Abstract
The pharmaceutical industry has been desperately searching for efficient drug discovery methods. Organ-on-a-Chip, a cutting-edge technology that can emulate the physiological environment and functionality of human organs on a chip for disease modeling and drug testing, shows great potential in revolutionizing the drug development pipeline. However, successful translation of this novel engineering platform into routine pharmacological and medical scenarios remains to be realized. This review discusses how the Organ-on-a-Chip technology can play critical roles at different preclinical stages of drug development and highlights the current challenges in translation and commercialization of this technology for the pharmacological and medical end-users. Moreover, this review sheds light on the future developmental trends and need for a next-generation Organ-on-a-Chip platform to bridge the gap between animal studies and clinical trials for the pharmaceutical industry.
Keywords: Organ-on-a-Chip, microphysiological system, microfluidics, drug development, precision medicine
Microfluidic Organ-on-a-Chip: A paradigm shift in drug development
The pharmaceutical industry has continuously sought a productive and efficient research and development (R&D, see Glossary) framework for drug discovery. However, the current in vitro two-dimensional (2D) or three-dimensional (3D) cell culture and in vivo animal experimentation platforms (Figure 1) remain unsatisfactory for an efficient and accurate preclinical evaluation of drug efficacy and toxicity before clinical trials can be approved for testing in human subjects [1, 2]. To date, animal studies remain the gold standard for the preclinical validation of drugs in pharmaceutical development; however, the accuracy and reproducibility of the testing results obtained from animal studies are undermined in humans owing to species differences between the animal and human systems [3, 4]. Because of variable responses and unexpected toxicity in humans, approximately 40% of the newly developed drugs fail clinical trials even after accomplishing preclinical evaluation with animal models [5]. Drug development involves assessment of the physiological and toxicological effects of numerous compounds and their derivatives to identify the most effective and safe drug candidates. However, the limitations of low-throughput in vivo animal studies largely contribute to the prolonged drug development life cycle and increased development cost. For emergent cases, such as the coronavirus disease (COVID-19) pandemic, rapid drug screening platforms are urgently required to accelerate the development of new therapeutics and vaccines [6]. In addition, although in vitro cell culture in Petri dishes is a simple and high-throughput method for basic drug screening and testing, these cellular models generally lack the in vivo tissue microarchitecture and physiological functionality. Therefore, alternative tissue models with biomimetic human pathophysiology are urgently required to bridge the gap between animal studies and clinical trials involving human subjects in the drug development pipeline [7].
Figure 1. Microfluidic Organ-on-a-Chip platform.
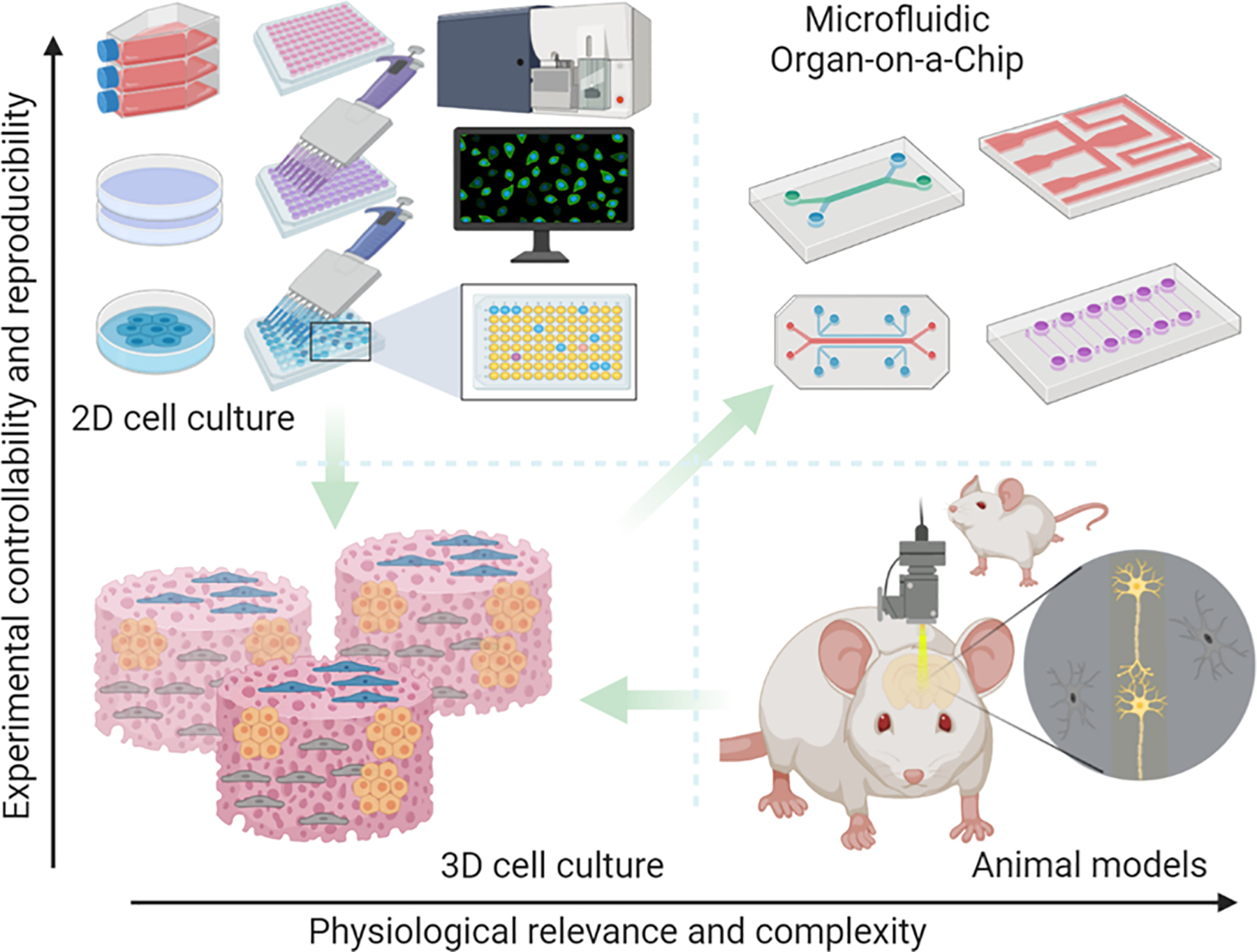
Preclinical studies rely on major tools, i.e. 2D or 3D in vitro cell cultures, and in vivo animal models, for drug development. 2D in vitro culture offers a rapid and reproducible way to analyze drug response; however, they lack the 3D physiological tissue environment. Conventional 3D cell culture in the hydrogel matrix though can provide a 3D culture environment, it still falls short to controllably recapitulate the in vivo physiology and pathology in the human body. Animal models enable in vivo analysis, yet the species of differences between animal and human physiological mechanisms and complexity of the in vivo physiology weakens the accuracy and reproducibility of experimental results. Microfluidic Organ-on-Chip platform that enables controllable cell culture within an organotypic microarchitectural environment provides a simple yet more physiologically relevant platform to controllably and systematically interrogate human biology. Figure generated in BioRender (BioRender.com).
Recent advances in the microfluidics-based Organ-on-a-Chip technique, also termed as the microphysiological system that mimics the physiology and functionality of human organs on a chip, have been envisaged to foster a paradigm shift in drug development and personalized medicine by replacing animal testing [8]. The origin of the Organ-on-a-Chip can be traced to three decades ago, beginning with the application of microfluidic devices for cell culture and biological analysis [9–12]. As opposed to tissue engineering, being designed from the viewpoint of reductionism, Organ-on-a-Chip does not strive to reproduce the whole tissues or organs at the original scale for clinical replacement of their human counterparts [12, 13]. Instead, this technique aims at mimicking the key organotypic cellular architecture and functionality, 3D extracellular matrix (ECM), biochemical factors, and biophysical cues at a smaller scale, which serves the purpose for disease modeling and drug screening. As a type of microfluidic device, Organ-on-a-Chip is fabricated with the silicon-based organic polymer polydimethylsiloxane (PDMS) using the standard soft lithography technique; as such, the chip has a compact size and microchannels to precisely pattern cells and manipulate various fluidic and chemical parameters, such as flow rate, pressure, oxygen, and pH, providing controllable culture conditions [9–12] (Box 1). This reflects the in vivo microstructural and functional characteristics of human tissues and organs, thus enabling effective and accurate research in medicine, biology, and pharmacology. Despite the revolution that the Organ-on-a-Chip technique may bring to the pharmaceutical industry, its overall impact remains to be determined, with grand challenges existing in the transition from basic research to preclinical integration of this platform into the drug development pipeline.
Box 1: General fabrication processes of Organ-on-a-Chip device.
Organ-on-a-Chip is a microfluidic culture system fabricated with polydimethylsiloxane (PDMS) using the soft lithography technique. PDMS polymer has become a dominantly used structure material in microfluidics due to its high elasticity, gas permeability, optical transparency, and biocompatibility, since the PDMS-based replica modeling process (soft lithography) was developed by the Whitesides group [9–12]. Normally, master molds of the designed structure components are first fabricated with the photolithography-based protocol. Then different layers of the microfluidic device are generated with PDMS polymer using soft lithography which replicates the micro-size features from photolithographic molds into PDMS slabs, followed by assembly with glass slides via oxygen plasma-assisted bonding. Therefore, the PDMS-based microfluidic chips enable the ease of fabrication, handling, and integration, long-term cell culture, real-time imaging and monitoring of Organ-on-a-Chip cultures [11, 12]. The overall steps to manufacture Organ-on-a-Chip devices are similar, though different structural designs exist to properly imitate characteristics of different tissues/organs.
Single- and multiple-Organ-on-a-Chip systems
Since the early 2000s, researchers attempted to apply various microfluidic devices and lab-on-a-chip systems to enable controllable and organotypic cell culture for in vitro biochemical and pharmacological analyses [11, 12], which incubated the concept of Organ-on-a-Chip system. In 2010, the Ingber group at the Harvard Medical School reported a Lung-on-a-Chip model built on Huh’s early work from the Takayama group [14, 15], which attracted enormous attention from both the biology and engineering communities and was deemed to usher in the blossoming development of Organ-on-a-Chip. By co-culturing the lung alveolar and capillary cells on the two sides of a porous membrane in the microfluidic channels of the Lung-on-a-Chip, researchers can study the breathing mechanisms occurring at the alveoli–capillary interface of the human lungs as well as the environmental effects on lung cells in vitro, providing a biomimetic model to decipher the pathological mechanisms underlying various pulmonary or other respiratory diseases, such as COVID-19 [6]. Since then, numerous single-organ chips, such as liver chips [4, 16, 17], kidney chips [18, 19], pancreas chips [20, 21], heart chips [22–26], intestine and gut chips [27–29], blood–brain barrier (BBB) chips [30, 31], and bone and bone marrow chips [32–34], have been successfully developed for investigating disease progression and analyzing adverse drug reactions. These single-organ chip assays can help identify critical biological mechanisms as well as test drug efficiency and toxicity in target organs at the preclinical development stage, thus providing a reliable reference for clinical trials.
While single-organ chips focus on mimicking individual organ functions, multi-organ chips integrating multiple organ units, such as the gut compartment for drug absorbance, liver compartment for drug metabolism, and kidney compartment for drug elimination, in a single chip have recently become prevalent to enable more comprehensive studies [35, 36]. For instance, a heart–liver–skin three-organ system was developed by Pires de Mello et al. [35] to analyze the effects of acute and chronic drug exposure on both heart and liver functions. In addition, a four-organ chip integrated with sequentially connected intestine, liver, skin, and kidney compartments, with stable homeostasis across different organ compartments, was developed for testing the systemic toxicity of drug candidates [36]. Currently, development of an even advanced version, termed “Body-on-a-Chip” or “Human-on-a-Chip”, is underway to mirror the physiology of the entire human body using a single platform for drug pharmacokinetic and pharmacodynamic analyses [37–39]. For instance, Miller and Shuler [40] developed a proof-of-concept 13-organ system with various cell lines representing the main parenchymal organs and physiological barrier tissues of humans, demonstrating a physical framework to investigate the inter-organ commutation in response to drug challenges at the human level. Undoubtedly, the Human-on-a-Chip platform has the potential to serve as an alternative model system to replace animal models in drug development, ultimately revolutionizing the pharmaceutical industry; however, there are numerous technical challenges to be addressed given the complexity of the human system.
Organ-on-a-Chip embraces drug development: A perfect match
The Organ-on-a-Chip technology, aping human physiology, can be organically incorporated into the drug development pipeline (Box 2) from early drug discovery to preclinical screening, testing, and translation before the approval of a drug by the US Food and Drug Administration (FDAi) for use in clinical trials and finally in the market (Figure 2, Key Figure).
Box 2: Drug development pipeline.
The entire process of drug development ranges from early drug discovery through basic biological research, disease modeling, and target discovery, preclinical studies with in vitro, ex vivo, and in vivo models, and clinical development with human subjects (phase I, II, and III trials), to finally FDA review and approval and post-market monitoring (Figure I) [65, 108]. Preclinical development encompasses activities that link drug discovery in the laboratory to the initiation of clinical trials involving human subjects. Typically, preclinical drug development can be divided into three principal stages: (1) early drug discovery stage: target identification, disease modeling, and drug discovery; (2) preclinical screening and testing stage: lead optimization and pharmacokinetic and pharmacodynamic (PK/PD) studies; and (3) preclinical trial and translational stage: validation of drug toxicity and efficacy. Each stage aims at eliminating ineligible drug candidates, which are either ineffective or toxic. Specifically, the early drug discovery stage seeks to understand the biological and pathophysiological mechanisms underlying a specific disease and identify druggable targets, followed by drug modeling and screening to consequently discover candidate compounds. Subsequently, the preclinical screening and testing stage mainly focuses on the delineation of the PK and PD profiles of the drug candidates using conventional mammalian models and aims to determine the initial drug regimens for clinical trials. The preclinical trial and translational stage includes toxicological and safety studies, since drugs exhibiting no adverse effects in the preclinical stage may lead to hepatic, cardiac, or neural impairment during clinical trials as well as produce unexpected toxicity induced by drug–drug interactions. Therefore, the balance between toxicity and efficacy is an important decision-making process during the late preclinical stage before approving the drug for use in clinical trials involving human subjects. These clinical data regarding the safety and efficiency of developed drugs will be critical for FDA review and approval and as well the drugs entering the market will be under surveillance for any potential adverse drug reactions that were not identified during preclinical and clinical studies.
Figure I. Drug development pipeline.
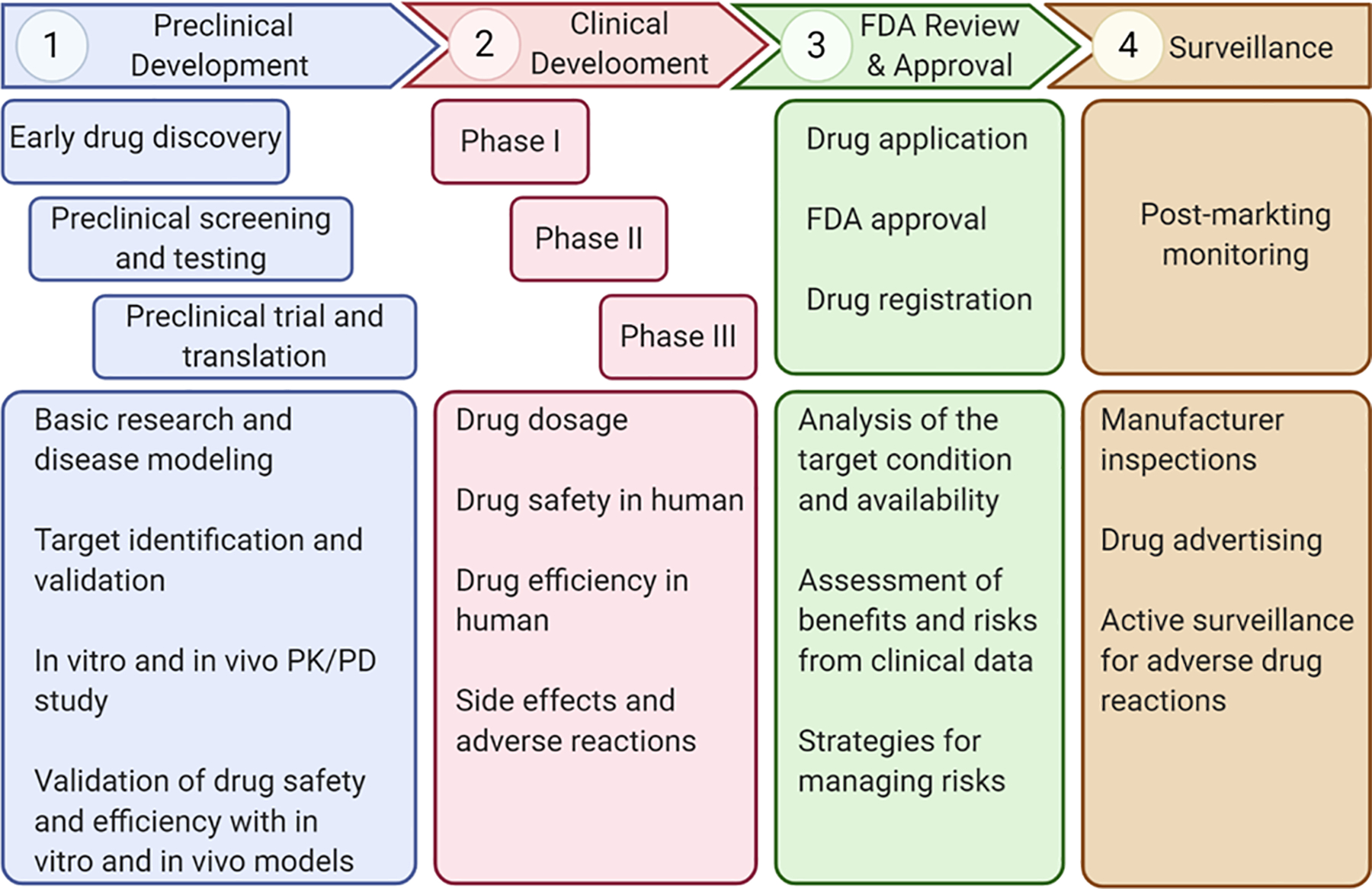
The whole drug development process starts from early drug discovery through basic biological research, disease modeling, and target discovery to search for potential drugs. These drug candidates identified during early stage are then screened and tested with various preclinical studies by in vitro, ex vivo, and in vivo models before being approved for subsequent human trials, such as phase I, II, and III trials, during clinical development. Finally, the developed drug that is determined both effective and safe in human is submitted to FDA for regulatory review and commercial approval, after which post-market measures will be implemented for monitoring potential adverse drug reactions. Figure generated in BioRender (BioRender.com).
Figure 2. Organ-on-a-Chip platforms in preclinical drug development.
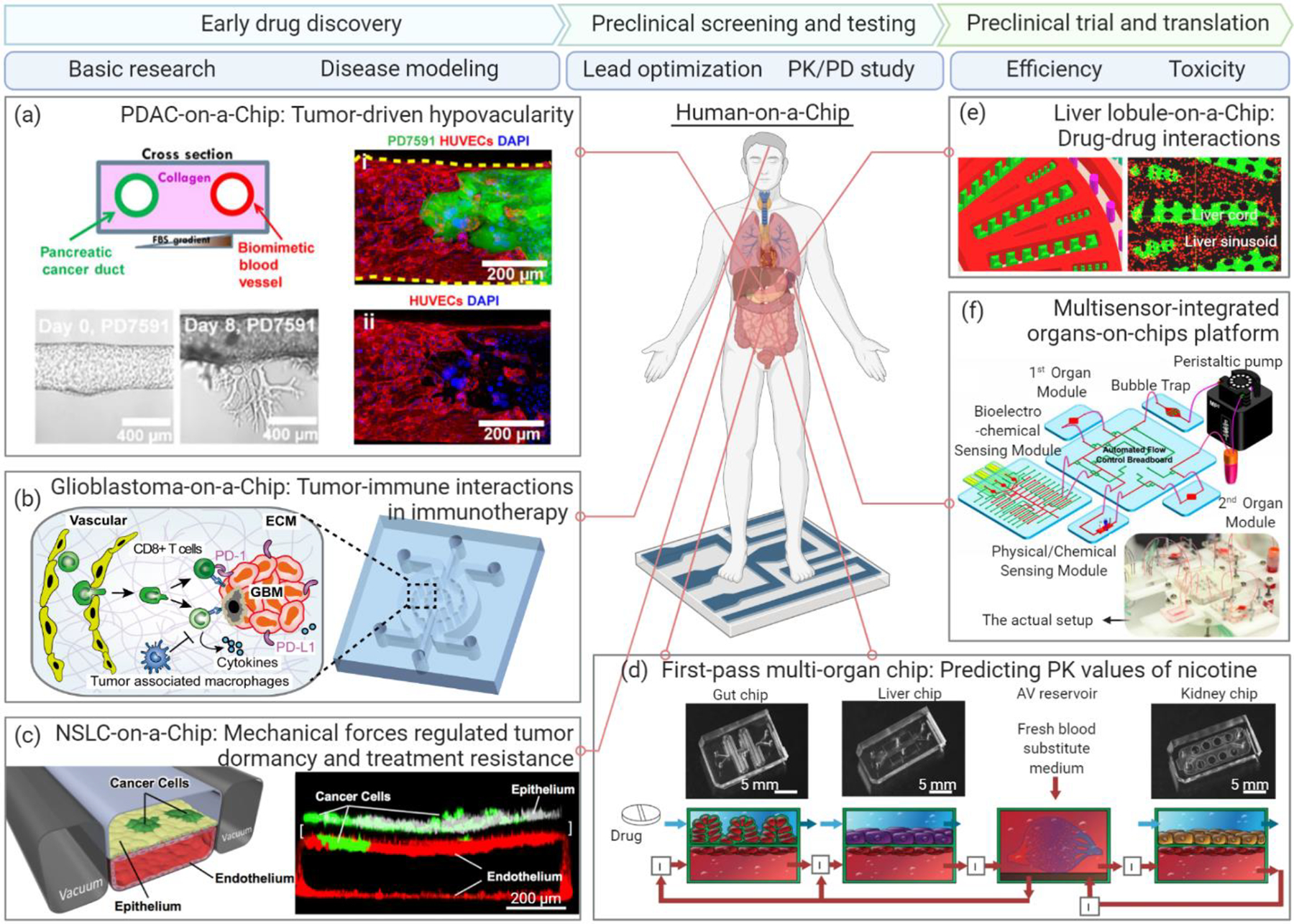
Preclinical drug development includes three main stages: early drug discovery, preclinical screening and testing, and preclinical trial and translation. (a) A PDAC-on-a-Chip (left) with a biomimetic vascular network (right) (HUVECs, red) and pancreatic cancer duct (PD7591 cells, green) revealed the Activin-ALK7 pathway as a hypovascularity mechanism for PDAC. Figure adapted with permission from ref. [41], AAAS. (b) A bioengineered glioblastoma brain tumor model with biomimetic tumor-immune-vascular interactions demonstrated that blockade of immunosuppression contributed by tumor-associated macrophage improved anti-PD-1 immunotherapy. Figure adapted with permission from ref. [48], eLife. (c) An NSCLC microenvironment model study found that the mechanical forces during lung breath (vacuum-driven in two side channels) may promote dormancy and drug resistance of NSCLC cells. Figure adapted with permission from ref. [43], Elsevier. (d) A first-pass multi-organ system was applied to predict the PK parameters of nicotine with a linked gut-liver-kidney chip. Figure adapted with permission from ref. [58], Springer Nature. (e) A liver lobule-on-a-chip consisted of a liver cord (green) and a liver sinusoid (red) was applied to analyze adverse drug reactions induced by unexpected drug-drug interactions. Figure adapted with permission from ref. [16], ACS. (f) A multi-organ platform integrated with a multiplex biomarker analysis module was developed to noninvasively monitor liver toxicity, as well as cardiotoxicity mediated by inter-organ metabolism. Figure adapted with permission from ref. [67], PNAS. Abbreviations: NSCLC, non-small-cell lung cancer; PDAC, pancreatic ductal adenocarcinoma. Figure generated in BioRender (BioRender.com).
Early drug discovery: Basic research and disease modeling
A central reason for the high failure rate of new drugs in clinical trials is our insufficient understanding of the fundamental human pathophysiology and the underlying mechanisms. Organ-on-a-Chip can better model the human system to pinpoint the drug targets in a controllable and traceable manner than animal models. For instance, the Organ-on-a-Chip platform is a powerful tool for studying the multifaceted processes and mechanisms contributing to cancer progression and treatment, such as cancer cell migration and invasion, extracellular signaling, biophysical factors in the tumor microenvironment, and tumor heterogeneity [32, 41–45]. In particular, many 3D cancer-on-a-chip models have been developed to mimic various types of solid and liquid tumor microenvironments involving different stromal components, immune suppressor cells, and chemokines to elucidate the mechanisms of resistance to chemotherapy and immunotherapy [46–48]. An organotypic pancreatic cancer chip model (Figure 2a) was developed to investigate the interactions of pancreatic ductal adenocarcinoma (PDAC, a major exocrine pancreatic cancer) with the tumor vascular network, and the activin–ALK7 pathway identified to be the mechanism of PDAC hypovascularity, leading to poor drug delivery and chemotherapeutic outcomes of pancreatic cancer treatment [41]. Using a glioblastoma-on-a-chip brain tumor microenvironment model (Figure 2b), researchers revealed the immunological mechanisms, such as macrophage-mediated angiogenesis and immunosuppression, underlying the regulation of resistance to both chemotherapy and immunotherapy [45, 48]. In addition to solid tumor models, a 3D B-cell acute lymphoblastic leukemia bone marrow niche model was bioengineered by Ma et al. [47, 49] at the New York University to clarify the contributions of genetically different leukemia bone marrow niches to chemotherapeutic resistance. Using this leukemia liquid tumor niche model, the researchers revealed that the stromal niche cell-mediated chemokine CXCL12, vascular cell adhesion protein-1 signal, and leukemia intrinsic NF-κB signaling as well as the nonclassical monocytic immune cell subsets may be the key mediators of and targets for regulating the response of leukemia to chemotherapy. More importantly, multi-organ chip systems linked with the vasculature and circulatory system are crucial for elucidating local and distant disease development, such as cancer initiation and metastasis [50]. For instance, a four-organ chip that recapitulated lung cancer metastasis to the brain, bone, and liver revealed tumor-induced tissue damage in the targeted bone and liver compartments [51].
In addition to revealing the underlying biological signaling and interactions, Organ-on-a-Chip can be applied to study the contributions of mechanobiological factors (Mechanobiology) to disease progression and therapeutic resistance [32, 43, 52]. By applying the lung chip for constructing a non-small-cell lung cancer (NSCLC) microenvironment model, Hassell et al. [43] found that mechanical forces during breathing may promote dormancy and drug resistance of NSCLC cells to tyrosine kinase inhibitor therapy via the epidermal growth factor receptor and mesenchymal–epithelial transition protein kinase (Figure 2c). Tumor dormancy and chemoresistance regulated by extracellular physical factors have also been demonstrated in a bone perivascular niche model of metastasized breast cancer, which experienced controllable interstitial flow, oxygen gradient, and shear stress [32]. These studies, as well as many others, have thereby built confidence in the justification that Organ-on-a-Chip can serve as a replacement or alternative modeling platform to the current animal models to help uncover critical pathological mechanisms and identify therapeutic biomarkers and targets for improving disease outcomes.
Preclinical screening and testing: Pharmacokinetics and pharmacodynamics
Pharmacokinetic (PK) and pharmacodynamic (PD) studies follow the identification of drug candidates and targets. Typically, PK studies profile the drug concentrations at different organ sites during the metabolic processes, termed the ADME (i.e., absorption, distribution, metabolism, and elimination) characterization of a drug candidate, whereas PD studies examine the effects of the drug (e.g., the relationship between drug dose and pharmacological or toxicological response) on target organs or tissues. Multi-organ chip systems comprising major metabolically related organ compartments are particularly suitable for systematic and in situ PK–PD studies across diverse human organ sites and under various drug administration conditions [37, 53–55]. Of particular interest are the liver and kidney, which play pivotal roles in drug metabolism, as well as the heart, which is strongly affected by drug toxicity. Given the various delivery modes of drugs, such as transdermal delivery, oral administration, and intravenous injection, the skin, gut, and bone marrow compartments can be integrated [37, 53]. A PK–PD study using a 3D tumor–liver–bone marrow multi-organ chip system was first introduced by the Shuler group at the Cornell University to assess the toxicity and mechanism of action of the anticancer drug 5-fluorouracil; the authors found that the liver compartment was more resistant to the drug than the bone marrow and tumor compartments [56, 57]. Similarly, gut–liver–kidney and bone marrow–liver–kidney multi-organ systems have been developed by Herland et al. [58] to predict the PK parameters of nicotine (an orally administered drug for aiding smoking cessation) and cisplatin (an intravenously injected anticancer drug), such as the maximum nicotine concentration in the arteriovenous reservoir and the time to reach the maximum level, which were consistent with clinical data (Figure 2d). The bone marrow–liver–kidney multi-organ system further confirmed the pharmacological responses to cisplatin: when administered at a dose of 160 μM for 24 h, cisplatin exhibited no hepatotoxicity in the liver chip, but exhibited myeloid toxicity and nephrotoxicity in the bone marrow and kidney chips, respectively, recapturing the in vivo PDs of cisplatin [58]. The consistency of the in vitro quantified and predicted PK and PD parameters with the corresponding clinical data highlights the high functionality of these integrated multi-organ chips, which may help optimize the drug regimens for phase I clinical trials. Furthermore, a human-on-a-chip platform containing a functional human immune component (circulating monocytic cells) has been developed to evaluate the tissue-specific immune responses of the cardiac, skeletal, and hepatic compartments to amiodarone (an anti-arrhythmic drug) treatment, highlighting the potential application of this system to assess tissue-specific responses of a given drug in the PK–PD profile [59].
To map the processes of drug transport and delivery across different organs on a chip, a fluidic circulatory system should be established between multiple organ compartments, and the physiological barriers between the vasculature and parenchymal tissues need to be modeled as well [50, 60, 61]. For example, a vascularized multi-organ chip with the intestine, liver, kidney, heart, lung, skin, BBB, and brain organ compartments fluidically connected via endothelialized vascular microchannels and periodically perfused with a common blood substitute has been developed [54]. This platform was successfully applied to timely capture and quantitatively predict drug distribution across multi-organ compartments using an inulin tracer. In addition to the vasculature and circulatory system, physiological barriers in other tissues or organs, such as BBB [30, 31], blood–air barrier in the lung [14, 15], glomerular filtration barrier in the kidney [62], human placental barrier [63, 64] and other tissue–tissue interfaces between the vascular endothelium and the parenchymal cells [65], are also essential for studying drug transport and delivery kinetics. For instance, BBB is a highly selective physiological barrier formed by the brain capillary endothelial cells, astrocytes, and pericytes, which regulates the penetration of biochemical molecules, such as glucose, from the blood into the brain microenvironment through specific transport proteins [30, 31]. Likewise, either drugs themselves or their metabolites must be assessed during drug development to ensure that they are either able to penetrate through the BBB to treat neural disorders or restricted by the BBB to prevent the off-target brain damage. A microfluidic BBB model infused with endothelial cells, neuroblastoma cells, microglia, and astrocytes has been applied to study organophosphate toxicity to BBB, such as barrier integrity damage, acetylcholinesterase inhibition, and cellular viability reduction [31]. Such a BBB platform can be functionally coupled with chips for other parenchymal organs, such as the jejunum, liver, and kidney, to study the sequential metabolism of drug across multiple organ compartments and the BBB, highlighting a systematic platform to validate therapeutics for neural diseases [55]. Overall, it remains technically challenging to reconstruct and integrate these blood–tissue and tissue–tissue interfaces into multi-organ chips and thus requires a close collaboration among engineers, biologists, pharmacologists, and computational scientists.
Preclinical trial and translation: Evaluation of drug safety and efficiency
Many drugs may show no adverse effects on animals during the preclinical stage but unpredictably exhibit hepatic, cardiac, or renal impairments in patients during clinical trials [3, 4]. Therefore, the evaluation of toxicity and efficacy is a paramount decision-making process during the late stage of preclinical development and clinical trials [66]. Human Organ-on-a-Chip can thus serve as a useful tool for efficient and accurate assessment of drug toxicity before the drug is approved for use in clinical trials. For instance, a biomimetic human liver chip with lobule-like microarchitectures was employed to analyze the adverse reactions induced by drug–drug interactions during liver metabolism, offering a screening platform for drug toxicity and safety during combinational therapies (Figure 2e) [16]. In addition, multi-organ chips can be specifically applied to study both on- and off-target effects as well as the inter-organ metabolism of drugs [67–69]. For instance, a multi-organ platform can allow the integration of a multiplex, automated, noninvasive biomarker analysis module to monitor drug toxicity in the liver and heart (Figure 2f). The results validated that anticancer drugs, such as capecitabine, exhibited hepatotoxicity when metabolized by hepatocytes into the active form as well as cardiotoxicity [67]. More recently, McAleer et al. [68] noted variable on- and off-target effects of anticancer drugs in a multi-organ system, possibly due to liver cell-mediated drug metabolism. Notably, construction of the multi-organ chips and advanced human-on-a-chip requires comprehensive and systematic consideration of various biological and technical factors, such as organ scaling and integration, during conceptualization, but the multidimensionality of the human body makes this a challenging task. Thus, while a complex multi-organ chip can provide a more physiologically relevant scenario for drug toxicity testing, a balance between feasibility and complexity of the system should be taken into account during development.
Due to genetic and microenvironmental heterogeneities, responses of patients to drugs are often variable, necessitating the accurate evaluation of therapeutic efficacy and optimization for individual patients. Organ-on-a-Chip integrates primary cells derived from healthy and patient donors, demonstrating the feasibility of assessing patient-specific drug responses in an organotypic human pathophysiological environment [31, 70]. For instance, a human small airway chip infused with cells from patients with chronic obstructive pulmonary disease reliably reproduced the relevant clinical symptoms [70]. Recently, cancer immunotherapies, such as PD-1-based immune checkpoint blockade and adoptive T-cell transfer [i.e., T-cell receptor- and chimeric antigen receptor (CAR)-engineered T-cells], have demonstrated encouraging outcomes in clinical trials of patients with various cancers [71, 72]. However, most patients continued to exhibit suboptimal response and even experienced disease relapse, suggesting the need for the stratification and selection of patients to achieve effective therapeutic response and disease management [73]. Accordingly, 3D glioblastoma chips using different molecular subtypes of patient-derived tumor cells [48] or organotypic tumor spheroids [74] were engineered to recapture patient tumor immunity, evaluate patient-specific responsiveness to anti-PD-1 immunotherapy, and screen for additional therapeutic combinations. Moreover, a micropatterned tumor array was developed to dynamically monitor CAR T-cell trafficking and evaluate its killing activity, highlighting significant differences across CAR T-cell products of different donors as well as across various CAR T-cell constructs, indicating that this chip may serve as a preclinical screening platform for the quality check of CAR T-cell products [75]. Taken together, these platforms demonstrate the feasibility of ex vivo modeling of the tumor immune niche and predict therapeutic efficiency in a given patient, suggesting the superiority of these models over animal models which lack human immunity. Nevertheless, current studies on Organ-on-a-Chips with a human immune component in drug development remain at the infant stage and require significant effort in the future [76–78]. Most Organ-on-a-Chip systems developed thus far utilize allogeneic cells, and further incorporation of autologous patient-derived immune and tumor cells is imperative to reliably evaluate and predict patient outcome during or even prior to immunotherapy administration in clinical trials.
Organ-on-a-Chip marches toward the market
Major players, costumers, and business models
Dramatic growth of the market and need for drug development are anticipated once the Organ-on-a-Chip products pave their way into the drug discovery segment. Because of the emerging Organ-on-a-Chip market, demands are dynamic and influencing factors are diverse. For example, academic researchers may require complex microphysiological systems to reveal pathophysiological mechanisms at the early drug discovery stage; biotech and pharmaceutical companies may prefer a high-throughput yet low-cost platform for rapid screening of drug candidates, PK–PD studies, and toxicity and efficiency evaluations at the preclinical drug development stage; and other end-users, such as clinics, may prefer a standardized system or customized service for the screening of personalized drugs. We herein gathered publicly accessible information on major players (Table 1) from various sources, including company websites, Crunchbaseii, and other publications, with permission [8, 79, 80]. At present, most Organ-on-a-Chip startups prefer to provide standard liver and cancer chips because of their high demand in drug PK–PD and toxicity studies at the preclinical stage. In general, device type and its reliability are the contributing factors for market positioning of Organ-on-a-Chip startups [80, 81]. Of note, although most current Organ-on-a-Chip devices share similar fabrication methods, constant upgrade and differentiation of products while ensuring unique or highly integrated solutions to address the end-users’ needs would help these startups succeed in a competitive market. For instance, Mimetas OrganoPlate® offers a unique solution with Phaseguides™ for passive liquid handling of cells and gel loading. This platform is manufactured to support up to 96 tissue models on a single industry-standard 384-well plate to enable compatibility with the standard liquid handling and readout equipment, facilitating high-throughput drug screening. A more integrated solution, called “Human Emulation System,” comprising organ chips, hardware, and software applications, sold by Emulate Inc., offers a highly standardized organ chip platform, which has garnered much attention from the scientific, pharmaceutical, and industrial communities, as well as from the venture capital industry.
Table 1.
A brief list of Organ-on-a-Chip startups worldwide.
| Company | Found Year | University Spin-off | Scientific Founders | Major Products | Region |
|---|---|---|---|---|---|
| HμREL | 2006 | Cornell University | Greg Baxter Robert Freedman | Liver chip | United States |
| Kirkstall | 2006 | University of Pisa | John Wilkinson | Quasi Vivo® system | British |
| Hepregen, (merged into BioIVT) | 2007 | MIT | Sangeeta Bhatia | Liver, Islet, Cancer model, Accessory devices | United States |
| CN-Bio Innovations | 2009 | MIT | Linda G Griffith | Liver, Gut, Skin, Heart, Lung, Kidney, Brain chip, | British |
| InSphero | 2009 | N/A | Jan Lichtenberg Jens M. Kelm Wolfgang Moritz | Liver, Islet, Tumor cell culture | Switzerland |
| TissUse | 2010 | Berlin Institute of Technology | Uwe Marx | Multi-organ-chip, Accessory devices | Germany |
| Nortis | 2012 | Washington University | Thomas Neumann | Kidney, Liver, Multi-organ-chip, Accessory devices | United States |
| Emulate | 2013 | Harvard University | Donald Ingber | Liver, Kidney, Lung, Intestine chip, Accessory devices | United States |
| Mimetas | 2013 | Leiden University | Jos Joore Paul Vulto Thomas Hankermeier | Kidney, Gut, Tumors, Liver, Lung, Intestine, Blood vessel, Neuronal models, Accessory devices | The Netherland |
| Axosim | 2014 | Tulane University | Michael Moore | Nerve-on-chip | United States |
| SynVivo | 2014 | N/A | Kapil Pant B. Prabhakar Pandian | SynTumor, SynBBB, SynRAM, SynTox | United States |
| Tara Biosystems | 2014 | Toronto University | Milica Radisic Gordana Vunjak-Novakovic Robert Langer John M. Baldoni | Biowire™ II platform | United States |
| Alveolix | 2015 | University of Bern | Olivier Guenat | Lung-on-chip | Switzerland |
| ANANDA Devices | 2015 | N/A | Margaret Magdesian | Neuro Device | Canada |
| Hesperos | 2015 | Cornell University Central Florida University | Michael Shuler James Hickman | Heart, Liver, Lung, Brain, Skin, and Kidney chip, Multi-organ-chip | United States |
| Altis BioSystems | 2016 | University of North Carolina, Chapel Hill | Nancy Allbritton | RepliGut Kits | United States |
| MesoBioTech | 2016 | N/A | Yong Chen | Microfluidics Lung chip | France |
| BiomimX | 2017 | The Polytechnic University of Milan | Alberto Redaelli | Heart-on-chip | Italy |
| BI/OND | 2017 | Delft University of Technology | Cinzia Silvestri | Organoids cultivation, Tissue-tissue interface | The Netherland |
| Jiksak Bioengineering | 2017 | N/A | Jiro Kawada Keita Shibuya Norihiro Yumoto Shinji Tokunaga | Nerve Organoids | Japan |
| DAXIANG | 2018 | N/A | Yu Zhou | Liver chip, cancer chip | China |
| Aracari Bio | 2019 | University of California, Irvine | G. Wesley Hatfield Christopher C.W. Hughes Steven C. George Abraham P. Lee | Vascularized micro-organ chip | United States |
| REVIVO Biosystems | 2019 | Agency for Science, Technology and Research | Massimo Alberti | Microfluidic Skin-on-a-Chip | Singapore |
A clear and proper business model would help Organ-on-a-Chip startups survive in a competitive market. Currently, three business models exist for Organ-on-a-Chip startups: (1) to provide ready-for-culture microfluidic devices, (2) to provide fully operational, ready-to-use Organ-on-a-Chip, and (3) to offer a holistic full-service solution ranging from the initial design to post-sale training and maintenance [81]. For instance, several versions of HUMIMIC™ chip offered by TissUse allow the users to culture two or more organ compartments of interest to study inter-organ communications, and the ready-for-culture OrganoPlate® from Mimetas has been adapted by several research groups to establish their own on-chip vascularized barrier tissues, as discussed above [30, 31]. The gut model product OrganoReady Caco-2™ from Mimetas is a ready-to-use intestine barrier chip with biomimetic Caco-2 epithelial tubules. Similar intestine chips are also available from Emulate Inc., which allow the end-users to perform assays on drug toxicity and transport and study intestinal diseases. In addition to directly selling devices, TissUse offers service contracts to help create a customized organ chip platform for drug toxicity evaluation and human disease modeling through their established proprietary rapid prototyping procedure. Furthermore, CN-Bio Innovations provides a rapid study service for the on-chip disease modeling of human nonalcoholic steatohepatitis, validation of disease mechanisms of interest, and therapeutic screening using their PhysioMimix™ OOC system.
Commercialization of Organ-on-a-Chip
The translation and commercialization of Organ-on-a-Chip emphasize the industrial perspective, including technology standardization and reliability, ease of operation, cost-effectiveness, and compliance with government regulations [8, 79, 80]. Thus, further analytical validations of the diagnostic and therapeutic effectiveness, reproducibility, and safety of these chips for fulfilling the practical needs of pharmacological and medical application as well as the FDA regulations are warranted. Early and close collaboration between academic institutes, industrial R&D departments, and healthcare agencies during each stage of Organ-on-a-Chip development will fulfill different interests and needs and generate a positive feedback loop to corroborate the effectiveness of Organ-on-a-Chip platforms and maximize their utility in the actual healthcare industry. For instance, Emulate Inc. has partnered with AstraZeneca, Johnson & Johnson, Merck, Takeda, and FDA for the effectiveness validation of their various products by assessing the safety and efficacy of the drug candidates for human use in an industrial environment.
Another commercialization challenge results from the limited venture capital investment in the organ chip field despite the huge potential of this technology, with only several Organ-on-a-Chip startups receiving substantial investments, such as Emulate Inc. ($142.3M), InSphero ($35.2M), and Mimetas ($34.2M), according to the public information on Crunchbase. National healthcare and research systems can play a vital role in creating partnerships with academic institutes and companies, supporting proprietary protection and providing funding opportunities. For instance, the US National Center for Advancing Translational Science has cooperated with other agencies to launch a series of Organ-on-a-Chip programs to foster the use of this technology for practical drug development. The US federal agencies such as the National Institute of Health, National Science Foundation, and Department of Defense provide seed funds through the Small Business Innovation Research and Small Business Technology Transfer programs to foster the development, standardization, and commercialization of the Organ-on-a-Chip technology for use in the drug development pipeline. Outside the US, several universities and research centers across Europe initiated an open project (Organ-on-Chip in Development, ORCHIDiii) in 2017 and subsequently established the European Organ-on-Chip Society (EUROoCSiv) to facilitate a collaboration network across academic, research, industrial, and regulatory institutions to advance the Organ-on-a-Chip technology and its general application. Notably, the Asia-Pacific region (primarily China, Singapore, South Korea, and Japan) is deemed to be the emerging market owing to government support for healthcare technologies. For instance, the Chinese Academy of Science introduced a 5-year initiative of “Organ Reconstruction and Manufacturingv” in 2018. With consistently increasing investment, the technologies are expected to continue to improve but the cost continues to decrease. Market needs for Organ-on-a-Chip products are anticipated to grow rapidly and be well accepted by the pharmaceutical industry.
The future advances in Organ-on-a-Chip: Challenges and opportunities
The initial development of Organ-on-a-Chip platforms over the past two decades have demonstrated its great potential as a new tool for drug discovery and development. Moving to the next decade, new Organ-on-a-Chip platforms with significant improvements in functionality, integration, automation, manufacture, and personalized precision medicine has just started emerging to meet the growing need for better preclinical models for drug development.
First, the future Organ-on-a-Chip platforms will demonstrate a physiologically relevant and spatiotemporally responsive microenvironment for solving biological and pharmaceutical problems of interest. The technological functions of future Organ-on-a-Chip platforms will allow for real-time, in situ, and dynamic maintenance and monitoring of a large array of biological parameters, such as shear stress, pH, oxygen, cytokines, and chemokines, as well as downstream and off-chip analyses of molecular signature, cellular physiology, and tissue pathology with using traditional analytical tools, such as enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), and single-cell mRNA sequencing (scRNA-seq). Such a series of functionalities can be achieved with more advanced incorporation of in situ optical, electrical, chemical, and biological biosensors as integrative microfluidic components to detect the key signals in a spatiotemporal manner, which is challenging in animal experimentation [25, 67, 82, 83]. For instance, the integration of label-free and multiplexed nanoplasmonic sensors for in situ analysis of cytokine secretion during drug and pro-inflammatory stimulation in a biomimetic microfluidic adipose-tissue-on-a-chip platform has highlighted its potential as a high-throughput and an integrated preclinical readout system for drug testing [83]. Development and future integration of new multi-omics detecting approaches, such as deterministic barcoding in tissue for spatial omics sequencing (DBiT-seq), in microfluidic Organ-on-a-Chip platforms will allow for spatial barcoding and sequencing of massive molecular information from tissues at a genome-scale resolution [84]. We believe that integration with novel concepts and technologies will continuously improve the performance of Organ-on-a-Chip, endorsing its wider application in drug development.
Second, the future Organ-on-a-Chip platforms require improvement and standardization of the product manufacturing process as well as categorizing of the system designs, configurable modules, and interfaces. Currently, most Organ-on-a-Chip devices are manually fabricated with PDMS in research laboratories using the soft lithography technique. The throughput and reproducibility of fabrication are questionable for the large-scale production of devices for the market, necessitating a standardized and high-throughput yet low-cost manufacturing process. Implementation of advanced additive manufacturing methods (e.g., 3D printing) or current standard manufacturing materials and methods (e.g., injection modeling and laser cutting) as well as use of a more standardized, modular format with biologically inert materials (e.g., plastics to replace PDMS) should be considered [39]. For example, 3D bioprinting is a promising method for fabricating Organ-on-a-Chip devices, using which sophisticated tissue architectures, complex scaffolds, or templates of a device can be programmed in advance and automatically printed with high fidelity and controllability [82, 85–87]. By offering a one-step approach of tissue reconstruction and culture platform engineering, 3D bioprinting and other additive manufacturing methods are expected to extensively transform the Organ-on-a-Chip fabrication protocol in the near future. Moreover, the operation of Organ-on-a-Chip platforms should be materialized in a more automated, high-throughput and parallelized manner through a standardized user-friendly interface to enable compatibility with the routine biological laboratory experiments and work mode of the pharmaceutical industry [54, 88, 89]. Early attempts of using robotic interrogator have succeeded to empower, such as automated cell culture, intercompartmental fluidic coupling, repeated sample collection, and in situ microscopic imaging for weeks, in an integrated eight-organ chip [54]. Most recently, a microfluidic platform that is named IFlowPlate and built on a 384-well plate enabled in vitro perfusable culture and vascularization of patient-derived colon organoids, providing a higher throughput capacity as compared to current organ chips [89]. Such an upgrade of the Organ-on-a-Chip platforms will be critical for their commercialization and promotion for acceptance by the end-users.
Last but not the least, the future Organ-on-a-Chip platform will be developed based on patient-derived materials, such as patient tissue, decellularized ECM, and other biological materials for personalized precision medicine, in which patient selection and stratification biomarkers will be critical factors leading to successful drug development [73]. In many cases, patient tissue cells are limited in number and have low proliferative potential or invasive sample collection is required; such an unavailability and unreliability of patient cell sources present a significant barrier. For instance, lack of functional human podocytes can hinder the on-chip structural formation of glomerulus with selective filtration characteristics [18, 19], as also evidenced for human neural and cardiac chips [90–93]. Recent studies have shown that patient induced pluripotent stem cells (iPSCs), such as those generated from skin-derived fibroblasts, serve as an alternative and unlimited cell source to produce autologous target organs or tissues, thus enabling the construction of patient-specific organ chips for personalized disease modeling and drug screening [94]. For instance, a human BBB chip constructed with patient iPSC-derived neurons, astrocytes, and brain microvascular endothelial-like cells demonstrated patient-specific disruption of barrier integrity and blood-to-brain permeability of pharmacologics [95]. Additionally, using healthy or patient iPSC-derived cardiomyocytes, researchers have achieved in vitro modeling of cardiovascular diseases, screening of potential drugs, and evaluation of cardiac toxicity resulting from drug interactions and nanomaterials, all of which could not be achieved because of the lack of human cardiac cells [96–101]. Furthermore, the integration of iPSC-based organoids—another concept in which an ex vivo organotypic microtissue is developed via self-organization and differentiation of stem cells in a 3D matrix—into the Organ-on-a-Chip platform resulted in the construction of a powerful hybrid tool, “Organoids-on-a-Chip” [102–105]. Microfluidic kidney and retinal organoid chips, for instance, have been demonstrated to be more physiologically relevant in terms of tissue maturity and functionality [104, 105]. Although not all of studies could be enumerated due to space limit, attempts aimed at developing patient iPSC-derived organ chips would unsurprisingly overcome the inadequacy of the traditional “one-size-fits-all” therapeutics, providing an ideal treatment for individual patients across large populations for the same disorder. Furthermore, several future advantages are envisioned, particularly in the modeling and analysis of rare human diseases, which are restricted by available biological studies and subsequent high R&D cost [106, 107].
Concluding remarks
Although a strong notion for paradigm shift in drug development has emerged in order to improve the overall pass rate of newly developed drugs, the industrial drug development process is quite standardized [108]. Since the entire drug development process may involve going back and forth from disease modeling to drug testing, the design and incorporation of the Organ-on-a-Chip platforms into the drug development pipeline would be evidently beneficial at all preclinical stages and even realize trial-on-chips for clinical validation [109]. Given the rapidly rising concerns about animal welfare and rights in biological experiments, Organ-on-a-Chip platforms can be a promising alternative to avoid ethical issues related to animal use and live up to the guiding 3R principles (i.e., replacement, reduction, and refinement) [110]. However, the Organ-on-a-Chip systems are still marginalized in the pharmaceutical industry owing to the current challenges in meeting the practical needs of rapid drug discovery and accurate preclinical evaluation (see “Outstanding Questions”). From the long-term viewpoint, incessant integration of novel concepts and techniques into the Organ-on-a-Chip platform is expected to bridge the biological and technical gaps between translational, preclinical, and clinical studies. In summary, we are enthusiastic regarding the potential of Organ-on-a-Chip in the pharmaceutical industry and its increasingly promising future in personalized precision medicine.
Outstanding Questions.
What are the existing grand challenges in preventing the integration of Organ-on-a-Chip system into the drug development pipeline?
How can Organ-on-a-Chip system accurately reproduce human immunology and discern ‘self’ between ‘non-self’ to screen and assess the novel immunotherapeutic, such as immune checkpoint blockade and most recently CAR T-cell immunotherapy?
How can Organ-on-a-Chip system become a preclinical and/or clinical tool for personalized precision medicine for patient selection and stratification, as well as screening of customized therapeutics?
How can Organ-on-a-Chip platform outcompete conventional animal experimentations for preclinical drug development and thereby fulfill the 3R principle?
Highlights.
Organ-on-a-Chip is a promising interdisciplinary technique emulating the in vivo physiology and pathology for in vitro disease modeling, drug screening, and precision medicine.
The Organ-on-a-Chip technology can be organically incorporated into the drug development pipeline from early drug discovery to preclinical screening, testing, and translation of new drugs, which bridges the gap between animal studies and clinical trials involving human subjects.
The future development of personalized Organ-on-a-Chip and continuous integration of novel engineering tools (e.g. automation handling, 3D printing and in situ multi-sensors) and biological concepts (e.g. patient-specific iPSCs and organoid) into Organ-on-a-Chip platform will unprecedentedly promote its biomedical applications.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (R21EB025406 and R35GM133646).
Glossary
- 3D Bioprinting
A biofabrication strategy that precisely prints bioinks (e.g. cells, hydrogels and biocompatible materials) to reconstruct the structures and functions of living systems.
- 3R principle
The 3R stands for Replacement, Reduction, and Refinement, which is a guiding principle to minimize the number of animal experimentation worldwide.
- ADME
An abbreviation for ‘absorption, distribution, metabolism, and excretion’, which describes pharmacokinetics of a compound within an organism.
- BBB, Blood-Brain Barrier
A multiple cell layer structure consisted of brain endothelial cells, astrocytes, and pericytes, and its selective permeability prevents drugs from non-selectively entering the brain tissue.
- Body-on-a-Chip or Human-on-a-Chip
A type of multi-organ chip to recapitulate the whole human physiology within a single platform.
- COVID-19
Coronavirus disease 2019, is an infectious disease caused by severe acute respiratory syndrome coronavirus 2.
- Extracellular matrix
A complex molecular network of non-cellular components to provide physical support and biochemical/biophysical cues for tissue development and homeostasis.
- iPSCs, induced pluripotent stem cells
A type of pluripotent stem cell that can be generated from somatic cells by direct introduction of four Yamanaka factors, i.e. Myc, Oct3/4, Sox2 and Klf4.
- Mechanobiology
An emerging research field interrogating the contribution of the hemostasis and dysfunction of mechanical properties of cells and tissues to maintain cell function, tissue development, and pathogenesis.
- Microfluidics
A science and technology of manufacturing microminiaturized devices for manipulating and controlling a small volume of fluids (typically microliters to femtoliters) in a network of micro-channels.
- Multi-organ Chip
A type of Organ-on-a-Chip that reproduces organotypic functions of at least two types of tissues/organs to study inter-organ reactions.
- Organ-on-a-Chip
A microfluidic in vitro culture system upon which single or multiple cell types were controllably cultured within a 3D extracellular matrix to recapitulate the physiology and/or pathophysiology of in vivo tissues/organs.
- Organoids-on-a-Chip
A conceptual technology merging organoids with Organ-on-a-Chip to recapitulate the complexity of human organs by application of intrinsic tissue development process and external engineering method.
- PK, Pharmacokinetics
PK study profiles the dynamic movement of a drug in the body over time, such as the kinetics of ADME processes.
- PD, Pharmacodynamics
PD study describes the quantitative relationship between drug concentrations and the biochemical and physiologic responses.
- Photolithography
A manufacturing process transfers micrometric patterns from a photomask to a light-sensitive chemical photoresist.
- R&D
The abbreviation for research and development, is the process by which the pharmaceutical industry explores and develops new treatments or medications.
- Single-Organ Chip
A type of Organ-on-a-Chip to mainly recapitulate the structure and physiological functions of one specific tissue or organ.
- Soft lithography
A family of patterning techniques to reproduce structures into a soft polymer material, mostly PDMS, from a silicon mold with micro/nanoscale features.
Footnotes
Disclaimer Statement
The authors declare no competing financial interests.
Resources
FDA, www.fda.gov/home
Crunchbase, www.crunchbase.com
ORCHID, https://h2020-orchid.eu/
European Organ-on-Chip Society, www.euroocs.eu
Chinese Academy of Sciences, www.cas.cn
References
- 1.Cook D et al. (2014) Lessons learned from the fate of AstraZeneca’s drug pipeline: a five-dimensional framework. Nat Rev Drug Discov 13 (6), 419–31. [DOI] [PubMed] [Google Scholar]
- 2.Dugger SA et al. (2018) Drug development in the era of precision medicine. Nat Rev Drug Discov 17 (3), 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day CP et al. (2015) Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163 (1), 39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang KJ et al. (2019) Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med 11 (517). [DOI] [PubMed] [Google Scholar]
- 5.Van Norman GA (2019) Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl Sci 4 (7), 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si L et al. (2020) Human organs-on-chips as tools for repurposing approved drugs as potential influenza and COVID19 therapeutics in viral pandemics.
- 7.Franzen N et al. (2019) Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov Today 24 (9), 1720–1724. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B et al. (2018) Advances in organ-on-a-chip engineering. Nature Reviews Materials 3 (8), 257–278. [Google Scholar]
- 9.Xia Y and Whitesides GM (1998) Soft Lithography. Angew Chem Int Ed Engl 37 (5), 550–575. [DOI] [PubMed] [Google Scholar]
- 10.Duffy DC et al. (1998) Rapid Prototyping of Microfluidic Systems in Poly(dimethylsiloxane). Anal Chem 70 (23), 4974–84. [DOI] [PubMed] [Google Scholar]
- 11.Whitesides GM (2006) The origins and the future of microfluidics. Nature 442 (7101), 368–373. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia SN and Ingber DE (2014) Microfluidic organs-on-chips. Nat Biotechnol 32 (8), 760–72. [DOI] [PubMed] [Google Scholar]
- 13.Langer R and Vacanti J (1993) Tissue engineering. Science 260 (5110), 920–926. [DOI] [PubMed] [Google Scholar]
- 14.Huh D et al. (2007) Acoustically detectable cellular-level lung injury induced by fluid mechanical stresses in microfluidic airway systems. Proceedings of the National Academy of Sciences of the United States of America 104 (48), 18886–18891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huh D et al. (2010) Reconstituting Organ-Level Lung Functions on a Chip. Science 328 (5986), 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma C et al. (2016) On-Chip Construction of Liver Lobule-like Microtissue and Its Application for Adverse Drug Reaction Assay. Anal Chem 88 (3), 1719–27. [DOI] [PubMed] [Google Scholar]
- 17.Ma C et al. (2016) Pneumatic-aided micro-molding for flexible fabrication of homogeneous and heterogeneous cell-laden microgels. Lab on a Chip 16 (14), 2609–2617. [DOI] [PubMed] [Google Scholar]
- 18.Mu X et al. (2013) Engineering a 3D vascular network in hydrogel for mimicking a nephron. Lab Chip 13 (8), 1612–8. [DOI] [PubMed] [Google Scholar]
- 19.Musah S et al. (2017) Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shik Mun K et al. (2019) Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun 10 (1), 3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glieberman AL et al. (2019) Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 19 (18), 2993–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn S et al. (2018) Mussel-inspired 3D fiber scaffolds for heart-on-a-chip toxicity studies of engineered nanomaterials. Anal Bioanal Chem 410 (24), 6141–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsano A et al. (2016) Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16 (3), 599–610. [DOI] [PubMed] [Google Scholar]
- 24.Ugolini GS et al. (2018) Generation of functional cardiac microtissues in a beating heart-on-a-chip. Methods Cell Biol 146, 69–84. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X et al. (2016) High-Throughput Assessment of Drug Cardiac Safety Using a High-Speed Impedance Detection Technology-Based Heart-on-a-Chip. Micromachines (Basel) 7 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehy SP et al. (2017) Toward improved myocardial maturity in an organ-on-chip platform with immature cardiac myocytes. Exp Biol Med (Maywood) 242 (17), 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shim KY et al. (2017) Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices 19 (2), 37. [DOI] [PubMed] [Google Scholar]
- 28.Poceviciute R and Ismagilov RF (2019) Human-gut-microbiome on a chip. Nat Biomed Eng 3 (7), 500–501. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y et al. (2018) A Biomimetic Human Gut-on-a-Chip for Modeling Drug Metabolism in Intestine. Artif Organs 42 (12), 1196–1205. [DOI] [PubMed] [Google Scholar]
- 30.Wevers NR et al. (2018) A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koo Y et al. (2018) Three-dimensional (3D) tetra-culture brain on chip platform for organophosphate toxicity screening. Sci Rep 8 (1), 2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marturano-Kruik A et al. (2018) Human bone perivascular niche-on-a-chip for studying metastatic colonization. Proc Natl Acad Sci U S A 115 (6), 1256–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao S et al. (2018) A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 14 (12), e1702787. [DOI] [PubMed] [Google Scholar]
- 34.Torisawa YS et al. (2016) Modeling Hematopoiesis and Responses to Radiation Countermeasures in a Bone Marrow-on-a-Chip. Tissue Eng Part C Methods 22 (5), 509–15. [DOI] [PubMed] [Google Scholar]
- 35.Pires de Mello CP et al. (2020) Microphysiological heart-liver body-on-a-chip system with a skin mimic for evaluating topical drug delivery. Lab Chip 20 (4), 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maschmeyer I et al. (2015) A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15 (12), 2688–99. [DOI] [PubMed] [Google Scholar]
- 37.Abaci HE and Shuler ML (2015) Human-on-a-chip design strategies and principles for physiologically based pharmacokinetics/pharmacodynamics modeling. Integr Biol (Camb) 7 (4), 383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang C et al. (2009) Towards a human-on-chip: culturing multiple cell types on a chip with compartmentalized microenvironments. Lab Chip 9 (22), 3185–92. [DOI] [PubMed] [Google Scholar]
- 39.Sung JH et al. (2019) Recent Advances in Body-on-a-Chip Systems. Anal Chem 91 (1), 330–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller PG and Shuler ML (2016) Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnol Bioeng 113 (10), 2213–27. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen DT et al. (2019) A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling. Sci Adv 5 (8), eaav6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao Y et al. (2019) Ex vivo Dynamics of Human Glioblastoma Cells in a Microvasculature-on-a-Chip System Correlates with Tumor Heterogeneity and Subtypes. Adv Sci (Weinh) 6 (8), 1801531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassell BA et al. (2017) Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep 21 (2), 508–516. [DOI] [PubMed] [Google Scholar]
- 44.Toh YC et al. (2018) A 3D Microfluidic Model to Recapitulate Cancer Cell Migration and Invasion. Bioengineering (Basel) 5 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui X et al. (2018) Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 161, 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hassell BA et al. (2017) Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Reports 21 (2), 508–516. [DOI] [PubMed] [Google Scholar]
- 47.Ma C et al. (2020) Leukemia-on-a-chip: Dissecting the chemoresistance mechanisms in B cell acute lymphoblastic leukemia bone marrow niche. Sci Adv 6 (44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cui X et al. (2020) Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witkowski MT et al. (2020) Extensive Remodeling of the Immune Microenvironment in B Cell Acute Lymphoblastic Leukemia. Cancer Cell 37 (6), 867–882 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sontheimer-Phelps A et al. (2019) Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 19 (2), 65–81. [DOI] [PubMed] [Google Scholar]
- 51.Xu Z et al. (2016) Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Appl Mater Interfaces 8 (39), 25840–25847. [DOI] [PubMed] [Google Scholar]
- 52.Asmani M et al. (2018) Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat Commun 9 (1), 2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prantil-Baun R et al. (2018) Physiologically Based Pharmacokinetic and Pharmacodynamic Analysis Enabled by Microfluidically Linked Organs-on-Chips. Annu Rev Pharmacol Toxicol 58, 37–64. [DOI] [PubMed] [Google Scholar]
- 54.Novak R et al. (2020) Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng 4 (4), 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vernetti L et al. (2017) Functional Coupling of Human Microphysiology Systems: Intestine, Liver, Kidney Proximal Tubule, Blood-Brain Barrier and Skeletal Muscle. Sci Rep 7, 42296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sung JH and Shuler ML (2009) A micro cell culture analog (microCCA) with 3-D hydrogel culture of multiple cell lines to assess metabolism-dependent cytotoxicity of anti-cancer drugs. Lab Chip 9 (10), 1385–94. [DOI] [PubMed] [Google Scholar]
- 57.Sung JH et al. (2010) A microfluidic device for a pharmacokinetic-pharmacodynamic (PK-PD) model on a chip. Lab Chip 10 (4), 446–55. [DOI] [PubMed] [Google Scholar]
- 58.Herland A et al. (2020) Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng 4 (4), 421–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasserath T et al. (2020) Differential Monocyte Actuation in a Three-Organ Functional Innate Immune System-on-a-Chip. Adv Sci (Weinh) 7 (13), 2000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park D et al. (2020) Integrating Organs-on-Chips: Multiplexing, Scaling, Vascularization, and Innervation. Trends Biotechnol 38 (1), 99–112. [DOI] [PubMed] [Google Scholar]
- 61.van der Helm MW et al. (2016) Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 4 (1), e1142493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qu YY et al. (2018) A nephron model for study of drug-induced acute kidney injury and assessment of drug-induced nephrotoxicity. Biomaterials 155, 41–53. [DOI] [PubMed] [Google Scholar]
- 63.Blundell C et al. (2016) A microphysiological model of the human placental barrier. Lab Chip 16 (16), 3065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin FC et al. (2019) A 3D human placenta-on-a-chip model to probe nanoparticle exposure at the placental barrier. Toxicology in Vitro 54, 105–113. [DOI] [PubMed] [Google Scholar]
- 65.Sakolish CM et al. (2016) Modeling Barrier Tissues In Vitro: Methods, Achievements, and Challenges. EBioMedicine 5, 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cirit M and Stokes CL (2018) Maximizing the impact of microphysiological systems with in vitro-in vivo translation. Lab Chip 18 (13), 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang YS et al. (2017) Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc Natl Acad Sci U S A 114 (12), E2293–E2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McAleer CW et al. (2019) Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci Transl Med 11 (497). [DOI] [PubMed] [Google Scholar]
- 69.Esch EW et al. (2015) Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov 14 (4), 248–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benam KH et al. (2016) Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods 13 (2), 151–7. [DOI] [PubMed] [Google Scholar]
- 71.Binnewies M et al. (2018) Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24 (5), 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ando Y et al. (2019) Evaluating CAR-T Cell Therapy in a Hypoxic 3D Tumor Model. Adv Healthc Mater 8 (5), e1900001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Berg A et al. (2019) Personalised organs-on-chips: functional testing for precision medicine. Lab Chip 19 (2), 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jenkins RW et al. (2018) Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov 8 (2), 196–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X et al. (2019) Dynamic Profiling of Antitumor Activity of CAR T Cells Using Micropatterned Tumor Arrays. Adv Sci (Weinh) 6 (23), 1901829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boussommier-Calleja A et al. (2016) Microfluidics: A new tool for modeling cancer-immune interactions. Trends Cancer 2 (1), 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polini A et al. (2019) Towards the development of human immune-system-on-a-chip platforms. Drug Discov Today 24 (2), 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller CP et al. (2020) Engineering Microphysiological Immune System Responses on Chips. Trends in Biotechnology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livingston CA et al. (2016) Facilitating the commercialization and use of organ platforms generated by the microphysiological systems (Tissue Chip) program through public-private partnerships. Comput Struct Biotechnol J 14, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang B and Radisic M (2017) Organ-on-a-chip devices advance to market. Lab Chip 17 (14), 2395–2420. [DOI] [PubMed] [Google Scholar]
- 81.Mastrangeli M et al. (2019) Organ-on-chip in development: Towards a roadmap for organs-on-chip. ALTEX 36 (4), 650–668. [DOI] [PubMed] [Google Scholar]
- 82.Lind JU et al. (2017) Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat Mater 16 (3), 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J et al. (2018) An integrated adipose-tissue-on-chip nanoplasmonic biosensing platform for investigating obesity-associated inflammation. Lab Chip 18 (23), 3550–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu Y et al. (2019) High-Spatial-Resolution Multi-Omics Atlas Sequencing of Mouse Embryos via Deterministic Barcoding in Tissue. bioRxiv, 788992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang YS et al. (2016) Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials 110, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li J et al. (2020) Improving Bioprinted Volumetric Tumor Microenvironments In Vitro. Trends Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi HG et al. (2019) A bioprinted human-glioblastoma-on-a-chip for the identification of patient-specific responses to chemoradiotherapy. Nat Biomed Eng 3 (7), 509–519. [DOI] [PubMed] [Google Scholar]
- 88.Benam KH et al. (2016) Matched-Comparative Modeling of Normal and Diseased Human Airway Responses Using a Microengineered Breathing Lung Chip. Cell Syst 3 (5), 456–466 e4. [DOI] [PubMed] [Google Scholar]
- 89.Rajasekar S et al. (2020) IFlowPlate-A Customized 384-Well Plate for the Culture of Perfusable Vascularized Colon Organoids. Adv Mater, e2002974. [DOI] [PubMed] [Google Scholar]
- 90.Yi Y et al. (2015) Central Nervous System and its Disease Models on a Chip. Trends Biotechnol 33 (12), 762–776. [DOI] [PubMed] [Google Scholar]
- 91.Mathur A et al. (2015) Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep 5, 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y et al. (2019) A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176 (4), 913–927 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Motallebnejad P et al. (2019) An isogenic hiPSC-derived BBB-on-a-chip. Biomicrofluidics 13 (6), 064119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu LP et al. (2019) Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Reports 27 (2), 455-+. [DOI] [PubMed] [Google Scholar]
- 95.Vatine GD et al. (2019) Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 24 (6), 995–1005 e6. [DOI] [PubMed] [Google Scholar]
- 96.Millard DC et al. (2016) Identification of Drug-Drug Interactions In Vitro: A Case Study Evaluating the Effects of Sofosbuvir and Amiodarone on hiPSC-Derived Cardiomyocytes. Toxicological Sciences 154 (1), 174–182. [DOI] [PubMed] [Google Scholar]
- 97.Ellis BW et al. (2017) Human iPSC-derived myocardium-on-chip with capillary-like flow for personalized medicine. Biomicrofluidics 11 (2), 024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ulmer BM et al. (2018) Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes. Stem Cell Reports 10 (3), 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Acun A and Zorlutuna P (2019) CRISPR/Cas9 Edited Induced Pluripotent Stem Cell-Based Vascular Tissues to Model Aging and Disease-Dependent Impairment. Tissue Engineering Part A 25 (9–10), 759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Acun A et al. (2019) In vitro aged, hiPSC-origin engineered heart tissue models with age-dependent functional deterioration to study myocardial infarction. Acta Biomater 94, 372–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goldfracht I et al. (2019) Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater 92, 145–159. [DOI] [PubMed] [Google Scholar]
- 102.Park SE et al. (2019) Organoids-on-a-chip. Science 364 (6444), 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Takebe T et al. (2017) Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell 21 (3), 297–300. [DOI] [PubMed] [Google Scholar]
- 104.Homan KA et al. (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16 (3), 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Achberger K et al. (2019) Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Low LA and Tagle DA (2016) Tissue Chips to aid drug development and modeling for rare diseases. Expert Opin Orphan Drugs 4 (11), 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Mello CPP et al. (2019) A human-on-a-chip approach to tackling rare diseases. Drug Discov Today 24 (11), 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaitin KI (2010) Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther 87 (3), 356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blumenrath SH et al. (2020) Tackling rare diseases: Clinical trials on chips. Exp Biol Med (Maywood), 1535370220924743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.P. F (2002) Replacement, Reduction and Refinement. ALTEX 19 (2), 73–78. [PubMed] [Google Scholar]


