Abstract
Herein partially summarizes one scientist-clinician’s wanderings through the jungles of primate aqueous humor outflow over the past ~45 years. Totally removing the iris has no effect on outflow facility or its response to pilocarpine, whereas disinserting the ciliary muscle (CM) from the scleral spur/trabecular meshwork (TM) completely abolishes pilocarpine’s effect. Epinephrine increases facility in CM disinserted eyes. Cytochalasins and latrunculins increase outflow facility, subthreshold doses of cytochalasins and epinephrine given together increase facility, and phalloidin, which has no effect on facility, partially blocks the effect of both cytochalasins and epinephrine. H-7, ML7, Y27632 and nitric oxide – donating compounds all increase facility, consistent with a mechanosensitive TM/SC. Adenosine A1 agonists increase and angiotensin II decrease facility. OCT and optical imaging techniques now permit visualization and digital recording of the distal outflow pathways in real time. Prostaglandin (PG) F2α analogues increase the synthesis and release of matrix metalloproteinases by the CM cells, causing remodeling and thinning of the interbundle extracellular matrix (ECM), thereby increasing uveoscleral outflow and reducing IOP. Combination molecules (one molecule, two or more effects) and fixed combination products (two molecules in one bottle) simplify drug regimens for patients. Gene and stem cell therapies to enhance aqueous outflow have been successful in laboratory models and may fill an unmet need in terms of patient compliance, taking the patient out of the delivery system. Functional transfer of genes inhibiting the rho cascade or decoupling actin from myosin increase facility, while genes preferentially expressed in the glaucomatous TM decrease facility. In live NHP, reporter genes are expressed for 2+ years in the TM after a single intracameral injection, with no adverse reaction. However, except for one recent report, injection of facility-effective genes in MOCAS have no effect in live NHP. While intracameral injection of an FIV.BOVPGFS-myc.GFP PGF synthase vector construct reproducibly induces an ~2mmHg reduction in IOP, the effect is much less than that of topical PGF2⍺ analogue eyedrops, and dissipates after 5 months. The turnoff mechanism has yet to be defeated, although proteasome inhibition enhances reporter gene expression in monkey organ cultured anterior segments (MOCAS). Intracanalicular injection might minimize off-target effects that activate turn-off mechanisms. An AD-P21 vector injected sub-tenon is effective in ‘right-timing’ wound healing after trabeculectomy in live laser-induced glaucomatous monkeys. In human (H)OCAS, depletion of TM cells by saponification eliminates the aqueous flow response to pressure elevation, which can be restored by either cultured TM cells or by IPSC-derived TM cells.
There were many other steps along the way, but much was accomplished, biologically and therapeutically over the past half century of research and development focused on one very small but complex ocular apparatus. I am deeply grateful for this award, named for a giant in our field that none of us can live up to.
Keywords: Glaucoma, Aqueous humor outflow, Intraocular pressure, Actin cytoskeleton, Gene therapy, Stem cell therapy, Nitric oxide, Mechanosensitivity
1. Introduction
I am honored and grateful to receive the 2018 Endré A. Balázs Prize, and to present the lecture named for him. Endré A. Balázs was a distinguished scientist and entrepreneur, whose outstanding contributions provided far-reaching progress in experimental eye research and in clinical ocular surgery (“Endre Balazs,” 2015). Dr. Balazs was one of a group of scientists who came together in 1968 to establish an international organization to support vision research, which eventually became ISER. Dr. Balázs was the founding editor of Experimental Eye Research, which eventually became the official journal of ISER. He also co-founded the International Society for Hyaluronan Sciences. Dr. Balazs was a world leader in ophthalmic biochemistry and was instrumental in the development of products that revolutionized eye surgery, notably high-molecular weight hyaluronan, commercially known as Healon®. Importantly, he founded several companies and a philanthropic foundation dedicated to exploring and promoting the therapeutic potential of hyaluronic acid. Dr. Balazs’s complete biography is easily found (“Endre-A-Balazs-doctor-who-found-acid-to-treat-arthritic-knees,” 2015) and is an inspiring story well worth reading.
2. Personal History
Mine is a 45-year story filled with amazing people and fascinating and often unexpected science, that redefined how we think about glaucoma, the world’s most common cause of irreversible vision loss. I always knew that I wanted to be both a physician and a scientist, but scientist-physician was a better descriptor for me than was physician-scientist. However, I approached my senior year of medical school with no idea of what my next step should be. Fortunately, the mandatory military service requirement in the US for physicians at that time provided two years of post-internship training and service at the NIH, where I learned a lot about biostatistics, epidemiology and clinical trials design, became engaged and married, and had time for my pin-the-tail-on-the-donkey tentative decision to do my residency training in ophthalmology to jell. By what I can only ascribe to a stroke of good fortune or the hand of a guardian angel, I landed at Washington University in St. Louis, under the tutelage of the great Bernard Becker and the terrific team he had assembled. Although the clinical demands of the residency did not allow much time for research, I was able to design and execute two small studies of the biochemical makeup of subretinal fluid as a predictor of surgical and visual outcome in patients with rhegmatogenous retinal detachments (Kaufman, 1976, 1975; Kaufman and Podos, 1973; Kaufman PL, 1974, 1973). Small though they were, these studies taught me the joy of asking the “how” and “why” of pathophysiology and therapeutics.
Glaucoma and physiology/pharmacology seemed like the ideal intellectual setting for me. Dr. Becker; his protégé Steven Podos; Carl Kupfer, then Director of the National Eye Institute; and Ernst Bárány, the great ocular pharmacologist/physiologist and the world’s leading authority on aqueous humor dynamics, planned a two-year fellowship for me in Prof. Bárány’s lab at the University of Uppsala in Sweden, where he was the Chair of the Department of Medical Pharmacology. The NEI Study Section enthusiastically voted to support this, but then the application was “administratively withdrawn” when the Nixon Administration suspended all NIH training funds. I managed to secure a Seeing Eye Fellowship of $12,000, and another $2,000 from ALZA, the manufacturer of the pilocarpine Ocusert (Lee et al., 1975) and the Alzet mini pumps (Greenshaw, 1986).
In 1973, this princely sum of $14,000 did not go very far in Sweden. The 1973 Middle East War and oil embargo combined to drive down the value of the US dollar, but with a 6-month old child and minimal resources even for a 1-year fellowship, my wife and I made the final decision to go just three weeks before our scheduled departure shortly after the end of my residency training. Dr. Kupfer provided the security of a 2-year staff fellowship at the NEI when I returned if I had not secured an academic position by that time.
Life overall was good for us in Sweden. Wife Margaret, daughter Alison and I lived in a large if somewhat stark partially university-subsidized apartment. Laundry facilities were an outdoor quarter-mile walk away – lots of fun in the winter - with a drying room rather than automatic dryers, where everything was hung on drying racks and you then waited for magic to happen. We had old bicycles and a hand-me-down baby carriage, a rattletrap ancient car in our second year, and no discretionary money. Indeed, we dipped into savings to make ends meet. Despite the Spartan lifestyle, the apartment complex and the department were both collegial and we made friends - American, Canadian, German, Finnish and Swedish - that we have to this day, 45 years later.
3. Aqueous Humor Dynamics
3.1. Physiology & Pharmacology
The work environment was off-the-charts phenomenal, after getting off to a slow start. It took about six months and a truly great laboratory instrument machine shop to work out the surgical techniques. We worked exclusively in non-human primates (and still do so), partnering with Prof. Anders Bill for certain boutique physiological experiments and Profs. Johannes Rohen and Elke Lütjen-Drecoll for light and electron microscopic studies.
3.1.a. Conventional (trabecular meshwork/Schlemm’s canal) outflow pathway
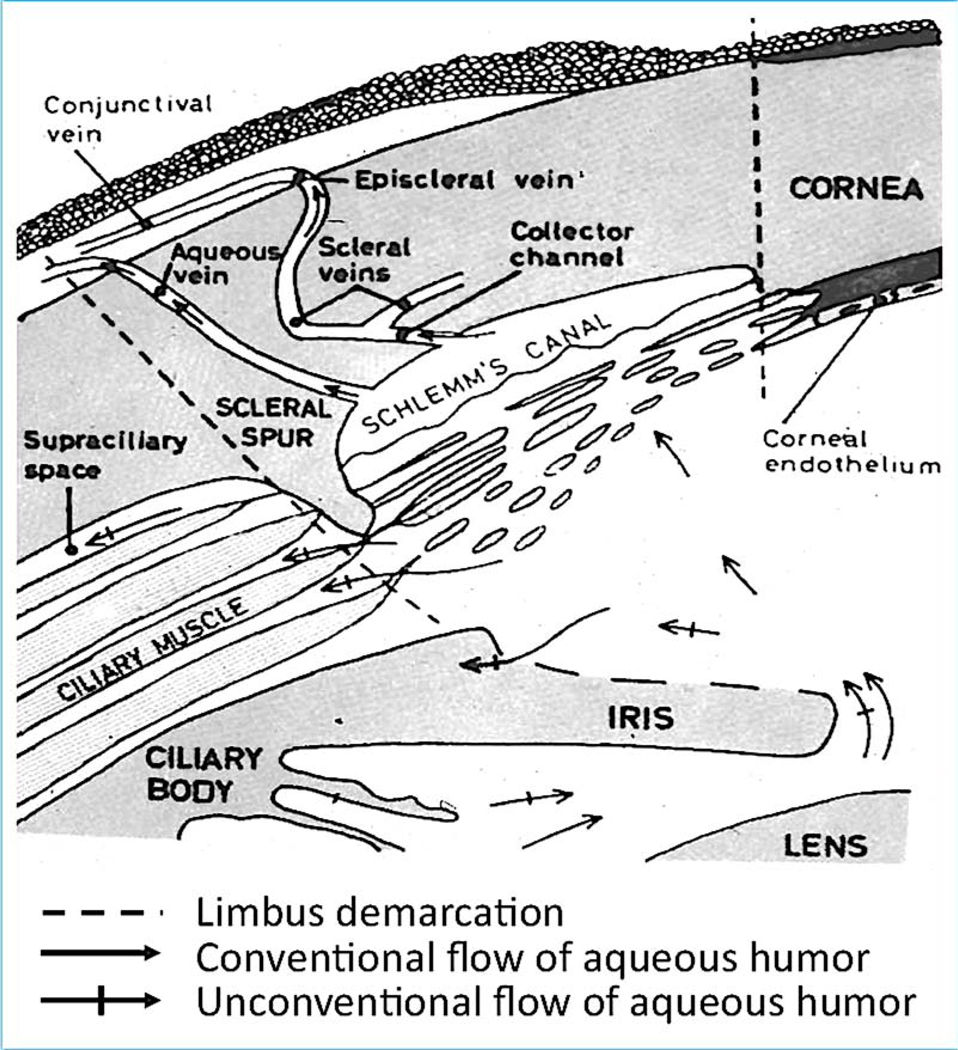
This pathway encompasses outflow from the anterior chamber through the various regions of the trabecular meshwork (uveal, trabecular, juxtacanalicular (cribriform), then across or through the inner wall endothelium of Schlemm’s canal into the canal lumen, then through an intrascleral vascular plexus (aqueous collector channels, intrascleral veins) that empty into the episcleral veins and from there eventually into the general venous circulation (Fig. 1).
Fig. 1.
Aqueous humor outflow pathways. Aqueous humor, produced by the ciliary body, exits the eye through the conventional trabecular meshwork/ Schlemm’s canal pathway (plain arrows), and the unconventional uveoscleral pathway (crossed arrows). Aqueous humor production and outflow are always equal, and the resistance in the trabecular meshwork/Schlemm’s canal determinestheintraocularpressure(IOP). Increasedresistanceresultsinelevation in IOP, a causal risk factor for glaucoma. From Svedbergh, 1976 with permission.
3.1.a.1. Muscarinic cholinomimetics
We completely removed the iris via a small transcorneal incision in the live monkey eye (Kaufman and Lütjen-Drecoll, 1975). After a short post-surgical recovery, the eyes were quiet and resting perfusion outflow facility and the facility response to intravenous pilocarpine were identical in the surgically aniridic and the contralateral iridic eyes, proving that the iris has no functional role in regulating trabecular meshwork outflow facility or its response to muscarinic cholinergic agonists (Kaufman, 1979). Thus, to say that miotic drugs are a treatment for open angle glaucoma is somewhat a misnomer, as miosis has nothing to do with their facility-increasing effect. There is, however an intimate anatomic relationship between the ciliary muscle and the trabecular meshwork, with some of the muscle’s anterior tendons mingling with the elastic network of the trabecular meshwork, which in turn sends fibers that insert into special surface adaptations of the Schlemm’s canal inner wall endothelial cells (Suppl Fig. 1) (Rohen et al., 1981, 1967).
When the ciliary muscle contracts as in response to muscarinic cholinergic agonist drugs, the meshwork is expanded, relaxed, and less stiff, and Schlemm’s canal dilates (Lütjen-Drecoll, 1973), all contributing to increased outflow facility and reduced intraocular pressure (IOP). To prove that ciliary muscle contraction was mediating the facility increase, we used a transcameral goniotomy-type surgical approach to cut the ciliary muscle from the scleral spur over the entire 360-degree circumference at one sitting (Kaufman and Bárány, 1976). This allowed the muscle’s elastic posterior attachments to retract posteriorly so that the posteriorly-pulled anterior tips reattached firmly to the inner scleral wall about 2 mm posterior to the scleral spur (Lütjen-Drecoll et al., 1977). In the non-inflamed eyes, now absent the iris and with the ciliary muscle disinserted from the scleral spur and trabecular meshwork/Schlemm’s canal (TM/SC) [Fig. 2], resting outflow facility was modestly reduced (Kaufman and Bárány, 1976), as expected if the meshwork became more compact and the canal narrowed absent the tonic pull of the ciliary muscle. Intravenous and intracameral pilocarpine had essentially no effect on outflow facility (Kaufman and Bárány, 1976) [Suppl Fig. 2]. This constellation of results proved that contraction of the ciliary muscle increased outflow facility, that pilocarpine’s facility-increasing effect was mediated solely by ciliary muscle contraction affecting TM and SC geometry with no primary drug effect on the TM/SC, and that the iris was completely uninvolved (Kaufman, 1979; Kaufman and Bárány, 1976). It now became feasible to assess the effects of other drugs on outflow facility, free from potential confounding effects on ciliary muscle contraction/relaxation. There was still the hope that the miosis, accommodative and outflow facility effects of cholinomimetics could be decoupled, as the first two were hard-stop side effects in elderly folks with even early cataracts, and younger patients who still retained significant accommodative capacity, respectively. However, it turned out that all three functions were mediated by the same m3 muscarinic receptor subtype, and no way of pharmacologically separating them was found (Gabelt and Kaufman, 1992).
Fig. 2.
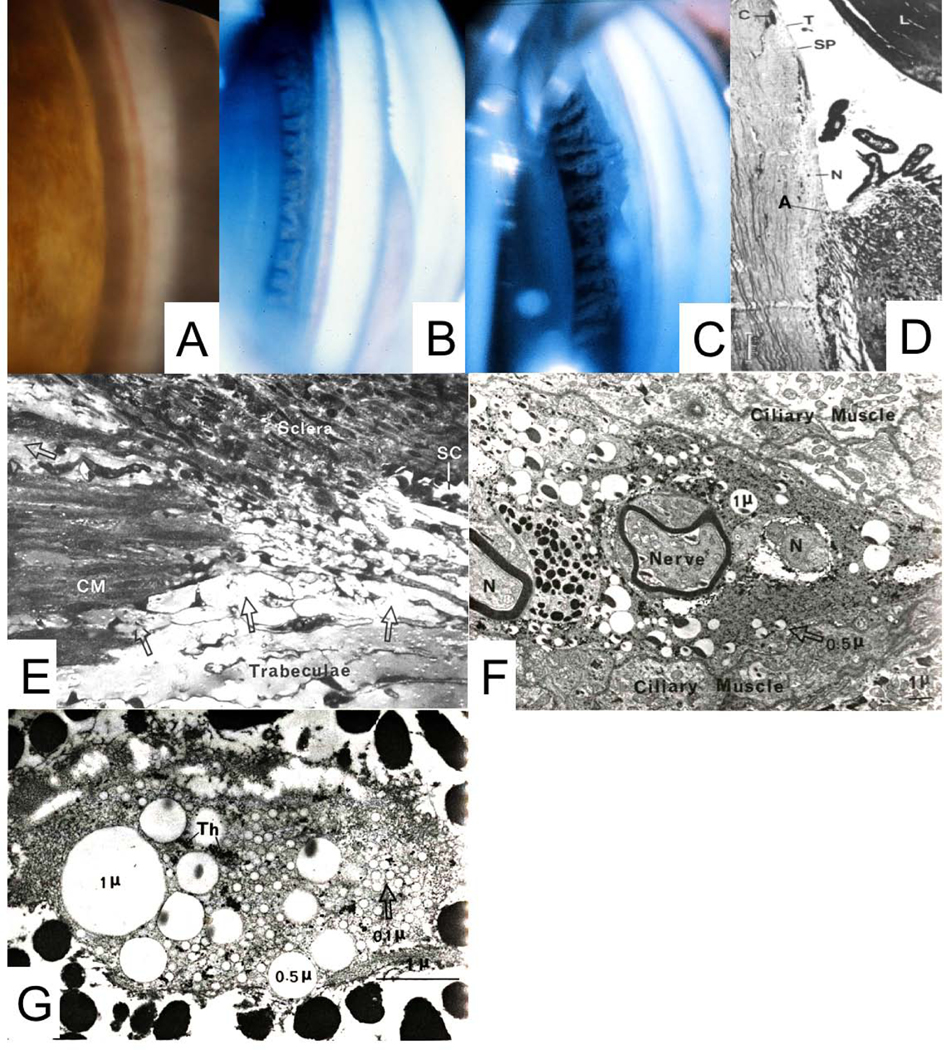
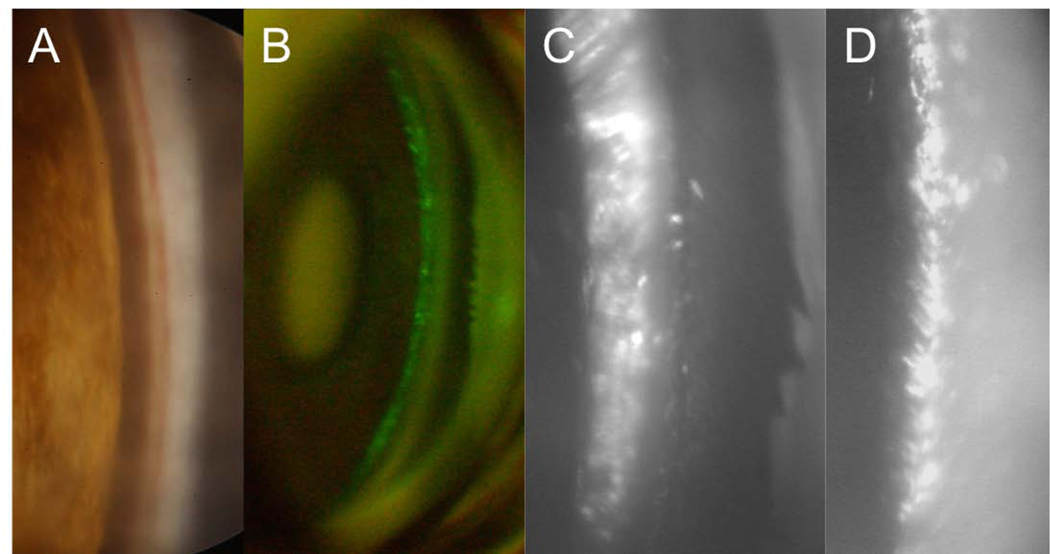
A: Normal cynomolgus monkey anterior chamber angle. B: Gonioscopic appearance of surgically aniridic eye. C: Surgical aniridia and ciliary muscle disinsertion and retrodisplacement. Deep, fairly uniform retrodisplacement of ciliary body. Thinbandofmuscleremainsattachedtoscleralspur. D: Typical histologic appearance ofanterior chamber angle following ciliary muscle disinsertion; C = canal of Schlemm, filled with blood in lower half of the image; A = adhesion of retrodisplaced muscle to sclera; L = lens; N = naked sclera; SP=scleralspur;T=trabecularmeshwork.E:Light micrograph of the posterior portion of the trabecular meshwork/anterior portion of the ciliary muscle after perfusion with three sizes of latex spheres and thorotrast in gelatin. Streams of homogeneous gelatin, containing tracer particles (arrows), enter the ciliary body from the posterior portion of the trabecular meshwork and pass through the connective tissue between bundles of the ciliary muscle. CM: Ciliary muscle; SC: Schlemm’s canal (x 460). F: Monkey ciliary muscle intermuscular space showing latex spheres that had been injected into the anterior chamber. N: Nerve fiber (x 5500). G: Monkey suprachoroid at the posterior pole, showing 3 sizes of latex spheres that were injected into the anterior chamber. Th: Thorotrast. (X22,000). From: B - Kaufman PL, Lütjen-Drecoll 1975; C - Kaufman PL, Bárány EH 1976; D - Lütjen-Drecoll E, et al 1977; E, F, G - Inomata H, et al 1972; all with permission.
3.1.a.2. Adrenergics
Epinephrine, norepinephrine and 3’,5’-cyclic adenosine monophosphate (cAMP) (but not the inactive metabolite 5’AMP) increased facility similarly in iridic and aniridic eyes, and in CM-disinserted and non-CM-disinserted eyes. This proved that the TM/SC was not ‘dead’, and that epinephrine and its second messenger cAMP were acting directly on the TM/SC (Kaufman, 1987a, 1986a, 1986b; Kaufman et al., 1981; Kaufman and Rentzhog, 1981). We then defined other classes of compounds that provided significant insights into TM biology and regulation, and that might be ‘druggable’ for glaucoma therapy by upregulating or downregulating an outflow facility – related pathway (Croft and Kaufman, 1995a, 1995b; Hubbard et al., 1997, 1996; Kaufman, 1987b; Kaufman et al., 1982, 1977; Kaufman and Erickson, 1982; Menage et al., 1995; Robinson and Kaufman, 1992; Svedbergh et al., 1978; Tian et al., 1997).
3.1.a.3. Actin cytoskeleton
3.1.a.3.a. Cytochalasins
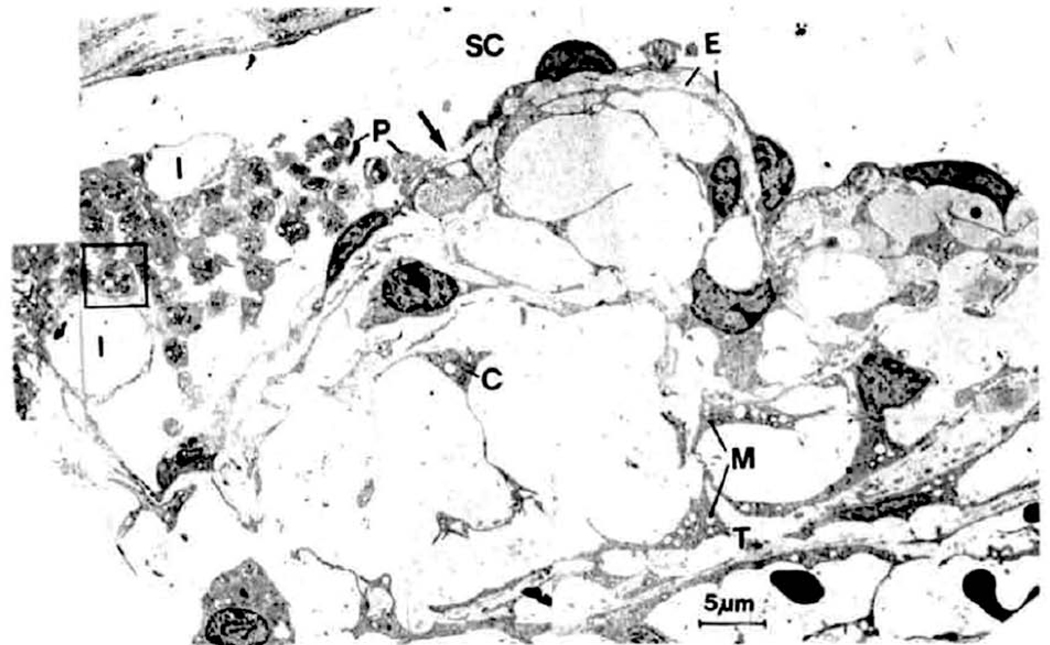
Cytochalasins are fungal metabolites that disrupt actin microfilaments and the actin cytoskeleton. In essence they prevent the addition of actin monomers to one end of the microfilament without affecting the dissolution of actin monomers at the other end. (Kaufman and Erickson, 1982) The process of monomer addition and deletion has been termed the “F-actin treadmill”, and keeps the filaments refreshed and stable. With cytochalasins present, the filaments lose but do not replenish monomers, and thus become shorter and weaker. This disrupts the actin microfilament system, impairs acto-myosin contractility, degrades and weakens cell-cell adherens attachments, as well as cell-ECM attachments (focal contacts), and relaxes the cells (Kaufman et al., 1977; Kaufman and Erickson, 1982; Svedbergh et al., 1978; Takano et al., 1975). In cells with a robust actin cytoskeleton such as HTM and Schlemm’s canal inner wall cells this may have important functional consequences (Inomata et al., 1972a; Stamer et al., 2015). In live monkeys, the entire meshwork relaxes and expands and Schlemm’s canal dilates, allowing fluid to flow through the entire system more easily, i.e., outflow resistance decreases / outflow facility increases (Gabelt and Kaufman, 2012). Unfortunately, given the two compartment – two pressure proximal conventional outflow system that comprises the anterior chamber, trabecular meshwork and Schlemm’s canal, the inner canal wall was ruptured and platelets attempted to plug the rupture as in blood vessels (Fig. 3) (Gabelt and Kaufman, 2012; Kaufman, 1987b; Kaufman et al., 1977; Kaufman and Erickson, 1982; Robinson and Kaufman, 1991; Svedbergh et al., 1978; Tian et al., 1999).
Fig. 3.
Schlemm’s canal (SC) and cribriform meshwork approximately 30 min after intracameral infusion of 5 μg of cytochalasin B. Inner wall endothelium demonstrates ruptures (arrow) and abnormally large invaginations (I). Extracellular material (E) has been lost from some areas between the inner wall endothelium and the first subendothelial cell layer (and replaced by plasma (asterisk)) and is completely absent from most parts of the cribriform meshwork. The cribriform (juxtacanalicular) meshwork is greatly expanded. P, degranulated platelets; C, cellsof cribriform meshwork; M, swollen mitochondria; T, first corneoscleral trabeculum. From Svedbergh et al., 1978, with permission.
While these findings were of great mechanistic interest, this was not a viable therapeutic approach. However, it did provide valuable therapeutic insights that utilized carefully developed epinephrine (Kaufman, 1986a) and cytochalasin B (Kaufman and Erickson, 1982) dose – outflow facility response relationships. Sub-threshold doses of epinephrine and cytochalasin B given together intracamerally induced significant facility increases that were dose-dependent within the subthreshold range for either drug (Robinson and Kaufman, 1991). Furthermore, addition of subthreshold doses of cytochalasin B to a maximal dose of epinephrine gave an additional response (Robinson and Kaufman, 1991). Finally, phalloidin, which stabilizes actin microfilaments and protects them against the disruptive actions of cytochalasins, partially inhibited the facility-increasing effect of epinephrine (Robinson and Kaufman, 1994). Thus, epinephrine was actually the first and the original cytoskeletal drug for increasing outflow facility, other claims notwithstanding. Indeed, epinephrine predated other cytoskeletal / actomyosin contractility-inhibiting drugs by well over half a century – even though its effector mechanism was completely unknown! Of course, and as is often the case, clues were there from other areas of science. Epinephrine acts as, among other things, a ß2-adrenergic receptor agonist, via the cAMP signaling cascade.(Bazrafkan et al., 2010; Neufeld and Sears, 1974). As noted above, in live monkeys, cAMP, but not its inactive metabolite 5’AMP, increases outflow facility, both in normal and in iridectomized and ciliary muscle inserted eyes (Kaufman, 1986b). Macrophage migration through blood vessel walls and lymphocyte adhesion to vessel walls are altered by epinephrine (Petty and Martin, 1989) and those effects are inhibited by timolol, a non-selective ß1, ß2 adrenergic receptor antagonist (Petty and Martin, 1989). Thus, non-innervated white blood cells have ß-adrenergic receptors mediating cytoskeleton/actomyosin contractility-related functional effects. Welcome to the TM of the 21st century!
3.1.a.3.b. Latrunculins
Recognizing that cytochalasin-based ‘war’ on the TM was not a viable therapeutic strategy, but believing that we had identified an important and potentially therapeutically useful control mechanism, we were drawn to work involving latrunculins, marine macrolides that are metabolites of Red Sea sponges. Latrunculins bind 1:1 with globular free actin monomers in cell cytoplasm, thus ‘drying up’ the pool of free actin available to add to the F-actin treadmill (Spector et al., 1989, 1983). Both latrunculin A and B, delivered intracamerally or topically to live monkey eyes, dramatically increased outflow facility by ~100–200% in normal (Suppl Fig 3) and aniridic/ciliary muscle disinserted eyes (Peterson et al., 2000, 1999; Sabanay et al., 2006). Latrunculin B was carried forward into a phase 1 human clinical trial, which confirmed its safety and modest efficacy (Rasmussen et al., 2014). It was not developed further because the company, Inspire Pharmaceuticals, was bought by Merck for other reasons, and Merck had no glaucoma drug development program.
3.1.a.4. Actomyosin contractility
3.1.a.4.a. Kinase inhibitors
Concurrently we approached the system from the actomyosin contractility aspect. Broad spectrum serine-threonine kinase inhibitors such as H-7, that can inhibit both the Rho kinase (RK) and myosin light-chain kinase (MLCK) pathways, prevent phosphorylation and activation of the myosin light chain and thereby inhibit actomyosin contractility (Fig.4). This leads to degeneration of actin microfilaments and vinculin-rich cell-ECM junctions (focal contacts) (Iizuka et al., 1999; Sabanay et al., 2004; Somlyo and Somlyo, 1994; Traube, 2006), and ultimately to expansion of the JCT and dilation of SC but without SCIWE breaks (Fig 5, Suppl Fig 4). In live monkeys, H-7, Y-27362 (selective RK inhibitor, Suppl Fig 5) and ML-7 (selective MLCK inhibitor) all increase outflow facility by 100–200% in live monkeys (Honjo et al., 2001; Tian et al., 2004, 2000; Tian and Kaufman, 2005; Vasantha Rao et al., 2001). RKIs were selected for development as glaucoma therapeutics perhaps because pharmaceutical companies were more familiar with them, and many companies had them in their libraries. At present, one such agent entered the commercial market in Japan in 2014 (ripasudil) (Honjo and Tanihara, 2018; Inazaki et al., 2017; Inoue and Tanihara, 2017, 2013) and another entered the USA market in 2018 (netarsudil) (Kahook et al., 2019; Serle et al., 2018).
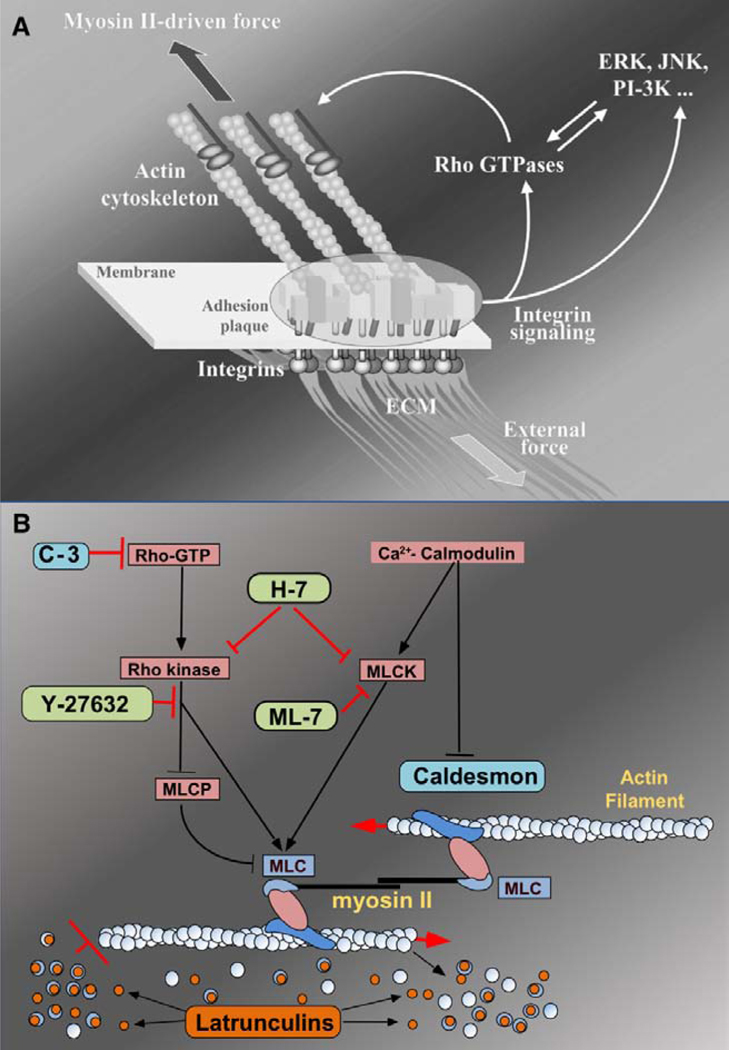
Fig. 4.
A: Focal adhesions (FAs) as a mechanosensors. FAs are multi-molecular complexes connectingthe extracellular matrix with the actin cytoskeleton. Heterodimeric trans membrane integrin receptors (pink) bind matrix proteins via their extracellular domains,while theircytoplasmicdomains are associated with a dense submembrane plaque containing more than 50 different proteins (“boxes” enclosed in the oval area) including structural elements as well as signal transduction proteins such as FAK, Src, ILK, etc. The plaque, in turn, is connected to the termini of actin filament bundles. The assembly and maintenance of FAs depend on local mechanical forces. These forces may be generated by myosin II-driven isometric contraction of the actin cytoskeleton, or by extracellular perturbations such as matrix stretching or fluid shear stress. Force-induced assembly of the adhesion plaque leads to the activation of a variety of signaling pathways that control cell proliferation, differentiation, and survival (e.g., MAP kinase and PI 3- kinase pathways) as well as the organization of the cytoskeleton (e.g., Rho family GTPase pathways). Rho, in particular, is an indispensable regulator of FA assembly affecting, via its immediate targets Dia1 and ROCK, actin polymerization and myosin II-driven contractility. Geiger and Bershadsky, 2002 with permission. B: Schematic drawing illustrating targets for agents known to disrupt the actin cytoskeleton to enhance outflow facility. C-3, Y-27632 and H-7 block the Rho cascade, inhibiting actomyosin contraction and disrupting actin stress fibers; H-7 and ML-7 block myosin light chain kinase phosphorylation of the myosin light chain to interfere with actinmyosin interactions; latrunculin sequesters monomeric G actin leading to microfilament disassembly; caldesmon negatively regulates actin-myosin interactions. Modified from original by Alexander Bershadsky, with permission.
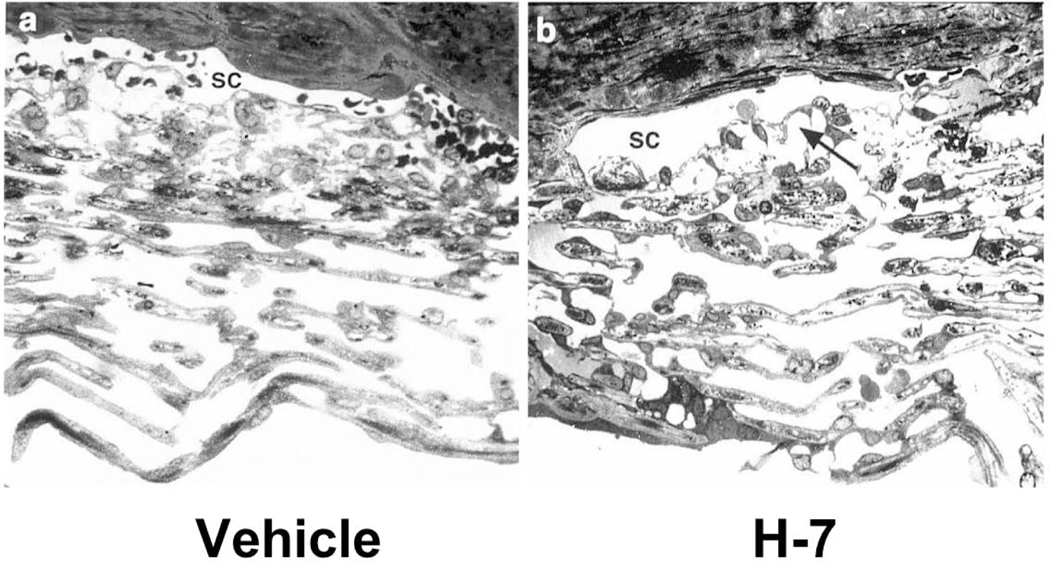
Fig. 5.
Light micrographs of trabecular meshwork (TM) and Schlemm’s canal (SC) in monkey eyes treated with vehicle (a) or H-7 (1-[5-isoquinoline sulfonyl]-2-methyl piperazine), 300 μmol/L (b). The juxtacanalicular area (arrow in b) and intercellular spaces are extended. Inner wall endothelial cells in H-7–treated eyes were thinner than in controls, and cell-cell junctions were intact (not shown). Inner uveal TM were not significantly affected (b). From Sabanay et al., 2000 with permission.
3.1.a.4.b. Nitric oxide
Nitric oxide (NO) can be both a toxic free radical and an important signaling molecule (Becquet et al., 1997). Acting through soluble guanylyl cyclase (cGMP) as the second messenger (Suzuki et al., 2009), NO relaxes vascular endothelial cells and vascular smooth muscle by inhibiting various aspects of the rho cascade, (Nathanson and McKee, 1995; Stamer et al., 2011) thereby dilating blood vessels. Compounds that contain a nitric oxide-donating moiety relax the TM and dilate SC and increase outflow facility after intracameral injection in non-human primates (Gabelt et al., 2011; Heyne et al., 2013). cGMP itself injected into the anterior chamber also increases outflow facility (Kee et al., 1994). Latanoprostene bunod, a single molecule with a PGF2a backbone and an NO-donating moiety (Garcia et al., 2016; Kaufman, 2017), lowers IOP in ocular hypertensive and POAG human subjects (Kawase et al., 2016; Medeiros et al., 2016; Weinreb et al., 2015) by ~ 1 mmHg more than latanoprost (Weinreb et al., 2015), and entered the US commercial market in 2018 (Weinreb et al., 2018) after FDA approval in December 2017. Since NO inhibits the rho cascade, additivity between RKI and NO-donating compounds is uncertain.
3.1.a.4.c. TM/SC mechanosensitivity
Trabecular meshwork/ Schlemm’s canal inner wall (TM/SCIW) cells have an intrinsic actomyosin contractility mechanism that may be the efferent arm of an IOP-regulating mechano-sensitivity reflex. IOP, shear stress, various hormones and cytokines may be the afferent arms; endothelial nitric oxide synthase (eNOS) – NO may be a signaling arm (Stamer et al., 2011); and cellular relaxation / contractility and cell-ECM / cell-cell adherens junction formation/degradation may be the efferent arms (Kaufman and Rasmussen, 2012). Carreon and Johnstone have elegantly hypothesized and described the movements of the system at the macro level (Carreon et al., 2017; Johnstone, 2014).
3.1.a.5. Adenosine agonists
Adenosine A1 receptor agonists injected into the anterior chamber increase outflow facility in monkeys (Tian et al., 1997), perhaps by upregulating the synthesis/release of various metalloproteinases affecting the TM / SCIW (Crosson et al., 2005; Husain et al., 2006; Shearer and Crosson, 2002). A Phase III clinical trial of an adenosine A1R agonist yielded only modest IOP reduction, and its clinical future is uncertain (Liebmann and Lee, 2017; Myers et al., 2016).
3.1.a.6. Conventional outflow facility-decreasing agents
There are several facility-decreasing molecular pathways in addition to glucocorticosteriods (Montecchi-Palmer et al., 2017; Tripathi et al., 1994). For example, TGFß2, found in excess in the aqueous humor and TM of patients with POAG, decreases outflow facility in MOCAS (Bhattacharya et al., 2009; Goel et al., 2012) while ergotamine and angiotensin II decrease facility in live monkeys (Kaufman et al., 1981; Kaufman and Rentzhog, 1981) and angiotensin antagonists have been tested as potential anti-glaucoma agents (Costagliola et al., 2000; Hirooka and Shiraga, 2007; Lotti and Pawlowski, 2009; Shah et al., 2000). These and other strategies discussed below may allow development of experimental molecular models for glaucoma in MOCAS or live monkeys.
3.1.a.7. ‘Distal’ conventional outflow pathway
Decades-old studies and mantra have attributed ~25% of conventional outflow resistance as residing beyond (i.e., downstream from) the inner wall endothelium of Schlemm’s canal, and likely beyond the canal itself (Grant, 1963, 1958). There was awareness of what is now called the distal conventional outflow pathway – the labrynth of intrascleral vessels connecting Schlemm’s canal and the episcleral veins (Hamanaka et al., 1992), but their complex anatomy and physiology was poorly understood, and virtually nothing was known about their putative pathophysiology, if any. Recent studies have shown that the collector channels emanating from the outer wall of Schlemm’s canal may have contractile and perhaps sphincter-like properties (Rohen and Rentsch, 1968), and even more distal intrascleral venules may have a contractile apparatus, so that resistance in this pathway under some conditions may be higher than previously thought (de Kater et al., 1992; Gonzalez et al., 2017). Recent advances and future developments in optical coherence tomographic imaging e.g. OCT angiography (Huang et al., 2018) may help unravel these mysteries.
3.1.b. Unconventional (uveoscleral) outflow pathway
This pathway encompasses the connective tissue-filled spaces between the sheaths surrounding the ciliary muscle bundles (~12 muscle fibers within each bundle) that communicate with the anterior chamber at the anterior face of the ciliary muscle, and with the supraciliary and suprachoroidal spaces all the way back to the macular and optic nerve head region (Inomata et al., 1972b), so that aqueous humor and ciliary process and choroidal stromal fluid and protein can be carried out of the eye (with debate as to how much is carried away trans-sclerally (Anders Bill, 1967; Bill, 1971, 1966; Inomata et al., 1972b, 1972a), by the choroidal vasculature (Pederson et al., 1977), and by recently described ciliary muscle/choroidal putative lymphatics (Yücel et al., 2009). Intense ciliary muscle contraction by cholinimimetic drugs can constrict or even obliterate the spaces between the ciliary muscle bundles (Rohen et al., 1967), impeding uveoscleral outflow dramatically (A Bill, 1967) (Suppl Fig 6), (Crawford and Kaufman, 1987; Nilsson et al., 1989). This was utilized in the late 1980s to help delineate the mechanism by which PGF2⍺ reduced IOP – see section 3.1.b.1 below.
3.1.b.1. Prostaglandin analogues
PGF2α increases uveoscleral outflow (Crawford et al., 1987; Crawford and Kaufman, 1987; Gabelt and Kaufman, 1989; Nilsson et al., 1989)(Table 1) by increasing the synthesis and release of various matrix metalloproteinases and consequently decreasing and remodeling the collagenous extracellular matrix in the ciliary muscle (Gaton et al., 2001). Increased uveoscleral outflow has been reported in monkey eyes with experimental autoimmune uveitis (Mermoud et al., 1994; Toris and Pederson, 1987). Histologic studies have shown enlarged ciliary muscle inter-muscular and suprachoroidal spaces in eyes with experimental uveitis induced by various methods (Liu et al., 1994; Toris and Pederson, 1987). The density of collagen type I in the extracellular matrix (ECM) of monkey ciliary muscle was reduced during anterior segment inflammation (Sagara et al., 1999a),indicating that reduction of ciliary muscle ECM may contribute to enhanced uveoscleral outflow during anterior segment inflammation. Twice daily topical treatment of cynomolgus monkey eyes with 2 μg PGF2α-isopropyl ester increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway (Sagara et al., 1999a) and also reduced collagen types I, III and IV immunoreactivity in the ciliary muscle (OCKLIND, 1998; Sagara et al., 1999a, 1999b). PGF2α analogs have become the most widely used anti-glaucoma medications worldwide (Stein et al., 2015, 2007).
Table 1.
Effect of topical PGF2α on uveoscleral outflow in monkeys 5th day of treatment bid with 2 μg PGF2α – IE
| PGF2α | Control | PG/Cont |
|---|---|---|
| 1.101 | 0.489 | 2.329* |
| ± | ± | ± |
| 0.141 | 0.07 | 0.235 |
Data are mean ± s.e.m. uveoscleral outflow (μl/min) for 9 animals, each contributing one treated and one control eye, following the ninth unilateral dose of PGF2α on day 5.
P<0.00001. Ratio significantly different from 1.0 by the two-tailed paired t-test. From Gabelt, 1989, with permission.
3.1.c. Novel products: combinatorial molecules - one molecule, two effects; and combination products - two molecules, two effects, one or more effects for each molecule
The FDA recently approved two novel products. Latanoprostene bunod (Vyzulta®, Bausch & Lomb) is a combinatorial molecule; one molecule with two effects. It has a latanoprost backbone and an NO-donating esterified side chain (Weinreb et al., 2018). Rocklatan®, (Aerie) is a fixed combination product; it contains two separate molecules, and conveys least two different effects, one or more for each molecule. It combines the rho kinase inhibitor Netarsudil (Rhopressa®, Aerie) with the prostaglandin PGF2⍺ analogue latanoprost. (Kahook et al., 2019; Serle et al., 2018).
4. Gene Therapy for Enhancing or Inhibiting Aqueous Outflow
4.1. Background / Unmet Medical Need
Topical self-administered eye drops have become problematic for clinical glaucoma therapy, as they rely on the patient to be an accurate, reliable, reproducible part of the delivery system. Studies have shown that the latter is not the case (Budenz, 2009; McKinnon et al., 2008; Nordstrom et al., 2005; Tsai, 2009). While various sustained release delivery systems are under development (NCT02371746, 2015; Vinod and Gedde, 2017; Walters et al., 2017), we have tried to develop a biological rather than a mechanical approach, namely gene transfer. This has nothing to do with hunting for and replacing a defective gene. Rather, the goal is to have relevant cells in the inflow or outflow pathways make more or less of something that affects an IOP–relevant physiological parameter so as to reduce IOP – whether or not an abnormality in that pathway was present. Thus, we have attempted to transfer genes that interfere with the rho pathway in the JCTM/SCIW to enhance conventional outflow facility, and genes that upregulate the PGF2α – MMP pathway in the ciliary muscle to enhance uveoscleral outflow.
4.2. Reporter gene transfer to the TM and CM
We started with a self-complimentary AAV (scAAV) viral vector carrying the green fluorescent protein (GFP) gene injected into the anterior chamber of live monkeys, and achieved GFP expression in the TM easily visible gonioscopically at the slit lamp for over two years, with no clinically visible ocular inflammation (Buie et al., 2010). A feline immunodeficient (FIV) viral vector carrying the GFP gene achieved the same outcome (Barraza et al., 2009). Immunohistochemically, GFP was localized primarily to the TM and the most anterior part of longitudinal ciliary muscle (Barraza et al., 2009; Buie et al., 2010) (Fig. 6).
Fig. 6.
A, B, C: Monkey chamber angle after intracameral administration of scAAV.GFP vector, day 641 post injection, SLE - quiet, IOP normal. Panel A: A digital camera with a 175-W xenon nova light source and a 3-mm-6-cm 0° teleotoscope probe (Hopkins II; Karl Storz Endoscopy-America, Inc., Culver City, CA) captured the image. Panel B: A retinal camera (50EX; Topcon, Tokyo, Japan), fitted with a digital SLR color camera body (D1X (Nikon Instruments, Inc., Melville, NY); with standard clinical fluorescein exciter and barrier filters, and a gonioprism, captured the image. Panel C: A customized microscope (Nikon) with a 12-bit, monochromatic, cooled-CCD camera (Retiga, 2000RV; QImaging Burnaby, BC, Canada), a specific GFP exciter and barrier filter set and a gonioprism captured the image. D: Monkey anterior chamber angle after intracameral administration of FIV. GFP vector, day 515 post injection, SLE - quiet, IOP normal.
4.3. Functional gene transfer to the TM
4.3.a. MOCAS
We then progressed to genes that, when overexpressed, would inhibit the rho cascade (the C3 exotoxin of Clostridium) or interfere with the actin/myosin interaction (caldesmon) in both cases inhibiting actomyosin contractility with consequent cellular relaxation and degradation of the actin cytoskeleton and cell-ECM attachments (Gabelt et al., 2006; Grosheva et al., 2006; Liu X, Wang N, 2005; Liu et al., 2005; Slauson et al., 2015). An adenoviral vector carrying either of these transgenes, infused into our MOCAS system for freshly enucleated monkey eyes, induced a doubling of outflow facility (Gabelt et al., 2006; Liu et al., 2005; Slauson et al., 2015) (Table 2).
Table 2.
The effect of adenoviral vectors on outflow facility in monkey organ cultured anterior segment.
| A | B | ||||||
|---|---|---|---|---|---|---|---|
| N=8 | Treated | Control | T/C | N=4 | Treated | Control | T/C |
| Baseline | 0.35±0.04 | 0.48±0.07 | 0.78±0.08* | Baseline | 0.31±0.09 | 0.41±0.10 | 0.82±0.16 |
| AdGFPCald or AdGFP | 1.03±0.30 | 0.67±0.17 | 1.47±0.17* | AdGFPC3 or AdGFP | 0.66±0.14 | 0.39±0.06 | 1.79±0.38 |
| Ad/BL | 2.63±0.47* | 1.34±0.20 | 2.01±0.19* | Ad/BL | 2.44±0.54 | 1.09±0.20 | 2.25±0.28* |
| Caldesmon | C3 | ||||||
A: Caldesmon (1.5v107 plaque forming units, 20 μl); B: C3 (1.2×108 viral particles, 80 μl). Ad, Adenoviral vector; GFP, green fluorescent protein; Cald, caldesmon; Data are mean ± s.e.m. outflow facility; ratios are unitless. Ratio significantly different from 1.0 by the two-tailed paired t-test;
P<0.05. From Gabelt et al., 2006: B: From Liu et al., 2005, with permission.
Several genes are known to drive outflow facility down in rodents and have also been implicated in the pathophysiology of human POAG. Examples among many are cochlin, SFRP1, TGFß2, and CTGF (Fuchshofer and Tamm, 2012; Goel et al., 2012; Junglas et al., 2012; Lee et al., 2010; Montecchi-Palmer et al., 2017; Pang et al., 2015; Tamm et al., 2015; Webber et al., 2016). This opens the possibility of creating a local genetic molecular model of ocular hypertension/POAG in NHP, absent the outflow tissue disruption, inflammation and scarification that accompanies current laser (Gaasterland and Kupfer, 1974) and trauma models, or the gross mechanical obstruction of microbead models (Chan et al., 2018; Cone et al., 2012; Sappington et al., 2010). Indeed, it is possible that one or more of these genes may underlie human POAG pathophysiology. Locally overexpressing one of more of these genes in the NHP JCT/SCIWE may provide the long sought-after molecular model of NHP POAG (Bhattacharya et al, 2009; (Suppl Fig. 8), and a stable platform for testing new potential ocular hypertensive and glaucomatous optic neuroprotective therapies going forward.
4.3.b. Live monkeys
4.3.b.1. Intracameral injection
However, when FIV or scAAV vectors carrying either gene were injected intracamerally into live monkeys there was, with only one exception (high-titer scAAV.C3, that induced corneal endothelium dysfunction and consequent corneal edema, but no IOP or outflow changes (unpublished data), no physiological effect, no inflammation beyond that from the injection procedure itself, or any evidence at all that the eyes had been touched, save for the transcorneal needle tracks (unpublished data). One may hypothesize that in the live animal, where most of the aqueous humor drainage is through the TM/SC and the remainder is via the ciliary muscle, the ‘system’ shrugs its shoulders at GFP, which doesn’t really affect the cellular machinery. However, caldesmon and C3 exoenzyme, which significantly affect the TM cellular machinery, may elicit a strong turn-off response (Aktas et al., 2014). Further, many cell types are exposed to the vector - corneal endothelium, scleral fibroblasts, TM, SC inner/outer wall, ciliary muscle, lens epithelium, iris pigment epithelium, etc. This might mount an ‘all hands-on deck’ turn-off response. Except for one recent report (Tan et al., 2019), injection of facility-effective genes in MOCAS have no effect in live NHP. While intracameral injection of an FIV.BOVPGFS-myc.GFP PGF synthase vector construct reproducibly induces an ~2mmHg reduction in IOP, the effect is much less than that of topical PGF2⍺ analogue eyedrops, and dissipates after 5 months. The turnoff mechanism has yet to be defeated, although proteasome inhibition enhances reporter gene expression in monkey organ cultured anterior segments (MOCAS). In MOCAS, there is of course no vascular circulation and no connection to any systemic ‘assistance’, and there are also only a few cell types – corneal endothelium, scleral fibroblasts, TM and Schlemm’s canal cells - and a vastly reduced (and injured) number of ciliary muscle cells with no posterior attachments.
4.3.b.2. Intracanalicular injection
This difference, as well as a study showing that direct injection of adenoviral vectors into Schlemm’s canal was feasible in rat and human donor eyes and had potential in glaucoma treatment (Hudde et al., 2005), suggested a live animal strategy of catheterizing Schlemm’s canal and passing the catheter 360 degrees around the entire circumference as in ABiC and GATT procedures (Francis et al., 2017; Gallardo et al., 2018; Grover et al., 2018; Rahmatnejad et al., 2017). This has required modifications of the human ABiC catheter design, with generous collaboration from the engineers at Ellex iTrack. Both ab externo and ab interno cannulation of SC over 360 degrees has been achieved, with injection of trypan blue dye visible for up to four clock hours from the catheter tip during insertion (Aktas et al., 2014) (Fig. 7). The entire canal can be decorated blue by passing the catheter 360 degrees and then injecting slowly while withdrawing the catheter (unpublished data), with only a few small leaks/ruptures into the AC. We are currently administering cytoskeleton/contractility-modifying constructs via this route and are also looking at molecular modifications of both the vectors and the transgenes.
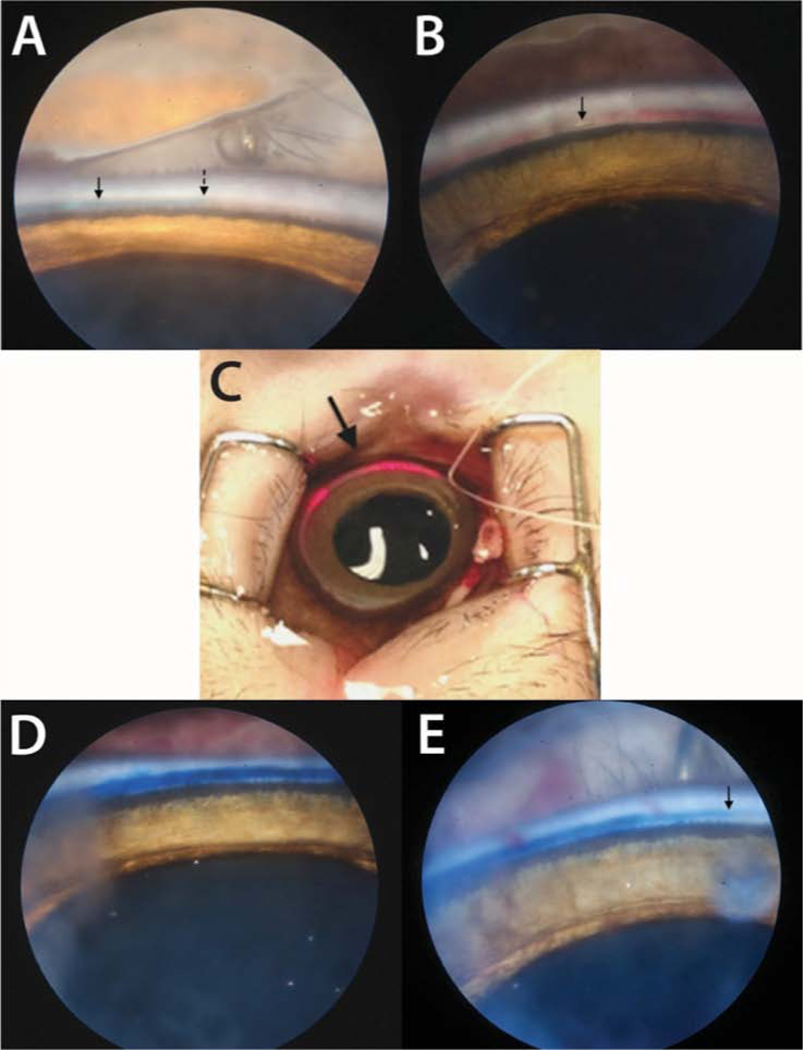
Fig. 7.
Drug delivery via catheterization of Schlemm’s Canal in monkeys A: Endoscopic camera image showing 6–0 prolene suture (solid arrow) and its end (dotted arrow) in Schlemm’s canal. B: Microcatheter in Schlemm’s canal (arrow). Note the blood in the canal blocking the gonioscopic view of thecatheterattherightofthepicture.C:LEDlightof the microcatheter in Schlemm’s canal. D: Endoscopic camera image of the temporal side of left eye at 3:00–4:00 o’clock showing trypan blue in Schlemm’s canalafterinjectionbycatheter(ostiumwasat12:00 o’clock, catheter tip at 1:00 o’clock). E: Infero-temporal site at 3:00–4:00 o’clock and the point where the visible dye column ended (arrow) at 5 o’clock.
4.3.b.3. Proteasome inhibition
We are also exploring proteasome inhibition. Short-term exposure of TM cells to the proteasome inhibitor MG132 increases the transduction efficiency of FIV-mediated reporter gene delivery to TM cells in culture and in the MOCAS model. A more even distribution and intensity of GFP transduction in the MOCAS TM was noted, but no transduction was seen in SCIW endothelium (Aktas et al., 2018).
4.4. Prostaglandin F2⍶ pathway gene transfer to the ciliary muscle
Currently, the most widely prescribed outflow drugs approved for clinical use are prostaglandin F2α analogues. The delivery of genes for the prostaglandin F2α and FP receptor biosynthetic pathways to the anterior chamber angle, and thence to the ciliary muscle, of live cats and monkeys has been achieved using lentiviral vectors, resulting in consistent low single digit IOP reduction, but far less than what is seen following topical administration of PGF2⍺ analogue eyedrops. (Barraza et al., 2010; Lee et al., 2014; Loewen et al., 2004). The IOP lowering effect in NHPs lasted for 5 months (Suppl Fig. 9), (Lee et al., 2014). In cats, the experiments were ended after 5 months, by design.
Although encouraging, these studies have identified a number of challenges to be overcome before prostaglandin gene therapy can be translated into the clinic: the IOP effect needs to be of greater magnitude, possibly by determining the mechanism whereby transgene expression is lost; engineering the vector to resist species-specific restriction factors or using proteasome inhibition to increase the transduction efficiency (Aktas et al., 2018); identifying alternative promoters to better drive gene expression, including more ciliary muscle - and/or trabecular meshwork - specific promoters; and determining if species-specific PGF synthase or codon optimization is effective (Aktas et al., 2018; Brandt et al., 2015; Lee et al., 2014; Slauson et al., 2015).
4.5. Post-glaucoma filtration surgery wound healing/antifibrosis
Glaucoma filtration surgery, commonly used for IOP lowering when medical and laser trabeculoplasty fail, uses a guarded sub conjunctival transscleral incision into the anterior chamber to allow the continuous long-term release of aqueous humor into the subconjunctival space, from whence it is resorbed. Antimetabolites, such as mitomycin C (MMC), are deployed subconjunctivally during surgery to block scarring of the ostomy during wound healing. Unfortunately, their use can result in undesirable side effects, such as tissue degeneration / cellular destruction leading to wound leaks, hypotony and ocular surface or intraocular infection. The human p21WAF-1/Cip-1 gene (rAd-p21), delivered via a recombinant adenovirus, was developed as a therapeutic alternative, (Heatley et al., 2004; Perkins et al., 2002) causing cell cycle arrest rather than destruction of surrounding cells and invading fibroblasts. In vitro, treatment of Tenon fibroblasts with rAd.p21 resulted in a dose-dependent inhibition of DNA synthesis and cell growth (Perkins et al., 2002). In vivo, in rabbits, rAd.p21 was comparable to mitomycin in inhibition of wound healing and fibroproliferation after filtration surgery without the complications of hypopyon and hyalitis that were associated with mitomycin (Perkins et al., 2002). When delivered to live ocular hypertensive (following laser scarification of the TM) monkey eyes, sub-Tenon / episcleral rAd-p21 treatment resulted in open surgical ostomies by both functional (normalized IOP) and histological criteria for at least 9 months (Heatley et al., 2004), without the tissue destruction seen in control animals treated with MMC (Heatley et al., 2004).
5. Stem cell therapy
As with gene therapy, stem cell strategies have the potential to provide patients with one-time long term solutions. With age and glaucoma, trabecular meshwork and JCT cell counts decrease (Alvarado et al., 1984, 1981; Grierson and Howes, 1987). The goal with stem cell approaches is to replace or regenerate lost tissue in the outflow pathways, restoring TM function. TM stem cells have been identified and localized in the Schwalbe’s line / insert region of the TM in the primate eye. (Braunger et al., 2014; Yun et al., 2016). Mesenchymal stem cells (MSC) and induced pluripotent stem cells (iPSC) have both been used in TM cell differentiation and regeneration applications. An advantage to these strategies is the ability to use autologous stem cells. Contractility and phagocytosis differ between TM cells and MSCs and can be used as a method to determine the extent to which a functional TM phenotype has been attained (Snider et al., 2018). In a laser-damaged trabecular meshwork mouse model, intracamerally transplanted human trabecular meshwork stem cells (TMSCs) preferentially homed and integrated to the damaged TM region and expressed differentiated cell markers at 2 and 4 weeks. TMSC transplantation resulted in both ultrastructural and functional restoration. (Yun et al., 2018) MSCs isolated from rat bone marrow, injected into the anterior chamber, significantly reduced IOP in rat eyes made hypertensive by episcleral vein cauterization. MSCs were localized in the ciliary processes and the TM (Roubeix et al., 2015). Transplantation of induced pluripotent stem cell (iPSC) derived TM cells (iPSC-TM ) restored IOP and outflow facility in young (Zhu et al., 2016) and aged ((Zhu et al., 2017) transgenic mice expressing a pathogenic form of human myocilin (Tg-MYOCY437H ) glaucoma. At 12 weeks after transplantation, IOP in iPSC-TM recipients was statistically lower and outflow facility was significantly improved compared to untreated controls (Zhu et al., 2017). An ex vivo HOCAS system was developed to study outflow pathway cell loss effects on IOP homeostatic function. iPSCs were used to repopulate the cell depletion model. The differentiated cells (TM-like iPSCs) became similar to TM cells in both morphology and expression patterns and were able to restore IOP homeostatic function (Abu-Hassan et al., 2015). Collectively, these studies show the potential for stem cell therapeutics targeted to the conventional TM outflow pathway.
6. Conclusion
We have come a long way in understanding and manipulating NHP and human aqueous humor outflow over the past half century. Yet, we still do not really understand the basic pathophysiology of the outflow dysfunction leading to ocular hypertension and POAG, nor even much of the normal physiology much less any putative pathophysiology of the distal conventional outflow pathway.
Finally, let me again thank ISER for bestowing this great honor upon me; my many friends, mentors, and close collaborators ‘in the business’ without whom this work would never have happened; and of course my intensely devoted, loyal and always supportive family who illuminate and inspire the long days and nights underpinning it.
Supplementary Material
Highlights.
Cholinomimetics increase outflow facility solely via CM contraction
Epinephrine, RKIs and NO ↑ facility by inhibiting TM contractility, relaxing TM and dilating SC
PGF2⍺ ↑ Fu by ↑ CM cell MMP synthesis/release, ↓ and remodeling CM ECM collagen
Viral vectors (VV) transfer reporter genes expressing for > 2yrs in live monkey TM
VV transfer genes inhibiting TM contractility and ↑ facility in MOCAS
VV transfer genes that ↓ facility in MOCAS; molecular models for human POAG?
iPSC cell-derived TM cells populated TM & rescued outflow in TM cell-depleted HOCAS
Acknowledgements:
This work was supported in part by the Core Grant for Vision Research from the NIH to the University of Wisconsin-Madison (P30 EY016665), and by other grants from the NIH, the Glaucoma Research Foundation, and Research to Prevent Blindness.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ, 2015. Induced pluripotent stem cells restore function in a human cell loss model of open-angle glaucoma. Stem Cells 33, 751–761. 10.1002/stem.1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas Z, Rao H, Slauson SR, Gabelt BT, Larsen IV, Sheridan RTC, Herrnberger L, Tamm ER, Kaufman PL, Brandt CR, 2018. Proteasome inhibition increases the efficiency of lentiviral vector-mediated transduction of trabecular meshwork. Investig. Ophthalmol. Vis. Sci 59, 298–310. 10.1167/iovs.17-22074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas Z, Tian B, McDonald J, Yamamato R, Larsen C, Kiland J, Kaufman PL, Rasmussen CA, 2014. Application of Canaloplasty in Glaucoma Gene Therapy: Where Are We? J. Ocul. Pharmacol. Ther 30, 277–282. 10.1089/jop.2013.0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R, 1984. Trabecular Meshwork Cellularity in Primary Open-angle Glaucoma and Nonglaucomatous Normals. Ophthalmology 91, 564–579. 10.1016/S0161-6420(84)34248-8 [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R, 1981. Age-related changes in trabecular meshwork cellularity. Investig. Ophthalmol. Vis. Sci 21, 714–727. 10.1155/2013/295204 [DOI] [PubMed] [Google Scholar]
- Barraza RA, McLaren JW, Poeschla EM, 2010. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol. Ther 18, 491–501. 10.1038/mt.2009.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza RA, Rasmussen CA, Loewen N, Cameron JD, Gabelt BT, Teo W-L, Kaufman PL, Poeschla EM, 2009. Prolonged Transgene Expression with Lentiviral Vectors in the Aqueous Humor Outflow Pathway of Nonhuman Primates. Hum. Gene Ther 20, 191–200. 10.1089/hum.2008.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazrafkan M, Panahi M, Saki G, Ahangarpour A, Zaeimzadeh N, 2010. Effect of aqueous extract of Ruta graveolens on spermatogenesis of adult rats. Int. J. Pharmacol 6, 926–929. 10.3923/ijp.2010.926.929 [DOI] [Google Scholar]
- Becquet F, Courtois Y, Goureau O, 1997. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv. Ophthalmol 42, 71–82. 10.1016/S0039-6257(97)84043-X [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Gabelt BT, Ruiz J, Picciani R, Kaufman PL, 2009. Cochlin expression in anterior segment organ culture models after TGFβ2 treatment. Investig. Ophthalmol. Vis. Sci 50, 551–559. 10.1167/iovs.08-2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill A, 1971. Aqueous humor dynamics in monkeys (Macaca irus and Cercopithecus ethiops). Exp. Eye Res 11, 195–206. 10.1016/s0014-4835(71)80023-4 [DOI] [PubMed] [Google Scholar]
- Bill Anders, 1967. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus). Exp. Eye Res 6, 120-IN3. 10.1016/S0014-4835(67)80062-9 [DOI] [PubMed] [Google Scholar]
- Bill A, 1967. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus). Exp. Eye Res 6, 120–5. 10.1016/s00144-835(67)80062-9 [DOI] [PubMed] [Google Scholar]
- Bill A, 1966. Conventional and uveo-scleral drainage of aqueous humour in the cynomolgus monkey (Macaca irus) at normal and high intraocular pressures. Exp. Eye Res 5, 45–54. 10.1016/s0014-4835(66)80019-2 [DOI] [PubMed] [Google Scholar]
- Brandt CR, Rao H, Gabelt BT, Aktas Z, Slauson SR, 2015. Genome deficient lentiviral particles improve FIV transduction efficiency of trabecular meshwork. Investig. Ophthalmol. Vis. Sci, 2015 Annual Meeting of the Association for Research in Vision and Ophthalmology, ARVO 2015. United States. 56, 3280. [Google Scholar]
- Braunger BM, Ademoglu B, Koschade SE, Fuchshofer R, Gabelt BT, Kiland JA, HennesBeann EA, Brunner KG, Kaufman PL, Tamm ER, 2014. Identification of adult stem cells in Schwalbe’s line region of the primate eye. Invest. Ophthalmol. Vis. Sci 55, 7499–7507. 10.1167/iovs.14-14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budenz DL, 2009. A Clinician’s Guide to the Assessment and Management of Nonadherence in Glaucoma. Ophthalmology 116, S43–S47. 10.1016/j.ophtha.2009.06.022 [DOI] [PubMed] [Google Scholar]
- Buie LKK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, Samulski RJ, Kaufman PL, Borrás T, 2010. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Investig. Ophthalmol. Vis. Sci 51, 236–248. 10.1167/iovs.09-3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreon T, van der Merwe E, Fellman RL, Johnstone M, Bhattacharya SK, 2017. Aqueous outflow - A continuum from trabecular meshwork to episcleral veins. Prog. Retin. Eye Res 57, 108–133. 10.1016/j.preteyeres.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Lynn MN, Tun SBB, Barathi VA, Aung T, 2018. Bilateral intraocular pressure (IOP) changes in a non human primate (NHP) microbead model of chronic IOP elevation: Can both eyes achieve similar elevations for therapeutics evalution? Invest. Ophthalmol. Vis. Sci [Google Scholar]
- Cone FE, Steinhart MR, Oglesby EN, Kalesnykas G, Pease ME, Quigley HA, 2012. The effects of anesthesia, mouse strain and age on intraocular pressure and an improved murine model of experimental glaucoma. Exp. Eye Res 99, 27–35. 10.1016/j.exer.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costagliola C, Verolino M, Leonarda De Rosa M, Iaccarino G, Ciancaglini M, Mastropasqua L, 2000. Effect of oral losartan potassium administration on intraocular pressure in normotensive and glaucomatous human subjects. Exp. Eye Res 71, 167–171. 10.1006/exer.2000.0866 [DOI] [PubMed] [Google Scholar]
- Crawford K, Kaufman PL, 1987. Pilocarpine antagonizes prostaglandin f2α-induced ocular hypotension in monkeys: Evidence for enhancement of uveoscleral outflow by prostaglandin f2α. Arch. Ophthalmol 105, 1112–1116. 10.1001/archopht.1987.01060080114039 [DOI] [PubMed] [Google Scholar]
- Crawford K, Kaufman PL, Gabelt BT, 1987. Effects of topical PGF2α on aqueous humor dynamics in cynomolgus monkeys. Curr. Eye Res 6, 1035–1044. 10.3109/02713688709034874 [DOI] [PubMed] [Google Scholar]
- Croft MA, Kaufman PL, 1995a. Effect of daily topical ethacrynic acid on aqueous humor dynamics in monkeys. Curr. Eye Res 14, 777–781. 10.3109/02713689508995799 [DOI] [PubMed] [Google Scholar]
- Croft MA, Kaufman PL, 1995b. Effect of daily topical ethacrynic acid on aqueous humor dynamics in monkeys. Curr. Eye Res 14, 777–781. 10.3109/02713689508995799 [DOI] [PubMed] [Google Scholar]
- Crosson CE, Sloan CF, Yates PW, 2005. Modulation of conventional outflow facility by the adenosine A1 agonist N6-cyclohexyladenosine. Investig. Ophthalmol. Vis. Sci 46, 3795–3799. 10.1167/iovs.05-0421 [DOI] [PubMed] [Google Scholar]
- de Kater AW, Shahsafaei A, Epstein DL, 1992. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest. Ophthalmol. Vis. Sci 33, 424–9. [PubMed] [Google Scholar]
- Duan JC, Fulop A, 2015. Density-Tempered Marginalized Sequential Monte Carlo Samplers. J. Bus. Econ. Stat 33, 192–202. 10.1080/07350015.2014.940081 [DOI] [Google Scholar]
- Endre-A-Balazs-doctor-who-found-acid-to-treat-arthritic-knees [WWW Document], 2015. . NY Times. [Google Scholar]
- Balazs Endre [WWW Document], 2015. . Harvard Eye. URL https://eye.hms.harvard.edu/endrebalazs (accessed 11.10.18). [Google Scholar]
- Francis BA, Akil H, Bert BB, 2017. Ab interno Schlemm’s Canal Surgery. Dev. Ophthalmol 59, 127–146. 10.1159/000458492 [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER, 2012. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 10.1007/s00441-011-1274-7 [DOI] [PubMed] [Google Scholar]
- Gaasterland D, Kupfer C, 1974. Experimental glaucoma in the rhesus. Invest. Ophthalmol. Vis. Sci 13, 4208801. [PubMed] [Google Scholar]
- Gabelt BT, Hu Y, Vittitow JL, Rasmussen CR, Grosheva I, Bershadsky AD, Geiger B, Borrás T, Kaufman PL, 2006. Caldesmon transgene expression disrupts focal adhesions in HTM cells and increases outflow facility in organ-cultured human and monkey anterior segments. Exp. Eye Res 82, 935–944. 10.1016/j.exer.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL, 2012. Production and Flow of Aqueous Humor, in: (Eds: Kaufman PL, Alm A, Levin L, Nilsson S, VerHoeve J, W.S. (Ed.), Adler’s Physiology of the Eye. Saunders/Elsevier, pp. 274–307. 10.1016/b978-0-323-05714-1.00011-x [DOI] [Google Scholar]
- Gabelt BT, Kaufman PL, 1992. Inhibition of outflow facility and accommodative and miotic responses to pilocarpine in rhesus monkeys by muscarinic receptor subtype antagonists. J. Pharmacol. Exp. Ther 263, 1133–9. [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL, 1989. Prostaglandin F2 alpha increases uveoscleral outflow in the cynomolgus monkey. Exp. Eye Res 49, 389–402. [DOI] [PubMed] [Google Scholar]
- Gabelt BT, Kaufman PL, Rasmussen CA, 2011. Effect of nitric oxide compounds on monkey ciliary muscle in vitro. Exp. Eye Res 93, 321–327. 10.1016/j.exer.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo MJ, Supnet RA, Ahmed IIK, 2018. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: Ab-interno vs ab-externo canaloplasty with tensioning suture. Clin. Ophthalmol 12, 2493–2498. 10.2147/OPTH.S178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GA, Ngai P, Mosaed S, Lin KY, 2016. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin. Ophthalmol 10.2147/OPTH.S103985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaton DD, Sagara T, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN, 2001. Increased matrix metalloproteinases 1, 2, and 3 in the monkey uveoscleral outflow pathway after topical prostaglandin F2α-isopropyl ester treatment. Arch. Ophthalmol 119, 1165–1170. 10.1001/archopht.119.8.1165 [DOI] [PubMed] [Google Scholar]
- Goel M, Sienkiewicz AE, Picciani R, Wang J, Lee RK, Bhattacharya SK, 2012. Cochlin, intraocular pressure regulation and mechanosensing. PLoS One 7. 10.1371/journal.pone.0034309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Ko MK, Hong Y-K, Weigert R, Tan JCH, 2017. Deep tissue analysis of distal aqueous drainage structures and contractile features. Sci. Rep 7, 17071. 10.1038/s41598-017-16897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant WM, 1963. Experimental Aqueous Perfusion in Enucleated Human Eyes. Arch. Ophthalmol 69, 783–801. 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- Grant WM, 1958. Further studies on facility of flow through the trabecular meshwork. AMA. Arch. Ophthalmol 60, 523–33. [DOI] [PubMed] [Google Scholar]
- Greenshaw AJ, 1986. Osmotic mini-pumps: A convenient program for weight-adjusted filling concentrations. Brain Res. Bull 16, 759–761. 10.1016/0361-9230(86)90150-4 [DOI] [PubMed] [Google Scholar]
- Grierson I, Howes RC, 1987. Age-related depletion of the cell population in the human trabecular meshwork. Eye 1, 204–210. 10.1038/eye.1987.38 [DOI] [PubMed] [Google Scholar]
- Grosheva I, Vittitow JL, Goichberg P, Gabelt BT, Kaufman PL, Borrás T, Geiger B, Bershadsky AD, 2006. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp. Eye Res 82, 945–958. 10.1016/j.exer.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Grover DS, Smith O, Fellman RL, Godfrey DG, Gupta A, De Oca IM, Feuer WJ, 2018. Gonioscopy-Assisted transluminal trabeculotomy: An ab interno circumferential trabeculotomy: 24 months follow-up. J. Glaucoma 27, 393–401. 10.1097/IJG.0000000000000956 [DOI] [PubMed] [Google Scholar]
- Hamanaka T, Bill A, Ichinohasama R, Ishida T, 1992. Aspects of the development of Schlemm’s canal. Exp. Eye Res 55, 479–488. 10.1016/0014-4835(92)90121-8 [DOI] [PubMed] [Google Scholar]
- Heatley G, Kiland J, Faha B, Seeman J, Schlamp CL, Dawson DG, Gleiser J, Maneval D, Kaufman PL, Nickels RW, 2004. Gene therapy using p21 WAF-1/Cip-1 to modulate wound healing after glaucoma trabeculectomy surgery in a primate model of ocular hypertension. Gene Ther. 11, 949–955. 10.1038/sj.gt.3302253 [DOI] [PubMed] [Google Scholar]
- Heyne GW, Kiland JA, Kaufman PL, Gabelt BT, 2013. Effect of nitric oxide on anterior segment physiology in monkeys. Investig. Ophthalmol. Vis. Sci 54, 5103–5110. 10.1167/iovs.12-11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka K, Shiraga F, 2007. Potential role for angiotensin-converting enzyme inhibitors in the treatment of glaucoma. Clin. Ophthalmol 1, 217–23. [PMC free article] [PubMed] [Google Scholar]
- Honjo M, Inatani M, Kido N, Sawamura T, Yue BYJT, Honda Y, Tanihara H, 2001. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch. Ophthalmol 119, 1171–1178. 10.1001/archopht.119.8.1171 [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, 2018. Impact of the clinical use of ROCK inhibitor on the pathogenesis and treatment of glaucoma. Jpn. J. Ophthalmol 62, 109–126. 10.1007/s10384-018-0566-9 [DOI] [PubMed] [Google Scholar]
- Huang AS, Francis BA, Weinreb RN, 2018. Structural and functional imaging of aqueous humour outflow: a review. Clin. Experiment. Ophthalmol 46, 158–168. 10.1111/ceo.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard WC, Johnson M, Gong H, Gabelt BT, Peterson JA, Sawhney R, Freddo T, Kaufman PL, 1997. Intraocular pressure and outflow facility are unchanged following acute and chronic intracameral chondroitinase ABC and hyaluronidase in monkeys. Exp. Eye Res 65, 177–190. 10.1006/exer.1997.0319 [DOI] [PubMed] [Google Scholar]
- Hubbard WC, Kee C, Kaufman PL, 1996. Aceclidine effects on outflow facility after ciliary muscle disinsertion. Ophthalmologica 210, 303–307. 10.1159/000310729 [DOI] [PubMed] [Google Scholar]
- Hudde T, Apitz J, Bordes-Alonso R, Heise K, Johnson KTM, Steuhl KP, Geerling G, Pützer BM, 2005. Gene transfer to trabecular meshwork endothelium via direct injection into the Schlemm canal and in vivo toxicity study. Curr. Eye Res 30, 1051–1059. 10.1080/02713680500323350 [DOI] [PubMed] [Google Scholar]
- Husain S, Shearer TW, Crosson CE, 2006. Mechanisms Linking Adenosine A1 Receptors and Extracellular Signal-Regulated Kinase 1/2 Activation in Human Trabecular Meshwork Cells. J. Pharmacol. Exp. Ther 320, 258–265. 10.1124/jpet.106.110981 [DOI] [PubMed] [Google Scholar]
- Iizuka K, Yoshii A, Samizo K, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M, 1999. A major role for the Rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea. Br. J. Pharmacol 128, 925–933. 10.1038/sj.bjp.0702864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inazaki H, Kobayashi S, Anzai Y, Satoh H, Sato S, Inoue M, Yamane S, Kadonosono K, 2017. One-year efficacy of adjunctive use of Ripasudil, a rho-kinase inhibitor, in patients with glaucoma inadequately controlled with maximum medical therapy. Graefe’s Arch. Clin. Exp. Ophthalmol 255, 2009–2015. 10.1007/s00417-017-3727-5 [DOI] [PubMed] [Google Scholar]
- Inomata H, Bill A, Smelser GK, 1972a. Aqueous humor pathways through the trabecular meshwork and into Schlemm’s canal in the cynomolgus monkey (Macaca irus). An electron microscopic study. Am. J. Ophthalmol 73, 760–789. 10.1016/0002-9394(72)90394-7 [DOI] [PubMed] [Google Scholar]
- Inomata H, Bill A, Smelser GK, 1972b. Unconventional Routes of Aqueous Humor Outflow in Cynomolgus Monkey (Macaca Irus). Am. J. Ophthalmol 73, 893–907. 10.1016/0002-9394(72)90459-X [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanihara H, 2017. Ripasudil hydrochloride hydrate: targeting Rho kinase in the treatment of glaucoma. Expert Opin. Pharmacother 18, 1669–1673. 10.1080/14656566.2017.1378344 [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanihara H, 2013. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog. Retin. Eye Res 10.1016/j.preteyeres.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Johnstone MA, 2014. Intraocular Pressure Regulation: Findings of Pulse-Dependent Trabecular Meshwork Motion Lead to Unifying Concepts of Intraocular Pressure Homeostasis. J. Ocul. Pharmacol. Ther 30, 88–93. 10.1089/jop.2013.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bösl M, Bosserhoff A, Köstler J, Wagner R, Tamm ER, Fuchshofer R, 2012. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol 180, 2386–2403. 10.1016/j.ajpath.2012.02.030 [DOI] [PubMed] [Google Scholar]
- Kahook MY, Serle JB, Mah FS, Kim T, Raizman MB, Heah T, Ramirez-Davis N, Kopczynski CC, Usner DW, Novack GD, ROCKET-2 Study Group, 2019. Long-term Safety and Ocular Hypotensive Efficacy Evaluation of Netarsudil Ophthalmic Solution: Rho Kinase Elevated IOP Treatment Trial (ROCKET-2). Am. J. Ophthalmol 200, 130–137. 10.1016/j.ajo.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 2017. Latanoprostene bunod ophthalmic solution 0.024% for IOP lowering in glaucoma and ocular hypertension. Expert Opin. Pharmacother 18, 433–444. 10.1080/14656566.2017.1293654 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1987a. Adenosine 3′,5′-cyclic-monophosphate and outflow facility in monkey eyes with intact and retrodisplaced ciliary muscle. Exp. Eye Res 44, 415–423. 10.1016/S0014-4835(87)80175-6 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1987b. Non-additivity of maximal pilocarpine and cytochalasin effects on outflow facility. Exp. Eye Res 44, 283–291. 10.1016/S0014-4835(87)80012-X [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1986a. Epinephrine, norepinephrine, and isoproterenol dose-outflow facility response relationships in cynomolgus monkey eyes with and without ciliary muscle retrodisplacement. Acta Ophthalmol. 64, 356–363. 10.1111/j.1755-3768.1986.tb06933.x [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1986b. Total iridectomy does not alter outflow facility responses to cyclic AMP in cynomolgus monkeys. Exp. Eye Res 43, 441–447. 10.1016/S0014-4835(86)80079-3 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1979. Aqueous humor dynamics following total iridectomy in the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci 18, 870–875. [PubMed] [Google Scholar]
- Kaufman PL, 1976. Prognosis of primary rhegmatogenous retinal detachments: 2. Accounting for and predicting final visual acuity in surgically reattached cases. Acta Ophthalmol. 54, 61–74. 10.1111/j.1755-3768.1976.tb00419.x [DOI] [PubMed] [Google Scholar]
- Kaufman PL, 1975. Prognosis of primary rhegmatogenous retinal detachments: 1. Associations between clinical detachment characteristics, subretinal fluid butyrylcholinesterase and visual outcome following scleral buckling procedures. Acta Ophthalmol. 53, 660–671. 10.1111/j.1755-3768.1975.tb01785.x [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Bárány EH, 1976. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodisplacement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci 15, 793–807. [PubMed] [Google Scholar]
- Kaufman PL, Barany EH, Bárány EH, 1981. Adrenergic drug effects on aqueous outflow facility following ciliary muscle retrodisplacement in the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci 20, 644–651. [PubMed] [Google Scholar]
- Kaufman PL, Bárány EH, Erickson KA, 1982. Effect of serotonin, histamine and bradykinin on outflow facility following ciliary muscle retrodisplacement in the cynomolgus monkey. Exp. Eye Res 35, 191–199. 10.1016/S0014-4835(82)80066-3 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Bill A, Bárány EH, 1977. Effect of cytochalasin B on conventional drainage of aqueous humor in the cynomolgus monkey. Exp. Eye Res 25, 411–414. 10.1016/S0014-4835(77)80037-7 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Erickson KA, 1982. Cytochalasin B and D dose - outflow facility response relationships in the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci 23, 646–650. [PubMed] [Google Scholar]
- Kaufman PL, Lütjen-Drecoll E, 1975. Total iridectomy in the primate in vivo: surgical technique and postoperative anatomy. Invest. Ophthalmol 14, 766–71. [PubMed] [Google Scholar]
- Kaufman PL, Podos SM, 1973. Subretinal fluid butyrylcholinesterase. 1. Source of the enzyme and factors affecting its concentration in subretinal fluid from primary rhegmatogenous retinal detachments. Am. J. Ophthalmol 75, 627–636. 10.1016/0002-9394(73)90814-3 [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Rasmussen CA, 2012. Advances in glaucoma treatment and management: Outflow drugs. Investig. Ophthalmol. Vis. Sci 53, 2495–2500. 10.1167/iovs.12-9483m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PL, Rentzhog L, 1981. Effect of total iridectomy on outflow facility responses to adrenergic drugs in cynomolgus monkeys. Exp. Eye Res 33, 65–74. 10.1016/S0014-4835(81)80082-6 [DOI] [PubMed] [Google Scholar]
- Kaufman PL PS, 1974. Subretinal fluid butyrylcholinesterase. 2. Reoperated rhegmatogenous detachments and diabetic traction detachments. Am J Ophthalmol 19–24. [PubMed] [Google Scholar]
- Kaufman PL PS, 1973. The subretinal fluid in primary rhegmatogenous retinal detachment. Surv. Ophthalmol 100–116. [DOI] [PubMed] [Google Scholar]
- Kawase K, Vittitow JL, Weinreb RN, Araie M, Hoshiai S, Hashida S, Iwasaki M, Kano K, Kato T, Kuwayama Y, Muramatsu T, Mitsuhashi M, Matsuzaki S, Nakajima T, Sato I, Yoshimura Y, 2016. Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv. Ther 33, 1612–1627. 10.1007/s12325-016-0385-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee C, Kaufman PL, Gabelt BT, 1994. Effect of 8-Br cGMP on aqueous humor dynamics in monkeys. Invest. Ophthalmol. Vis. Sci 35, 2769–73. [PubMed] [Google Scholar]
- Lee ES, Gabelt BT, Faralli JA, Peters DM, Brandt CR, Kaufman PL, Bhattacharya SK, 2010. COCH transgene expression in cultured human trabecular meshwork cells and its effect on outflow facility in monkey organ cultured anterior segments. Investig. Ophthalmol. Vis. Sci 51, 2060–2066. 10.1167/iovs.09-4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Rasmussen CA, Filla MS, Slauson SR, Kolb AW, Peters DM, Kaufman PL, Gabelt BT, Brandt CR, 2014. Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Curr. Eye Res 10.3109/02713683.2014.884593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Shen Y, Eberle M, 1975. The long-acting Ocusert-pilocarpine system in the management of glaucoma. Invest. Ophthalmol 14, 43–6. [PubMed] [Google Scholar]
- Liebmann JM, Lee JK, 2017. Current therapeutic options and treatments in development for the management of primary open-angle glaucoma. Am. J. Manag. Care 23, S279–S292. [PubMed] [Google Scholar]
- Liu GJ, Mizukawa A, Okisaka S, 1994. Mechanism of intraocular pressure decrease after contact transscleral continuous-wave Nd:YAG laser cyclophotocoagulation. Ophthalmic Res. 26, 65–79. 10.1159/000267395 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang N, K.P., 2005. Experimental Gene Therapy for Glaucoma with C3 Transferase. China Biotechnol. 25, 109–117. [Google Scholar]
- Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL, 2005. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol. Vis 11, 1112–21. [PubMed] [Google Scholar]
- Loewen N, Fautsch MP, Teo WL, Bahler CK, Johnson DH, Poeschla EM, 2004. Longterm, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Investig. Ophthalmol. Vis. Sci 45, 3091–3098. 10.1167/iovs.04-0366 [DOI] [PubMed] [Google Scholar]
- Lotti VJ, Pawlowski N, 2009. Prostaglandins Mediate the Ocular Hypotensive Action of the Angiotensin Converting Enzyme Inhibitor MK-422 (Enalaprilat) in African Green Monkeys . J. Ocul. Pharmacol. Ther 6, 1–7. 10.1089/jop.1990.6.1 [DOI] [PubMed] [Google Scholar]
- Lütjen-Drecoll E, 1973. Structural Factors Influencing Outflow Facility and its Changeability Under Drugs A Study in Macaca Arctoides. Investig. Ophthalmol. Vis. Sci 12, 280–294. [PubMed] [Google Scholar]
- Lutjen-Drecoll E, Kaufman PL, Barany EH, 1977. Light and electron microscopy of the anterior chamber angle structures following surgical disinsertion of the ciliary muscle in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci 16, 218–225. [PubMed] [Google Scholar]
- McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ, 2008. Current management of glaucoma and the need for complete therapy. Am. J. Manag. Care https://doi.org/10022 [pii] [PubMed] [Google Scholar]
- Medeiros FA, Martin KR, Peace J, Scassellati Sforzolini B, Vittitow JL, Weinreb RN, 2016. Comparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: The LUNAR Study. Am. J. Ophthalmol 168, 250–259. 10.1016/j.ajo.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Menage MJ, Croft MA, Gange SJ, Kaufman PL, 1995. ε-Aminocaproic acid does not inhibit outflow resistance washout in monkeys. Investig. Ophthalmol. Vis. Sci 36, 1745–1749. [PubMed] [Google Scholar]
- Mermoud A, Baerveldt G, Mickler DS, Wu GS, Rao NA, 1994. Animal model for uveitic glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol 232, 553–560. 10.1007/BF00181999 [DOI] [PubMed] [Google Scholar]
- Montecchi-Palmer M, Bermudez JY, Webber HC, Patel GC, Clark AF, Mao W, 2017. TGFβ2 induces the formation of cross-linked actin networks (CLANs) in human trabecular meshwork cells through the smad and non-smad dependent pathways. Investig. Ophthalmol. Vis. Sci 58, 1288–1295. 10.1167/iovs.16-19672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JS, Sall KN, DuBiner H, Slomowitz N, McVicar W, Rich CC, Baumgartner RA, 2016. A Dose-Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of 2 and 4 Weeks of Twice-Daily Ocular Trabodenoson in Adults with Ocular Hypertension or Primary Open-Angle Glaucoma. J. Ocul. Pharmacol. Ther 32, 555–562. 10.1089/jop.2015.0148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JA, McKee M, 1995. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Invest. Ophthalmol. Vis. Sci 36, 1765–73. [PubMed] [Google Scholar]
- NCT02371746, 2015. Safety and Efficacy of ENV515 Travoprost Extended Release (XR) in Patients With Bilateral Ocular Hypertension or Primary Open Angle Glaucoma. Https://clinicaltrials.gov/show/nct02371746. [Google Scholar]
- Neufeld AH, Sears ML, 1974. Cyclic-AMP in ocular tissues of the rabbit, monkey, and human. Invest. Ophthalmol 13, 475–7. [PubMed] [Google Scholar]
- Nilsson SFE, Samuelsson M, Bill A, Stjernschantz J, 1989. Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2α−1-isopropylester in the cynomolgus monkey. Exp. Eye Res 48, 707–716. 10.1016/0014-4835(89)90011-0 [DOI] [PubMed] [Google Scholar]
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM, 2005. Persistence and adherence with topical glaucoma therapy. Am. J. Ophthalmol 140, 598.e1–598.e11. 10.1016/j.ajo.2005.04.051 [DOI] [PubMed] [Google Scholar]
- Ocklind A, 1998. Effect of Latanoprost on the Extracellular Matrix of the Ciliary Muscle. A Study on Cultured Cells and Tissue Sections. Exp. Eye Res 67, 179–191. 10.1006/exer.1998.0508 [DOI] [PubMed] [Google Scholar]
- Pang IH, Millar JC, Clark AF, 2015. Elevation of intraocular pressure in rodents using viral vectors targeting the trabecular meshwork. Exp. Eye Res 141, 33–41. 10.1016/j.exer.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson JE, Gaasterland DE, MacLellan HM, 1977. Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Invest. Ophthalmol. Vis. Sci 16, 1008–7. [PubMed] [Google Scholar]
- Perkins TW, Faha B, Ni M, Kiland JA, Poulsen GL, Antelman D, Atencio I, Shinoda J, Sinha D, Brumback L, Maneval D, Kaufman PL, Nickells RW, 2002. Adenovirus-mediated gene therapy using human p21WAF-1/Cip-1 to prevent wound healing in a rabbit model of glaucoma filtration surgery. Arch. Ophthalmol 120, 941–949. 10.1001/archopht.120.7.941 [DOI] [PubMed] [Google Scholar]
- Peterson JA, Tian B, Bershadsky AD, Volberg T, Gangnon RE, Spector I, Geiger B, Kaufman PL, 1999. Latrunculin-A increases outflow facility in the monkey. Investig. Ophthalmol. Vis. Sci 40, 931–941. [PubMed] [Google Scholar]
- Peterson JA, Tian B, Geiger B, Kaufman PL, 2000. Effect of Latrunculin-B on outflow facility in monkeys. Exp. Eye Res 70, 307–313. 10.1006/exer.1999.0797 [DOI] [PubMed] [Google Scholar]
- Petty HR, Martin SM, 1989. Combinative ligand-receptor interactions: Effects of CAMP, epinephrine, and met-enkephalin on RAW264 macrophage morphology, spreading, adherence, and microfilaments. J. Cell. Physiol 138, 247–256. 10.1002/jcp.1041380205 [DOI] [PubMed] [Google Scholar]
- Rahmatnejad K, Pruzan NL, Amanullah S, Shaukat BA, Resende AF, Waisbourd M, Zhan T, Moster MR, 2017. Surgical Outcomes of Gonioscopy-assisted Transluminal Trabeculotomy (GATT) in Patients with Open-angle Glaucoma. J. Glaucoma 26, 1137–1143. 10.1097/IJG.0000000000000802 [DOI] [PubMed] [Google Scholar]
- Rasmussen CA, Kaufman PL, Ritch R, Haque R, Brazzell RK, Vittitow JL, 2014. Latrunculin B Reduces Intraocular Pressure in Human Ocular Hypertension and Primary Open-Angle Glaucoma. Transl. Vis. Sci. Technol 3, 1. 10.1167/tvst.3.5.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JC, Kaufman PL, 1994. Phalloidin Inhibits Epinephrine’s and Cytochalasin B’s Facilitation of Aqueous Outflow. Arch. Ophthalmol 112, 1610–1613. 10.1001/archopht.1994.01090240116035 [DOI] [PubMed] [Google Scholar]
- Robinson JC, Kaufman PL, 1992. The effect of 12(R)-hydroxyeicosatetraenoic acid on aqueous humor dynamics in the rabbit and cynomolgus monkey. Exp. Eye Res 54, 813–5. [DOI] [PubMed] [Google Scholar]
- Robinson JC, Kaufman PL, 1991. Cytochalasin B potentiates epinephrine’s outflow facility-increasing effect. Investig. Ophthalmol. Vis. Sci 32, 1614–1618. [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lütjen-Drecoll E, 1981. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest. Ophthalmol. Vis. Sci 21, 574–85. [PubMed] [Google Scholar]
- Rohen JW, Lütjen E, Bárány E, 1967. The relation between the ciliary muscle and the trabecular meshwork and its importance for the effect of miotics on aqueous outflow resistance. Albr. von Graefes Arch. für Klin. und Exp. Ophthalmol 172, 23–47. 10.1007/bf00577152 [DOI] [PubMed] [Google Scholar]
- Rohen JW, Rentsch FJ, 1968. [Morphology of Schlemm’s canal and related vessels in the human eye]. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol 176, 309–29. 10.1007/bf00421587 [DOI] [PubMed] [Google Scholar]
- Roubeix C, Godefroy D, Mias C, Sapienza A, Riancho L, Degardin J, Fradot V, Ivkovic I, Picaud S, Sennlaub F, Denoyer A, Rostene W, Sahel JA, Parsadaniantz SM, Brignole-Baudouin F, Baudouin C, 2015. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther 6, 177. 10.1186/s13287-015-0168-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL, 2006. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp. Eye Res 82, 236–246. 10.1016/j.exer.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL, 2004. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp. Eye Res 78, 137–150. 10.1016/j.exer.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN, 1999a. Reduction of collagen type I in the ciliary muscle of inflamed monkey eyes. Investig. Ophthalmol. Vis. Sci 40, 2568–2576. [PubMed] [Google Scholar]
- Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN, 1999b. Topical prostaglandin F(2α) treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch. Ophthalmol 117, 794–801. 10.1001/archopht.117.6.794 [DOI] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ, 2010. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci 51, 207–216. 10.1167/iovs.09-3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serle JB, Katz LJ, McLaurin E, Heah T, Ramirez-Davis N, Usner DW, Novack GD, Kopczynski CC, 2018. Two Phase 3 Clinical Trials Comparing the Safety and Efficacy of Netarsudil to Timolol in Patients With Elevated Intraocular Pressure: Rho Kinase Elevated IOP Treatment Trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol 186, 116–127. 10.1016/j.ajo.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Shah GB, Sharma S, Mehta AA, Goyal RK, 2000. Oculohypotensive effect of angiotensin-converting enzyme inhibitors in acute and chronic models of glaucoma. J. Cardiovasc. Pharmacol 36, 169–175. 10.1097/00005344-200036001-00075 [DOI] [PubMed] [Google Scholar]
- Shearer TW, Crosson CE, 2002. Adenosine A1receptor modulation of MMP-2 secretion by trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci 43, 3016–3020. [PubMed] [Google Scholar]
- Slauson SR, Peters DM, Schwinn MK, Kaufman PL, Gabelt BT, Brandt CR, 2015. Viral vector effects on exoenzyme C3 transferase-mediated actin disruption and on outflow facility. Investig. Ophthalmol. Vis. Sci 56, 2431–2438. 10.1167/iovs.14-15909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider EJ, Vannatta RT, Schildmeyer L, Stamer WD, Ethier CR, 2018. Characterizing differences between MSCs and TM cells: Toward autologous stem cell therapies for the glaucomatous trabecular meshwork. J. Tissue Eng. Regen. Med 12, 695–704. 10.1002/term.2488 [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV, 1994. Signal transduction and regulation in smooth muscle. Nature. 10.1038/372231a0 [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A, 1983. Latrunculins: Novel marine toxins that disrupt microfilament organization in cultured cells. Science (80-. ). 219, 493–495. 10.1126/science.6681676 [DOI] [PubMed] [Google Scholar]
- Spector I, Shorlet NR, Blasberger D, Kashman Y, 1989. Latrunculins - novel marine macrolides that disrupt microfilament organization and affert cell growth: I. Comparison with cytochalasin D. Cell Motil. Cytoskeleton 13, 127–144. 10.1002/cm.970130302 [DOI] [PubMed] [Google Scholar]
- Stamer WD, Braakman ST, Zhou EH, Ethier CR, Fredberg JJ, Overby DR, Johnson M, 2015. Biomechanics of Schlemm’s canal endothelium and intraocular pressure reduction. Prog. Retin. Eye Res 10.1016/j.preteyeres.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ross Ethier C, 2011. eNOS, a pressure-dependent regulator of intraocular pressure. Investig. Ophthalmol. Vis. Sci 52, 9438–9444. 10.1167/iovs.11-7839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JD, Shekhawat N, Talwar N, Balkrishnan R, 2015. Impact of the introduction of generic latanoprost on glaucoma medication adherence. Ophthalmology 122, 738–747. 10.1016/j.ophtha.2014.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JD, Sloan FA, Lee PP, 2007. Rates of Glaucoma Medication Utilization Among Older Adults with Suspected Glaucoma, 1992 to 2002. Am. J. Ophthalmol 143. 10.1016/j.ajo.2006.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kimura K, Shirai H, Eguchi K, Higuchi S, Hinoki A, Ishimaru K, Brailoiu E, Dhanasekaran DN, Stemmle LN, Fields TA, Frank GD, Autieri MV, Eguchi S, 2009. Edothelial nitric oxide synthase inhibits G 12/13 and Rho-Kinase activated by the angiotensin II type-1 receptor implication in vascular migration. Arterioscler. Thromb. Vasc. Biol 29, 217–224. 10.1161/ATVBAHA.108.181024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedbergh B, Lutjen-Drecoll E, Ober M, Kaufman PL, 1978. Cytochalasin B-induced structural changes in the anterior ocular segment of the cynomolgus monkey. Investig. Ophthalmol. Vis. Sci 17, 718–734. [PubMed] [Google Scholar]
- Takano E, Kaufman PL, Bárány EH, 1975. [Impression of the 1975 General Meeting of the Japan Nursing Association. Disappointing attitude of the Assembly toward the nursing system]. Hokenfu. Zasshi 31, 339–41. [PubMed] [Google Scholar]
- Tamm ER, Braunger BM, Fuchshofer R, 2015. Intraocular Pressure and the Mechanisms Involved in Resistance of the Aqueous Humor Flow in the Trabecular Meshwork Outflow Pathways, in: Progress in Molecular Biology and Translational Science. pp. 301–314. 10.1016/bs.pmbts.2015.06.007 [DOI] [PubMed] [Google Scholar]
- Tian B, Brumback LC, Kaufman PL, 2000. ML-7, chelerythrine and phorbol ester increase outflow facility in the monkey eye. Exp. Eye Res 71, 551–566. 10.1006/exer.2000.0919 [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Crosson CE, Kaufman PL, 1997. Effects of adenosine agonists on intraocular pressure and aqueous humor dynamics in cynomolgus monkeys. Exp. Eye Res 64, 979–989. 10.1006/exer.1997.0296 [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Geiger B, Kaufman PL, 1999. Combined effects of H-7 and cytochalasin B on outflow facility in monkeys. Exp. Eye Res 68, 649–655. 10.1006/exer.1998.0647 [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL, 2005. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp. Eye Res 80, 215–225. 10.1016/j.exer.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Tian B, Wang RF, Podos SM, Kaufman PL, 2004. Effects of topical H-7 on outflow facility, intraocular pressure, and corneal thickness in monkeys. Arch. Ophthalmol 122, 1171–1177. 10.1001/archopht.122.8.1171 [DOI] [PubMed] [Google Scholar]
- Toris CB, Pederson JE, 1987. Aqueous humor dynamics in experimental iridocyclitis. Investig. Ophthalmol. Vis. Sci 28, 477–481. [PubMed] [Google Scholar]
- Traube M, 2006. Physiology of the Gastrointestinal Tract, in: Johnson L. (Ed.), Journal of Clinical Gastroenterology. Raven Press, p. 607. 10.1097/00004836-198910000-00040 [DOI] [Google Scholar]
- Tripathi RC, Li J, Chan WFA, Tripathi BJ, 1994. Aqueous humor in glaucomatous eyes contains an increased level of TGF-β2. Exp. Eye Res 59, 723–728. 10.1006/exer.1994.1158 [DOI] [PubMed] [Google Scholar]
- Tsai JC, 2009. A Comprehensive Perspective on Patient Adherence to Topical Glaucoma Therapy. Ophthalmology 116, S30–S36. 10.1016/j.ophtha.2009.06.024 [DOI] [PubMed] [Google Scholar]
- Vasantha Rao P, Deng PF, Kumar J, Epstein DL, 2001. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Investig. Ophthalmol. Vis. Sci 42, 1029–1037. [PubMed] [Google Scholar]
- Vinod K, Gedde SJ, 2017. Clinical investigation of new glaucoma procedures. Curr. Opin. Ophthalmol 10.1097/ICU.0000000000000336 [DOI] [PubMed] [Google Scholar]
- Walters TR, Lee SS, Goodkin ML, Whitcup SM, Robinson MR, 2017. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. Am. J. Ophthalmol 175, 137–147. 10.1016/j.ajo.2016.11.020 [DOI] [PubMed] [Google Scholar]
- Webber HC, Bermudez JY, Sethi A, Clark AF, Mao W, 2016. Crosstalk between TGFβ and Wnt signaling pathways in the human trabecular meshwork. Exp. Eye Res 148, 97–102. 10.1016/j.exer.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Liebmann JM, Martin KR, Kaufman PL, Vittitow JL, 2018. Latanoprostene Bunod 0.024% in Subjects with Open-angle Glaucoma or Ocular Hypertension: Pooled Phase 3 Study Findings. J. Glaucoma 27, 7–15. 10.1097/IJG.0000000000000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Ong T, Sforzolini BS, Vittitow JL, Singh K, Kaufman PL, Ackerman S, Branch J, Cottingham A, Day D, Depenbusch M, El-Hazari S, Firozvi A, Jorizzo P, Ou R, Peace J, Rotberg M, Schenker H, Smith S, Tyson F, Zaman F, Madzharova L, Toshev R, Vassilveva P, Misiuk-Hojło M, Kocięcki J, Liehneova I, Růžičková E, Scassellati Sforzolini B, Vittitow JL, Singh K, Kaufman PL, 2015. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: The VOYAGER study. Br. J. Ophthalmol 99, 738–745. 10.1136/bjophthalmol-2014-305908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücel YH, Johnston MG, Ly T, Patel M, Drake B, Gümüş E, Fraenkl SA, Moore S, Tobbia D, Armstrong D, Horvath E, Gupta N, 2009. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp. Eye Res 89, 810–819. 10.1016/j.exer.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Yun H, Wang Y, Zhou Y, Wang K, Sun M, Stolz DB, Xia X, Ethier CR, Du Y, 2018. Human stem cells home to and repair laser-damaged trabecular meshwork in a mouse model. Commun. Biol 1, 216. 10.1038/s42003-018-0227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Zhou Y, Wills A, Du Y, 2016. Stem Cells in the Trabecular Meshwork for Regulating Intraocular Pressure. J. Ocul. Pharmacol. Ther 32, 253–260. 10.1089/jop.2016.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gramlich OW, Laboissonniere L, Jain A, Sheffield VC, Trimarchi JM, Tucker BA, Kuehn MH, 2016. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl. Acad. Sci 113, E3492–E3500. 10.1073/pnas.1604153113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH, 2017. Restoration of aqueous humor outflow following transplantation of iPSC-derived trabecular meshwork cells in a transgenic mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci 58, 2054–2062. 10.1167/iovs.16-20672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.