Abstract
Insulin secretion from beta cells is crucial for maintaining euglycaemia and preventing type 2 diabetes, a disease correlated with ageing. Therefore, understanding the functional changes that beta cell function undergoes with age can reveal new therapeutic targets and strategies to delay or revert the disease. Herein, a systematic review of the literature agrees that, as humans age, their beta cell function declines, independently of peripheral insulin resistance, BMI and waist circumference. Rodent studies reveal that, with age, basal insulin secretion increases with either no change or an increase in stimulated insulin secretion, but the biological significance of this is unclear. The accumulation of senescent beta cells could explain some of these functional changes: transcriptional analysis of senescent and aged beta cells revealed parallel downregulation of several steps along the pathway linking glucose stimulation and insulin secretion. Moreover, specific deletion of senescent cells (senolysis) improved residual beta cell function, gene expression profile and blood glucose levels. In conclusion, cellular senescence could underlie the functional decline of beta cells during ageing and could represent a novel and promising approach for recovering insulin secretion.
Keywords: Ageing, Beta cells, Function, Human, Insulin secretion, Review, Rodents, Senescence, Senolysis, Type 2 diabetes
Age is one of the main risk factors for type 2 diabetes, a disease where peripheral insulin resistance and insufficient beta cell insulin secretion converge and result in longstanding hyperglycaemia and its complications. The majority of individuals with type 2 diabetes are over 50 years of age [1], emphasising the importance of understanding the disease from an ageing point of view.
Herein, the functional changes in beta cells during ageing are reviewed, with an emphasis on senescence, one of the mechanisms through which beta cells respond to age and stress [2, 3]. In addition, molecular changes induced by age are explored as potential new targets to preserve and enhance beta cell function during ageing and senescence, which represents a new way to understand and treat type 2 diabetes.
The literature was systematically reviewed on MEDLINE through freely accessible PubMed as a search engine for the terms ‘aging’, ‘insulin-secretion’, ‘senescence’ and ‘β-cell function’; the most relevant and significant studies were included. Only studies that specifically measured insulin secretion/beta cell function in differently aged groups were included. For mechanisms, studies that addressed different steps in stimulus–secretion coupling mechanisms were included as were those that looked at beta cell senescence or ageing pathways. Although only findings on type 2 diabetes have been included in this review it is likely that some of these changes are also relevant in type 1 diabetes.
Age and insulin secretion
Human studies
Evaluation of beta cell function in humans, in vivo and in vitro, indicate that insulin secretion decreases with age (Table 1), an observation consistent with the increased incidence of type 2 diabetes in the ageing population. In the remainder of this section a number of representative studies supporting this statement are discussed.
Table 1.
Summary of effects of ageing on insulin secretion in humans and rodents
| Age | Evaluation method | Effect of age on insulin secretion | Reference | |
|---|---|---|---|---|
| Humans | ||||
| In vivo | Young 20–39 years Middle-aged 40–59 years Older ≥60 |
OGTT and insulinogenic index | Decreased beta cell function | [4] |
| In vivo | 35–84 years | HOMA-B | Decreased beta cell function | [5] |
| In vivo | 23 years vs 70 years | i.v. glucose | Decreased beta cell function | [6] |
| In vivo | <35 years vs >60 years | i.v. glucose | Decreased beta cell function | [7] |
| In vivo | 18–85 years | Clamp | Increased and decreased stimulated insulin secretion | [8] |
| In vitro | Islets; 16–70 years | GSIS | Lower GSIS and incomplete reversal of diabetes | [9] |
| In vitro | Islets; 19–64 years | GSIS | Decreased insulin secretion in response to high glucose | [10] |
| In vitro | Islets; donors 16–61 years | Perifusion | Decreased insulin secretion in response to high glucose | [11] |
| Rodents | ||||
| Mice | Young: 1 month Aged: 16–20 months |
In vivo GSIS | Higher basal and increased stimulated insulin secretion | [13] |
| Mice | Ages: 1 month, 6 months, 11 months, 27 months |
In vitro GSIS | Increased stimulated insulin secretion between 1 and 6 months. No change between 6 and 27 months | [14] |
| Mice | Young: 6 months Middle: 18 months Aged: >24 months |
In vivo and in vitro GSIS | In vivo: Increased basal and stimulated insulin secretion in middle and aged groups In vitro: Increased basal and stimulated insulin secretion in islets from aged groups |
[10] |
| Rats | Young: 3 months Aged: 12 months |
In vitro GSIS | No changes in basal or stimulated insulin secretion | [15] |
| Mice | Young: 3 months Aged: 18 months |
In vitro GSIS; RHPA | Increased basal insulin secretion | [16] |
| Mice | Aged: 14–20 months | In vivo GSIS | Increased basal insulin secretion | [2] |
A large study performed OGTTs on 32,245 Chinese individuals without diabetes and used the Matsuda index to estimate insulin sensitivity and the insulinogenic index to evaluate beta cell function. There was a higher proportion of post-challenge hyperglycaemia with older age (≥60 years). In addition, a decrease in the insulinogenic index was observed in the older group relative to the young participants (20–39 years) and middle-aged group (40–59 years). Insulin sensitivity was higher in the older group and results were independent of BMI and waist circumference [4].
Along the same lines, a 2 year follow-up study with 1750 participants (35–84 years old) revealed a significant decrease in beta cell function as determined by HOMA-B after adjusting for BMI and waste circumference (p < 0.0001) [5]. A second study corroborated lower indices of insulin secretion in elderly patients (mean 70 years), even after compensating for changes in adiposity and insulin resistance [6]. This progressive impairment of insulin secretion was corroborated by results generated by more exhaustive measures, such as acute insulin response to i.v. glucose, disposition index and beta cell sensitivity to glucose in people older than 60 years [7].
A retrospective study looking at clamp data from a large group of nondiabetic Europeans aged 18–85 years found that the fasting post-hepatic insulin delivery rate (IDR) gradually increased with age, indicating a progressive increase in basal insulin secretion. In this case, it was positively related to BMI and negatively related to insulin sensitivity. After controlling for these factors, IDR showed an inverse correlation with age (p < 0.0001) [8].
In vitro measurement of insulin secretion from isolated islets is a complementary approach to the in vivo measurement of insulin secretion, which, in spite of correction methods, is inevitably influenced by peripheral insulin resistance. A number of in vitro studies have measured insulin secretion from islets of differently aged donors. One of these studies analysed human islets from donors aged 16–70 years of age and used glucose-stimulated insulin secretion (GSIS) to evaluate beta cell function. It found that islets from younger donors (<40 years of age) had significantly higher GSIS. In transplantation experiments into a mouse model of diabetes, diabetes was reversed in 96% of recipients of islets from younger donors, compared with 68% of recipients of transplants from older donors [9]; these results correlated with the capacity of islets to generate ATP.
In addition, two separate studies reported an age-dependent loss of insulin secretion. The first one used static GSIS and found a decline of beta cell function with age characterised by impaired insulin secretion in response to high glucose levels (p < 0.0001) with no correlation to donor BMI [10]. The second found an age-dependent decline in insulin secretion dynamics when using a perifusion system and the decline of insulin secretion at high glucose concentrations was corroborated by evaluation using static GSIS [11].
In summary, these and other studies (reviewed in [12]) consistently point to an age-dependent decline of beta cell function in humans independent of peripheral insulin resistance.
Rodent studies
Attempts to reach a consensus about the effects of age on insulin secretion from rodent islets have been unsuccessful. Studies have reported conflicting data, with some claiming improvement and others finding a decline or no change in insulin secretion with age (Table 1).
An improvement in function was detected using islets from adolescent (1 month of age) and very old (16–20 months old) mice where in vivo insulin secretion assays revealed that older mice tended to have higher fasting blood insulin levels, consistent with higher basal insulin secretion, and increased insulin secretion after 3 min but not after 7 min of glucose injection [13]. A second study compared insulin secretion by GSIS between islets isolated from 1-, 6-, 11- and 27-month-old mice. Stimulated insulin secretion was found to increase between 1 and 6 months but not thereafter [14].
A study comparing beta cell function in young (6 months old), middle-aged (18 months old) and aged (>24 months old) mice found an increase in fasting insulin levels in vivo in middle-aged and aged mice. This was also reflected in vitro, where insulin secretion at 2 mmol/l glucose was significantly higher in islets from aged mice. After glucose stimulation, aged animals exhibited an augmented insulin secretory response both in vivo and in vitro [10]. Yet, a separate study found no changes in beta cell function between islets of 3- and 12-month-old rats by GSIS [15].
Our group has found a consistent age-dependent impairment of insulin secretion from mouse islets using different models and techniques. We compared islets from young (3 months old) and aged (18 months old) mice with static GSIS and found higher basal insulin secretion from aged islets, with no changes in stimulated conditions. This was confirmed using a mouse strain that develops insulin resistance with age (C57BL/6J) and a model that remains insulin sensitive with age (INK-ATTAC) [16]. In addition, we used the reverse haemolytic plaque assay (RHPA) to measure insulin secretion from individual beta cells [17] and found a significant increase in basal and a decrease in stimulated insulin secretion from beta cells of aged mice. Due to the potential effects of changes in islet composition or in the function of other islet cell types, the RHPA provides a unique model to evaluate cell-autonomous functional changes. In a subsequent publication, beta cell function was evaluated in vivo and we confirmed an elevation of fasting insulin levels in 14–20-month-old mice, and in 9-month-old animals treated with a high-fat diet, an intervention that accelerates the beta cell ageing process [2].
While there is no evident reason for the differences among mouse studies, the explanation might lie in the animal strains used, insulin resistance status of the animals, rate of mitochondrial damage and changes in the glucose stimulus–insulin secretion coupling mechanism as suggested by some studies [18]. Another source of variation is the wide age range considered ‘young’ and ‘aged’ in each study (Table 1). Methodological differences in the measurement of insulin secretion, such as culturing overnight vs immediately after isolation, can also affect metabolism and gene expression in beta cells. Key differences between mice and humans need to be considered when comparing insulin secretion, such as [19]: (1) circadian rhythmicity (humans are diurnal whereas mice are nocturnal); (2) eating patterns (mice eat non-stop, while humans feed in discrete episodes); (3) differences in diet composition; and (4) molecular differences in insulin secretory machinery.
Finally, lack of a uniform definition as to what constitutes impaired vs enhanced insulin secretion can be a major confounder in the interpretation of results. For example, does increased basal insulin secretion constitute impaired function? Within the field, it is necessary to define what constitutes a healthy vs an unhealthy insulin secretory profile, both in vivo and in vitro. Additional methods and models can help us understand this key issue further.
Mechanisms of insulin secretion change with age
Beta cell functional changes can be attributed to variations at three levels: (1) cell-autonomous changes in beta cells, such as senescence and stimulus–secretion coupling; (2) changes in beta cell mass and proliferation; and (3) changes in insulin action, such as insulin resistance. In the following sections the potential contribution of each of these will be evaluated in the context of insulin secretion and ageing.
Cellular senescence
Ageing is defined as a time-dependent decline in cellular function. However, different tissues and cell types age through different mechanisms, described as the hallmarks of ageing [20]. Out of the nine hallmarks described, cellular senescence and the senescence-associated secretory phenotype (SASP) are one of the main mechanisms through which beta cells age [2]. By understanding senescent beta cells, we can further understand beta cell ageing. Cellular senescence is a stress response to an array of effects, such as DNA damage, ER stress and oncogene activation. The senescence state is characterised by a lack of cellular proliferation, increased β-galactosidase (βGal) activity and SASP secretion. SASP proteins include soluble and insoluble factors, such as chemokines, cytokines and extracellular matrix remodelling factors. These factors can induce dysfunction in surrounding cells and precipitate their entry into the senescence process [21], and by recruiting immune cells can favour a proinflammatory microenvironment. In addition, senescent cells upregulate anti-apoptotic pathways, which make them resistant to apoptosis. Since it is a stress response, senescence can occur at any time. But with age, cellular stressors increase and the immune response decreases, leading to an accumulation of senescent cells in tissues of aged animals (Fig. 1). We have shown that senescent beta cells accumulate in the islets of aged mice and humans and their proportion is further increased by insulin resistance, high BMI and type 2 diabetes [2]. To further understand the biology of the senescent beta cell subpopulation and its impact on insulin secretion, a beta cell senescence signature was generated through RNA sequencing (RNA-seq) comparing senescent (βGal+) and non-senescent (βGal−) beta cells from the same pool of islets [22]. It revealed a downregulation of beta cell identity genes (Ins1, Pdx1, Mafa, Neurod1), upregulation of genes that are usually suppressed (Cat, Ldha) and increased expression of markers of ageing (Igf1r, Bambi), senescence (p16Ink4a [also known as Cdkn2a] and p21Cip1 [also known as Cdkn1a]) and SASP (Ccl2, Il1a, Il6, Tnf) along with increased βGal activity. Conditions with a higher percentage of senescent beta cells, such as ageing and insulin resistance, were characterised by impaired beta cell function with higher basal insulin secretion.
Fig. 1.
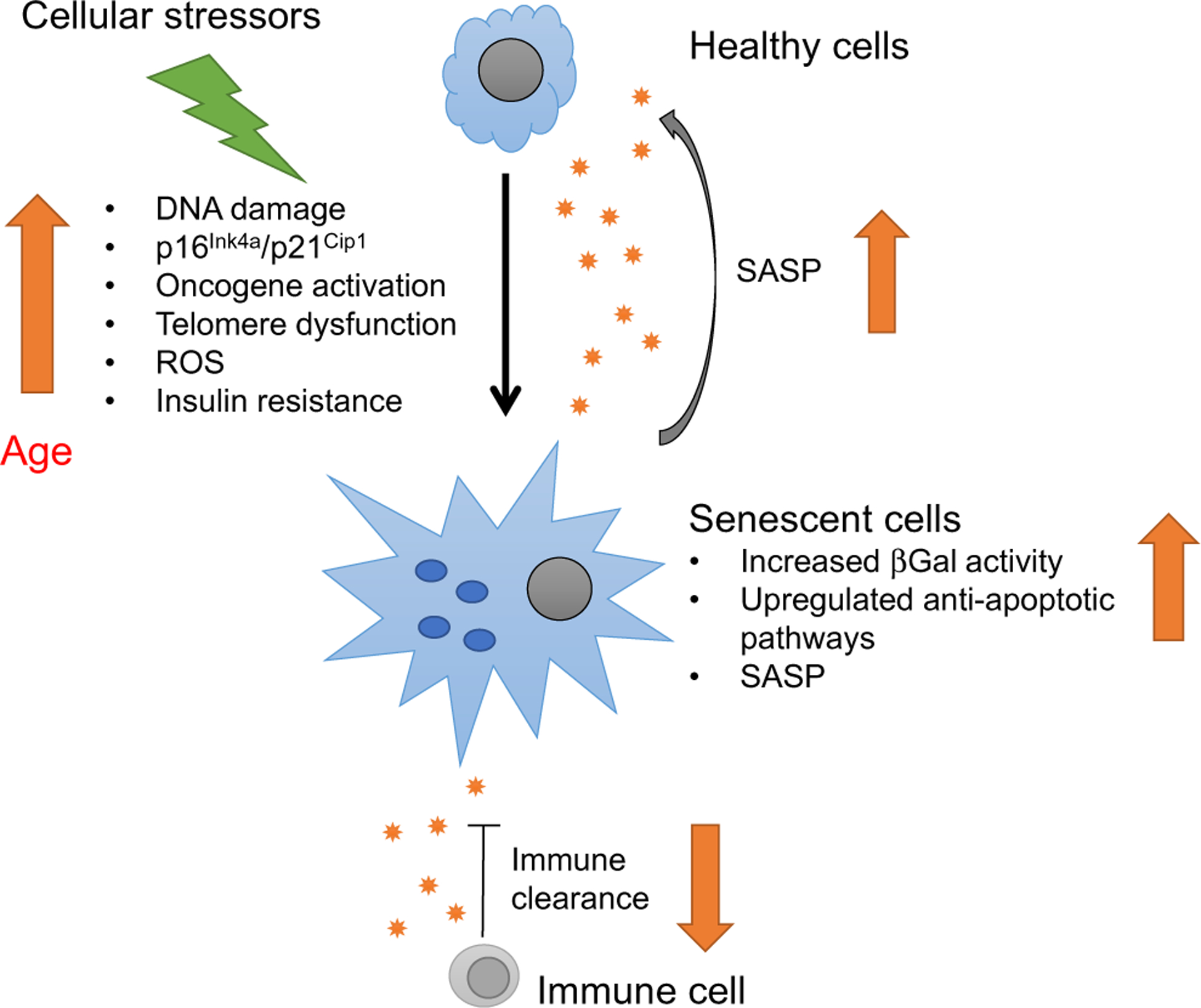
Cellular senescence is a stress response (to DNA damage, ER stress, oncogene activation that induces expression of senescence markers and effectors p16Ink4a and p21Cip1) that leads to a lack of proliferation in response to growth stimuli, increased activity of senescence-associated (SA-βGal) and to the secretion of an array of proteins specific to each cell type known as SASP. SASP proteins include soluble and insoluble factors, such as chemokines, cytokines and extracellular matrix remodelling factors. SASP can induce dysfunction in surrounding cells and promote their entry into senescence, i.e. they have a ‘contagious-like’ effect. Some SASP proteins can recruit immune cells for clearance of senescent cells. Since senescence is a cellular stress response, it can occur at any time, but with age, cellular stressors increase and the immune response decreases, leading to an accumulation of senescent cells.
Changes in stimulus–secretion coupling
The expression of genes involved in glucose stimulus–insulin secretion coupling was compared between senescent and non-senescent beta cells using the same RNA-seq data described above. Senescent beta cells were characterised by a significant downregulation of genes involved in glycolysis, cellular depolarisation (ATP-dependent K+ [KATP] channel, chloride channels, cation channels, voltage-gated calcium channels, sodium channels), incretin pathway receptors and components of insulin granules (Fig. 2a). These transcriptional changes mirror those observed during beta cell ageing, as seen in a microarray analysis of purified beta cells from 1- and 2-year-old MIP-GFP mice in which GFP expression is driven by the insulin gene promoter [16] (Fig. 2b). In both cases, there is a decrease in the transcription of genes involved in glucose metabolism, ionic channels, incretin signalling and insulin synthesis (Fig. 3), all of which predict a functional decline of beta cells with age and senescence. Some of these changes could also explain the functional characteristics of ageing beta cells such as increased basal insulin secretion. For example, decreased KATP channels would hypothetically lead to increased beta cell depolarisation at low glucose concentrations which would be reflected as higher insulin secretion in the basal state. Although this has not been proved experimentally, it represents a novel and interesting mechanism to be explored.
Fig. 2.
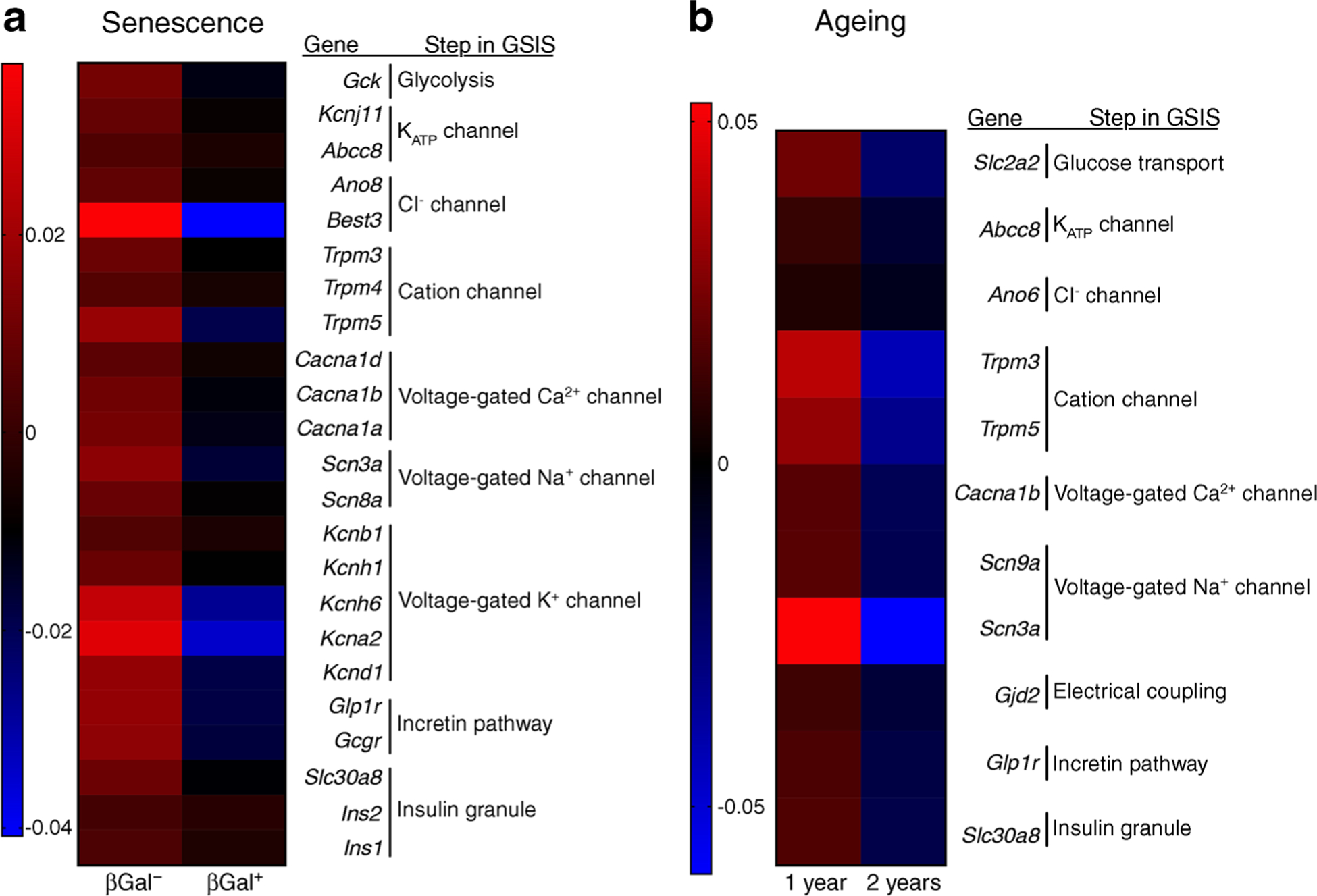
Beta cell gene expression changes due to senescence and chronological ageing are similar and reveal downregulation of key players in the stimulus–secretion coupling mechanism. (a) Islets isolated from 7–8-month-old C56Bl/6 J mice were FACS sorted into non-senescent (βGal−) and senescent (βGal+) for RNA-Seq; Mean expression is shown for n = 7 sets of paired samples. Data reported in this panel have been deposited in NCBI Gene Expression Omnibus (GEO accession number GSE121539) [2]. (b) Microarray data of purified beta cells of MIP-GFP mice 1 and 2 years of age. Mean expression is shown for n = 4 for 1 year of age, n = 3 for 2 years of age. Data reported in this panel have been deposited in the NCBI GEO database under accession number GSE72753 [16].
Fig. 3.
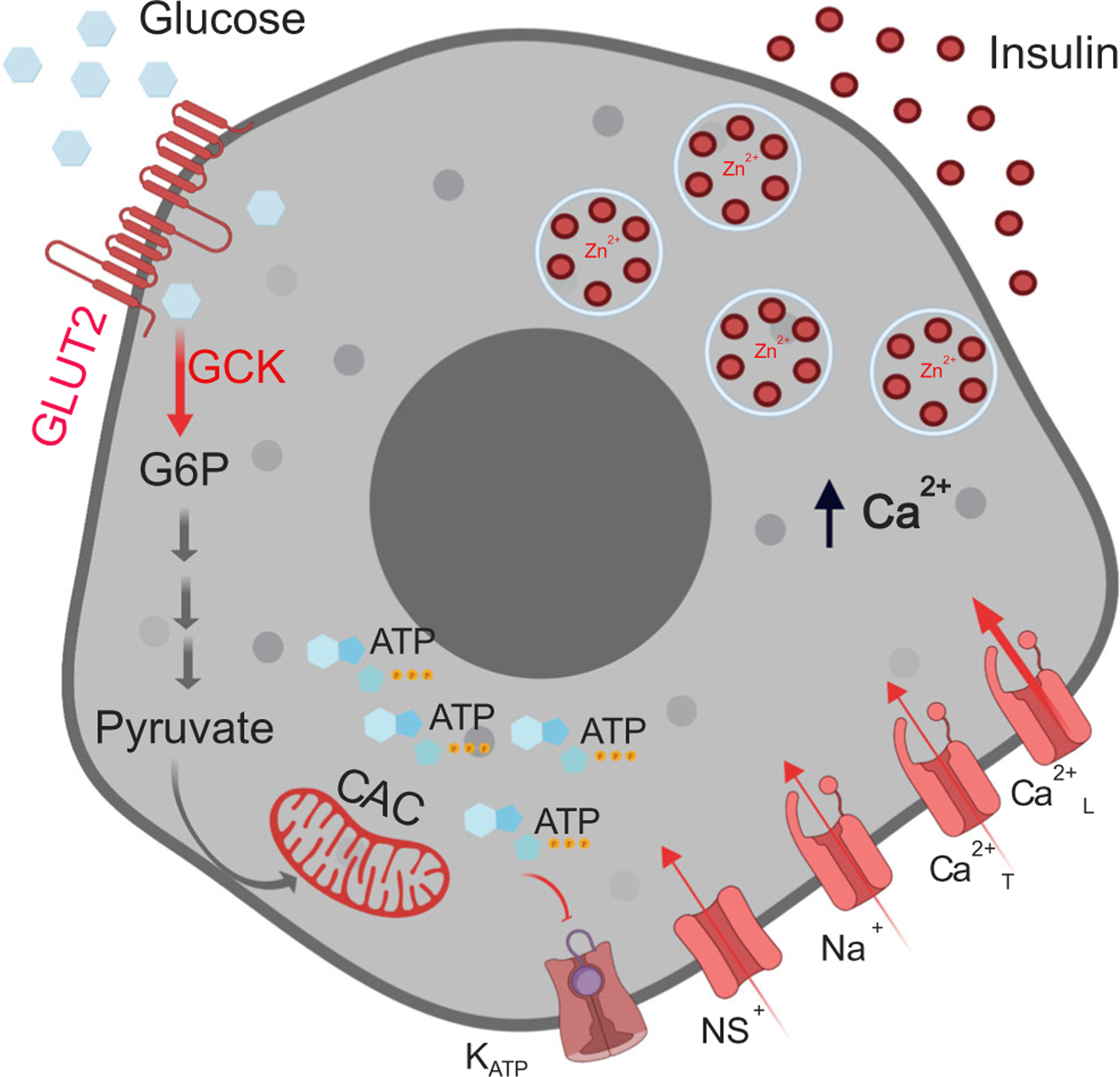
Glucose stimulus–insulin secretion coupling mechanism in beta cells. As extracellular glucose concentrations increase, glucose uptake via GLUT2 increases glucose metabolism via glycolysis (glucokinase-GCK), the citric acid cycle (CAC) and oxidative phosphorylation, increasing ATP production. This, in turn, leads to closure of the KATP channels, which, along with continued conductance through non-selective cationic channels (NS+), results in membrane depolarisation. When the membrane potential reaches a certain threshold, voltage-gated sodium (Na+) channels and T-type Ca2+ channels (Ca2+T) open increasing the membrane potential further to activate the L-type Ca2+ channels (Ca2+L). The associated Ca2+ influx triggers exocytosis of insulin-containing secretory granules. Shown in the different shades of red are components decreased in aged and senescent cells that could result in decreased insulin secretion. G6P, glucose 6-phosphate. Created in BioRender.com.
Other studies have provided supporting evidence for age-associated changes in important components of the stimulus–secretion coupling mechanism, such as a decline in the coordination of calcium dynamics, gap junction coupling and insulin secretion with age in humans [11]. Another key player of insulin secretion is mitochondrial activity, which is impaired in aged human islets as measured by NADPH fluorescence lifetime imaging [10, 23]. A study performed in single human beta cells suggested that the mechanistic deficit underlying changes in mitochondrial activity is located upstream of the citric acid cycle (CAC), thus altering the control of mitochondrial membrane potential [24]. Some of these changes in mitochondrial activity could be secondary to a decrease in mitochondrial number since a negative correlation between mtDNA copy number and islet donor age has been described [25]; accordingly, when this degree of mitochondrial depletion was simulated in beta cell-derived MIN6 cells, GSIS was impaired [26].
Another important player in age-related changes of insulin secretion could be insulinotropic hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1). Postprandial secretion of these hormones was shown to be increased in postmenopausal vs premenopausal women [27].
Age-associated changes in beta cell proliferation and mass
Although not a measure of function per se, changes in beta cell mass, due to the balance between proliferation and apoptosis, will have an impact on in vivo measurements of beta cell function.
In humans, beta cell mass in human does not change in response to ageing [28]. In addition, various studies indicate there is a functional reserve of beta cell mass that maintains overall insulin secretion and blood glucose levels unless a threshold is reached. This functional reserve has been estimated as 20–25% in rats [29], while in humans it ranges between 50% and 70% based on data on recent-onset type 1 [30, 31] and type 2 diabetes [32].
However, ageing does decrease the ability of beta cells to proliferate in response to higher metabolic demands in rodents [33] and humans [34], which is translated as a limited regenerative capacity of beta cells. In mice, beta cell regeneration is diminished by 12 months of age [35–38] and could be related to beta cell senescence [39–44]. This is suggested by studies knocking-out p16Ink4a (a marker and effector of senescence), which increased beta cell proliferation in older mice [40]. However, beta cell mass in rodents increases with age [45], which means the actual pool of replicating beta cells is greater in adults than in young animals due a greater number of cells [46, 47].
Taken together, we can conclude that age-related changes in beta cell mass and proliferation do not impact beta cell functional measures in nondiabetic settings.
Age-associated changes in insulin resistance
Peripheral insulin sensitivity plays a key role in determining beta cell function and development of type 2 diabetes. States of insulin resistance brought about by obesity or a sedentary lifestyle are initially accompanied by a compensatory increase in insulin secretion, which, in susceptible individuals, declines and develops into overt diabetes [48]. It is generally accepted that insulin resistance occurs with ageing [49], partly due to the accumulation of senescent cells in the adipose tissue and the subsequent local secretion of SASP, which promote sterile inflammation, a form of pathogen-free inflammation caused by mechanical trauma, ischaemia, stress or environmental conditions [50, 51]. However, some studies have found an improvement in insulin sensitivity with age [4], even in the context of a consistent worsening of insulin secretion. This highlights the heterogeneity of ageing in different individuals, a key concept of the pathophysiology of diabetes underscored by a recent publication that describes five subgroups of adult-onset diabetes, which vary according to the degree of beta cell dysfunction vs insulin resistance [52]. The clinical identification of the subgroup in which an individual lies should be used for personalised treatment.
Other mechanisms of cellular ageing
Changes in other cell types, such as blood vessels and exocrine cells, might also impact insulin secretion [53]. For example, it has been shown that revascularising islets from old mice with blood vessels from young ones restores their functional and proliferative capacity [54]. In addition, circulating factors provide another interesting and thought-provoking mechanism for regulation of insulin secretion. A study using parabiosis showed that the replicative capacity of islets in old mice was restored when parabiosed with young ones [55], suggesting the existence of a circulating factor(s) that confers rejuvenating properties to beta cells.
Preservation and enhancement of insulin secretion during ageing and senescence
Understanding changes in insulin secretion with ageing, and the mechanisms behind these, can lead to new strategies that preserve beta cell function. We have shown that senescent beta cells accumulate in mouse and human islets with age, and their appearance is accelerated in insulin resistance and type 2 diabetes [2, 16]. Their genetic profile (Fig. 2) predicts impaired function with downregulation of key genes in beta cells and upregulation of genes of senescence and SASP. When senescent cells were specifically deleted in a transgenic model or using senolytic drugs (which target anti-apoptotic pathways), insulin secretion improved, gene expression was restored and blood glucose levels improved in different models of insulin resistance and chronological ageing. The subpopulation of senescent beta cells represented between 2% and 10% of the total beta cell population in rodents and between 10% and 30% of the cell population in human donors without type 2 diabetes. Decreasing the senescent population by 30–50% was enough to see a positive effect [2] with no impact on beta cell mass.
In spite of these encouraging results and their potential translation into new therapies for diabetes, there remain questions that need addressing. One of them is the lack of tissue specificity, since the anti-apoptotic pathways upregulated by senescent cells are not tissue specific. In the setting of type 2 diabetes, this might not be entirely negative, since decreasing senescent cells in adipose tissue improves peripheral insulin sensitivity [56]. Other undesirable effects of senolytic therapies include interference in wound healing and pro-oncogenic effects, all of which will have to be fully understood before senolysis can move into clinical use.
In conclusion, the increased risk of type 2 diabetes with age could be related to an intrinsic age-related loss of beta cell function. Although there might be multiple additional mechanisms, cellular senescence is emerging as a promising and exciting target to counteract this phenomenon.
Funding
Work in the author’s laboratory is supported by the Institutional Start-Up funds, Joslin Diabetes Center and by a grant from NIH P30 DK036836 to Joslin Diabetes Research Center (Pilot and Feasibility Study award to CAM).
Abbreviations
- GSIS
Glucose-stimulated insulin secretion
- IDR
Insulin delivery rate
- KATP
ATP-dependent K+ (channel)
- RHPA
Reverse haemolytic plaque assay
- RNA-seq
RNA sequencing
- SASP
Senescence associated secretory phenotype
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00125-020-05185-6) contains a slideset of the figures for download, which is available to authorised users.
References
- 1.Koopman RJ, Mainous AG 3rd, Diaz VA, Geesey ME (2005) Changes in age at diagnosis of type 2 diabetes mellitus in the United States, 1988 to 2000. Ann Fam Med 3(1):60–63. 10.1370/afm.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguayo-Mazzucato C, Andle J, Lee TB Jr et al. (2019) Acceleration of beta cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab 30(1):129–142 e124. 10.1016/j.cmet.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson PJ, Shah A, Ntranos V, Van Gool F, Atkinson M, Bhushan A (2019) Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab 29(5):1045–1060 e1010. 10.1016/j.cmet.2019.01.021 [DOI] [PubMed] [Google Scholar]
- 4.Xiao J, Weng J, Ji L et al. (2014) Worse pancreatic beta-cell function and better insulin sensitivity in older Chinese without diabetes. J Gerontol A Biol Sci Med Sci 69(4):463–470. 10.1093/gerona/glt104 [DOI] [PubMed] [Google Scholar]
- 5.Hirose H, Takayama M, Iwao Y, Kawabe H (2016) Effects of aging on visceral and subcutaneous fat areas and on homeostasis model assessment of insulin resistance and insulin secretion capacity in a comprehensive health checkup. J Atheroscler Thromb 23(2):207–215. 10.5551/jat.30700 [DOI] [PubMed] [Google Scholar]
- 6.Basu R, Breda E, Oberg AL et al. (2003) Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52(7): 1738–1748. 10.2337/diabetes.52.7.1738 [DOI] [PubMed] [Google Scholar]
- 7.Chang AM, Smith MJ, Galecki AT, Bloem CJ, Halter JB (2006) Impaired beta-cell function in human aging: response to nicotinic acid-induced insulin resistance. J Clin Endocrinol Metab 91(9): 3303–3309. 10.1210/jc.2006-0913 [DOI] [PubMed] [Google Scholar]
- 8.Iozzo P, Beck-Nielsen H, Laakso M, Smith U, Yki-Jarvinen H, Ferrannini E (1999) Independent influence of age on basal insulin secretion in nondiabetic humans. European Group for the Study of Insulin Resistance. J Clin Endocrinol Metab 84(3):863–868. 10.1210/jcem.84.3.5542 [DOI] [PubMed] [Google Scholar]
- 9.Ihm SH, Matsumoto I, Sawada T et al. (2006) Effect of donor age on function of isolated human islets. Diabetes 55(5):1361–1368. 10.2337/db05-1333 [DOI] [PubMed] [Google Scholar]
- 10.Gregg T, Poudel C, Schmidt BA et al. (2016) Pancreatic β-cells from mice offset age-associated mitochondrial deficiency with reduced KATP channel activity. Diabetes 65(9):2700–2710. 10.2337/db16-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westacott MJ, Farnsworth NL, St Clair JR et al. (2017) Age-dependent decline in the coordinated [Ca2+] and insulin secretory dynamics in human pancreatic islets. Diabetes 66(9):2436–2445. 10.2337/db17-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang AM, Halter JB (2003) Aging and insulin secretion. Am J Physiol Endocrinol Metab 284(1):E7–E12. 10.1152/ajpendo.00366.2002 [DOI] [PubMed] [Google Scholar]
- 13.Avrahami D, Li C, Zhang J et al. (2015) Aging-dependent demethylation of regulatory elements correlates with chromatin state and improved beta cell function. Cell Metab 22(4):619–632. 10.1016/j.cmet.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helman A, Klochendler A, Azazmeh N et al. (2016) p16Ink4a-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med 22(4):412–420. 10.1038/nm.4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tugay K, Guay C, Marques AC et al. (2016) Role of microRNAs in the age-associated decline of pancreatic beta cell function in rat islets. Diabetologia 59(1):161–169. 10.1007/s00125-015-3783-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguayo-Mazzucato C, van Haaren M, Mruk M et al. (2017) β cell aging markers have heterogeneous distribution and are induced by insulin resistance. Cell Metab 25(4):898–910 e895. 10.1016/j.cmet.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomon D, Meda P (1986) Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res 162(2):507–520. 10.1016/0014-4827(86)90354-x [DOI] [PubMed] [Google Scholar]
- 18.Barker CJ, Li L, Kohler M, Berggren PO (2015) β-Cell Ca2+ dynamics and function are compromised in aging. Adv Biol Regul 57:112–119. 10.1016/j.jbior.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Rorsman P, Ashcroft FM (2018) Pancreatic beta-cell electrical activity and insulin secretion: of mice and men. Physiol Rev 98(1):117–214. 10.1152/physrev.00008.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153(6):1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5:99–118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimri GP, Lee X, Basile G et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92(20):9363–9367. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerencser AA (2018) Metabolic activation-driven mitochondrial hyperpolarization predicts insulin secretion in human pancreatic beta-cells. Biochim Biophys Acta Bioenerg 1859(9):817–828. 10.1016/j.bbabio.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerencser AA (2015) Bioenergetic analysis of single pancreatic beta-cells indicates an impaired metabolic signature in type 2 diabetic subjects. Endocrinology 156(10):3496–3503. 10.1210/en.2015-1552 [DOI] [PubMed] [Google Scholar]
- 25.Cree LM, Patel SK, Pyle A et al. (2008) Age-related decline in mitochondrial DNA copy number in isolated human pancreatic islets. Diabetologia 51(8):1440–1443. 10.1007/s00125-008-1054-4 [DOI] [PubMed] [Google Scholar]
- 26.Nile DL, Brown AE, Kumaheri MA et al. (2014) Age-related mitochondrial DNA depletion and the impact on pancreatic Beta cell function. PLoS One 9(12):e115433. 10.1371/journal.pone.0115433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ranganath L, Sedgwick I, Morgan L, Wright J, Marks V (1998) The ageing entero-insular axis. Diabetologia 41(11):1309–1313. 10.1007/s001250051070 [DOI] [PubMed] [Google Scholar]
- 28.Saisho Y, Butler AE, Manesso E, Elashoff D, Rizza RA, Butler PC (2013) β-Cell mass and turnover in humans: effects of obesity and aging. Diabetes Care 36(1):111–117. 10.2337/dc12-0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsson R, Carlsson PO (2011) A low-oxygenated subpopulation of pancreatic islets constitutes a functional reserve of endocrine cells. Diabetes 60(8):2068–2075. 10.2337/db09-0877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Damond N, Engler S, Zanotelli VRT et al. (2019) A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab 29(3):755–768 e755. 10.1016/j.cmet.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YJ, Traum D, Schug J et al. (2019) Multiplexed in situ imaging mass cytometry analysis of the human endocrine pancreas and immune system in type 1 diabetes. Cell Metab 29(3):769–783 e764. 10.1016/j.cmet.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52(1):102–110. 10.2337/diabetes.52.1.102 [DOI] [PubMed] [Google Scholar]
- 33.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S (1997) Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138(4):1736–1741 [DOI] [PubMed] [Google Scholar]
- 34.Gregg BE, Moore PC, Demozay D et al. (2012) Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab 97(9):3197–3206. 10.1210/jc.2012-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rankin MM, Kushner JA (2009) Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 58(6):1365–1372. 10.2337/db08-1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA (2005) Very slow turnover of beta-cells in aged adult mice. Diabetes 54(9):2557–2567 [DOI] [PubMed] [Google Scholar]
- 37.Stolovich-Rain M, Hija A, Grimsby J, Glaser B, Dor Y (2012) Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. J Biol Chem 287(33):27407–27414. 10.1074/jbc.M112.350736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan R, Kang Z, He L, Chan J, Xu G (2011) Exendin-4 improves blood glucose control in both young and aging normal non-diabetic mice, possible contribution of beta cell independent effects. PLoS One 6(5):e20443. 10.1371/journal.pone.0020443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnamurthy J, Torrice C, Ramsey MR et al. (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114(9):1299–1307. 10.1172/JCI22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krishnamurthy J, Ramsey MR, Ligon KL et al. (2006) p16INK4a induces an age-dependent decline in islet regenerative potential. Nature 443(7110):453–457. 10.1038/nature05092 [DOI] [PubMed] [Google Scholar]
- 41.Dhawan S, Tschen SI, Bhushan A (2009) Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev 23(8):906–911. 10.1101/gad.1742609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tschen SI, Dhawan S, Gurlo T, Bhushan A (2009) Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 58(6):1312–1320. 10.2337/db08-1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ (2011) Cell cycle control of beta-cell replication in the prenatal and postnatal human pancreas. Am J Phys Endocrinol Metab 300(1):E221–E230. 10.1152/ajpendo.00496.2010 [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Gu X, Su IH et al. (2009) Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 23(8):975–985. 10.1101/gad.1742509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montanya E, Nacher V, Biarnes M, Soler J (2000) Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes 49(8):1341–1346. 10.2337/diabetes.49.8.1341 [DOI] [PubMed] [Google Scholar]
- 46.Aguayo-Mazzucato C, Bonner-Weir S (2018) Pancreatic beta cell regeneration as a possible therapy for diabetes. Cell Metab 27(1): 57–67. 10.1016/j.cmet.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chintinne M, Stange G, Denys B et al. (2010) Contribution of post-natally formed small beta cell aggregates to functional beta cell mass in adult rat pancreas. Diabetologia 53(11):2380–2388. 10.1007/s00125-010-1851-4 [DOI] [PubMed] [Google Scholar]
- 48.Weir GC, Bonner-Weir S (2004) Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes 53(Suppl 3): S16–S21. 10.2337/diabetes.53.suppl_3.s16 [DOI] [PubMed] [Google Scholar]
- 49.Ehrhardt N, Cui J, Dagdeviren S et al. (2019) Adiposity-independent effects of aging on insulin sensitivity and clearance in mice and humans. Obesity (Silver Spring) 27(3):434–443. 10.1002/oby.22418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stout MB, Justice JN, Nicklas BJ, Kirkland JL (2017) Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 32(1):9–19. 10.1152/physiol.00012.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gustafson B, Nerstedt A, Smith U (2019) Reduced subcutaneous adipogenesis in human hypertrophic obesity is linked to senescent precursor cells. Nat Commun 10(1):2757. 10.1038/s41467-019-10688-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahlqvist E, Storm P, Karajamaki A et al. (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 6(5):361–369. 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 53.Xiao X, Chen C, Guo P et al. (2017) Forkhead box protein 1 (FoxO1) inhibits accelerated beta cell aging in pancreas-specific SMAD7 mutant mice. J Biol Chem 292(8):3456–3465. 10.1074/jbc.M116.770032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almaca J, Molina J, Arrojo EDR et al. (2014) Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci U S A 111(49):17612–17617. 10.1073/pnas.1414053111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salpeter SJ, Khalaileh A, Weinberg-Corem N, Ziv O, Glaser B, Dor Y (2013) Systemic regulation of the age-related decline of pancreatic beta-cell replication. Diabetes 62(8):2843–2848. 10.2337/db13-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palmer AK, Xu M, Zhu Y et al. (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18(3):e12950. 10.1111/acel.12950 [DOI] [PMC free article] [PubMed] [Google Scholar]


