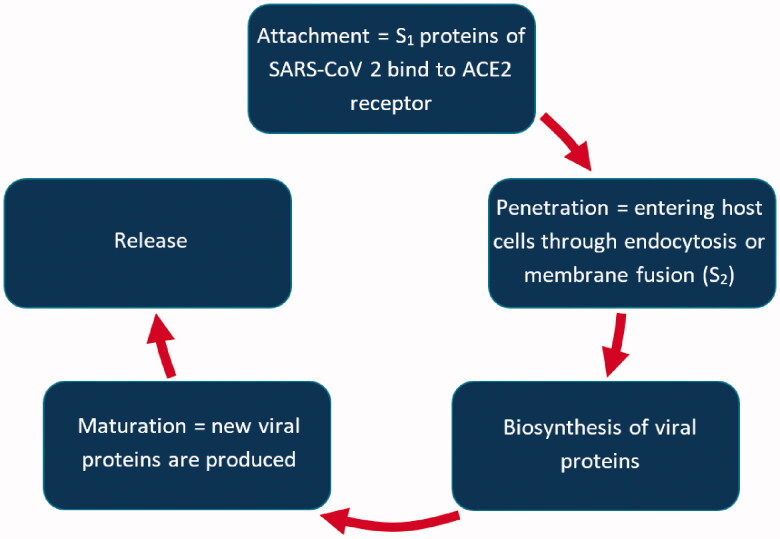
The life cycle of SARS-CoV-2 comprises five stages: attachment, penetration, biosynthesis, maturation, and release, as presented in Figure 1. Infection is initiated through the interaction of the viral particle with the proteins on the cell surface. After completion of the first step, S1 protein binds to the host one and spike protein starts the cleavage. The penetration (fusion) process involves large conformational changes of the spike protein. The coronavirus spike is different from others because many proteases can cleave and activate it (Belouzard et al. 2012), due to the existence of the furin cleavage site (‘RPPA’ sequence), making SARS-CoV-2 very pathogenic. Besides the furin precleavage, TMPRSS2 - cellular serine protease is mandatory to process SARS-CoV-2 spike protein and determine virus-host cell entry (Hoffmann et al. 2020). The envelope of the viruses fuses with the host cell membrane so that the nucleocapsid is delivered to the cell.
Figure 1.
The life cycle of SARS-CoV-2.
2.2. Host response to SARS-CoV-2 invasion
When our immune system detects a viral infection, it begins to respond with antibodies and proteins that try to prevent virus penetration into the cell (Nüssing et al. 2018). Circulant leukocytes make viruses harmless. Infected host cells secrete interferon, which is released to neighboring cells, as this has the role to slow down the virus multiplication. Interferon also attracts natural killer (NK) cells that aim to detect and eliminate infected cells (Schmidt et al. 2018; Gyurova et al. 2020). Age or immune system diseases make subjects more prone to develop viral infections, as in over 60 years of age patients, B and T lymphocytes formation is reduced. In patients diagnosed with obesity, an impairment in the functionality of NK cells has been demonstrated (Bähr et al. 2020).
The demonstrated transmission pathway of SARS-CoV-2 is via respiratory droplets, the fecal-oral transmission pathway is not yet demonstrated. After infection, there is an incubation time of 4-5 days before the first signs and symptoms are present (Li et al. 2020). Five to 6 days after symptoms onset, SARS-CoV-2 viral load reaches the peak, earlier than SARS-CoV, where the viral peak is about 10 days after symptoms onset (Peiris et al. 2003; Zou et al. 2020). Eight to nine days after symptoms onset, severe cases of SARS-CoV-2 progress to acute respiratory distress (ARDS) (Wang et al. 2020). Some of these ARDS cases may complicate to secondary bacterial or fungal infections (Chen et al. 2020), or respiratory failure, recognized as the cause of death of 70% of COVID-19 cases (Zhang et al. 2020).
The host responds to infection with an aggressive inflammatory reaction, implicated in the damage of the airways (Wong et al. 2004). A vast release of cytokines by the immune system occurs, leading to a cytokine storm associated with symptoms of sepsis, associated with 28% of fatal COVID-19 case (Onaivi and Sharma 2020). Uncontrolled inflammation affects multiple organs, leading to organ cardiac, renal, or hepatic failure.
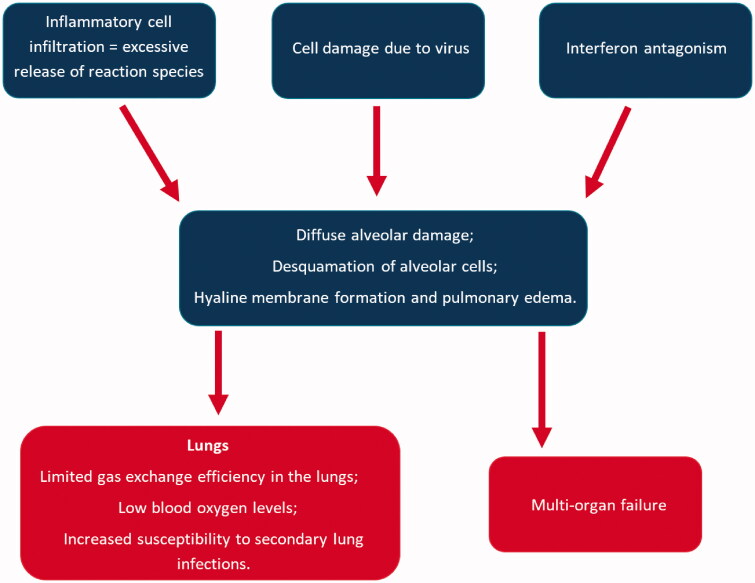
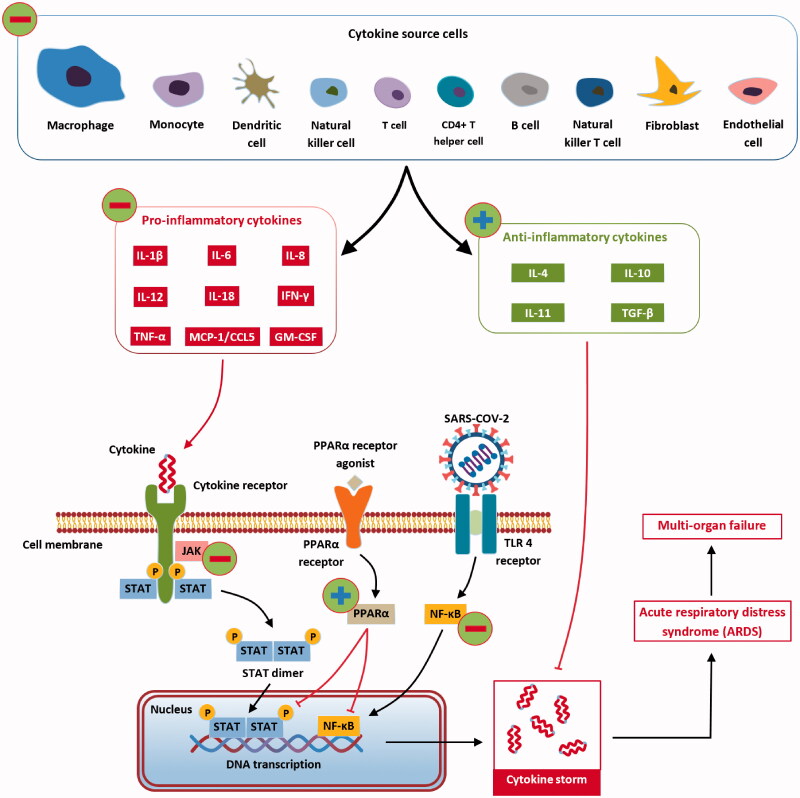
As previously presented, the first step of infection is the binding of S protein of SARS-CoV-2 to ACE-2, especially targeted being the airways epithelial cells, the alveolar epithelial cells, the vascular endothelial cells, and the macrophages in the lungs (Hamming et al. 2004; Xu et al. 2020). After infection, ACE-2 expression in lung cells is reduced, which is associated with acute lung injury. Downregulation of ACE-2 is associated with a dysfunction of the renin-angiotensin system, impacting blood pressure, the fluid/electrolyte balance and stimulateing the inflammation process and the vascular permeability in the airways. Besides that lung cell infection, SARS-CoV-2 triggers the recruitment of macrophages and monocytes, that release cytokine, as well as T and B cells. In most cases, this limits the spread of the infection, but in other cases, a modified (dysfunctional) response is installed. Viruses infected cells and tissues die, a process called pyroptosis. This process is highly associated with cytopathic viruses, such as SARS-CoV-2 (Park et al. 2020). Pyroptosis might be the trigger for the inflammatory response (Yang 2020), associated with increased secretion of cytokines and chemokines: IL-1 β, IL-6, IFNγ, MCP1, and IP-10 (Huang et al. 2020). The release of these cytokines and chemokines attracts immune cells into the infected site (T lymphocytes, monocytes) (Tian et al. 2020). The agglomeration of immune cells and lymphocytes in the pulmonary tissue might be an explanation for lymphopenia (Guan et al. 2020; Qin et al. 2020). At this stage, in most patients, recruited cells limit the infection and patients recover. In some patients, a cytokine storm (IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (G-CSF), IP-10, MCP1, macrophage inflammatory protein 1α (MIP1α), and tumor necrosis factor (TNF) (Huang et al. 2020) occurs, that triggers extensive lung inflammation. It is demonstrated that patients with severe forms of COVID-19 present higher inflammatory monocyte-derived macrophages in the bronchoalveolar fluids (Liao et al. 2020) and CD14+CD16+ inflammatory monocytes in peripheral blood (Zhou et al. 2020). The previously mentioned cells secrete cytokines that contribute to the cytokine storm (Figure 2).
Figure 2.
The immune system response for SARS-CoV-2 infection (Cabral and Griffin-Thomas 2009).
The mechanism by which SARS-CoV-2 destroys the cytokine antiviral response is not elucidated yet. One explanation might be that the antagonism of the interferon response supports viral replication, which leads to an increase in pyroptosis products. On the other hand, lung inflammatory cells can secrete proteases and reactive oxygen species, which can be linked to lung damage (Xu et al. 2020).
A research group suggested that stimulating the immune system could be a good approach to prevent viral infections (Hui et al. 2018). As SARS-CoV-2 is associated with the over-reaction of the immune system and a cytokine storm (Tay et al. 2020), a combined therapeutic approach is recommended to block the host’s excessive response to SARS-CoV-2 invasion.
3. Current treatment options for COVID-19
The lack of specific treatment for COVID-19 is the main reason for the important morbidity and high mortality rate associated with the disease. The only treatments available today are represented by supportive care (Song et al. 2020). The treatment options include antivirals, corticosteroids, immunoglobulins, antimalarials, interleukin-6 inhibitors, anti-GM-CSF, convalescent plasma, immunotherapy, antibiotics, oxygen therapy, and circulation support (Song et al. 2020; Vijayvargiya et al. 2020).
3.1. Antivirals
Remdesivir was developed for the Ebola virus and it disrupts the viral RNA transcription (Song et al. 2020). Remdesivir was proven efficient against SARS-CoV-2 during in vitro and animal model studies (Song et al. 2020). It is a well-tolerated agent, leading to few adverse reactions such as nausea, hypotension, liver enzyme elevation (Song et al. 2020). Although it can improve oxygenation and reduce the overall recovery time, the mortality rate is not significantly reduced with the remdesivir treatment, according to Song Y et al. (Song et al. 2020).
Lopinavir/ritonavir is a protease inhibitor developed for the treatment of human immunodeficiency virus (HIV) (Song et al. 2020). The issue of lopinavir is the impaired pharmacodynamics of the drug to achieve an efficient plasma concentration (Song et al. 2020). The role of ritonavir is to inhibit cytochrome P450 4 A to increase the plasma concentration of lopinavir (Song et al. 2020). It showed a cytopathic effect on SARS-CoV during in vitro studies (Song et al. 2020). When used during the SARS virus, it reduced the mortality rate (Song et al. 2020). A clinical trial on COVID-19 did not show any significant difference regarding mortality or clinical improvement (Song et al. 2020).
Ribavirin is effective against multiple RNA viruses due to the interference with the RNA polymerase and viral-specific protein synthesis (Song et al. 2020). Apart from promising results during in vitro studies, a clinical trial on COVID-19 on 127 patients where ribavirin was associated with lopinavir/ritonavir and interferon, showed a shorter time to negative RT-PCR test and a faster clinical improvement (Song et al. 2020). Considering the associated treatments, it is impossible to conclude that ribavirin was responsible for the beneficial effects.
Favipiravir also inhibits RNA polymerase and viral protein synthesis (Vijayvargiya et al. 2020). Although favipiravir could reach higher concentrations compared to remdesivir, the lack of clinical trials limits its use in the COVID-19 patients (Vijayvargiya et al. 2020).
Interferon enhances RNA lysis and transcription (Song et al. 2020). In the case of the SARS outbreak, clinical studies showed faster recovery and shorter intubation time, mainly when associated with corticosteroids (Song et al. 2020). Regarding the SARS-CoV-2 pandemic, interferon use is limited due to variable pharmacokinetics during the nebulization, high risk of infection with aerosols, and lack of clinical results.
Umifenovir is only limited to several markets around the world (Song et al. 2020). Although considered inefficient when used alone, in association with lopinavir/ritonavir it showed a lung injury improvement and a faster viral clearance (Song et al. 2020). More well-designed clinical trials are needed to confirm the impact of umifenovir on COVID-19.
3.2. Corticosteroids
These anti-inflammatory drugs are used in a wide range of diseases such as autoimmune diseases, cancers, or septic shock (Song et al. 2020). Corticosteroids have been used in most intensive care unit patients (Song et al. 2020). The current use of corticosteroids to limit the injury produced by the ‘cytokine storm’ is controversial due to the lack of well-designed clinical trials (29).
3.3. Immunoglobulins
The immunoglobulins enhance the host’s immune system and are administered intravenously (Song et al. 2020). Currently, there is a lack of clinical trials to support the positive effect of immunoglobulins on the coronaviruses, despite some promising results during animal model experiments (Song et al. 2020).
3.4. Antimalarials
Chloroquine and hydroxychloroquine are antimalarial drugs acting as antivirals by inhibiting the endosome mediated viral entry and the viral fusion to the cell membrane (Song et al. 2020; Vijayvargiya et al. 2020). It is also supposed to decrease ACE-2’s affinity for SARS-CoV-2 (Vijayvargiya et al. 2020). These drugs can be poorly tolerated due to their adverse reactions (Song et al. 2020). During in vitro studies, both chloroquine and hydroxychloroquine showed a good antiviral effect (Song et al. 2020). There are conflicting results between clinical trials on the antimalarials effects on COVID-19 (Song et al. 2020; Vijayvargiya et al. 2020).
3.5. Interleukin-6 (IL-6) inhibitors
Tocilizumab is approved in case of cytokine release syndrome, which also occurs in COVID-19 patients, leading to severe complications (Song et al. 2020). The current clinical trials showed a beneficial effect of tocilizumab on oxygen intake, lung injury, or lymphopenia (Song et al. 2020).
Sarilumab also inhibits the IL-6 action by binding to its receptor (Song et al. 2020). Currently, no clinical trial on sarilumab was finished to recommend for or against its use for the COVID-19 patients (Song et al. 2020).
Siltuximab reduced the CRP level and improved the clinical outcome in a small clinical trial on COVID-19 patients (Song et al. 2020). Larger clinical trials are needed in order to determine its efficiency (Song et al. 2020).
3.6. Anti GM-CSF
The granulocyte-macrophage colony-stimulating factor (GM-CSF) is a pro-inflammatory cytokine that plays an important part in lung injury, macrophage homeostasis, and immunological diseases (Vijayvargiya et al. 2020). There are no available clinical data regarding its use in COVID-19 patients (Vijayvargiya et al. 2020).
3.7. Convalescent plasma
The mechanism of action is a direct antibody neutralization of the SARS-CoV-2 (Vijayvargiya et al. 2020). One clinical study showed an improvement in the hospital stay and viral load (Vijayvargiya et al. 2020). Nevertheless, the limitations of the study included associated treatments and a low number of patients (Vijayvargiya et al. 2020).
4. Cannabinoids interaction with the pathophysiology of SARS-CoV-2
4.1. Cannabinoid system
Cannabinoids are generally known for their psychotropic effects on the central nervous system ( Apostu et al. 2019; Pacher et al. 2006; x32). This perception lowered the research interest in the field of cannabinoids, although it is nowadays generally accepted that some cannabinoids can have multiple beneficial effects in the human organism, without any psychotropic effects (Apostu et al. 2019). The endocannabinoid system is comprised of endogenous cannabinoids, cannabinoid receptors, and enzymes involved in the production or degradation of endocannabinoids (Lu and Mackie 2016). This system is involved in all of the human bodys’ internal interactions, including the immune component (Pacher et al. 2006; Lu and Mackie 2016; Apostu et al. 2019).
Type of cannabinoids. There are three main types of cannabinoids: phytocannabinoids, endocannabinoids, and drugs containing either natural or synthetic cannabinoids (Apostu et al. 2019; Petrescu et al. 2020). The phytocannabinoids are obtained by decarboxylation of the cannabinoid acids within the plants and are represented by cannabigerol (CBG), cannabidivarin (CBGV), cannabidiol (CBD), cannabichromene (CBC), cannabidivarin (CBDV), Δ9-tetrahydrocannabivarin (Δ9-THCV), and cannabichromevarin (CBCV) (Apostu et al. 2019). Endocannabinoids are ligands of the cannabinoid receptors and are synthesized by specific enzymes (Apostu et al. 2019). The main endocannabinoids of the human body are N-arachidonoylethanolamide (anandamide or AEA) and 2-arachidonoylglycerol (2-AG) (Apostu et al. 2019). Drugs containing natural cannabinoids are represented by Sativex™ or Epidiolex™, which contain cannabidiol and/or THC (Apostu et al. 2019). Synthetic cannabinoid drugs consist in cannabinoid agonists and are represented by Marinol™ (dronabinol), Syndros™ (dronabinol), or Cesamet™ (nabilone), (Apostu et al. 2019). By activating CB-1 or CB-2 receptors, cannabinoids modulate systems such as immune, cardiovascular, musculoskeletal, pulmonary, digestive, and central nervous system (Apostu et al. 2019).
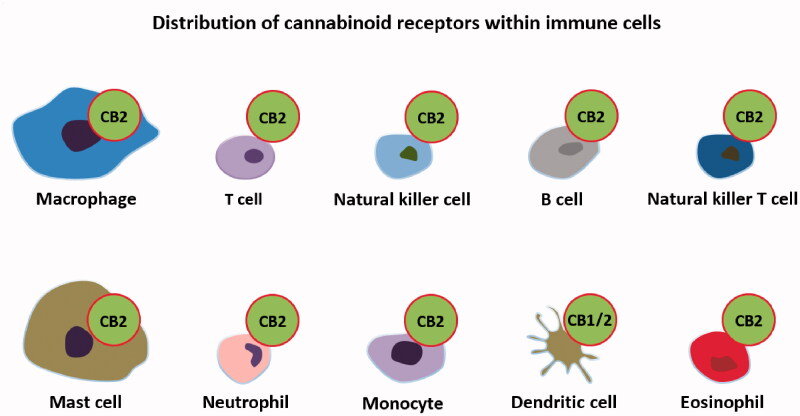
Cannabinoid receptors are generally represented by CB-1 and CB-2. CB-1 is mostly found in the central nervous system and is the main receptor responsible for the psychotropic effects, while CB-2 is responsible for the actions on the immune system (Apostu et al. 2019) (Figure 3). Other receptors such as G-protein-coupled receptor 55 (GPR55), G-protein-coupled receptor 18 (GPR18), peroxisome proliferator-activated receptors alpha and gamma (PPARα and PPARβ) or transient receptor potential vanilloid 1 (TRPV1) are also activated by cannabinoids but are not generally considered to be cannabinoid receptors (Apostu et al. 2019).
Figure 3.
Cannabinoid receptor distribution in the immune system’s cells.
Cannabinoid and immune system. The immune system is defined as a complex network of proteins and cells that protect the body against infections. The cells found within the immune system are macrophages, T cells, B cells, mast cells, basophils, neutrophils, eosinophils, dendritic cells, natural killer cells, natural killer T cells, CD4+ cells, and CD8+ cells (Apostu et al. 2019; Nichols and Kaplan 2020). The main proteins found in the immune systems are signaling proteins (cytokines), complement proteins, and antibodies (Nichols and Kaplan 2020). This system is regulated by other systems as well, such as the endocrine and nervous. The cannabinoid system also plays a part in the immune response (Nichols and Kaplan 2020). Cannabinoid receptors are present throughout the immune system as they are found in B lymphocytes, T4 lymphocytes, T8 lymphocytes, leukocytes, macrophages, microglia, mononuclear cells, mast cells, natural killer cells, spleen, thymus, tonsils, or lymph nodes (Cabral and Griffin-Thomas 2009).
As described by Nichols and Kaplan, the cannabinoids have multiple effects on the immune system as following: decreased cytokines levels, increased apoptosis of immune cells, and decreased migration of immune cells (Nichols and Kaplan 2020). These actions induce an immune suppression by cannabidiol (Nichols and Kaplan 2020). The following parameters proved a lower level when the endocannabinoid system was activated: IL-2, IL-4, IL-6, IFN-γ, TNF-α, NF-Ƙb, IL-17A, CCL2, IL-1β, IL-5, IL-13, IL-23A, IL-23R, serum antibodies, leukocytes, lymphocytes, neutrophils, natural killer cells, C cells and T cells (Nichols and Kaplan 2020). Moreover, the level of anti-inflammatory cytokine IL-10 was found to increase in most cases (Nichols and Kaplan 2020). This outlines the anti-inflammatory effect of the cannabinoid system, which could help prevent de ‘cytokine storm’ found in COVID-19 patients.
4.2. Cannabinoid system in SARS-CoV-2 pathophysiology
4.2.1. Immune cells
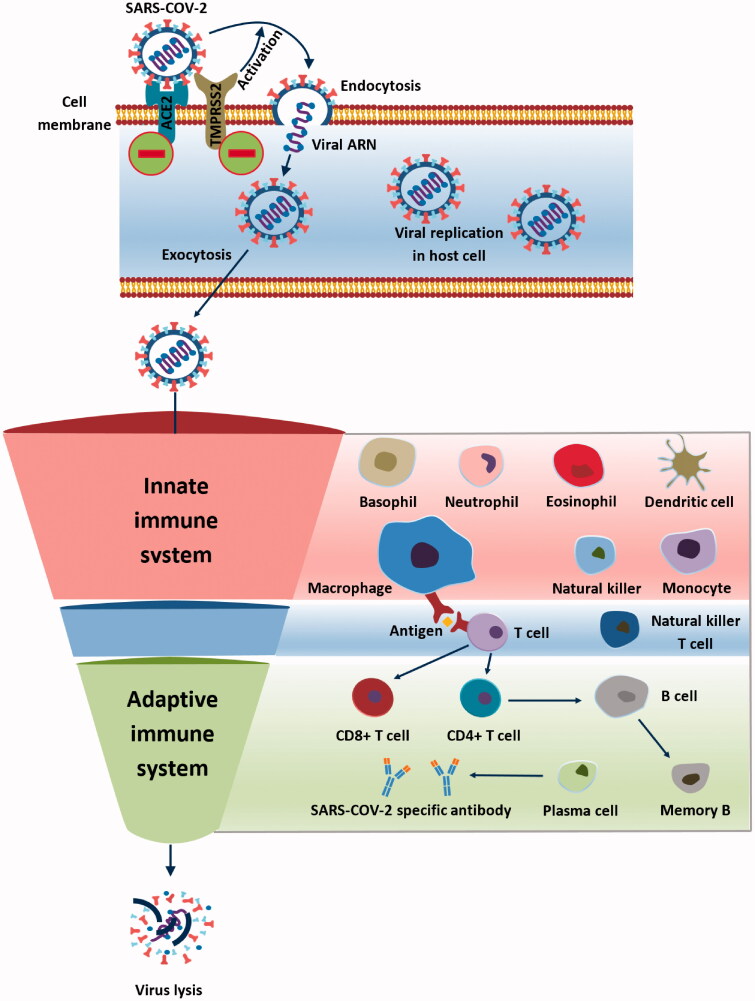
Macrophages migration into the lungs is decreased by CBD administration, as shown in human bronchoalveolar lavage supernatant (Pisanti et al. 2017). Another experimental study showed that Poly(I:C) administration to murine models decreased the level of macrophages (Khodadadi et al. 2020). Furthermore, CB-2 activation inhibits the formation of M1 phenotype macrophages, which release pro-inflammatory cytokines and in return, more M2 phenotype macrophages are produced, leading to anti-inflammatory IL-10 and TGF-β production (Almogi-Hazan and Or 2020; Rossi et al. 2020) (Figure 4).
Figure 4.
The immune system and SARS-CoV-2 infection (Cabral and Griffin-Thomas 2009).
Neutrophil migration to the lung tissue is downregulated by CBD administration, as found in the human bronchoalveolar lavage supernatant (Pisanti et al. 2017). An experimental study on the murine model showed that Poly(I:C) administration decreased the level of neutrophils in the lung tissue (Khodadadi et al. 2020). Moreover, the endocannabinoid system is supposed to decrease the mobilization of neutrophils to the site of inflammation (Almogi-Hazan and Or 2020; Nichols and Kaplan 2020) (Figure 4).
Lymphocytes’ relocation into the lungs is decreased by CBD administration, as a clinical study suggests, in the human bronchoalveolar lavage supernatant (Pisanti et al. 2017). CB-2 receptor activation reduces both lymphocytes B and T (Rossi et al. 2020). Furthermore, CB-2 receptor activation retains the immature B cells in the bone tissue and prevents future migration (Almogi-Hazan and Or 2020). Also, blood lymphocyte levels are lowered in the animal model when the cannabinoid system is activated (Nichols and Kaplan 2020) (Figure 4).
T cell activation is when CB-2 activation by endocannabinoids occurs during in vivo and in vitro studies (Eisenstein and Meissler 2015; Almogi-Hazan and Or 2020; Beji et al. 2020). A review by Eisenstein showed the overall immunosuppressive effect of cannabinoid receptors on T cells (Eisenstein and Meissler 2015). Moreover, Th1 cells are decreased by THC and CBD during in vivo and in vitro studies, while Th2 cells were promoted (Mamber et al. 2020; Mohammed et al. 2020) (Figure 4).
Both natural killer T cells and natural killer cells are suppressed by cannabinoid system activation in an animal model (Nichols and Kaplan 2020) (Figure 4).
Dendritic cells’ migration is reduced by CB-2, while their functions have been reduced under THC treatment (Lu et al. 2006; Almogi-Hazan and Or 2020) (Figure 4).
4.2.2. Receptors and transmembranal proteins
ACE-2 receptor. As stated before, the angiotensin-converting enzyme (ACE) receptor is the main receptor in the pathophysiology for the SARS-CoV-2 (Sainz-Cort and Heeroma 2020). Despite the lack of experimental studies, the hypotensive effect of cannabinoids could be explained by their agonist effect on the ACE receptor (Sainz-Cort and Heeroma 2020). Another paper pointed out that some Cannabis sativa lines could downregulate the expression of ACE-2 (Wang et al. 2020). Although no clear data exist, this hypothesis should be considered in future experimental studies due to its important impact on SARS-CoV-2 pathophysiology (Figure 4).
TMPRSS2 protein activation plays a key part in SARS-CoV-2 endocytosis and future replication. Wang et al. demonstrated that some Cannabis sativa lines downregulate the TMPRRSS2 protein expression (x2). Nevertheless, more studies are needed to prove the cannabinoid system impact on TMPRSS2 transmembranal protein (Figure 4).
4.2.3. Cytokines
IFN-γ pro-inflammatory cytokine is decreased by THC in multiple animal model studies, including ones with acute respiratory distress syndrome model or Staphylococcal enterotoxin B exposure mice (x3, Byrareddy and Mohan 2020; Rossi et al. 2020; Mohammed et al. 2020; Mamber et al. 2020; Nagarkatti et al. 2009; Nichols and Kaplan 2020; Khodadadi et al. 2020). Moreover, CBD strongly inhibits IFN-γ in LPS-stimulated microglial cells (Pisanti et al. 2017) (Figure 5).
Figure 5.
The impact of the cannabinoid system on the immune system in SARS-CoV-2 infection.
TNF-α pro-inflammatory cytokine is decreased by THC in an animal model with acute respiratory distress syndrome, under Poly(I:C) administration or in Staphylococcal enterotoxin B exposure mice (Khodadadi et al. 2020; Mohammed et al. 2020). Other studies showed that CB-1 and CB-2 receptor activation decreases the TNF-α levels (Nagarkatti et al. 2009; Tahamtan et al. 2016; Byrareddy and Mohan 2020; Costiniuk and Jenabian 2020; Nichols and Kaplan 2020). CBD has been shown to decrease the level of TNF-α in bronchoalveolar lavage supernatant (Pisanti et al. 2017) (Figure 5).
CCL5 pro-inflammatory cytokine is decreased by THC in an animal model with acute respiratory distress syndrome and Staphylococcal enterotoxin B exposure mice (Mohammed et al. 2020) (Figure 5).
MCP-1 pro-inflammatory cytokine is decreased by THC in an animal model with acute respiratory distress syndrome (Byrareddy and Mohan 2020; Mohammed et al. 2020; Nichols and Kaplan 2020). CBD has also been shown to decrease the level of MCP-1 in human bronchoalveolar lavage supernatant (Pisanti et al. 2017) (Figure 5).
IL-1 pro-inflammatory cytokine production is decreased by activation of the CB2 receptor (Costiniuk and Jenabian 2020). On the other hand, other studies suggest that THC increases IL-1 levels (Nagarkatti et al. 2009) (Figure 5).
IL-1β pro-inflammatory cytokine production in monocytes is suppressed by CB-2 activation in an animal model (Tahamtan et al. 2016; Nichols and Kaplan 2020). Moreover, in a murine model with the HIV-1 virus, IL-1β production is impaired by CB1/2 agonist (Beji et al. 2020). Also, CBD strongly inhibits IL-1β in LPS-stimulated microglial cells and Staphylococcal enterotoxin B exposure mice (Pisanti et al. 2017; Mohammed et al. 2020) (Figure 5).
IL-2 pro-inflammatory cytokine is decreased by THC administration in Staphylococcal enterotoxin B exposure mice (Almogi-Hazan and Or 2020; Mohammed et al. 2020) (Figure 5).
IL-4 anti-inflammatory cytokine is increased when the CB-2 receptor is activated in a murine model (Tahamtan et al. 2016; Mamber et al. 2020; Nichols and Kaplan 2020) (Figure 5).
IL-6 pro-inflammatory cytokine level, an essential protein in the ‘cytokine storm’ following SARS-COV-2 infection, was decreased by CBD in bronchoalveolar lavage supernatant (Pisanti et al. 2017). Moreover, CBD strongly inhibits IL-6 in LPS-stimulated microglial cells, in Staphylococcal enterotoxin B exposure mice or following Poly(I:C) administration on the murine model (Pisanti et al. 2017; Khodadadi et al. 2020; Mohammed et al. 2020). Multiple papers also show the cannabinoids (e.g. AEA) potential to decrease the IL-6 levels (Nagarkatti et al. 2009; Byrareddy and Mohan 2020; Costiniuk and Jenabian 2020; Mamber et al. 2020; Rossi et al. 2020) (Figure 5).
IL-8 pro-inflammatory cytokine production is suppressed by CBD in vitro (Rossi et al. 2020). On the contrary, some studies showed that cannabinoid signaling increases the level of IL-8 (Mormina et al. 2006; Nagarkatti et al. 2009). Therefore, the cannabinoid effect on this cytokine is still controversial (Figure 5).
IL-10 anti-inflammatory cytokine is increased by THC in an animal model with acute respiratory distress syndrome (Mohammed et al. 2020; Nichols and Kaplan 2020). Another study showed that CB-2 activation increases IL-10 production in the central nervous system (Tahamtan et al. 2016). On the other hand, other studies found a decrease of IL-10 levels after THC administration (Nagarkatti et al. 2009). The general opinion is that the cannabinoid system increases the level of the anti-inflammatory IL-10 (Figure 5).
IL-12 pro-inflammatory cytokine production is decreased in the macrophages found in the central nervous system (Tahamtan et al. 2016). Moreover, THC and CB2 activation inhibit the release of IL-12 according to multiple studies (Nagarkatti et al. 2009; Costiniuk and Jenabian 2020; Nichols and Kaplan 2020; Rossi et al. 2020) (Figure 5).
IL-17 pro-inflammatory cytokine level is reduced by THC and CBD in multiple animal studies, following a CB-2 activation (Kozela et al. 2013; Guillot et al. 2014; Pisanti et al. 2017; Nichols and Kaplan 2020) (Figure 5).
TGF-β anti-inflammatory cytokine is increased by THC administration in an animal model with acute respiratory distress syndrome (Mohammed et al. 2020). These results are generally accepted in the current literature (Figure 5).
GM-CSF pro-inflammatory cytokine level is lowered by CBD administration in an animal model, but few research data currently exist on this topic (Nagarkatti et al. 2009; Pisanti et al. 2017).
4.2.4. Signaling pathways
NF-ƘB signaling pathway is decreased by CBD administration in LPS-stimulated microglial cells (Pisanti et al. 2017). It is important to mention that THC did not produce reduced signaling using the NF-ƘB pathway. The pathway activity is also decreased in other animal model studies when the cannabinoid system is activated (Apostu et al. 2019; Nichols and Kaplan 2020) (Figure 5).
PPAR receptor pathway is downregulated by CBD in LPS-treated mice (Pisanti et al. 2017). Multiple cannabinoids activate the PPARγ such as anandamide, 2-arachidonoylglycerol, ajulemic acid, JU 210, and others (O'Sullivan and Kendall 2010; Mamber et al. 2020) (Figure 5).
Other mechanisms of cannabinoids in Covid physiopathology include the activation of the adenosine A2A receptor, leading to an improved lung function (Esposito et al. 2020). Furthermore, THC increases the resistance of epithelial cells in Staphylococcal enterotoxin B exposure mice (Mohammed et al. 2020) (Figure 5).
4.2.5. Currently-approved drugs and SARS-CoV-2 pathophysiology
The currently FDA approved cannabinoid drugs are Marinol (dronabinol), Syndros (dronabinol), Cesamet (nabilone) and Epidiolex (cannabidiol), but there are no clinical trials involving these drugs on SARS-CoV-2 patients. Nevertheless, we can deduce their effects by searching the current literature for their active ingredient.
As described by Esposito et al., cannabidiol can down-regulate ACE-2 receptor and TMPRSS2, therefore decreasing the viral entry. In addition, cannabidiol decreases PPARγ and adenosine A2A receptor activation, thus limiting the pulmonary fibrosis (Esposito et al. 2020; Anil et al. 2021). A recent study on an ARDS animal model, showed important antiinflammatory properties in the lung tissue by down-regulating IL-6 and IL-8 expression (Salles et al. 2020; Anil et al. 2021). Moreover, in a recent in vitro research, cannabidiol proved to be a more potent antiviral than other reference drugs such as remdesivir, chloroquinone and lopinavir (Raj et al. 2021). This study suggests that cannabidiol could be used in combination to treat SARS-CoV-2 patients (Raj et al. 2021). Another in vitro study by Anil et al. showed that a combination of cannabidiol, cannabigerol and tetrahtdrocannabivarin reduced IL-6, IL-8 and ACE-2 expresions. These in vitro results underline the cannabidiol potential effect in SARS-CoV-2 patients. We have found no data regarding dronabinol and nabilone. On the other side, Nelson et al. stated that cannabinoids’ clinical effects on the SARS-CoV-2 lack scienfitic foundation, therefore they should not currently be indicated in the case of SARS-CoV-2 infection.
4.2.6. Cannabinoid limitations for the SARS-CoV-2 patient
Considering the presence of cannabinoid receptors in many of the human cells, off-target effects are present. There are three types of receptors that cannabinoids can activate: CB-1, CB-2 and GPR55.
CB-1 receptor. Cannabinoids can have either an agonistic or antagonistic efect on the CB-1 receptor, depending on the type of cannabioid. In the case of CB-1 activation, the effects on the patient can involve:
the central nervous system (loss of appetite, increased or decreased excitotoxicity),
the liver, the adipose tissue and the skeletal muscle (increased fat synthesis and storage, increased insulin resistance, decreased glucose tolerance),
the cardiovascular system (increased cardiovascular dysfunction, increased pro-inflammatory response, decreased myocardial contractility) or
the kidney (increased pro-inflammatory response and increased kidney dysfunction) (Paloczi et al. 2018).
When CB-2 receptor is activated, the most important effects include an increased bone metabolism, decreased cytokine release, reduced fibrosis remodeling, reduced inflammation and an enhanced immune response overall (Apostu et al. 2019). In the case of GPR55 receptor effects such as increased angiogenesis, anti-inflammatory effects, decreased osteoclast formation or increased glucose tolerance are mainly represented (Simcocks et al. 2018).
Due to the well-known psychotropic effect of cannabis sativa, there are particular challenges in conducting a cannabinoid research in the SARS-COV-2 patients. In the United States, depending on the state, conducting a research on cannabinoids may require approvals from the U.S. Food and Drug Administration (FDA), U.S. Drug Enforcement Administration (DEA), National Institute on Drug Abuse (NIDA), institutional review boards (IRB) or different departments in the stat government (National Academies of Sciences, Engineering, and Medicine 2017). Additionally, a limited cannabinoid supply and the limited funding from the governmental organizations lead to lack of support and therefore a low interest in conducting research on cannabinoids (National Academies of Sciences, Engineering, and Medicine 2017).
Another limitation is represented by the unknown effect of the cannabinoids’ interaction with the currently used drugs in the treatment of SARS-COV-2 patients, such as antivirals, corticosteroids, immunoglobulins, antimalarials, interleukin inhibitors or convalescent plasma. Moreover, the lack of cannabinoids dosing information which could produce the effects described above is another limitation for future clinical trials.
4.2.7. Directions for future research
We consider that the key targets indicated for immediate research are regarding the viral entry and cytokine storm, due to their importance in the SARS-CoV-2 morbidity and the lack of efficient alternatives in these situations. The viral entry can be decreased by the cannabinoid’s effect on the cell membrane receptors and transmembrane proteins such as ACE-2 and TMPRSS2. Moreover, clinical research to validate the IL-6 cytokine down-regulation by the cannabinoids would be a valuable tool in preventing the cytokine storm.
5. Conclusion
Overall, the cannabinoid system can potentially reduce pulmonary inflammation, increase the immunomodulatory effect, decrease PMN infiltration, reduce fibrosis, and decrease viral replication, as well as decrease the ‘cytokine storm.’ Although the cannabinoid system has many mechanisms to provide certain benefits in the treatment of SARS-CoV-2 infected patients, research in this field is needed for a better understanding of the cannabinoid impact in this situation.
Funding Statement
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS – UEFISCDI, project number PN-III-P1-1.1-PD-2019-0124, within PNCDI III.
Disclosure statement
All the authors have approved the manuscript and no conflict of interest is declared.
References
- Almogi-Hazan O, Or R.. 2020. Cannabis, the endocannabinoid system and immunity-the journey from the bedside to the bench and back. IJMS. 21(12):4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anil SM, Shalev N, Vinayaka AC, Nadarajan S, Namdar D, Belausov E, Shoval I, Mani KA, Mechrez G, Koltai H.. 2021. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci Rep. 11(1):1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostu D, Lucaciu O, Mester A, Benea H, Oltean-Dan D, Onisor F, Baciut M, Bran S.. 2019. Cannabinoids and bone regeneration. Drug Metab Rev. 51(1):65–75. [DOI] [PubMed] [Google Scholar]
- Bähr I, Spielmann J, Quandt D, Kielstein H.. 2020. Obesity-associated alterations of natural killer cells and immunosurveillance of cancer. Front Immunol. 11:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beji C, Loucif H, Telittchenko R, Olagnier D, Dagenais-Lussier X, van Grevenynghe J.. 2020. Cannabinoid-induced immunomodulation during viral infections: a focus on mitochondria. Viruses. 12(8):875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S, Millet JK, Licitra BN, Whittaker GR.. 2012. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 4(6):1011–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch BJ, van der Zee R, De Haan CA, Rottier PJ.. 2003. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 77(16):8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrareddy SN, Mohan M.. 2020. SARS-CoV2 induced respiratory distress: can cannabinoids be added to anti-viral therapies to reduce lung inflammation? Brain Behav Immun. 87:120–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L.. 2009. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 11 :e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, et al. . 2020. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan China: a descriptive study. Lancet. 395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costiniuk CT, Jenabian MA.. 2020. Acute inflammation and pathogenesis of SARS-CoV-2 infection: cannabidiol as a potential anti-inflammatory treatment? Cytokine Growth Factor Rev. 53:63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein TK, Meissler JJ.. 2015. Effects of cannabinoids on T-cell function and resistance to infection. J Neuroimmune Pharmacol. 10(2):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Corpetti C, Sarnelli G.. 2020. The potential of cannabidiol in the COVID-19 pandemic. Br J Pharmacol. 177(21):4967–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui D, China Medical Treatment Expert Group for Covid-19, et al. . 2020. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot A, Hamdaoui N, Bizy A, Zoltani K, Souktani R, Zafrani ES, Mallat A, Lotersztajn S, Lafdil F.. 2014. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology. 59(1):296–306. [DOI] [PubMed] [Google Scholar]
- Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y.. 2020. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 7(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurova IE, Ali A, Waggoner SN.. 2020. Natural killer cell regulation of B cell responses in the context of viral infection. Viral Immunol. 33(4):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H.. 2004. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 203(2):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, et al. . 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. . 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet (London England). 395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Lee N, Chan PK, Beigel JH.. 2018. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. 150:202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G.. 2020. Virology epidemiology pathogenesis and control of COVID-19. Viruses. 12(4):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi H, Salles ÉL, Jarrahi A, Chibane F, Costigliola V, Yu JC, Vaibhav K, Hess DC, Dhandapani KM, Baban B.. 2020. Cannabidiol modulates cytokine storm in acute respiratory distress syndrome induced by simulated viral infection using synthetic RNA. Cannabis Cannabinoid Res. 5(3):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Juknat A, Kaushansky N, Rimmerman N, Ben-Nun A, Vogel Z.. 2013. Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol. 8(5):1265–1276. [DOI] [PubMed] [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung K, Lau E, Wong JY, et al. . 2020. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 382(13):1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, et al. . 2003. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Liu Y, Yuan J, Wen Y, Xu G, Zhao J, Chen K, Li J, Wang X, Wang F, et al. . 2020. The landscape of lung bronchoalveolar immune cells in COVID-19 revealed by single-cell RNA sequencing. MedRxiv. DOI: 10.1101/2020.02.23.20026690. [DOI] [Google Scholar]
- Lu HC, Mackie K.. 2016. An introduction to the endogenous cannabinoid system. Biol Psychiatry. 79(7):516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, Friedman H, Klein TW.. 2006. Cannabinoid treatment suppresses the T-helper cell-polarizing function of mouse dendritic cells stimulated with Legionella pneumophila infection. J Pharmacol Exp Ther. 319(1):269–276. [DOI] [PubMed] [Google Scholar]
- Lucaciu O, Tarczali D, Petrescu N.. 2020. Oral Healthcare during the COVID-19 pandemic. J Dent Sci. 15:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamber SW, Krakowka S, Osborn J, Saberski L, Rhodes RG, Dahlberg AE, Pond-Tor S, Fitzgerald K, Wright N, Beseme S, et al. . 2020. Can unconventional immunomodulatory agents help alleviate COVID-19 symptoms and severity? mSphere. 5(3):E00288–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A, Alghetaa HK, Zhou J, Chatterjee S, Nagarkatti P, Nagarkatti M.. 2020. Protective effects of Δ9 -tetrahydrocannabinol against enterotoxin-induced acute respiratory distress syndrome are mediated by modulation of microbiota. Br J Pharmacol. 177(22):5078–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormina ME, Thakur S, Molleman A, Whelan CJ, Baydoun AR.. 2006. Cannabinoid signalling in TNF-alpha induced IL-8 release. Eur J Pharmacol. 540(1–3):183–190. [DOI] [PubMed] [Google Scholar]
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M.. 2009. Cannabinoids as novel anti-inflammatory drugs. Future Med Chem. 1(7):1333–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine ; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. 2017. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington (DC): National Academies Press (US). Challenges and barriers in conducting cannabis research. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425757/. [Google Scholar]
- Nichols JM, Kaplan B.. 2020. Immune responses regulated by cannabidiol. Cannabis Cannabinoid Res. 5(1):12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüssing S, Sant S, Koutsakos M, Subbarao K, Nguyen T, Kedzierska K.. 2018. Innate and adaptive T cells in influenza disease. Front Med. 12(1):34–47. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Sharma V.. 2020. Cannabis for COVID-19: can cannabinoids quell the cytokine storm? Future Sci Oa. 6(8):FSO625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA.. 2010. Cannabinoid activation of peroxisome proliferator-activated receptors: potential for modulation of inflammatory disease. Immunobiology. 215(8):611–616. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G.. 2006. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 58(3):389–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloczi J, Varga ZV, Hasko G, Pacher P.. 2018. Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid Redox Signal. 29(1):75–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WB, Kwon NJ, Choi SJ, Kang CK, Choe PG, Kim JY, Yun J, Lee GW, Seong MW, Kim NJ, Seo JS, et al. . 2020. Virus isolation from the first patient with SARS-CoV-2 in Korea. J Korean Med Sci. 35(7):e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, HKU/UCH SARS Study Group, et al. 2003. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 361(9371):1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu NB, Jurj A, Sorițău O, Lucaciu OP, Dirzu N, Raduly L, Berindan-Neagoe I, Cenariu M, Boșca BA, Campian RS, et al. . 2020. Cannabidiol and vitamin D3 impact on osteogenic differentiation of human dental mesenchymal stem cells. Medicina (Kaunas Lithuania). 56(11):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M, Faggiana G, Proto MC, Fiore D, Laezza C, et al. . 2017. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther. 175:133–150. [DOI] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, et al. . 2020. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD.. 2020. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens (Basel Switzerland). 9(3):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V, Park JG, Cho KH, Choi P, Kim T, Ham J, Lee J.. 2021. Assessment of antiviral potencies of cannabinoids against SARS-CoV-2 using computational and in vitro approaches. Int J Biol Macromol. 168:474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Tortora C, Argenziano M, Di Paola A, Punzo F.. 2020. Cannabinoid receptor type 2: a possible target in SARS-CoV-2 (CoV-19) infection? IJMS. 21(11):3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainz-Cort A, Heeroma JH.. 2020. The interaction between the endocannabinoid system and the renin angiotensin system and its potential implication for COVID-19 infection. J Cannabis Res. 2(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles ÉL, Khodadadi H, Jarrahi A, Ahluwalia M, Paffaro VAJr, Costigliola V, Yu JC, Hess DC, Dhandapani KM, Baban B.. 2020. Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome. J Cell Mol Med. 24(21):12869–12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Tramsen L, Rais B, Ullrich E, Lehrnbecher T.. 2018. Natural killer cells as a therapeutic tool for infectious diseases - current status and future perspectives. Oncotarget. 9(29):20891–20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcocks AC, O’Keefe L, Hryciw DH, Mathai ML, Hutchinson DS, McAinch AJ.. 2018. GPR55. In: Choi S, editor. Encyclopedia of signaling molecules. Cham: Springer. [Google Scholar]
- Song Y, Zhang M, Yin L, Wang K, Zhou Y, Zhou M, Lu Y.. 2020. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int J Antimicrob Agents. 56(2):106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahamtan A, Tavakoli-Yaraki M, Rygiel TP, Mokhtari-Azad T, Salimi V.. 2016. Effects of cannabinoids and their receptors on viral infections. J Med Virol. 88(1):1–12. [DOI] [PubMed] [Google Scholar]
- Tay MZ, Poh CM, Rénia L, MacAry PA, Ng L.. 2020. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 20(6):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY.. 2020. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 15(5):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayvargiya P, Esquer Garrigos Z, Castillo Almeida NE, Gurram PR, Stevens RW, Razonable RR.. 2020. Treatment considerations for COVID-19: a critical review of the evidence (or lack thereof). Mayo Clin Proc. 95(7):1454–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Kovalchuk A, Li D, Rodriguez-Juarez R, Ilnytskyy Y, Kovalchuk I, Kovalchuk O.. 2020. In search of preventive strategies: novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging (Albany NY). 12(22):22425–22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. . 2020. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CK, Lam CWK, Wu AKL, Ip WK, Lee NLS, Chan IHS, Lit LCW, Hui DSC, Chan MHM, Chung SSC, et al. . 2004. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clinical and Experimental Immunology. 136(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q.. 2020. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. 2020. Cell pyroptosis A potential pathogenic mechanism of 2019-nCoV infection. SSRN. DOI: 10.2139/ssrn.3527420. [DOI] [Google Scholar]
- Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, Song Q, Jia Q, Wang J.. 2020. Clinical characteristics of 82 cases of death from COVID-19. PloS One. 15(7):e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Litvinova M, Wang W, Wang Y, Deng X, Chen X, Li M, Zheng W, Yi L, Chen X, et al. . 2020. Evolving epidemiology and transmission dynamics of coronavirus disease 2019 outseide Hubei province China: a descriptive and modelling study. Lancet. 20(7):793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H.. 2020. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 7(6):998–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, et al. . 2020. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 382(12):1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z.. 2020. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]