Abstract
Objectives:
The goal of the present study was to determine whether baseline mindful eating, general mindful awareness, or acceptance was most strongly associated with short- and long-term weight loss in a lifestyle modification program.
Methods:
Data were from 178 participants (baseline BMI=40.9±5.9 kg/m2, age=44.2±11.2 years; 87.6% female; 71.3% black) who enrolled in a two-phase trial. All participants attended an initial 14-week lifestyle modification program that included a meal replacement diet. Participants who had lost ≥5% of initial weight (N=137) were then randomized to 52 weeks of lifestyle modification with lorcaserin or placebo. Linear mixed models examined whether mindful eating (Mindful Eating Questionnaire) and general mindful awareness and acceptance (Philadelphia Mindfulness Scale) predicted short-term weight loss at week 14 in the full sample and long-term weight loss at the end of the trial in the subsample of randomized participants.
Results:
In the full sample, higher baseline acceptance predicted greater short-term weight losses (p=.004). At week 14, individuals low in acceptance (−1SD) lost an average of 8.7 kg (SE=0.6) compared to 11.2 kg (SE=0.6) among those high in acceptance (+1SD). In the subsample of participants who successfully lost weight in phase 1, the independent effect of acceptance on total losses at the end of the trial did not reach statistical significance (p=.058). Neither mindful eating nor general mindful awareness independently predicted weight loss at either time point.
Conclusions:
Acceptance was a stronger predictor than either general or eating-specific awareness of weight loss with lifestyle modification.
Keywords: Obesity, weight loss, mindfulness, acceptance
Mindfulness interventions have been increasingly applied to the treatment of obesity in the hopes that these skills will facilitate behavior change and improve weight loss. Mindfulness can be defined as “paying attention in a particular way: on purpose, in the present moment, and nonjudgmentally” (Kabat-Zinn, 1994, p. 4) and has been operationalized as having two components: awareness and acceptance (Bishop et al., 2004; Cardaciotto et al., 2008). Awareness refers to sustained attention to the present experience (rather than preoccupation with past or future events), while acceptance refers to taking a nonjudgmental or open stance towards those experiences, rather than attempting to change or avoid events as they occur (Bishop et al., 2004; Cardaciotto et al., 2008).
In recent systematic reviews, the efficacy of mindfulness interventions for weight loss has been mixed (Carrière et al., 2018; Katterman et al., 2014; Olson & Emery, 2015; Rogers et al., 2017; Ruffault et al., 2017). This may be due, in part, to heterogeneity of the intervention approaches. Some interventions, such as Mindfulness-Based Eating Awareness Training (MB-EAT; Kristeller & Wolever, 2014), focus specifically on improving mindful eating through exercises designed to increase attention to hunger, satiety, and triggers for overeating. Other interventions, including Mindfulness-Based Cognitive Therapy (MBCT; Teasdale et al., 2000) and Mindfulness-Based Stress Reduction (MBSR; Kabat-Zinn, 2013), focus on improving general mindfulness through formal meditation practice. Acceptance-based approaches, such as Acceptance and Commitment Therapy (ACT; Hayes et al., 1999), include specific exercises designed to promote the acceptance of internal experiences in addition to mindful awareness practice. The majority of acceptance-based weight loss interventions have incorporated both mindful eating and general mindfulness and acceptance approaches (Forman et al., 2013; Forman et al., 2016; Lillis et al., 2016).
It is unclear whether mindful eating, general mindful awareness, or acceptance is most important to weight loss. On the one hand, it may be most important to provide practice with applying mindful awareness to situations directly related to energy intake and expenditure. Awareness of hunger and satiety signals, the sensory properties of food, and environmental and emotional cues that may impact eating could help patients to reduce portion sizes, mindless eating, and emotional eating (Beshara et al., 2013; Framson et al., 2009; Van De Veer et al., 2016). Greater awareness of the sensory properties of food also may reduce consumption at subsequent meals due to enhanced recall of the eating episode (Higgs & Donohoe, 2011; Robinson et al., 2014). On the other hand, improving general mindfulness could have the greatest likelihood of increasing awareness of a broad range of experiences that may affect weight control (Schumacher et al., 2019). General mindful awareness also could facilitate weight-related behavior change through its independent associations with lower emotional distress (de Bruin et al., 2012; Hinterman et al., 2012; Masuda & Tully, 2011; Soysa & Wilcomb, 2015), higher self-compassion (Hollis-Walker & Colosimo, 2011; Raab, 2014), and stronger self-control (Howell & Buro, 2011).
If an individual, however, is unable to maintain a non-reactive approach, greater attention to food cues, cravings, or negative emotions could instead increase the likelihood of eating (Friese & Hoffman, 2016; Hendrikse et al., 2015). Acceptance may instead be the aspect of mindfulness that is most integral to successful weight loss. Acceptance is associated with lower impulsivity (Murphy & MacKillon, 2012; Peters et al., 2011), which could facilitate deliberate decision-making in the presence of eating cues. Openness towards one’s internal experiences (e.g., emotions, thoughts, urges, physical sensations) also may allow an individual to better maintain goal-consistent behaviors that are associated with unpleasant internal states. Individuals who take an accepting stance towards their weight control experiences may therefore be better able to tolerate reduced pleasure (e.g., choosing a low- vs high-calorie food) and any discomfort that occurs while changing their eating and activity behaviors (e.g., hunger, fatigue during exercise, negative emotions) (Carrière et al., 2018; Forman et al., 2013; Olson & Emery, 2015).
In one previous study, both higher general mindfulness and aspects of mindful eating were associated with less self-reported consumption of energy-dense foods per week (rs=−.20 to −.27; Beshara et al., 2013). Mindful eating mediated the relationship between general mindfulness and energy-dense food consumption, such that general mindfulness did not predict consumption when controlling for mindful eating. This finding suggests that general mindfulness only benefits eating behavior to the extent that it enhances eating-related mindfulness. However, the effects of mindful awareness and acceptance were not assessed separately. Additionally, the relationship between aspects of mindfulness and a single eating behavior may differ from their relationship to weight change, which is the product of a spectrum of eating and activity behaviors. Investigating mindful eating, general mindful awareness, and acceptance together as predictors of weight loss provides the opportunity to identify which aspects of mindfulness are most helpful to weight-related behavior change and may reveal relationships that are not apparent when mindfulness is measured as a single construct.
Our understanding of the relationship between mindfulness and behavior change may also be enhanced by determining whether components of mindfulness change during a standard lifestyle modification intervention. Recommendations to self-monitor eating and physical activity, identify eating and activity cues, and change habitual responses to cues, cravings, and emotions could foster greater awareness and acceptance. Evidence of changes in mindfulness components, particularly among people who successfully lose weight, would provide preliminary evidence that mindfulness is a mechanism through which health behavior change occurs. Although some studies have compared changes in general mindfulness or mindful eating between standard and mindfulness-based weight loss interventions (Daubemier et al., 2011; Mason et al., 2016), these results are difficult to interpret when one group has received training that might both allow them to more accurately report on mindfulness and may increase the perceived desirability of reporting its use (Grossman, 2011; Baer, 2011). Examining how mindfulness components change in a standard behavioral treatment would allow us to determine whether improvements in awareness and acceptance occur that are independent of increased exposure to these constructs.
The primary goal of the present study was to determine whether baseline mindful eating, general mindful awareness, or acceptance was most strongly associated with short- and long-term weight loss in a lifestyle modification intervention. This was a prespecified secondary analysis of data from a two-phase trial (Tronieri et al., 2017) in which all participants completed an initial 14-week, group lifestyle intervention that included the use of a meal-replacement diet (i.e., phase 1). Participants who lost ≥ 5% of their initial weight were then eligible to participate in a 52-week, double-blind randomized controlled trial (RCT) that compared the efficacy of lorcaserin, a selective serotonin 2C receptor agonist approved by the Food and Drug Administration in 2012 for chronic weight management, to placebo for weight loss maintenance (i.e., phase 2). (On February 13, 2020, lorcaserin was voluntarily withdrawn from the market because of concerns that it may increase the risk of various cancers.) We hypothesized that higher baseline levels of mindful eating and general acceptance would predict larger short-term weight losses at week 14 (end of phase 1) in the initial sample and larger long-term weight losses at week 52 of phase 2 (66 total weeks of treatment) among participants who lost ≥ 5% of their initial weight in phase 1. Due to previous evidence suggesting that the relationship between general mindful awareness and eating behavior could be accounted for by mindful eating (Beshara et al., 2013), we hypothesized that general mindful awareness would be associated with weight loss at these time points when included as the sole predictor but would not be an independent predictor of weight loss in the final model evaluating all three mindfulness components. We also explored whether mindful eating, general mindfulness, and general acceptance would improve among individuals who successfully lost weight during a standard lifestyle intervention.
Methods
Participants
Eligible participants were aged 21–65 years, had a body mass index (BMI) ≥33 kg/m2 and ≤ 55 kg/m2 (or ≥ 30 kg/m2 with an obesity-related comorbidity), and had no serious medical or psychological conditions (e.g., diabetes mellitus, recent cardiovascular disease, major depressive disorder) or contraindications to the use of lorcaserin (e.g., pregnancy). Participants were recruited from local media advertisements and completed a telephone prescreening and an in-person behavioral and medical screening. All participants provided written informed consent, and study procedures were approved by the university’s institutional review board. A total of 178 participants enrolled in the phase 1 lifestyle modification program, of whom 137 (77.0%) were randomized to lorcaserin or placebo in phase 2, the weight loss maintenance RCT. Due to the structure of the parent RCT, the present study could only include the participants who lost >5% of initial weight and enrolled in phase 2 in the analyses of long-term weight loss and of changes in mindfulness measures.
Procedure
The primary randomized controlled trial’s design, methods, and inclusion criteria have been described in detail previously (Tronieri et al., 2017). The randomized trial’s primary outcome was the effect of lorcaserin on weight loss maintenance from randomization (week 0) to week 52 of phase 2 in participants who lost ≥ 5% of their initial weight in phase 1, the initial 14-week group lifestyle intervention. Because the present secondary analysis was designed to evaluate baseline mindful eating, general awareness, and acceptance measured at week 1 of phase 1 as predictors of weight loss, the primary outcomes were prespecified as short- and long-term weight loss from the start of phase 1, rather than weight loss maintenance from week 0 to week 52 of phase 2. Phase 2 is still descried below as a “weight loss maintenance” intervention to maintain consistency with the primary study. We included an exploratory analysis of the relationship between baseline mindfulness variables (measured at the start of phase 1) and weight loss maintenance in phase 2 among the randomized participants.
Phase 1 Intervention: Weight loss induction with lifestyle modification and meal replacements.
The phase 1 intervention included 14 weekly, 90-minute group lifestyle modification sessions, delivered by registered dietitians or psychologists. The intervention content was based on previous studies (e.g., Wadden et al., 1997; Wadden et al., 2004) and included behavioral strategies such as self-monitoring of weight and calorie intake, stimulus control, goal setting, problem-solving, cognitive restructuring, and relapse prevention. All participants were prescribed a 1000–1200 kcal/day meal-replacement diet that included four servings of a liquid shake (Health Management Resources–HMR; 160 kcal/shake), a prepackaged entrée (250–300 kcal), 1–2 servings of fruit, and a salad. The use of shakes was gradually terminated by week 14. Participants were instructed to gradually increase their physical activity to 175 minutes/week.
Phase 2 Intervention: Weight loss maintenance with lifestyle modification and pharmacotherapy.
Participants who lost ≥ 5% of initial weight in phase 1 and enrolled in phase 2 were randomly assigned to lorcaserin (10 mg BID) or placebo and received group lifestyle modification for an additional 52 weeks. Sessions were provided every other week for the first 12 weeks (i.e., 6 sessions) and once every 4 weeks for the remainder of the 52 weeks (i.e., 10 sessions). Approximately every-other session was delivered via group conference call. Session topics included the behavioral principles described above, as well as dietary recommendations and weight loss maintenance strategies. One phase 2 session discussed mindful eating strategies. The intervention did not otherwise emphasize mindfulness or acceptance-based skills. All participants were prescribed a calorie goal of 1200–1800 kcal/day based on initial body weight. Participants were instructed to continue to self-monitor their weight and food intake and to increase their physical activity to 225 minutes/week by week 40. Participants could attempt to lose additional weight during this phase of treatment, if desired.
Measures
Body weight.
Body weight was measured using a digital scale (Tanita BWB-800) at all clinic visits and at four outcome assessments (i.e., phase 1 baseline and weeks 0 (randomization), 24, and 52 of phase 2).
Mindful eating.
The Mindful Eating Questionnaire (MEQ; Framson et al., 2009) consists of 28 items rated on a 5-point likert scale (“never/rarely” to “usually/always”). Its total score is the mean of five subscales that assess domains of mindful eating: disinhibition, awareness, external cues, emotional response, and distraction. Higher scores indicate higher levels of mindful eating. The scale and its subscales have been found to have good internal consistency and test-retest reliability (Apolzan et al., 2016; Framson et al., 2009). Cronbach’s alpha for our sample was 0.76 to 0.84 at each assessment.
General mindful awareness and acceptance.
The Philadelphia Mindfulness Scale (PHLMS; Cardaciotto et al., 2008) was constructed to independently measure two dimensions of mindfulness: awareness and acceptance. It includes 20 items rated on a 5-point likert scale (“never” to “very often”). The awareness subscale assesses the degree to which an individual notices ongoing internal and external experiences (e.g., “I am aware of what thoughts are passing through my mind”), and the acceptance subscale measures the degree to which a person experiences these events with a non-judgmental attitude as opposed to trying to avoid or change them (e.g., “If there is something I don’t want to think about, I’ll try many things to get it out of my mind”). The validity and internal consistency of both subscales has been demonstrated in both clinical and non-clinical samples (Cardaciotto et al., 2008). In the present study, Cronbach’s alpha was 0.81 to 0.91 for the awareness subscale and 0.89 to 0.90 to for the acceptance subscale at the different assessments.
Data Analyses
We used the initial sample of 178 participants who enrolled in phase 1 to examine relationships with short-term weight loss at week 14 and the sample of 137 participants who successfully lost ≥ 5% of their initial weight and enrolled in phase 2 to examine relationships with long-term weight loss at week 52 of phase 2. Only phase 2 participants were included in the exploratory analyses of changes in mindfulness components during treatment, because participants who were not eligible for phase 2 did not complete questionnaires at randomization (week 0) or week 52 of phase 2.
For the primary analyses, linear mixed models with residual maximum likelihood were used to determine the relationships of baseline mindful eating, general mindful awareness, and general acceptance with total weight loss at the end of phase 1 (week 14) and at week 52 of phase 2 (66 total weeks of treatment). Because our goal was to understand the relative benefit of each construct to successful weight loss, the primary analyses evaluated the independent effects of the three variables when analyzed together. We ran preliminary analyses exploring the relationship between each predictor and weight loss when analyzed separately to support our understanding of the impact of shared variance on the primary results. Unconditional models were used to determine the appropriate model shape (e.g., linear, quadratic, piecewise) and variance-covariance structure based on model fit criteria (Gallop & Tasca, 2009). All analyses were conducted using weight loss in kg as the outcome after controlling for participants’ heights and medication condition (for phase 2 analyses). Percent weight loss is also reported. Estimates of weight change at one standard deviation above and below the mean were calculated using least squared means. Dependent t-tests, repeated measures ANOVAs, and Pearson correlations were used to explore changes in mindfulness and acceptance during treatment and relationships to weight change.
Missing data.
Of the 178 initial participants, 150 (84.3%) provided a weight measurement at the end of phase 1. Of the 137 randomized participants, 112 (81.8%) provided a weight measurement at week 52 of phase 2. The use of linear mixed models allowed us to include data from all relevant participants regardless of missing weight data.
Questionnaire data were missing for additional participants due to non-completion or to skipped items. At baseline, 168–170 participants (94.4–95.5%) completed the PHLMS and MEQ. Of the 137 randomized participants, 132–134 (96.4–97.8%) completed these questionnaires following their completion of phase 1 (i.e., at randomization into phase 2), and 85–87 (62.0–63.5%) had complete questionnaire data at week 52 of phase 2. Missing values for the PHLMS subscales and MEQ total score were estimated with multiple imputation (MI) using chained equations. MI relies on the missing at random assumption. Twenty iterations were determined to be sufficient based on the fraction of missing data (γ = 0.02 to 0.38) (Graham et al., 2007). Treatment group, eligibility for phase 2, initial BMI, demographic characteristics (age, gender, race), depression (Beck Depression Inventory; Beck, Steer, & Brown, 1996), Eating Inventory scores (Stunkard & Messick, 1988), phase 1 weight loss, and phase 2 weight loss maintenance were entered as predictors in the imputation model.
Results
Phase 1 participants (N = 178) had a mean (± SD) initial weight of 114.4 ± 21.0 kg (BMI = 40.9 ± 5.9 kg/m2). Their mean age was 44.2 ± 11.2 years, 87.6% were female, and 71.3% were black (21.9% white). Baseline characteristics of the 137 participants who were later randomized in phase 2 were similar to the initial sample (Table 1).
Table 1.
Baseline characteristics of participants who enrolled in the 14-week weight loss induction program (phase 1) and of the subset of participants who successfully lost ≥ 5% of their weight and were later randomized to a weight loss maintenance condition (phase 2).
| Participants who enrolled in phase 1 (N=178) | Participants who later enrolled in phase 2 (N=137) | |
|---|---|---|
| Sex (female), n (%) | 156 (87.6%) | 118 (86.1%) |
| Race, n (%) | ||
| Black or African American | 127 (71.3%) | 94 (68.6%) |
| White | 39 (21.9%) | 33 (24.1%) |
| Asian | 4 (2.2%) | 4 (2.9%) |
| Multiracial or other | 8 (4.5%) | 6 (4.4%) |
| Ethnicity (Hispanic), n (%) | 11 (6.2%) | 7 (5.3%) |
| Age (years) | 44.2 ± 11.2 | 46.1 ± 10.1 |
| Weight (kg) | 114.4 ± 21.0 | 114.8 ± 22.5 |
| Height (cm) | 166.9 ± 8.7 | 167.2 ± 9.1 |
| BMI (kg/m2) | 40.9 ± 5.9 | 40.8 ± 5.9 |
Notes. Values are means ± SD, except as otherwise noted.
Participants’ mean mindfulness and acceptance scores at baseline of phase 1 and their intercorrelations are shown in Table 2. Mindful eating had a small association with general mindful awareness and a moderate association with acceptance. There was minimal correlation between general mindful awareness and acceptance. None of the mindfulness measures were associated with baseline weight
Table 2.
Intercorrelations among mindfulness variables and baseline weight at the start of phase 1 (N = 178).
| Mean ± SD | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. Baseline weight (kg) | 114.4 ± 21.0 | −.063 | .029 | −.073 |
| 2. General Mindful Awareness (PHLMS awareness) | 3.76 ± 0.71 | −.087 | .282*** | |
| 3. General Acceptance (PHLMS acceptance) | 3.19 ± 0.86 | .426*** | ||
| 4. Mindful Eating (MEQ total) | 2.71 ± 0.34 |
Notes.
= p < .05
= p < .01
= p < .001.
Predictors of Short-term Weight Loss in the Full Sample
The average estimated weight loss (± SE) of the initial phase 1 sample (N=178) was 10.0 ± 0.4 kg (−8.5 ± 0.3% of initial weight) at week 14 of phase 1. When analyzed in separate models, mindful eating (b=−0.049, SEb=0.080, t=−0.618, p=.537) and general mindful awareness (b=0.055, SEb=0.038, t=1.442, p=.149) did not predict weight loss at week 14. Higher acceptance was a significant predictor of greater weight loss (b=−0.099, SEb=0.031, t=−3.227, p=.001). In the combined model that included all three variables, neither mindful eating (b=0.045, SEb=0.093, t=0.483, p=.629) nor general mindful awareness (b=0.036, SEb=0.040, t=0.896, p=.370) independently predicted weight loss at week 14. Higher baseline acceptance was associated with a faster rate of weight loss (b=−0.104, SEb=0.036, t=−2.915, p=.004). As shown in Figure 1, at week 14, individuals low in acceptance (−1 SD) lost an average of 8.7 ± 0.6 kg (8.4 ± 0.5% of initial weight) compared to 11.2 ± 0.6 kg (10.2 ± 0.5%) among those high in acceptance (+1 SD).
Figure 1.
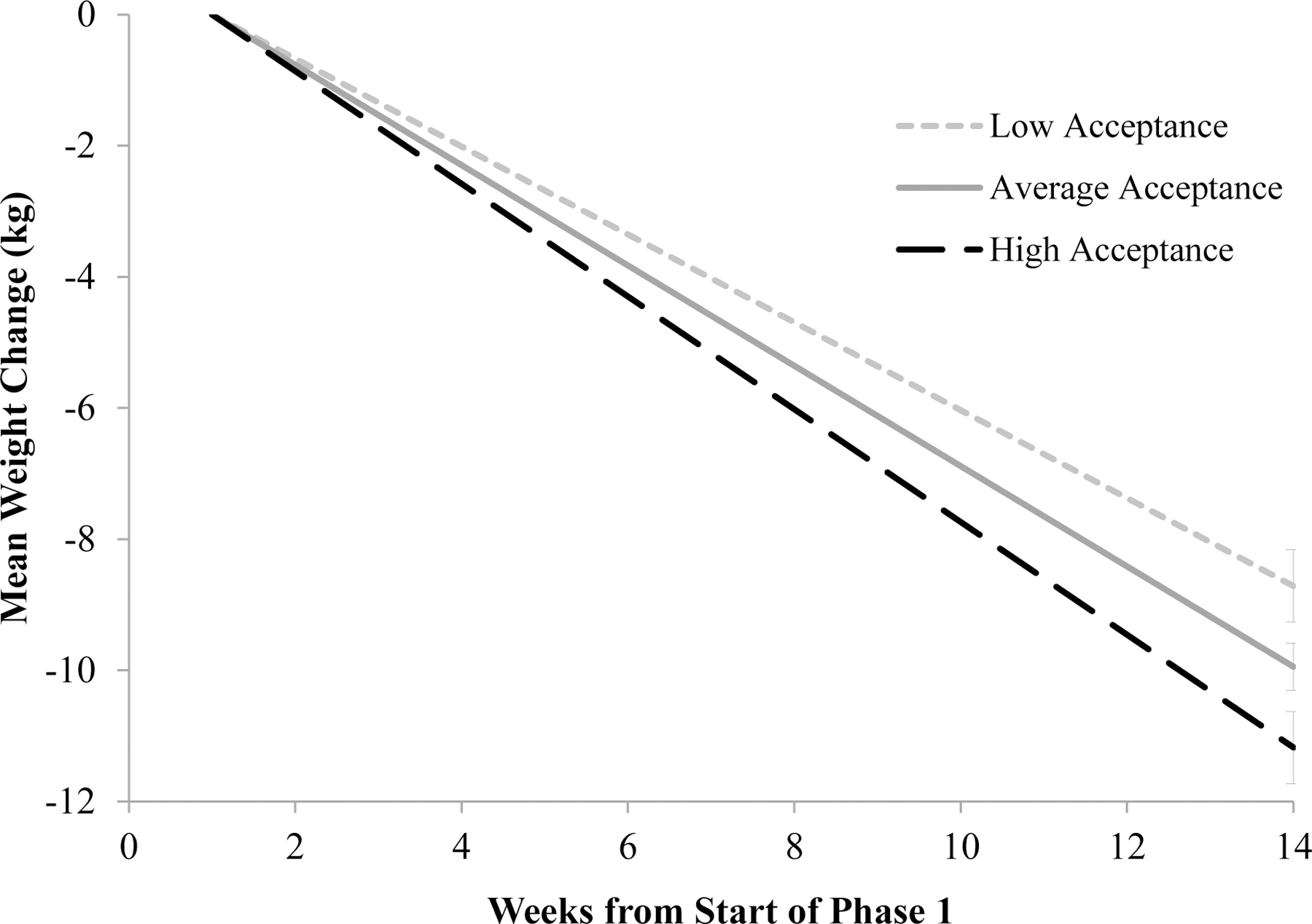
Effect of general acceptance on the induction of weight loss at week 14 of phase 1 in the full sample. Note. Data are estimated modeled means at the mean acceptance value and at +/−1 SD in acceptance for participants who enrolled in the initial weight loss induction phase (N=178). These results control for the (non-significant) effects of mindful eating and general mindfulness.
Predictors of Long-term Weight Loss and Weight Loss Maintenance Among Randomized Participants
As reported previously (Tronieri et al., 2018), the 137 participants who lost ≥5% of initial weight in phase 1 and were later randomized in phase 2 lost a mean of 10.7 ± 0.4 kg in phase 1 (9.3 ± 0.3% of initial weight). Lorcaserin-treated participants gained a mean of 2.0 ± 0.8 kg (1.8 ± 0.8% of initial weight) from randomization to week 52 of phase 2, which was not significantly different from the 2.5 ± 0.8 kg gain of participants assigned to placebo (2.2 ± 0.8%) (Tronieri et al., 2018). As measured from the start of phase 1, total long-term weight losses at week 52 (of phase 2) were 9.4 ± 0.9 kg (7.8 ± 0.8% of initial weight) for participants randomized to lorcaserin and 7.5 ± 1.0 kg (6.6 ± 0.9%) for those assigned to placebo.
In separated preliminary analyses, long-term weight change at week 52 of phase 2 (66 total weeks of treatment) was predicted by higher baseline mindful eating (b=−0.064, SEb=0.032, t=−2.004, p=.045) and acceptance (b=−0.033, SEb=0.013, t=−2.500, p=.012) but not by general mindful awareness (b=−0.001, SEb=0.015, t=−0.061, p=.951). In the combined model, none of the variables were significant independent predictors of total weight loss (mindful eating: b=−0.032, SEb=0.038, t=−0.845, p=.398; general mindful awareness: b=−0.001, SEb=0.016, t=−0.088, p=.930; acceptance: b=−0.029, SEb=0.015, t=−1.900, p=.058). As shown in Figure 2, at week 52, individuals low in acceptance (−1 SD) lost an estimated 6.9 ± 1.1 kg (6.0± 0.9% of initial weight) compared to 10.1 ± 1.1 kg (8.6% ± 0.9) among those high in acceptance (+1 SD). However, this difference did not reach statistical significance (p=.058).
Figure 2.
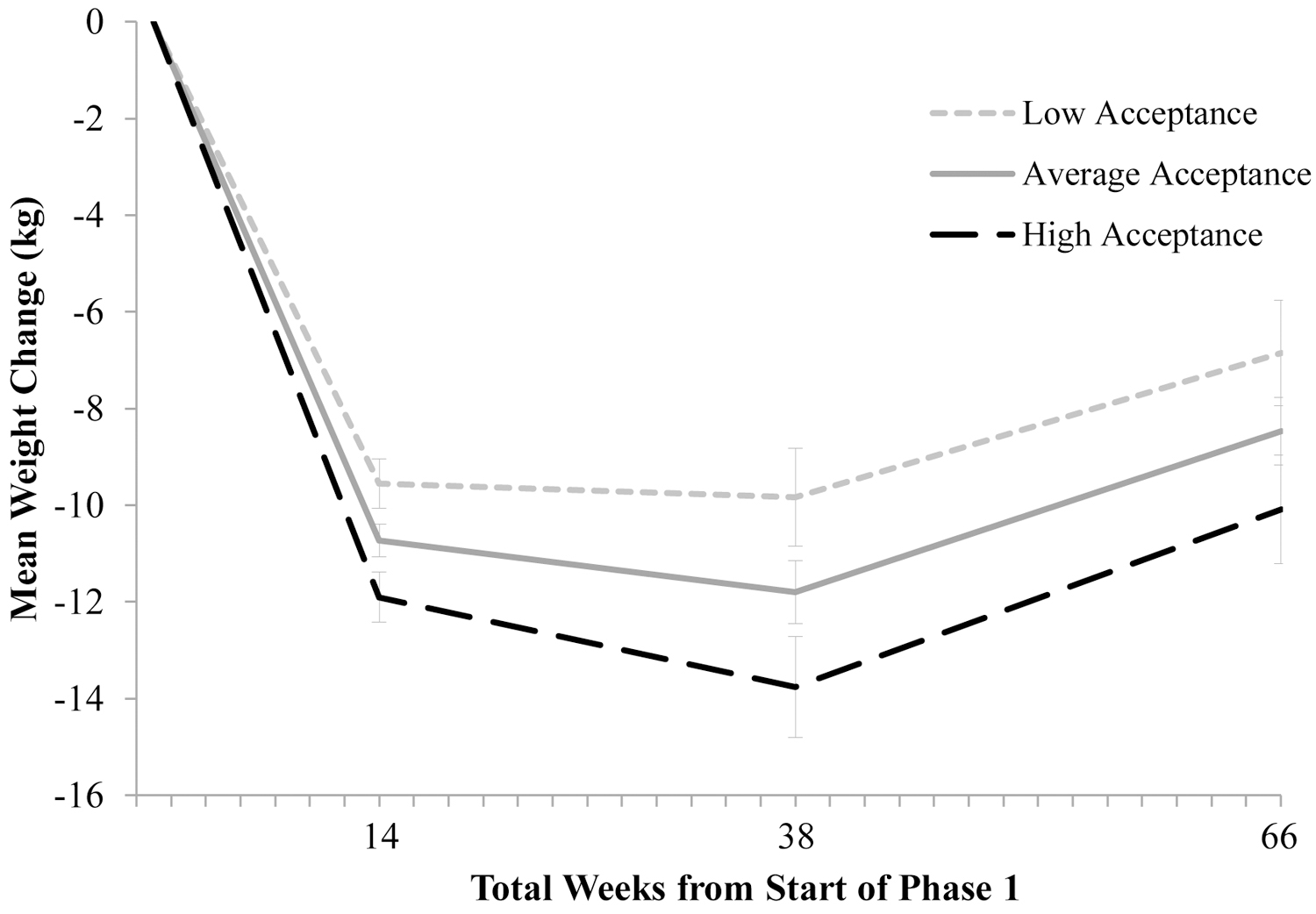
Effect of general acceptance on long-term weight loss at week 52 of phase 2 (66 total weeks of treatment) among participants who successfully lost weight. Note. Data are estimated modeled means at the mean acceptance value and at +/−1 SD in acceptance for successful weight losers who enrolled in the weight maintenance randomized trial (N=137). These results control for the (non-significant) effects of mindful eating and general mindfulness.
In an exploratory analysis, weight loss maintenance from randomization (week 0) to week 52 of phase 2 was not predicted by mindful eating (b=−0.043, SEb=0.038, t=−1.118, p=.264), general mindful awareness (b=−0.004, SEb=0.017, t=−0.214, p=.830), or acceptance (b=−0.010, SEb=0.016, t=−0.643, p=.520) as measured at baseline of phase 1.
Changes in Mindful Eating, General Mindful Awareness, and Acceptance Among Randomized Participants
Among the participants who lost ≥5% of their initial weight in phase 1 and then enrolled in phase 2 (N=137), mindful eating improved from baseline (M=2.70, SD=0.34) to week 14 of phase 1 (M=2.91, SD=0.34, p<.001), but decreased from the latter time to week 52 of phase 2 (M=2.84, SD=0.41, p=.019). At week 52 of phase 2, mindful eating scores were still significantly higher than baseline values at the start of phase 1 (p<.001). As shown in Table 3, there were no significant changes in general mindful awareness or acceptance during phase 1 or phase 2. Changes from baseline in these mindfulness components were not associated with total weight change at either time point, and all correlations were minimal in size (Table 3). The medication groups did not differ in changes in mindful eating (p=.665), general mindful awareness (p=.729), or acceptance (p=.785) from week 0 to week 52 of phase 2.
Table 3.
Changes in mindfulness components among participants who successfully lost ≥ 5% of their weight in phase 1 and enrolled in phase 2 (N = 137).
| Baseline | Week 14 of phase 1 | Week 52 of phase 2 | Change from baseline to week 14 of phase 1 | Correlation with phase 1 weight change (kg) | Change from baseline to week 52 of phase 2 | Correlation with total weight change (kg) at end of phase 2 | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | d | p | r | d | p | r | |
| General Mindful Awareness (PHLMS awareness) | 3.69 (0.72) | 3.77 (0.74) | 3.64 (1.07) | .139 | .104 | −.038 | −.053 | .537 | .099 |
| General Acceptance (PHLMS acceptance) | 3.20 (0.83) | 3.22 (0.81) | 3.26 (1.09) | .034 | .687 | −.082 | .054 | .530 | −.132 |
| Mindful Eating (MEQ total) | 2.70 (0.34) | 2.91 (0.34) | 2.84 (0.41) | .684 | <.001 | −.039 | .340 | <.001 | .056 |
Note: Positive r values reflect an association between larger increases on the measure and larger weight losses.
Discussion
In the present study, higher baseline acceptance independently predicted greater short-term weight loss at week 14 of phase 1. Among participants who successfully lost 5% or more of their initial weight, the effect of acceptance on total weight loss at week 52 of phase 2 (66 total weeks of lifestyle modification treatment) was statistically significant when this predictor was analyzed separately (p=.012), but was not significant when controlling for mindful eating and general mindful awareness (p=.058). In an exploratory analysis, acceptance did not independently predict how well weight loss was maintained after the initial 14-week low-calorie diet. Taken together, these findings suggest that any long-term benefit of baseline acceptance is likely attributable to its association with short-term weight loss, which strongly predicts total weight loss up to 8 years later (Unick et al., 2015). For each standard deviation increase in baseline acceptance, participants in the full sample were predicted to lose 1.2 kg more weight at week 14, and participants who successfully lost weight were predicted to lose 1.6 kg more at the end of treatment.
These results indicated that individuals who were more willing to tolerate the presence of negative or unwanted thoughts, emotions, and physical sensations were more successful with initial weight loss than those who typically attempted to change or avoid certain internal experiences. Maintaining a non-reactive, open approach to internal experiences may facilitate better adherence to weight control behaviors by increasing participants’ willingness to make and sustain changes that produce discomfort. Individuals with higher acceptance may therefore be better able to make healthy eating and activity choices in the presence of thoughts about tempting alternatives, feelings of reduced pleasure, or aversive physiological states (e.g., hunger, fatigue), whereas individuals who have low levels of acceptance might eat high-calorie foods or avoid physical activity in order to reduce these internal states. Additionally, acceptance may have allowed participants to make more deliberate decisions in the presence of internal and external eating cues through its associations with lower impulsivity (Murphy & MacKillon, 2012; Peters et al., 2011) or by limiting their reactivity towards resulting urges to eat. Future research should evaluate whether self-regulation and adherence to dietary and physical activity goals mediate the relationship between acceptance and weight loss.
Contrary to our hypotheses, mindful eating did not independently predict weight loss at either time point. General mindful awareness also was not associated with weight loss. These findings suggest that awareness alone is not sufficient to influence weight control behaviors, regardless of whether awareness is applied to the eating experience or more globally. Previous studies have suggested that higher awareness in the absence of an accepting stance is associated with higher levels of psychological distress (Cardaciotto et al., 2008; Hayes et al., 2006). Greater awareness of eating-related cues, in the absence of acceptance, could serve to increase the salience of those cues rather than to improve individuals’ ability to respond to them (Friese & Hoffman, 2016; Hendrikse et al., 2015).
This pattern of results may be attributable, in part, to the fact that the present study was conducted in a sample that had not been provided with mindfulness training. Although mindfulness questionnaires are designed to describe mindfulness-consistent and inconsistent experiences in ordinary language that an untrained individual can understand, several studies have shown that the interpretation of some items varies with mindfulness exposure (Baer, 2011). Experienced mindfulness practitioners tend to interpret questionnaire items about awareness as being experienced in a nonjudgmental way, whereas mindfulness-naïve individuals endorse these items even when they experience awareness that is judgmental and reactive (Baer, 2011). The minimal correlation between the PHLMS awareness and acceptance scales in this study and in other mindfulness-naïve samples (Cardaciotto et al., 2008; Siegling & Petrides, 2016) supports the idea that individuals without mindfulness training do not take their level of acceptance into account when responding to awareness items. A comparison of the strength of the relationship between these mindfulness components and weight loss in mindfulness-trained vs mindfulness-naive individuals would help to further clarify the role of mindful awareness in weight control.
It is also important to note that mindful eating was moderately correlated with both general mindful awareness and acceptance in this study, and the MEQ was not designed to separately measure these constructs. At week 52 of phase 2, there was a small bivariate association between mindful eating and greater total weight loss among participants initially who lost ≥ 5% of their weight. This effect remained statistically significant when general awareness alone was added to the model (data not presented), but not when acceptance was included. This pattern of results could suggest that the degree to which the MEQ measured eating-related acceptance was overshadowed by the more specific acceptance measure in this study. A scale specifically designed to assess eating-related acceptance could provide a more nuanced picture of the importance of general vs. eating-related acceptance. For example, a recent study found that changes in eating-related acceptance, but not general acceptance, correlated with 12-month weight loss in an acceptance-based intervention (Schumacher et al., 2019).
The standard lifestyle modification intervention provided in the present study produced medium-sized improvements in mindful eating, potentially through the use of behavioral techniques such as self-monitoring to increase awareness of food intake and eating-related cues. However, general mindful awareness and acceptance did not change with lifestyle modification alone. This outcome suggests that although higher acceptance may help people to change their eating or activity behaviors, the reverse is not true – changing one’s weight control behaviors alone does not foster an accepting attitude towards any unpleasant internal experiences that might arise. Although all mindfulness interventions include both awareness and acceptance components, the degree of emphasis varies (Tapper, 2017). A comparison of the extent to which the mindfulness interventions that have been applied to weight loss (e.g., MBCT, MBSR, MB-EAT, ACT) improve mindful eating, general awareness, and acceptance could be helpful for understanding differences in their efficacy. It also will be important to examine associations between changes in these constructs and weight change. The ability to detect such relationships in the present study was likely limited by the absence of mean changes in the general awareness and acceptance scales.
The present findings also highlight the potential benefit of a “bottom-up” approach to the development of mindfulness-based interventions for weight management, in which observational associations between mindfulness components and weight loss are used to inform intervention targets. Investigators might use an iterative approach, such as the Obesity-Related Behavioral Intervention Trials (ORBIT) model of intervention development, to first identify the aspects of mindfulness most relevant to weight loss and refine an intervention protocol to maximize its effect on those components prior to testing whether the treatment enhances weight loss (Czajkowski et al., 2015; Vieten et al., 2018).
Limitations and Future Research
Several aspects of the primary study’s design may impact the generalizability of these findings. Only the 77% of initial participants who had lost at least 5% of their weight in phase 1 could be included in the analyses related to prediction of long-term weight loss and changes in mindfulness variables across treatment. We cannot determine whether those results would apply to individuals who did not lose at least 5% of their initial weight in the first 14 weeks of treatment. Although the baseline acceptance scores of this subsample (M = 3.20, SD = 0.81) appeared to be similar to those of the full sample (M = 3.19, SD = 0.84), we also cannot rule out the possibility that the removal of participants with low initial weight loss from the data set affected the distribution of baseline acceptance scores (because they were associated with short-term weight loss). Additionally, although 81.8% of the randomized sample provided a weight measurement at the end of the trial, only 62–63.5% completed the questionnaire measures at that time, potentially due to trial fatigue. We attempted to use robust methods to account for missing weight and questionnaire data. However, it will be important to replicate these findings, particularly for the exploratory analyses of long-term changes in aspects of mindfulness.
All participants in this study were provided an initial 14-week meal replacement diet, followed by random assignment to lorcaserin or placebo, in addition to the lifestyle modification program. We observed that acceptance continued to be the strongest predictor of total weight loss after the 14-week meal replacement diet was completed, and we did not detect differences between the medication groups in mindfulness. However, we cannot determine whether the present results would generalize to programs that did not incorporate any meal replacements or medication. Therefore, a key next step will be to replicate these findings in a long-term lifestyle modification study that does not limit long-term enrollment or provide any adjunctive interventions.
In conclusion, higher acceptance predicted larger short-term weight losses in a lifestyle modification program, whereas general mindful awareness and mindful eating were not independently associated with weight loss. Further research is needed to replicate and extend these findings by evaluating behavioral pathways through which acceptance affects weight loss, assessing separately the importance of general and eating-related acceptance, and comparing the role of these constructs in mindfulness-trained and mindfulness-naïve samples.
Funding:
This research was supported by an Investigator-Initiated Study award (Wadden) from Eisai Inc. Dr. Tronieri was supported, in part, by a K23 Mentored Patient Oriented Research Award (K23DK116935) from the National Institutes of Health (NIH)/National Institute of Diabetes Digestive and Kidney Disease. Dr. Pearl’s collaboration was supported in part by a K23 Award from the NIH/National Heart, Lung, and Blood Institute (K23HL140176). Dr. Chao was supported in part by the National Institute of Nursing Research of the NIH (K23NR017209).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical statement: This study was approved by Institutional Review Board at the University of Pennsylvania and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All participants provided informed consent prior to their inclusion in the study.
Conflict of Interest Disclosures: JST and NA have served as consultants to Novo Nordisk. TAW reports serving on advisory boards for Novo Nordisk and WW (Weight Watchers). RIB serves as a consultant to Eisai Pharmaceutical, and AMC has consulted with Shire Pharmaceutical. The other authors declare no conflicts of interest.
Disclosures: Drs. Tronieri and Alamuddin have served as consultants to Novo Nordisk. Dr. Wadden reports serving on advisory boards for Novo Nordisk and WW (Weight Watchers). Dr. Berkowitz serves as a consultant to Eisai Pharmaceutical, and Dr. Chao has consulted with Shire Pharmaceutical. The other authors declare no conflicts of interest.
Data Accessibility:
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher for the purpose of replicating the analyses reported in the present manuscript. The datasets for this manuscript are not publicly available because the research team has not yet completed initial data analyses. Requests to access the datasets should be directed to TAW (wadden@pennmedicine.upenn.edu).
References
- Apolzan JW, Myers CA, Cowley AD, Brady H, Hsia DS, Stewart TM,Redman LM, & Martin CK (2016). Examination of the reliability and validity of the mindful eating questionnaire in pregnant women. Appetite, 100, 142–151. 10.1016/j.appet.2016.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer RA (2011). Measuring mindfulness. Contemporary Buddhism, 12(1), 241–261. 10.1080/14639947.2011.564842 [DOI] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck Depression Inventory-II (BDI-II) Manual. San Antonio, TX: Harcourt Brace. [Google Scholar]
- Beshara M, Hutchinson AD, & Wilson C (2013). Does mindfulness matter? Everyday mindfulness, mindful eating and self-reported serving size of energy dense foods among a sample of south Australian adults. Appetite, 67, 25–29. 10.1016/j.appet.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J, Segal ZV, Abbey S, Speca M, Velting D, & Devins G (2004). Mindfulness: A proposed operational definition. Clinical Psychology: Science and Practice, 11(3), 230–241. 10.1093/clipsy.bph077 [DOI] [Google Scholar]
- Cardaciotto L, Herbert JD, Forman EM, Moitra E, & Farrow V (2008). The assessment of present-moment awareness and acceptance: The Philadelphia Mindfulness Scale. Assessment, 15(2), 204–223. 10.1177/1073191107311467 [DOI] [PubMed] [Google Scholar]
- Carrière K, Khoury B, Günak M, & Knäuper B (2018). Mindfulness-based interventions for weight loss: A systematic review and meta-analysis. Obesity Reviews, 19(2), 164–177. 10.1111/obr.12623 [DOI] [PubMed] [Google Scholar]
- Czajkowski SM, Powell LH, Adler N, Narr-King S, Reynolds KD, Hunter CM, Laraia B, Olster DH, Perna FM, Peterson JC, Epel E, Boyington JE, & Charlson ME (2015). From ideas to efficacy: the ORBIT model for developing behavioral treatments for chronic diseases. Health Psychology, 34(10), 971–82. 10.1037/hea000016117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, Lustig RH, Kemeny M, Karan L, & Epel E (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. Journal of Obesity, 2011. 10.1155/2011/651936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EI, Topper M, Muskens JG, Bögels SM, & Kamphuis JH (2012). Psychometric properties of the Five Facets Mindfulness Questionnaire (FFMQ) in a meditating and a non-meditating sample. Assessment, 19(2), 187–197. https://doi.org/10.1177%2F1073191112446654 [DOI] [PubMed] [Google Scholar]
- Forman E, Butryn M, Juarascio A, Bradley L, Lowe M, Herbert J, & Shaw J (2013). The Mind Your Health project: A randomized controlled trial of an innovative behavioral treatment for obesity. Obesity, 21(6), 1119–1126. 10.1002/oby.20169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman EM, Butryn ML, Manasse SM, Crosby RD, Goldstein SP, Wyckoff EP, & Thomas JG (2016). Acceptance-based versus standard behavioral treatment for obesity: Results from the mind your health randomized controlled trial. Obesity, 24(10), 2050–2056. 10.1002/oby.21601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Framson C, Kristal AR, Schenk JM, Littman AJ, Zeliadt S, & Benitez D (2009). Development and validation of the mindful eating questionnaire. Journal of the American Dietetic Association, 109(8), 1439–1444. 10.1016/j.jada.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese M, & Hofmann W (2016). State mindfulness, self-regulation, and emotional experience in everyday life. Motivation Science, 2(1), 1. https://psycnet.apa.org/doi/10.1037/mot0000027 [Google Scholar]
- Gallop R, & Tasca GA (2009). Multilevel modeling of longitudinal data for psychotherapy researchers: II. The complexities. Psychotherapy Research, 19(4–5), 438–452. 10.1080/10503300902849475 [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, & Gilreath TD (2007). How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prevention Science, 8, 206–213. https://doi-org/10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- Grossman P (2011). Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology’s (re) invention of mindfulness: Comment on Brown et al.(2011). Assessment, 23(4), 1034–1040. https://psycnet.apa.org/doi/10.1037/a0022713 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, & Lillis J (2006). Acceptance and Commitment Therapy: Model, processes and outcomes. Behaviour Research and Therapy, 44(1), 1–25. 10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, & Wilson KG (1999). Acceptance and Commitment Therapy: An experiential approach to behavior change. Guilford Press. [Google Scholar]
- Hendrikse JJ, Cachia RL, Kothe EJ, McPhie S, Skouteris H, & Hayden MJ (2015). Attentional biases for food cues in overweight and individuals with obesity: a systematic review of the literature. Obesity reviews, 16(5), 424–432. 10.1111/obr.12265 [DOI] [PubMed] [Google Scholar]
- Higgs S, & Donohoe JE (2011). Focusing on food during lunch enhances lunch memory and decreases later snack intake. Appetite, 57(1), 202–206. 10.1016/j.appet.2011.04.016 [DOI] [PubMed] [Google Scholar]
- Hinterman C, Burns L, Hopwood D, & Rogers W (2012). Mindfulness: Seeking a more perfect approach to coping with life’s challenges. Mindfulness, 3(4), 275–281. 10.1007/s12671-012-0091-8 [DOI] [Google Scholar]
- Hollis-Walker L, & Colosimo K (2011). Mindfulness, self-compassion, and happiness in non-meditators: A theoretical and empirical examination. Personality and Individual differences, 50(2), 222–227. 10.1016/j.paid.2010.09.033 [DOI] [Google Scholar]
- Howell AJ, & Buro K (2011). Relations among mindfulness, achievement-related self-regulation, and achievement emotions. Journal of Happiness Studies, 12(6), 1007–1022. 10.1007/s10902-010-9241-7 [DOI] [Google Scholar]
- Kabat-Zinn J (2013). Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Bantam Books. [Google Scholar]
- Kabat-Zinn J (1994). Wherever you go, there you are: Mindfulness meditation in everyday life. Hachette Books. [Google Scholar]
- Katterman SN, Kleinman BM, Hood MM, Nackers LM, & Corsica JA (2014). Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: A systematic review. Eating Behaviors, 15(2), 197–204. 10.1016/j.eatbeh.2014.01.005 [DOI] [PubMed] [Google Scholar]
- Kristeller JL, & Wolever RQ (2014). Mindfulness-based eating awareness training: Treatment of overeating and obesity. In Baer RA (Ed.), Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications (pp. 119–139). Academic Press. [Google Scholar]
- Lillis J, Niemeier HM, Thomas JG, Unick J, Ross KM, Leahey TM, Kendra KE, Dorfman L, & Wing RR (2016). A randomized trial of an acceptance-based behavioral intervention for weight loss in people with high internal disinhibition. Obesity, 24(12), 2509–2514. 10.1002/oby.21680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Epel ES, Kristeller J, Moran PJ, Dallman M, Lustig RH, Acree M, Bacchetti P, Laraia BA, Hecht FM, & Daubenmier J (2016). Effects of a mindfulness-based intervention on mindful eating, sweets consumption, and fasting glucose levels in obese adults: data from the SHINE randomized controlled trial. Journal of Behavioral Medicine, 39(2), 201–213. 10.1007/s10865-015-9692-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A, & Tully EC (2012). The role of mindfulness and psychological flexibility in somatization, depression, anxiety, and general psychological distress in a nonclinical college sample. Journal of Evidence-Based Complementary & Alternative Medicine, 17(1), 66–71. https://doi.org/10.1177%2F2156587211423400 [Google Scholar]
- Murphy C, & MacKillop J (2012). Living in the here and now: interrelationships between impulsivity, mindfulness, and alcohol misuse. Psychopharmacology, 219(2), 527–536. 10.1007/s00213-011-2573-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson KL, & Emery CF (2015). Mindfulness and weight loss: A systematic review. Psychosomatic Medicine, 77(1), 59–67. 10.1097/PSY.0000000000000127 [DOI] [PubMed] [Google Scholar]
- Peters JR, Erisman SM, Upton BT, Baer RA, & Roemer L (2011). A preliminary investigation of the relationships between dispositional mindfulness and impulsivity. Mindfulness, 2(4), 228–235. 10.1007/s12671-011-0065-2 [DOI] [Google Scholar]
- Raab K (2014). Mindfulness, self-compassion, and empathy among health care professionals: a review of the literature. Journal of Health Care Chaplaincy, 20(3), 95–108. 10.1080/08854726.2014.913876 [DOI] [PubMed] [Google Scholar]
- Robinson E, Kersbergen I, & Higgs S (2014). Eating ‘attentively’ reduces later energy consumption in overweight and obese females. British Journal of Nutrition, 112(4), 657–661. 10.1017/S000711451400141X [DOI] [PubMed] [Google Scholar]
- Rogers JM, Ferrari M, Mosely K, Lang CP, & Brennan L (2017). Mindfulness-based interventions for adults who are overweight or obese: A meta-analysis of physical and psychological health outcomes. Obesity Reviews, 18(1), 51–67. 10.1111/obr.12461 [DOI] [PubMed] [Google Scholar]
- Ruffault A, Czernichow S, Hagger MS, Ferrand M, Erichot N, Carette C, Bboujut E, & Flahault C (2017). The effects of mindfulness training on weight-loss and health-related behaviours in adults with overweight and obesity: A systematic review and meta-analysis. Obesity Research & Clinical Practice, 11(5), 90–111. 10.1016/j.orcp.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Schumacher LM, Godfrey KM, Forman EM, & Butryn ML (2019). Change in domain-specific but not general psychological flexibility relates to greater weight loss in acceptance-based behavioral treatment for obesity. Journal of Contextual Behavioral Science, 12, 59–65. 10.1016/j.jcbs.2019.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegling AB, & Petrides KV (2016). Zeroing in on mindfulness facets: Similarities, validity, and dimensionality across three independent measures. PloS one, 11(4). 10.1371/journal.pone.0153073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soysa CK, & Wilcomb CJ (2015). Mindfulness, self-compassion, self-efficacy, and gender as predictors of depression, anxiety, stress, and well-being. Mindfulness, 6(2), 217–226. 10.1007/s12671-013-0247-1 [DOI] [Google Scholar]
- Stunkard AJ & Messick S (1998). Eating Inventory Manual. New York: Psychological Corporation. [Google Scholar]
- Tapper K (2017). Can mindfulness influence weight management related eating behaviors? If so, how?. Clinical Psychology Review, 53, 122–134. 10.1016/j.cpr.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, & Lau MA (2000). Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology, 68(4), 615. https://psycnet.apa.org/doi/10.1037/0022-006X.68.4.615 [DOI] [PubMed] [Google Scholar]
- Tronieri JS, Alfaris N, Chao AM, Pearl RL, Alamuddin N, Bakizada ZM, Berkowitz RI, & Wadden TA (2017). Lorcaserin plus lifestyle modification for weight loss maintenance: Rationale and design for a randomized controlled trial. Contemporary Clinical Trials, 59, 105–112. 10.1016/j.cct.2017.06.004 [DOI] [PubMed] [Google Scholar]
- Tronieri JS, Wadden TA, Berkowitz RI, Chao AM, Pearl RL, Alamuddin N, Leonard SM, Carvajal R, Bakizada ZM, Pinkasavage E, Gruber KA, Walsh OA, & Alfaris N (2018). A randomized trial of lorcaserin and lifestyle counseling for maintaining weight loss achieved with a low-calorie diet. Obesity, 26(2), 299–309. 10.1002/oby.22081 [DOI] [PubMed] [Google Scholar]
- Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, … & Look AHEAD Research Group. (2015). Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity, 23(7), 1353–1356. 10.1002/oby.21112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Veer E, Van Herpen E, & Van Trijp HC (2016). Body and mind: Mindfulness helps consumers to compensate for prior food intake by enhancing the responsiveness to physiological cues. Journal of Consumer Research, 42(5), 783–803. 10.1093/jcr/ucv058 [DOI] [Google Scholar]
- Vieten C, Laraia B, Kristeller J, Adler N, Coleman-Phox K, Bush NR, Duncan LG, & Epel E (2018). The mindful moms training: development of a mindfulness-based intervention to reduce stress and overeating during pregnancy. BMC Pregnancy Childbirth, 18(1), 201. 10.1186/s12884-018-1757-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Foster GD, Sarwer DB, Anderson DA, Gladis M, Sanderson RS, Letchak RV, Berkowitz RI, & Phelan S (2004). Dieting and the development of eating disorders in obese women: Results of a randomized controlled trial. The American Journal of Clinical Nutrition, 80(3), 560–568. 10.1093/ajcn/80.3.560 [DOI] [PubMed] [Google Scholar]
- Wadden TA, Vogt RA, Andersen RE, Bartlett SJ, Foster GD, Kuehnel RH, Wilk J, Weinstock R, Buckenmeyer P, Berkowitz RI, & Steen SN (1997). Exercise in the treatment of obesity: Effects of four interventions on body composition, resting energy expenditure, appetite, and mood. Journal of Consulting and Clinical Psychology. 65(2), 269–77. 10.1037//0022-006x.65.2.269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher for the purpose of replicating the analyses reported in the present manuscript. The datasets for this manuscript are not publicly available because the research team has not yet completed initial data analyses. Requests to access the datasets should be directed to TAW (wadden@pennmedicine.upenn.edu).


