Abstract
Background
Several recent genome-wide association studies suggested insomnia and anemia may share some common genetic components. We thus examined whether adults with anemia had higher odds of having insomnia relative to those without anemia in a cross-sectional study and a meta-analysis.
Methods
Included in this cross-sectional study were 12,614 Chinese adults who participated in an ongoing cohort, the Kailuan Study. Anemia was defined as hemoglobin levels below 12.0 g/dL in women and 13.0 g/dL in men. Insomnia was assessed using the Chinese version of the Athens Insomnia Scale (AIS). A total AIS score ≥6 was considered insomnia. The association between anemia and insomnia was assessed using a logistic regression model, adjusting for potential confounders such as age, sex, chronic disease status, and plasma C-reactive protein concentrations. A meta-analysis was conducted using the fixed effects model to pool results from our study and three previously published cross-sectional studies on this topic in adult populations.
Results
Individuals with anemia had greater odds of having insomnia (adjusted odds ratio [OR]: 1.32; 95% confidence interval [CI]: 1.03–1.70) compared with individuals without anemia. A significant association persisted after we excluded individuals with chronic inflammation, as suggested by C-reactive protein levels >1 mg/L (adjusted OR: 1.68; 95% CI: 1.22–2.32). The meta-analysis results, including 22,134 participants, also identified a positive association between anemia and insomnia (pooled OR: 1.39; 95% CI: 1.22–1.57).
Conclusions
The presence of anemia was significantly associated with a higher likelihood of having insomnia in adults. Due to the nature of the cross-sectional study design, results should be interpreted with caution.
Keywords: Anemia, Insomnia, C-reactive protein, Inflammation, Cohort
Introduction
Insomnia affects approximately 10% to 30% of the population, with 50% of those patients classified as chronic.[1] It is defined as subjective reports of difficulty falling or staying asleep and non-restorative sleep.[1] Insomnia has been linked to psychosocial, psychiatric, and medical disorders and poorer quality of life. It can impair daytime functioning and decrease memory performance.[2–4] It is thus of clinical significance to identify risk factors for insomnia.
Interestingly, several recent genome-wide association studies found that MEIS1, a gene associated with restless legs syndrome (RLS) and iron-deficiency anemia (IDA), also exhibits pleiotropy for insomnia.[5,6] Consistently, anemia, particularly IDA, has been found to be associated with sleep alterations in infancy and children.[7–10] However, to the best of our knowledge, only three studies to date have examined the relationship between anemia and insomnia in the adult population, and all three reported that anemia was associated with insomnia.[11–13] These studies are limited by failure to adjust for some important confounders (eg, other sleep parameters[11–13] and inflammation status[11,12]). Further, the dose-dependent relationship between hemoglobin levels and insomnia was not explored.[11–13]
Therefore, we analyzed a secondary data analysis based on a large-scale, community-based study, including over 10,000 participants to evaluate whether the presence of anemia status was associated with a higher likelihood of having insomnia. We further conducted a meta-analysis to combine our results with previous population-based studies on this topic.
Methods
Ethical approval
The study was approved by the Ethics Committee of the Kailuan Medical Group. All participants gave their written informed consent.
Participants
The current cross-sectional study was based on a subset of the population from the Kailuan cohort. This ongoing cohort consists of 101,510 Chinese adults (81,110 men and 20,400 women) aged 18 to 98 years living in Tangshan City, China.[14] In 2006 to 2007 when the cohort was first examined, all participants completed a baseline questionnaire assessing lifestyle habits, health status, and clinical and laboratory assessments. These assessments were reevaluated every 2 years. In 2012, sleep parameters such as insomnia, daytime sleepiness, snoring, and sleep duration were obtained among 12,990 participants at one of the participating 11 hospitals (ie, the Kailuan General Hospital) from the original cohort, as detailed previously.[15,16] The response rate was 48.1% (12,990 out of 26,980). We excluded 376 participants with an incomplete assessment of sleep parameters, hemoglobin levels, and other major covariates (eg, C-reactive protein and serum creatine), leaving 12,614 participants (10,392 men and 2222 women) for the current analysis.
Assessment of anemia (exposure)
Anemia was assessed using hemoglobin levels measured in 2006. Overnight fasting (8–12 h) venous blood samples were collected in the morning of the 2006 survey and analyzed in the Central Laboratory of Kailuan General Hospital on the same day. Hemoglobin was assessed using the sodium lauryl sulfate-hemoglobin method (SULFOLYSER, SYSMEX Medical Electronics [Shanghai] Co. Ltd, Shanghai, China), with a detection range of 0 to 25.0 g/dL. Laboratory variable coefficients for hemoglobin were ≤1.5%. Anemia was defined as hemoglobin levels below 12.0 g/dL in women and below 13.0 g/dL in men.[17] To test the dose-dependent relationship, participants were further classified as normal hemoglobin levels (≥13.0 g/dL in men and ≥12.0 g/dL in women), mild anemia (12.0–12.9 g/dL in men and 11.0–11.9 g/dL in women), moderate anemia (9.0–11.9 g/dL in men and 8.0–10.9 g/dL in women), and severe anemia (≤9.0 g/dL in men and <8.0 g/dL in women).[18]
Assessment of insomnia (outcome)
During the 2012 visit, insomnia was assessed using the Chinese version of the Athens Insomnia Scale (AIS), which was delivered by a trained healthcare professional at the Kailuan General Hospital. The questionnaire includes a total of eight self-reported questions. The first five items of the questionnaire investigate sleep procedure (sleep induction, night awakening, awakening in the early morning, total sleep duration, and quality of sleep). The last three items of the questionnaire assess a decreased sense of well-being, overall functioning, and daytime sleepiness. Each question is scored on a scale of 0 to 3, with 0 being no problem of the corresponding sleep parameter and 3 being a serious problem (occurrence of greater than three times a week in the past month). A total AIS score ≥6 was considered insomnia.[19] The AIS questionnaire has been validated in China with 83% test-retest readability, 96% sensitivity, and 76% specificity.[20]
Assessment of covariates
Information collected in 2006 via a questionnaire included age, sex, any former head injury, education level (primary, secondary, or university), income level (<600, 600–1000 and >1000 RMB/month), occupation (white-collar, blue-collar, or coalminer), physical activity status in a dedicated period (never, <4 times/week, or ≥4 times/week), smoking status (never, past smoker, or current smoker), and alcohol consumption (never, past drinker, or current drinker). History of myocardial infarction, stroke, and cancer was confirmed by review of medical records.[16,21]
In 2006, trained field workers measured height and weight to obtain body mass index. Blood pressure (BP) was measured twice in the seated position using a mercury sphygmomanometer; the average of the two readings was used for analyses. Hypertension was defined as ≥140 mmHg systolic and ≥90 mmHg diastolic BP readings or use of antihypertensive medication in the prior 2 weeks. Pre-hypertension was defined as systolic BP 120 to 129 mmHg and diastolic BP 80 to 89 mmHg. Fasting blood glucose was measured with the hexokinase/glucose-6-phosphate dehydrogenase method. Low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured using an enzymatic colorimetric method (Mind Bioengineering Co. Ltd, Shanghai, China). Serum creatinine was assessed using the sarcosine oxidase assay method (Creatinine kit, BioSino Bio-technology and Science Inc, Beijing, China). Diabetes was defined as a fasting blood glucose level ≥7 mmol/L or any active insulin-related medication. A fasting blood glucose level of 5.6 to 6.9 mmol/L was defined as impaired fasting glucose. Estimated glomerular filtration rate (eGFR) was computed using serum creatinine, sex, and age, according to the chronic kidney disease Epidemiology Collaboration equation.[21] Plasma high-sensitivity C reactive protein (hs-CRP) concentrations were measured using a high-sensitivity, particle-enhanced immunonephelometric assay (Cias Latex CRP-H, Kanto Chemical Co. Inc., Tokyo, Japan), as detailed previously.[22]
Statistical analyses
We used the SAS statistical package (version 9.4; SAS Institute, NC, USA) for the cross-sectional analyses and STATA (version SE15; College Station, TX, USA) for the meta-analysis.
Cross-sectional analysis
Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between anemia and insomnia. We adjusted for covariates, which could be associated with both the exposure and the outcome, including age, sex, education level, income level, occupation, physical activity, smoking status, alcohol status, body mass index, history of myocardial infarction history, stroke history, cancer history, hypertension, diabetes (yes/no for each), eGFR and blood concentrations of triglycerides, low-density lipoprotein, and high-density lipoprotein. We used a binary variable of anemia as the primary exposure. In a secondary analysis, we calculated the ORs for insomnia across four hemoglobin categories to explore the potential dose-dependent response relationship.
Because we did not have information on whether the anemia status was defined as iron-deficient or non-iron deficient, we conducted a sensitivity analysis by excluding individuals with chronic inflammation (hs-CRP ≥1 mg/L) although the authors are aware that anemia patients without inflammation could still be due to other reasons rather than iron-deficiency. Because poor kidney function is associated with both reduced hemoglobin production[23] and increased risk of insomnia,[21] we conducted other sensitivity analyses by excluding those participants with severe chronic kidney disease or kidney failure (eGFR <30 mL/min per 1.73 m2).
We explored potential interactions between anemia status and age (<60 vs. ≥60 years) and sex in relation to odds of having insomnia by including multiplicative terms in the logistic regression models with adjustment for aforementioned covariates.
Meta-analysis
We identified relevant studies by searching Medline and PubMed for all published studies from 1966 through June 2018, using the following search algorithm (MeSH terms included): (anemia OR iron-deficiency anemia OR hemoglobin) AND (insomnia OR sleep disorder). We also manually searched the reference lists of relevant publications to identify additional studies. The studies that were included in the meta-analysis had to (1) be human studies including at least 40 participants and (2) be conducted among adult populations (age >18 years). Three previously published studies were identified [Supplementary Table S1]. The pooled OR was calculated using the fixed-effects model because significant heterogeneity across studies was not detected by Q statistic (P for heterogeneity = 0.30). We also calculated I2 value, which describes the percentage of variation across the studies due to heterogeneity. Finally, we used the Begg test to evaluate the possible presence of publication bias.
Results
Cross-sectional analysis
Approximately 4.3% of the population was defined to have anemia based on hemoglobin levels and 15.2% of the population reported having insomnia [Table 1]. Among participants with insomnia, there were 1017 (9.8%) men and 343 (15.4%) women. The basic characteristics of the participants are presented in Table 1.
Table 1.
Basic characteristics of participants of the study according to anemia status (n=12,614).
| Anemia | ||
| Variables | No | Yes |
| Patients (n) | 12,076 | 538 |
| Age (years) | 54.5 ± 11.1 | 54.2 ± 13.1 |
| Men (%) | 10107 (83.70) | 287 (53.40) |
| Education level (%) | ||
| Primary school | 1075 (8.90) | 44 (8.18) |
| High school | 9878 (81.80) | 421 (78.30) |
| College | 1123 (9.30) | 73 (13.60) |
| Income level (RMB/month) | ||
| <600 | 4021 (33.30) | 171 (31.70) |
| 600–1000 | 6763 (56.00) | 296 (55.10) |
| >1000 | 1292 (10.70) | 71 (13.30) |
| Occupation (%) | ||
| White collar | 857 (7.10) | 67 (12.40) |
| Blue collar | 6183 (51.20) | 363 (67.40) |
| Coalminer | 5036 (41.70) | 108 (20.20) |
| Physical activity (each time more than 20 min) (%) | ||
| Never | 1050 (8.70) | 46 (8.60) |
| <4 times/week | 9323 (77.20) | 417 (77.50) |
| ≥4 times/week | 1703 (14.10) | 75 (13.90) |
| Smoking status (%) | ||
| Never | 5700 (47.20) | 380 (70.60) |
| Past smoker | 846 (7.00) | 28 (5.20) |
| Current smoker | 5531 (45.80) | 130 (24.16) |
| Alcohol consumption (%) | ||
| Never | 5250 (43.47) | 327 (60.78) |
| Past drinker | 520 (4.31) | 22 (4.09) |
| Current drinker | 6306 (52.22) | 189 (35.13) |
| Myocardial infarction history (%) | 134 (1.11) | 10 (1.87) |
| Stroke history (%) | 350 (2.90) | 14 (2.60) |
| Cancer (%) | 40 (0.33) | 2 (0.37) |
| Hypertension (%) | 5857 (48.50) | 189 (35.10) |
| Diabetes (%) | 1461 (12.10) | 38 (7.10) |
| Body mass index (kg/m2) | 25.2 ± 3.47 | 24.1 ± 3.63 |
| CRP (mg/L)∗ | 0.78 (0.76–0.80) | 0.85 (0.74–0.97) |
| LDL-C (mmol/L) | 2.23 ± 0.71 | 2.01 ± 0.84 |
| HDL-C (mmol/L) | 1.57 ± 0.39 | 1.58 ± 0.39 |
| Triglycerides (mmol/L) | 1.60 ± 1.29 | 1.21 ± 1.03 |
| eGFR (mL/min per 1.73 m2) | 81.7 ± 21.2 | 80.5 ± 15.6 |
Data are presented as median (range), mean ± standard deviation or n (%).
Mean and 95% confidence interval were presented due to non-normal distribution. CRP: C-reactive protein; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; eGFR: Estimated glomerular filtration rate.
Individuals with prior anemia had a higher prevalence of insomnia, relative to those without prior anemia (15.2% vs. 10.6%). The presence of prior anemia was associated with 32% increased odds (adjusted OR: 1.32; 95% CI: 1.03–1.70) of having insomnia 6 years later, relative to those without anemia [Table 2], after adjustment for potential confounders. The association became stronger after we excluded those with chronic inflammation, as suggested by hs-CRP concentration ≥1 mg/L [Table 2]. Excluding individuals with eGFR <30 mL/min per 1.73 m2 generated similar results [Table 2]. We observed a marginally significant dose-dependent response relationship between anemia severity and odds of having insomnia (P-trend = 0.06). Severe anemia, but not mild and moderate anemia, was significantly associated with higher odds of insomnia (adjusted OR: 1.95; 95% CI: 1.06–3.62) [Figure 1]. Participants with anemia were more likely to have difficulties in sleep induction (adjusted OR: 1.34; 95% CI: 1.08–1.67) and to awake in the early morning (adjusted OR: 1.31; 95% CI: 1.04–1.64), but not for night awakening (adjusted OR: 1.14; 95% CI: 0.92–1.42).
Table 2.
Odds ratios and 95% confidence intervals of insomnia according to anemia status (yes/no).
| Anemia | |||
| Variables | No | Yes | P |
| Model 1∗ | Ref | 1.52 (1.19–1.94) | <0.001 |
| Model 2† | Ref | 1.32 (1.03–1.70) | 0.040 |
| Excluding participants with CRP >1 mg/L∗ | Ref | 1.68 (1.22–2.32) | 0.002 |
| Excluding participants with eGFR <30 mL/min per 1.73 m2 | Ref | 1.30 (1.01–1.67) | 0.045 |
Adjusted for age and sex.
Adjusted for age, sex, education level (primary, middle, college), income level (<600, 600–1000, >1000 RMB/month), occupation (white collar, blue collar, coalminer), physical activity (never, <4, 4+ times/week), smoking status (never, past smoker, current smoker), alcohol status (never, past drinker, current drinker), myocardial infarction history (no, yes), stroke history (no, yes), cancer history (no, yes), hypertension (no, prehypertension, hypertension), diabetes (no, prediabetes, diabetes), body mass index (<24, 24–27.9, ≥28 kg/m2), triglyceride (<0.82, 0.82–1.21, 1.22–1.86, ≥1.87 mmol/L), low density lipoprotein (<1.76, 1.76–2.11, 2.12–2.64, ≥2.65 mmol/L), and high-density lipoprotein (<1.31, 1.31–1.53, 1.54–1.78, ≥1.79 mmol/L). CRP: C-reactive protein; eGFR: Estimated glomerular filtration rate.
Figure 1.
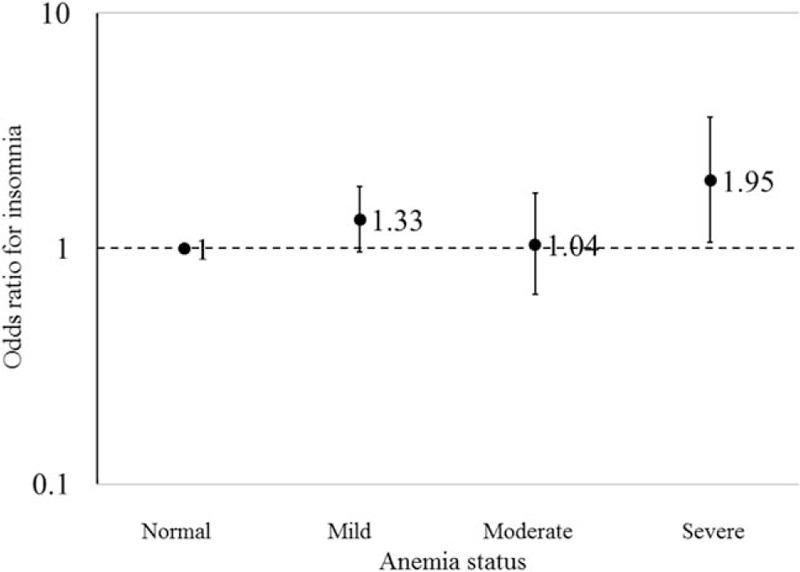
The ORs and 95% CIs of insomnia according to four anemia groups. (1) Normal was defined as the level of hemoglobin ≥13.0 (if man, g/dL)/12.0 (if woman, g/dL); mild anemia was defined as the level of hemoglobin ≥12.0 and <13.0 (if man, g/dL)/≥11.0 and <12.0 (if woman, g/dL); moderate anemia was defined as the level of hemoglobin ≥9 and <12 (if man, g/dL)/≥8 and <11 (if woman, g/dL); and severe anemia was defined as the level of hemoglobin <9 (if man, g/dL)/8 (if woman, g/dL). (2) Adjusted for age, sex, education level (primary, middle, and college), income level (<600, 600–1000, and >1000 RMB/month), occupation (white-collar, blue-collar, and coalminer), physical activity (never, <4, and 4+ times/week), smoking status (never, past smoker, and current smoker), alcohol status (never, past drinker, and current drinker), myocardial infarction history (no and yes), stroke history (no and yes), cancer history (no and yes), hypertension (no, prehypertension, and hypertension), diabetes (no, prediabetes, and diabetes), C-reactive protein (mg/L), body mass index (<24 kg/m2, 24–27.9 kg/m2, ≥28 kg/m2), triglyceride (<0.82 mmol/L, 0.82–1.21 mmol/L, 1.22–1.86 mmol/L, ≥1.87 mmol/L), low density lipoprotein (<1.76 mmol/L, 1.76–2.11 mmol/L, 2.12–2.64 mmol/L, ≥2.65 mmol/L), and high-density lipoprotein (<1.31 mmol/L, 1.31–1.53 mmol/L, 1.54–1.78 mmol/L, ≥1.79 mmol/L). CI: Confidence interval; OR: Odds ratio.
The association between anemia and insomnia was stronger in men (adjusted OR: 1.70; 95% CI: 1.22–2.36), but not significant in women (adjusted OR: 1.03; 95% CI: 0.69–1.52) (P-interaction = 0.04). In contrast, we did not find a significant interaction between anemia and age in relation to insomnia.
Meta-analysis
In the meta-analysis, we identified three previous studies with sample sizes ranging from 1053 to 6465 from the United States, South Korea, and England [Supplementary Figure S1]. The anemia ascertainment of two studies was based on hemoglobin concentrations, while one study used self-reported questionnaires. We pooled the current study with previous studies, including 9520 participants, and found an overall significant association between anemia and insomnia risk (OR: 1.39; 95% CI: 1.22–1.57; I2: 18.3%) [Figure 2]. There was no strong evidence of publication bias when evaluated by the Begg test (P = 0.09). However, the funnel plot showed some asymmetry, reflecting the relative absence of studies with both small numbers and small effects. This may lead to overestimating the association between anemia and insomnia [Figure 3].
Figure 2.
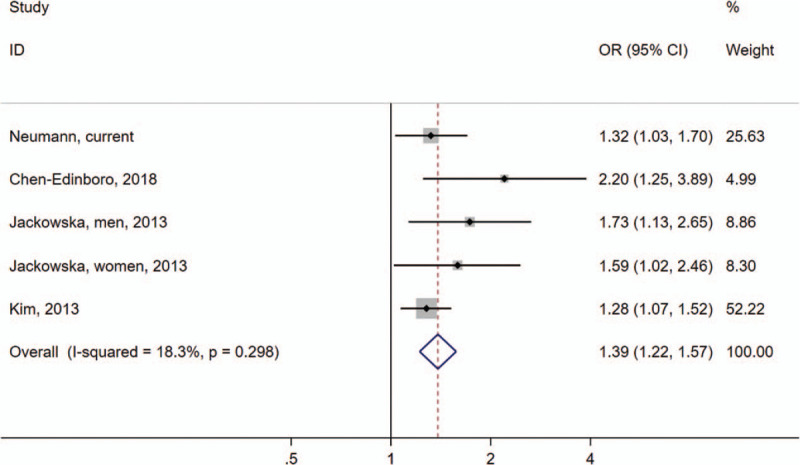
Meta-analysis of associations between anemia and insomnia. Squares indicate study-specific ORs; error bars indicate 95% CIs; diamond indicates OR and 95% CIs from pooled analysis; P = 0.30 for heterogeneity test. I2 = 18.3%. CI: Confidence interval; OR: Odds ratio.
Figure 3.
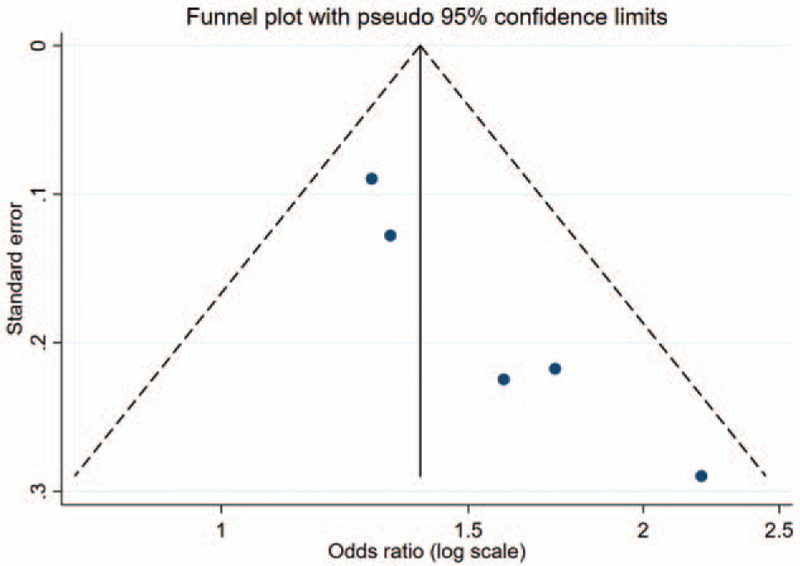
Funnel plot of all studies included in the meta-analysis.
Discussion
In this large-scale community-based study of over 12,000 participants, we found that anemia was associated with a higher risk of having insomnia. This finding was further supported by a subsequent meta-analysis on this topic. A sex-difference was also observed; the relationship was significant in men but not in women. This study has several strengths. To our knowledge, this is the largest study examining the relationship between anemia and insomnia that has been carried out. Further, the meta-analysis contributes a larger pooled sample size, offering greater support for the generalizability of our results. We adjusted for many potentials, including inflammation status, daytime sleepiness, sleep duration, and snoring status, suggesting that our results were independent of these factors. The study also differentiated between sex, age, and anemia status and included a validated insomnia questionnaire. This finding is of clinical and public health importance due to the high prevalence of anemia and insomnia. Due to the significance of our association, prospective studies are warranted to further clarify the temporal relationship between anemia and insomnia. If confirmed, a properly conducted clinical trial could be important to understand the causality by examining whether improving the treatment of anemia could improve symptoms of insomnia.
The current study is consistent with previous epidemiological studies on this topic. In a cross-sectional study using the Baltimore Aging Longitudinal Study, Chen-Edinboro et al[12] reported that patients with non-IDA had a higher likelihood and greater severity of insomnia compared with non-anemic participants. However, in this study, IDA could not be examined due to the low prevalence (0.9%). Kim et al[11] found a significant association between anemia and insomnia in a South Korean population of 2002 participants. In a cross-sectional study from England, Jackowska et al[13] discovered that anemia was more prevalent among men with disturbed sleep.
The exact underlying mechanisms for the observed anemia-insomnia relationship remain unclear. One potential explanation is that anemia could increase insomnia risk through a shared gene. The MEIS1 gene was reported to play a role in iron metabolism with a prominent effect on ferritin expression.[24] Interestingly, two rent genome-wide association studies showed that the MEIS1 gene, the one associated with iron status, was also associated with insomnia.[5,25] Consistent with this notion, we found that the association between anemia and insomnia became stronger after we restricted our analysis to those participants without chronic inflammation. Of note, we did not directly assess iron status in the current population. Nevertheless, this result might suggest that inflammation, another major cause of anemia, may not be the major contributor to the observed anemia-insomnia relationship. In sleep studies conducted in children, it has been postulated that IDA affects temporal organization during sleep. Peirano et al[10] found that children with IDA present a longer REM sleep episode in the first third of the night and shorter in the last third of the night compared with non-IDA children. The dopamine system plays an important role in REM sleep quantity, quality, and timing. In the basal ganglia, high concentrations of iron are highly connected to REM-regulatory structures, and supplementation of iron may not correct early iron deficiency in this area.[10] Further, disruption in iron availability or storage can affect its normal role in myelination, leading to a decreased efficiency in neural signals in sleep organization circuitry.[10]
Other pathways could also be involved in the observed association between anemia and insomnia. For example, fatigue as a symptom of anemia may induce sleep problems.[13] Another potential mechanism of this phenomenon may be explained by altered blood flow in the brain, which was associated with both anemia and insomnia.[12] A neuroimaging study found that cerebral blood flow in the frontotemporal region was linked to hemoglobin levels and anemia status,[26] and another neuroimaging study found that cortical thinning in the frontotemporal region was linked to shorter sleep duration.[27] The overlap of the same brain region linked to both hemoglobin levels and shorter sleep duration may suggest a common pathway in the development of anemia and insomnia. In addition, living with a chronic disease such as anemia may result in large stressors, leading to shorter sleep duration and more disturbed sleep. Further exploration into these proposals is required for verification.
We observed a significant sex-difference in the anemia–insomnia relationship, and the association was more pronounced in men, relative to women. This potential sex-difference was only examined in one previous study.[13] Consistently, a stronger association between anemia and disturbed sleep was observed in men (adjusted OR: 1.73; 95% CI: 1.13–2.65), relative to women (adjusted OR: 1.59; 95% CI: 1.02–2.46).[13] However, a significant level of interaction was not tested in this study. Interestingly, women are generally more likely to have anemia and insomnia than men due to possible biological, psychological, and social factors.[28–31] However, in older age, the decrease in the production of estrogen may be a protective factor in women for the development of anemia.[32] This may be a potential explanation for why our study found that the anemia-insomnia relationship in men was more pronounced. Further studies are needed to understand the biological mechanisms underlying this potential sex-difference.
Drawing an association between anemia and insomnia has important clinical implications. If anemia is a risk factor for insomnia, the clinical intervention can be focused on the treatment of anemia to relieve both conditions. Healthcare professionals should consider evaluating insomnia symptoms when caring for patients with anemia. In this context, further prospective studies are warranted to elucidate the temporal relationship between anemia and insomnia.
This study has several limitations. First, although the insomnia questionnaire was validated, it was self-reported. This subjects the data to recall bias and misclassification. Second, we did not measure any iron biomarkers and thus cannot directly classify if the anemia was iron-deficient or non-iron deficient. Third, residual confounding is of concern. We did not assess RLS status. It has been discovered that RLS patients have a high prevalence of iron deficiency and a decreased expression of the MEIS1 gene.[33] However, the prevalence of RLS in the Asian population is low (∼1%–2%).[34] For example, in a community-based study including 2609 Chinese older adults, only 0.69% of participants were found to have RLS after a face-to-face interview.[35] Another confounding factor could be the lack of information on the treatment of anemia. However, the education level of the studied population is mainly below the college level (90.5%), thus the effect of treatment misinformation is probably small to modest. In the current study, it is unknown whether those insomniacs had other causes. However, we adjusted for major chronic diseases such as myocardial infarction, stroke, cancer, hypertension, and diabetes in the analyses. We also conducted sensitivity analyses by excluding individuals with chronic inflammation (hs-CRP ≥1 mg/L) and those participants with severe chronic kidney disease or kidney failure (eGFR <30 mL/min per 1.73 m2) to reduce the impact of chronic diseases. We did not have information on psychological status, such as anxiety and depression, which could be a potential confounder. Further, the Kailuan study is not a representative population. For example, the prevalence of anemia was lower, relative to the estimated 9.7% in a national survey.[36] This would limit the generalizability of our findings. However, a subsequent meta-analysis reported similar results. Finally, although we used anemia status in 2006 to predict the likelihood of having insomnia in 2012, our study should be considered as cross-sectional due to lack of insomnia assessment at the baseline (2006), which prevents us from establishing a temporal relationship between anemia and insomnia. However, the use of exposures from 2006 to predict insomnia status in 2012 introduced a prospective component, improving the cross-sectional study design to some extent.
In conclusion, this large-scale community-based study found that individuals with anemia are at greater risk for insomnia. A prospective analysis with objective sleep measures and direct assessment of iron status is recommended for further verification. If our findings are confirmed, a properly designed clinical trial should be undertaken to see if suitable treatment of anemia can improve insomnia.
Funding
This research was supported by the start-up grant from the College of Health and Human Development and the Department of Nutritional Sciences, Penn State University, and the Institute for CyberScience Seed Grant Program, Penn State University.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Neumann SN, Li JJ, Yuan XD, Chen SH, Ma CR, Murray-Kolb LE, Shen Y, Wu SL, Gao X. Anemia and insomnia: a cross-sectional study and meta-analysis. Chin Med J 2021;134:675–681. doi: 10.1097/CM9.0000000000001306
Supplemental digital content is available for this article.
References
- 1.Buysse DJ. Insomnia. JAMA 2013; 309:706–716. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton EL. Insomnia. Med Clin North Am 2014; 98:565–581. doi: 10.1016/j.mcna.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Sutton EL. Psychiatric disorders and sleep issues. Med Clin North Am 2014; 98:1123–1143. doi: 10.1016/j.mcna.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 4.Levenson JC, Kay DB, Buysse DJ. The pathophysiology of insomnia. Chest 2015; 147:1179–1192. doi: 10.1378/chest.14-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerschlag AR, Stringer S, de Leeuw CA, Sniekers S, Taskesen E, Watanade K, Blanken TF, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet 2017; 49:1584–1592. doi: 10.1038/ng.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet 2019; 51:394–403. doi: 10.1038/s41588-018-0333-3. [DOI] [PubMed] [Google Scholar]
- 7.Kordas K, Siegel EH, Olney DK, Katz J, Tielsch JM, Chwaya HM, et al. Maternal reports of sleep in 6-18 month-old infants from Nepal and Zanzibar: association with iron deficiency anemia and stunting. Early Hum Dev 2008; 84:389–398. doi: 10.1016/j.earlhumdev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Peirano PD, Algarin CR, Garrido MI, Lozoff B. Iron deficiency anemia in infancy is associated with altered temporal organization of sleep states in childhood. Pediatr Res 2007; 62:715–719. doi: 10.1203/PDR.0b013e3181586aef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peirano P, Algarin C, Garrido M, Algarin D, Lozoff B. Iron-deficiency anemia is associated with altered characteristics of sleep spindles in NREM sleep in infancy. Neurochem Res 2007; 32:1665–1672. doi: 10.1007/s11064-007-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peirano PD, Algarín CR, Chamorro RA, Reyes SC, Duran SA, Garrido MI, et al. Sleep alterations and iron deficiency anemia in infancy. Sleep Med 2010; 11:637–642. doi: 10.1016/j.sleep.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim WH, Kim BS, Kim SK, Chang S, Lee D, Cho M, et al. Prevalence of insomnia and associated factors in a community sample of elderly individuals in South Korea. Int Psychogeriatr 2013; 25:1729–1737. doi: 10.1017/s1041610213000677. [DOI] [PubMed] [Google Scholar]
- 12.Chen-Edinboro LP, Murray-Kolb LE, Simonsick EM, Ferrucci L, Allen R, Payne ME, et al. Association between non-iron-deficient anemia and insomnia symptoms in community-dwelling older adults: the Baltimore Longitudinal Study of Aging. J Gerontol Ser A Biol Sci Med Sci 2018; 73:380–385. doi: 10.1093/gerona/glw332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackowska M, Kumari M, Steptoe A. Sleep and biomarkers in the English Longitudinal Study of Ageing: Associations with C-reactive protein, fibrinogen, dehydroepiandrosterone sulfate and hemoglobin. Psychoneuroendocrinology 2013; 38:1484–1493. doi: 10.1016/j.psyneuen.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris-Etherton PM, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open 2019; 2:e194758.doi: 10.1001/jamanetworkopen.2019.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong JC, Li J, Pavlova M, Chen S, Wu A, Wu S, et al. Risk factors for probable REM sleep behavior disorder: a community-based study. Neurology 2016; 86:1306–1312. doi: 10.1212/wnl.0000000000002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma C, Pavlova M, Liu Y, Liu Y, Huangfu C, Wu S, et al. Probable REM sleep behavior disorder and risk of stroke: a prospective study. Neurology 2017; 88:1849–1855. doi: 10.1212/wnl.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization; 2011. [Google Scholar]
- 18.Gilbertson DT, Ebben JP, Foley RN, Weinhandl ED, Bradbury BD, Collins AJ. Hemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol 2008; 3:133–138. doi: 10.2215/CJN.01610407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res 2000; 48:555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 20.Sun JL, Chiou JF, Lin CC. Validation of the Taiwanese version of the Athens Insomnia Scale and assessment of insomnia in Taiwanese cancer patients. J Pain Symptom Manage 2011; 41:904–914. doi: 10.1016/j.jpainsymman.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, et al. Sleep and CKD in Chinese adults: a cross-sectional study. Clin J Am Soc Nephrol 2017; 12:885–892. doi: 10.2215/cjn.09270816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Huang Z, Jin W, Rimm EB, Lichtenstein AH, Kris-Etherton PM, et al. Peripheral inflammatory biomarkers for myocardial infarction risk: a prospective community-based study. Clin Chem 2017; 63:663–672. doi: 10.1373/clinchem.2016.260828. [DOI] [PubMed] [Google Scholar]
- 23.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Primary Care 2008; 35:329–344. doi: 10.1016/j.pop.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, et al. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol 2011; 70:170–175. doi: 10.1002/ana.22435. [DOI] [PubMed] [Google Scholar]
- 25.Lane JM, Liang J, Vlasac I, Anderson SG, Bechtold DA, Bowden J, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet 2017; 49:274–281. doi: 10.1038/ng.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Sojkova J, Beason-Held LL, An Y, Longo DL, Ferrucci L, et al. Patterns of regional cerebral blood flow associated with low hemoglobin in the Baltimore Longitudinal Study of Aging. J Gerontol Ser A Biol Sci Med Sci 2012; 67:963–969. doi: 10.1093/gerona/gls121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spira AP, Gonzalez CE, Venkatraman VK, Wu MN, Pacheco J, Simonsick EM, et al. Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep 2016; 39:1121–1128. doi: 10.5665/sleep.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry 1979; 136:1257–1262. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 29.Mellinger GD, Balter MB, Uhlenhuth EH. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry 1985; 42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 30.Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med 1992; 152:1634–1637. [PubMed] [Google Scholar]
- 31.Piccinelli M, Wilkinson G. Gender differences in depression: critical review. Br J Psychiatry 2000; 177:486–492. [DOI] [PubMed] [Google Scholar]
- 32.Yang Q, Jian J, Katz S, Abramson SB, Huang X. 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 2012; 153:3170–3178. doi: 10.1210/en.2011-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Q, Li L, Chen Q, Foldvary-Schaefer N, Ondo WG, Wang QK. Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med 2011; 12:800–804. doi: 10.1016/j.sleep.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev 2012; 16:283–295. doi: 10.1016/j.smrv.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma JF, Xin XY, Liang L, Liu L, Fang R, Zhang Y, et al. Restless legs syndrome in Chinese elderly people of an urban suburb in Shanghai: a community-based survey. Parkinsonism Relat Disord 2012; 18:294–298. doi: 10.1016/j.parkreldis.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Chen J, Li M, Li WD, Piao JH. Study on the anemia status of Chinese urban residents in 2010-2012. Chin J Prev Med 2016; 50:213–216. doi: 10.3760/cma.j.issn.0253-9624.2016.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


