Abstract
Purpose of review:
to evaluate recent scientific research studies related to the changes in skeletal muscle after stroke and the presence of sarcopenia in stroke survivors to establish its incidence and effects on function.
Recent Findings:
Recently published findings on stroke-related sarcopenia are limited. This might be due to changes in the consensus definition of sarcopenia. Sarcopenia in stroke patients is estimated at 14 to 54%. The presence of sarcopenia at the time of a stroke can lead to worse recovery and functional outcomes.
Summary:
Presence of sarcopenia prior to a stroke may be more common than suspected and can lead to worse functional recovery. Clinicians should be aware of this to better identify and treat stroke-related sarcopenia. Future research should focus on larger population studies to more accurately establish correlation between stroke and sarcopenia.
Keywords: cerebrovascular accident, aging, secondary sarcopenia, skeletal muscle
Introduction
Sarcopenia is characterized by loss of muscle strength and mass and impaired function (1)(2). It is an age-related disease that predominantly affects older populations and is associated with disability, poor quality of life, increased risk of hospitalization, and increased risk of death, among other negative outcomes (3). Due to the increasing age of population in most countries of the world (4,5), the prevalence of sarcopenia is increasing. Similarly, stroke is an important and frequent cause of disability and death worldwide(4) Changes in skeletal muscle before and after stroke may contribute to functional deficits in stroke survivors (6,7). C. In this brief report, we will discuss these two conditions, sarcopenia and stroke, in relation to each other and describe changes in muscle mass, strength, fiber composition and functional performance in stroke survivors. We will also discuss the presence of sarcopenia before and after stroke and the possibility that sarcopenia contributes to stroke associated impairments and morbidity. It is important to note that there are few data regarding this issue. Furthermore, the definition and diagnostic criteria for sarcopenia has changed significantly in the last ten years suggesting that variability among studies may be due to discrepancy in diagnostic criteria and assessment tools or methods (8). For this reason, we have included studies that investigate changes in muscle even though they do not answer directly questions about the presence of sarcopenia prior to the stroke or the consequences of secondary sarcopenia in stroke survivors.
Stroke
Stroke is defined as an infarction of the brain attributable to ischemia or hemorrhage and based on neuropathological, neuroimaging, and/or clinical evidence of permanent injury. The term also broadly includes intracerebral hemorrhage and subarachnoid hemorrhage (4). In 2010, an estimated 16.9 million cases of first-ever stroke were reported worldwide (Figure 1). Notably, stroke incidence was markedly higher in in countries such as China, Russia, Mongolia and in some, but not all, African and Middle East countries. There were 33 million cases of stroke survivors worldwide with 5.9 million deaths related to the event in the same year (9). Stroke is the second leading cause of death in the world (4). The incidence and prevalence of stroke are strongly associated with age and more than 38% of new strokes and 55% of stroke-related deaths occur in people aged 75 years and older. Stroke survivors and stroke deaths are expected to reach 70 million and 12 million respectively by the year 2030 (10). In the United States, more than 795,000 people suffer a stroke each year (11). There are 140,000 yearly deaths related to stroke (10). It is a major source of economic burden in the United States economy with an estimated $34 billion on costs (4). Although stroke mortality rates have decreased, the global burden of stroke is increasing, with most of the burden in low-income and middle-income countries (1).
Fig. 1.
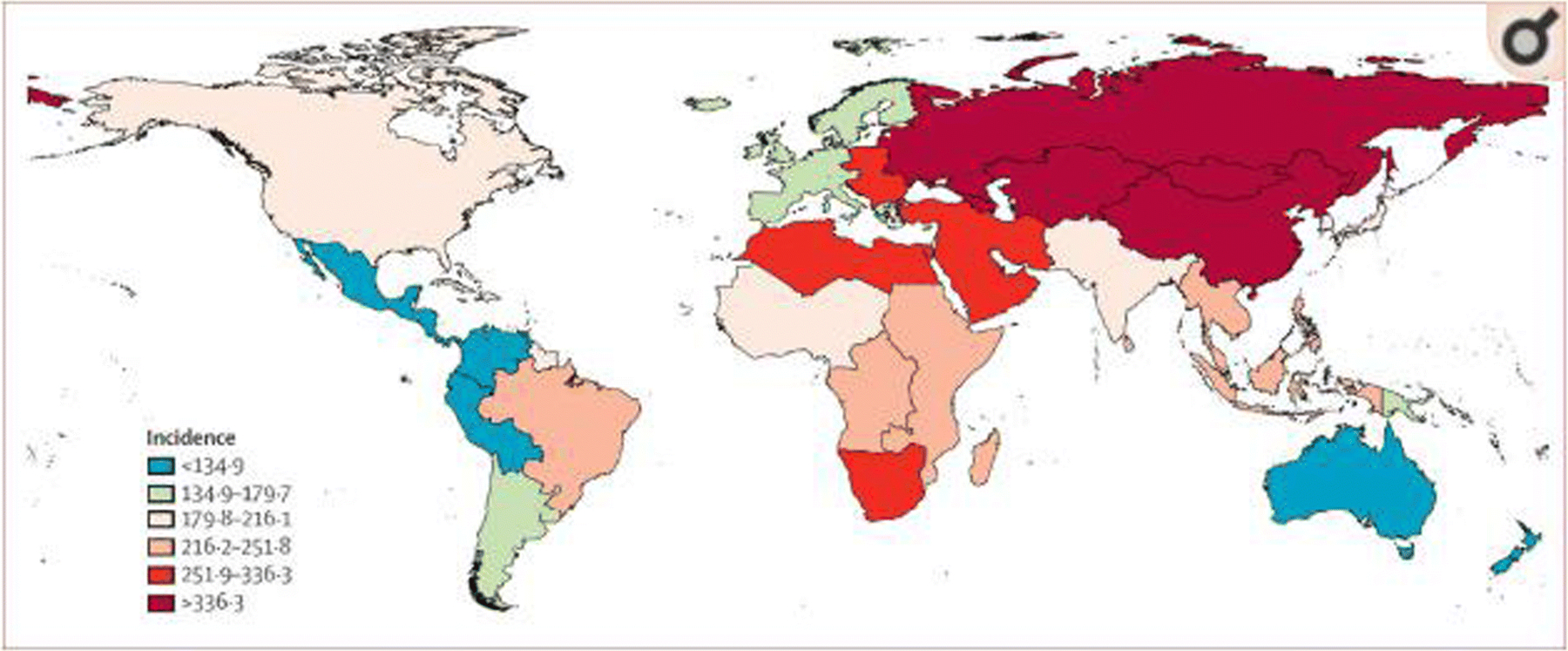
Age-standardized stroke incidence per 100,000 person-years for 2010 [4].
Stroke is also a global source of disability. Using disability-adjusted life-years (DALYs) as a measurement of disability burden, 102.2 million DALYs were lost worldwide due to stroke in 2010, 28% of these in the population aging 75 years and older (10). As with other countries, stroke is also a leading cause of long-term disability in the United States because it impairs motor performance in more than half of stroke survivors aged 65 and over (12). Stroke patients suffer from mobility and cognitive deficits, the most frequently reported types of disability in the United States (12). Notably, prevalence of any disability, and particularly mobility impairments, were higher among older age groups in the United States (4). Therefore, it is important to understand the effects of stroke on organs and systems responsible for mobility such as skeletal muscle. (1)
Skeletal Muscle Changes in Stroke
Stroke is the leading cause of disability in adult life (13). One quarter of stroke survivors suffer from severe motor disability at ninety days of onset (14). Although the brain is the main organ affected in a stroke, skeletal muscle is the main effector organ of disability following the event. A decrease in motor unit numbers in the affected limb can be observed as early as four hours following a stroke (15). Motor unit changes persist in the chronic phase. Yet, the non-paretic side is also affected with signs of weakness one week following the event (16). These changes are a result of the acute neurologic insult as well as of restricted mobility. The contribution of many factors to these changes is illustrated schematically in Figure 2.
Fig. 2.
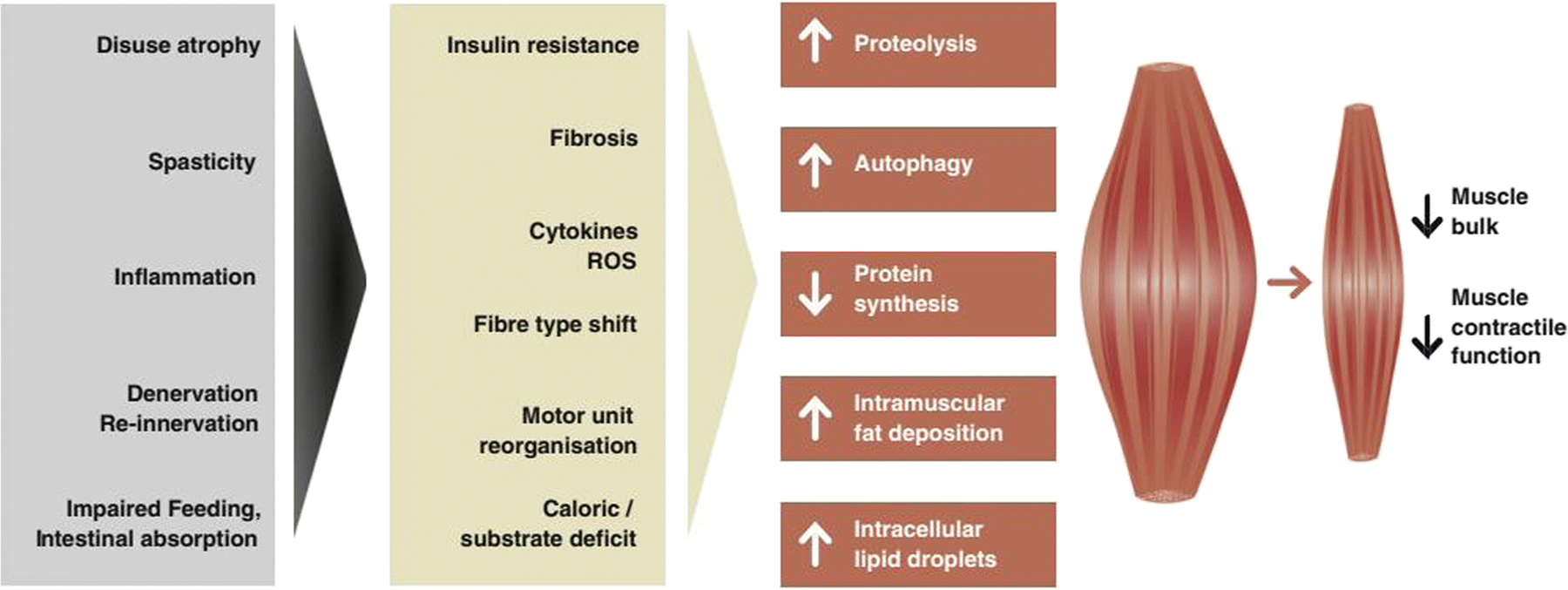
Mechanisms and pathways of muscle wasting in stroke-related sarcopenia [17]
Significant alterations at the level of skeletal muscle have been reported in various diseases of the central nervous system(17). For example, muscle fiber types change following a stroke. Contrary to normal aging, the prevalence of fast-twitch type 2 isoforms after stroke, more reliant on glycolytic metabolic pathways, increases (18). This is also observed in other chronic diseases that can cause muscle wasting, such as congestive heart failure (19). These findings suggest that disease-related muscle wasting occur independent of the underlying condition, perhaps sharing a common pathway (19). It has been argued that this switch to fast, anaerobic, muscle fiber types is a strong predictor of impaired functional capacity (20).
Loss of muscle mass is also very common after a cerebrovascular accident (21). There is close to a 24% reduction in muscle mass in the paretic side alone (19). This is accompanied by deposition of fat and connective tissue. Inactivity and immobilization following a stroke most likely contribute to these changes (22). For example, during hospitalization, acute stroke patients were only physically active for less than 40 minutes throughout the day (23,24). In healthy older adults, only ten days of bed rest led to a significant decrease in muscle protein synthesis and reduced lean muscle mass resulting in a 16% reduction of muscle strength (25). Yet, stroke leads to motor unit denervation, synaptic and motor system re-organization, and loss of local neuronal balance due to cerebral damage which are not seen in similar conditions that lead to muscle wasting (19). Thus, stroke-induced sarcopenia might be considered a specific and unique sequel of the disease (26). Although stroke is established as a cause of decreased skeletal muscle mass, the inverse may also be true. In fact, in a cross-sectional study, higher skeletal mass has been reported to be associated with fewer changes in brain white matter silent infarctions in brain matter (27).
Malnutrition can be another contributor to muscle changes in stroke patients. Present in 49% of stroke survivors (28), it can lead to decrease body weight, muscle mass loss and changes in muscle quality. Malnutrition can also affect stroke survivors prior to the event. In elderly patients, nutritional status is often reduced prior to hospitalization (29). After the event, stroke patients commonly present dysphagia which can, in turn, reduce the intake of nutrients and calories (30). Thus, events prior to and following the stroke can affect the nutritional status of patients. Nutritional optimization has been shown to improve outcomes following a stroke (31). This could potentially be linked to, among other things, improvement in muscle mass leading to reduced risk of post-stroke sarcopenia.
Spasticity is common following a stroke. After one year, 35% of stroke survivors suffer from spasticity (19). Spasticity is a velocity-dependent phenomenon and a result of upper motor neuron disease. It is characterized by muscle tightness impeding normal range of motion in the affected limbs. Although it could be counter-intuitive to think that a spastic muscle is a weak one, hypoactivity caused by the stroke can lead to decrease in muscle mass. Spastic muscles also suffer from changes in architecture and quality that can contribute to lower muscle strength (32). For these reasons, spasticity can be a potential contributor to sarcopenia following a stroke.
As seen in Figure 2, these and other factors present before and after a stroke can decrease muscle bulk and contractile function potentially causing post-stroke sarcopenia (33).
Sarcopenia
Sarcopenia is a progressive and generalized skeletal muscle disorder that, like stroke, is associated with an increase likelihood of adverse outcomes including falls, fractures, physical disability, hospitalizations and mortality (1). In the revised European consensus on its definition and diagnosis, sarcopenia is now considered a muscle disease with low muscle strength overtaking the role of walking speed as the initial test for its diagnosis (34,35). Sarcopenia is probable when low muscle strength is detected. The diagnosis is confirmed with the presence of low muscle quantity or quality and impaired performance (slow walking speed) makes it severe.
Case finding for sarcopenia should start based on specific patient reported symptoms. Questionnaires such as the SARC-F can aid in eliciting such self-reported symptoms (36). Criteria such as a being older than 65 years of age, frequent falls, recent loss of muscle strength, and living in a nursing home have been suggested as additional indications for screening and evaluation (37).
When indicated, tests of muscle strength, muscle mass and physical performance are administered to diagnose sarcopenia. Grip strength has been proposed as an accurate and simple measurement of muscle strength. Accurate measurements require a calibrated handheld dynamometer (38). The chair stand test is another acceptable way of measuring muscle strength, having the patient stand from a chair as many times as possible for 30 seconds (1). However, this test may reflect not only strength but also local muscular endurance. The assessment of muscle mass is also important in establishing the diagnosis of sarcopenia. Muscle mass can be assessed by computed tomography (CT), magnetic resonance image (MRI) or dual-energy X-ray absorptiometry (DXA) (39). Physical performance has been closely linked to locomotion where gait speed measurements (1,6,7) and tests such as the Timed-Up and Go test can aid in identifying any deficits in performance. The current consensus establishes an algorithm utilizing all these tests for case-finding, diagnosis, and determination of severity of sarcopenia.
As noted above, the definition of sarcopenia has evolved over time. Consensus statements published earlier and by various groups differ (40). This is particularly important when evaluating the medical and scientific literature regarding this condition as different authors have used different parameters, testing methods, and guidelines for the evaluation and diagnosis. Thus, the details on studies regarding sarcopenia used for this review, for example the prevalence of sarcopenia in stroke survivors, may vary depending on the year of publication and the diagnostic criteria used by the various authors.
Muscle mass and strength reach maximum levels in young adulthood, approximately in the fifth decade of life (41). Beyond, loss of muscle mass and strength is progressive (1). Sarcopenia can be categorized as primary (or age-related) and secondary (due to causal factors other than, or in addition to, aging) (42). Stroke-related sarcopenia is then qualified as that of the secondary type.
Physical activity (strength conditioning) and nutritional supplementation have been the cornerstones of sarcopenia treatment (42). In a systematic review, strengthening (also known as high-resistance and weight-training) exercises were noted to improve muscle mass and strength when compared to low-intensity home exercise and when administered for three to eighteen months (42). Yet, muscle strength and physical performance were more likely to improve than muscle mass in multiple studies evaluating either resistance-training exercises or combined exercise protocols (43–45).
Nutritional interventions usually focus on supplementation of either proteins, amino acids, B-hydroxy B-methylbutyric acid or fatty acids. However, the evidence for the first two is stronger than for the latter two interventions (46). The combination of both interventions, physical activity and nutrient supplementation, was also evaluated in a separate systematic review (47). In general, muscle mass, strength and physical performance generally improved with exercise. However, the combination of exercise with nutritional supplements did not always provide an additional benefit when compared to exercise alone. This comparison among studies may be limited by the use of different amounts of protein supplements. Some studies showed an additional effect on muscle mass when combining protein or creatine with exercise. Creatine and exercise in conjunction also had a positive effect on muscle strength (46). However, as noted above, most studies researching these combination protocols only focused on healthy older subjects and did not include stroke survivors. There might be an additional effect of dietary supplementation (protein and vitamin D) and exercise in stroke survivors that may be sarcopenic and frail that warrants future research (19).
Sarcopenia and Stroke
The many changes in skeletal muscle reviewed in the previous section may suggest, but not necessarily confirm, the presence of sarcopenia after stroke. Recent modifications in the definition of sarcopenia and shift in focus from performance to muscle strength change the way the condition is assessed in both the general population and in patients with chronic conditions. Prior to this change, however, assessing both physical performance and muscle strength was challenging after a stroke due to the inherent changes in function caused by the disease. For example, reduced gait speed has been used as a diagnostic criterion for sarcopenia (48). Assessment tools such as the 6-minute Walk Test have been used for this purpose, yet their applicability is limited in stroke patients because of the presence of mobility limitations. Patients who are initially unable to walk after a stroke, may not be able to walk again (15,49). As expected, even in stroke patients that can ambulate, paresis itself can affect gait and thus limit the validity and reliability of gait speed tests as a tool to monitor sarcopenia. However, lower limb muscle strength evaluation is possible in stroke patients and can be reliably performed from a sitting or supine position. Tests such as isometric knee extension measurement and bicycle ergometer with one lower limb have been used reliably in this patient population (50). Upper limb muscle strength has been reliably measured by way of isometric hand grip strength (51). It can be used in the non-paretic side to measure muscle strength and assess for sarcopenia in stroke patients.
A summary of selected recent studies on the topic of sarcopenia and stroke is presented in Table 1. To our knowledge, no longitudinal studies have been conducted to examine the possible contribution of pre-existing sarcopenia on the incidence, prevalence, and sequelae of stroke. Using various study designs, the prevalence of sarcopenia in patients after stroke, however, has been reported to range from 14 to 54%. Ryan et al reported a prevalence of 14 to 18% in stroke survivors (51). It is also more prevalent in stroke patients than in control-matched non-stroke counterparts (52). However, other studies have reported sarcopenia in stroke patients as high as 54% (53). This wide range in prevalence rates might be due to the use of different definitions of sarcopenia, measurement tools, or cutoff values. Ethnicity has also been shown to influence prevalence as well as cutoff values for measurements such as muscle strength, thus affecting sarcopenia reported rates (54).
Table 1:
Summary of selected recent studies of sarcopenia and skeletal muscle changes in stroke.
| Year | Journal | Name | Author | Study design | N | Results | Comments |
|---|---|---|---|---|---|---|---|
| 2019 | Nutrition | Pre-stroke sarcopenia and functional outcomes in elderly patients who have had an acute stroke: a prospective cohort study (54) | Nozoe M, et al. | Prospective observational cohort | 152 | Prevalence of pre-stroke sarcopenia was 18%. Pre-stroke sarcopenia was associated with unfavorable outcomes, with an odds ratio of 7.39 (95% CI, 1.47–37.21; P=0.02). | Sarcopenia was diagnosed using the SARC-F questionnaire score. Functional outcome evaluated only up to 3 months after stroke. About one third of the subjects had a previous stroke. |
| 2019 | J Stroke Cerebrovasc Dis | Pre-stroke sarcopenia and stroke severity in elderly patients with acute stroke (56) | Nozoe M, et al. | Cross-sectional study | 183 | Prevalence of pre-stroke sarcopenia was 15%. The group with sarcopenia scored higher in the NIH Stroke Scale. | Sarcopenia was diagnosed using the SARC-F questionnaire score. Physical activity previous to a stroke is also associated with stroke severity. Poor physical activity may confound results. |
| 2019 | Nutrition | Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation (57) | Yoshimura Y, et al. | Retrospective | 795 | Sarcopenia diagnosed on admission was independently and negatively associated with the rate of discharge home after stroke (OR=0.201; p<0.004), lower FIM-motor score at discharge (β=−0.240, p<0.002), and poor dysphagia status. | Sarcopenia was diagnosed using skeletal muscle index and grip strength, using cutoff values proposed by EWGSOP21. |
| 2019 | Geriatr Gerontol Int | Sarcopenia as a predictor of activities of daily living in stroke patients undergoing rehabilitation (58) | Matsushita T, et al | Retrospective | 267 | Patients with sarcopenia diagnosed on admission had a longer stay in the rehabilitation ward (116.9 vs 94.1; p<0.001) and a lower FIM score upon discharge (100 vs. 122; p<0.001). | Sarcopenia was defined using grip strength and skeletal muscle index based on the EWGSOP21 criteria. Cutoff values for grip strength and skeletal muscle index were based on those established by the AWGS2. |
| 2019 | J Clin Neurosci | Can initial sarcopenia affect post-stroke rehabilitation outcome? (55) | Jang Y, et al. | Retrospective | 194 | Patients with Modified Rankin Scale >3 had a higher rate of sarcopenia than those with score ≤3 (47.1% vs 27.8%, p<0.01) at 6 months. Sarcopenia was associated with a 2.71-fold higher risk of poor recovery. | Sarcopenia was assessed 2 weeks after stroke using non-hemiplegic handgrip strength and the cut-off values of the AWGS. Patients with Modified Rankin Scale >3 were also more likely to be on “nothing per mouth” restrictions. Malnutrition appears to be a confounding factor for sarcopenia. |
| 2019 | Ann Rehabil Med | Effects of decreased skeletal muscle index and hand grip strength on functional recovery in subacute ambulatory stroke patients (59) | Park JG, et al. | Prospective observational study | 39 | Patients with normal skeletal muscle index (measured by grip strength) after stroke showed greater gains in the 6-minute walk test. | Sarcopenia was diagnosed using the AWGS. Grip strength was measured by handheld dynamometer. Strength cutoff values were ≤26kg and ≤18kg for men and women, respectively. |
| 2018 | Clin Nutr | Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: implications for oral sarcopenia (52) | Shiraishi A, et al. | Cross-sectional | 202 | The prevalence of stroke-related sarcopenia was 53%. Of the 202 stroke patients included in the study, 166 (82.2%) had oral problems. Oral problems were independently associated with lower skeletal muscle mass index and handgrip strength. | Sarcopenia was diagnosed using handgrip strength and muscle mass. Oral status was measured with the Revised Oral Assessment Guide (ROAG). |
| 2018 | PLoS One | Muscle mass and intramuscular fat of the quadriceps are related to muscle strength in non-ambulatory chronic stroke survivors: A cross sectional study (61) | Akazawa N, et al. | Cross-sectional | 50 | In chronic stroke survivors, the correlation coefficients between quadriceps muscle strength and muscle thickness were 0.275 and 0.422 for paretic and non-paretic limbs respectively. The prevalence of sarcopenia was not assessed. | Quadriceps muscle strength was measured with handheld dynamometry. Muscle mass and intramuscular fat content of the rectus femoris and vastus intermedius were measured using ultrasonography. |
| 2017 | BMC Geriatr | Higher skeletal muscle mass may protect against ischemic stroke in community-dwelling adults without stroke and dementia: the PRESENT project (26) | Minn YK, Suk SH | Cross-sectional | 722 | More than 70% of those with white matter changes or silent infarctions were in the lower muscle mass subgroups. | Muscle mass was measured using bioelectrical impedance machine. Obesity data were not included in this study. |
| 2017 | Top Stroke Rehabil | Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals (24) | Hunnicut JL and Gregory C | Systematic review of literature | 375 | On average, muscle size (92%), thigh size (87%), knee extensors strength (64%), knee extensors power (57%) were lower in the paretic leg. | CT scan, MRI, ultrasound, and DEXA were used for the evaluation of muscle size and strength was primarily measured by dynamometry. |
| 2017 | Arch Phys Med Rehabil | Sarcopenia and physical function in middle-aged and older stroke survivors (51) | Ryan AS, et al. | Cross-sectional | 190 | Prevalence of sarcopenia in chronic stroke survivors survivors was reported to be between 14 and 18%. | Four definitions of sarcopenia were used to determine the prevalence of sarcopenia. Prevalence varied according to the definition used. The value of grip strength for the evaluation of sarcopenia may be decreased due to stroke. |
EWGSOP2 = European Working Group on Sarcopenia in Older People 2;
AWGS = Asian Working Group on Sarcopenia
Because of the changes mentioned above, it is reasonable to conclude that stroke-induced sarcopenia is a real phenomenon. In a study of 152 patients, the possible presence of sarcopenia before the stroke was estimated retrospectively on admission to a rehabilitation facility using the SARC-F questionnaire score and reported to be 18% (55,56). More importantly, the score was an independent predictor of functional outcomes 3 months after a stroke by a considerable margin. Pre-stroke sarcopenia was also established as an independent predictor of stroke severity as measured by initial National Institute of Health Stroke Scale score (NIHSS) (54). The presence of sarcopenia two weeks after a stroke, as measured by handgrip strength in the non-paretic limb, was also associated with poor functional outcomes at 6 months following the event (57,58). However, as previously noted, the non-paretic side can show signs of weakness one week after the stroke so this might not necessarily constitute pre-stroke sarcopenia. Sarcopenia in patients admitted to an inpatient rehabilitation unit following a stroke was a predictor of worse functional recovery and decreased likelihood of community discharge (59). Post-stroke ambulatory patients with diagnosed sarcopenia were also likely to have worse recovery when compared to non-sarcopenic stroke survivors (60).
It can be suggested that the treatment and rehabilitation of stroke-related sarcopenia should follow the same previously described management cornerstones including the use of nutritional interventions and strength conditioning. A stroke can greatly affect a patient’s nutritional status because of metabolic changes due to the inciting event or poor caloric intake due to dysphagia, among others. An early consult with a nutritionist can aid in preventing malnutrition and in the treatment of sarcopenia. Strengthening of both the paretic and non-paretic side is paramount in the functional treatment of stroke (61). Increasing muscle mass and decreasing intramuscular fat in chronic stroke survivors may increase muscle strength (56).
Conclusions:
Stroke and sarcopenia are frequent in the general population. The presence of sarcopenia prior to a stroke may be more common than suspected and can lead to worse functional recovery. Stroke can also lead to sarcopenia due to changes in skeletal muscle mass, quality and architecture. These changes are secondary to common issues following a stroke including malnutrition, decreased mobility, spasticity and motor system re-organization, among others. Nutritional optimization and muscle strengthening can potentially decrease stroke-related sarcopenia and improve functional recovery as well as increase the likelihood of community discharge. Clinicians following stroke patients should be aware of this condition and incorporate effective interventions in the rehabilitation plan (60).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Manuel Mas, Javier González and Walter Frontera declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Vol. 48, Age and Ageing. Oxford University Press; 2019. p. 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dicker D, Nguyen G, Abate D, Abate KH, Abay SM, Abbafati C, et al. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018. November 10;392(10159):1684–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Zhang W, Wang C, Tao W, Dou Q, Yang Y. Sarcopenia as a predictor of hospitalization among older people: A systematic review and meta-analysis. BMC Geriatrics. 2018. August 22;18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. The Lancet. 2014;383(9913):245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012. December 1;380(9859):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LK, Lee WJ, Peng LN, Liu LK, Arai H, Akishita M. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. Vol. 17, Journal of the American Medical Directors Association. Elsevier Inc.; 2016. p. 767.e1–767.e7. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis. Age and Ageing. 2010. April 13;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2013;44(7):2064–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012. December 1;380(9859):2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics’2017 Update: A Report from the American Heart Association. Vol. 135, Circulation. Lippincott Williams and Wilkins; 2017. p. e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Q, Tong X, Schieb L, Vaughan A, Gillespie C, Wiltz JL, et al. Vital Signs: Recent Trends in Stroke Death Rates — United States, 2000–2015. MMWR Morbidity and Mortality Weekly Report [Internet]. 2017. September 8 [cited 2018 Jun 26];66(35):933–9. Available from: http://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elizabeth ACL, Carroll DD, Zhang QC, Stevens AC, Griffin-Blake S, Armour BS, et al. Prevalence of disability and disability type among adults — United States, 2013. Morbidity and Mortality Weekly Report. 2015. July 31;64(29):777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004. March 6;363(9411):768–74. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Shin H, Zhou P, Niu X, Liu J, Rymer WZ. Power spectral analysis of surface electromyography (EMG) at matched contraction levels of the first dorsal interosseous muscle in stroke survivors. Clinical Neurophysiology [Internet]. 2014. May 1 [cited 2020 Jan 5];125(5):988–94. Available from: https://www.sciencedirect.com/science/article/pii/S1388245713011279 [DOI] [PubMed] [Google Scholar]
- 15.Harris ML, Polkey MI, Bath PM, Moxham J. Quadriceps muscle weakness following acute hemiplegic stroke. Clinical rehabilitation [Internet]. 2001. June [cited 2020 Jan 9];15(3):274–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11386397 [DOI] [PubMed] [Google Scholar]
- 16.Carda S, Cisari C, Invernizzi M. Sarcopenia or muscle modifications in neurologic diseases: A lexical or patophysiological difference? Vol. 49, European Journal of Physical and Rehabilitation Medicine. 2013. p. 119–30. [PubMed] [Google Scholar]
- 17.Canepari M, Pellegrino MA, D’Antona G, Bottinelli R. Single muscle fiber properties in aging and disuse. Scandinavian Journal of Medicine & Science in Sports [Internet]. 2010. February 1 [cited 2020 Jan 9];20(1):10–9. Available from: http://doi.wiley.com/10.1111/j.1600-0838.2009.00965.x [DOI] [PubMed] [Google Scholar]
- 18.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. The International Journal of Biochemistry & Cell Biology [Internet]. 2013. October 1 [cited 2020 Jan 9];45(10):2191–9. Available from: https://www.sciencedirect.com/science/article/pii/S1357272513001532 [DOI] [PubMed] [Google Scholar]
- 19.Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced Sarcopenia: muscle wasting and disability after stroke. International journal of cardiology [Internet]. 2013. December 10 [cited 2020 Jan 5];170(2):89–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24231058 [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen L, Jacobsen BK. Changes in muscle mass, fat mass, and bone mineral content in the legs after stroke: a 1 year prospective study. Bone [Internet]. 2001. June 1 [cited 2020 Jan 5];28(6):655–9. Available from: https://www.sciencedirect.com/science/article/pii/S8756328201004343 [DOI] [PubMed] [Google Scholar]
- 21.Ryan AS, Buscemi A, Forrester L, Hafer-Macko CE, Ivey FM. Atrophy and intramuscular fat in specific muscles of the thigh: associated weakness and hyperinsulinemia in stroke survivors. Neurorehabilitation and neural repair [Internet]. 2011. November 6 [cited 2020 Jan 5];25(9):865–72. Available from: http://journals.sagepub.com/doi/10.1177/1545968311408920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernhardt J, Dewey H, Thrift A, Donnan G. Inactive and alone: physical activity within the first 14 days of acute stroke unit care. Stroke [Internet]. 2004. April [cited 2020 Jan 9];35(4):1005–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14988574 [DOI] [PubMed] [Google Scholar]
- 23.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA [Internet]. 2007. April 25 [cited 2020 Jan 9];297(16):1772–4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17456818 [DOI] [PubMed] [Google Scholar]
- 24.Hunnicutt JL, Gregory CM. Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Topics in stroke rehabilitation [Internet]. 2017. [cited 2020 Jan 9];24(6):463–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28251861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones TA, Adkins DL. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology (Bethesda, Md) [Internet]. 2015. September [cited 2020 Jan 9];30(5):358–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26328881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minn Y-K, Suk S-H. Higher skeletal muscle mass may protect against ischemic stroke in community-dwelling adults without stroke and dementia: The PRESENT project. BMC geriatrics [Internet]. 2017. [cited 2020 Jan 9];17(1):45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28158989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley NC, Martin RE, Salter KL, Teasell RW. A review of the relationship between dysphagia and malnutrition following stroke. Vol. 41, Journal of Rehabilitation Medicine. 2009. p. 707–13. [DOI] [PubMed] [Google Scholar]
- 28.Pirlich M, Schütz T, Norman K, Gastell S, Lübke HJ, Bischoff SC, et al. The German hospital malnutrition study. Clinical Nutrition. 2006. August;25(4):563–72. [DOI] [PubMed] [Google Scholar]
- 29.Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database of Systematic Reviews. 2012. October 17; [DOI] [PubMed]
- 30.Dennis M, Lewis S, Cranswick G, Forbes J. FOOD: A multicentre randomized trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technology Assessment. 2006. January;10(2):1–91. [DOI] [PubMed] [Google Scholar]
- 31.Schinwelski MJ, Sitek EJ, Wąż P, Sławek JW. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurologia i neurochirurgia polska. 2019;53(6):449–57. [DOI] [PubMed] [Google Scholar]
- 32.Lieber RL, Fridén J. Muscle contracture and passive mechanics in cerebral palsy. Vol. 126, Journal of Applied Physiology. American Physiological Society; 2019. p. 1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. Journal of the American Medical Directors Association [Internet]. 2011. July [cited 2020 Jan 9];12(6):403–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21640657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: A symptom score to predict persons with sarcopenia at risk for poor functional outcomes. Journal of Cachexia, Sarcopenia and Muscle. 2016. March 1;7(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmstrom TK, Morley JE. SARC-F: A simple questionnaire to rapidly diagnose sarcopenia. Journal of the American Medical Directors Association. 2013;14(8):531–2. [DOI] [PubMed] [Google Scholar]
- 36.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: Consensus report of the Asian working group for sarcopenia. Vol. 15, Journal of the American Medical Directors Association. Elsevier Inc.; 2014. p. 95–101. [DOI] [PubMed] [Google Scholar]
- 37.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Vol. 40, Age and Ageing. 2011. p. 423–9. [DOI] [PubMed] [Google Scholar]
- 38.Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatrics. 2016;16(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility - Giving mobility clinical visibility: A mobility working group recommendation. Vol. 311, JAMA - Journal of the American Medical Association. American Medical Association; 2014. p. 2061–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, et al. Grip strength across the life course: Normative data from twelve British studies. PLoS ONE. 2014. December 4;9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, Ligaments and Tendons Journal. 2013. October;3(4):346–50. [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age and Ageing. 2014. November 1;43(6):48–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binder EF, Yarasheski KE, Steger-May K, Sinacore DR, Brown M, Schechtman KB, et al. Effects of progressive resistance training on body composition in frail older adults: Results of a randomized, controlled trial. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2005;60(11):1425–31. [DOI] [PubMed] [Google Scholar]
- 44.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: A randomized controlled trial. Journal of Applied Physiology. 2008. November;105(5):1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suetta C, Andersen JL, Dalgas U, Berget J, Koskinen S, Aagaard P, et al. Resistance training induces qualitative changes in muscle morphology, muscle architecture, and muscle function in elderly postoperative patients. Journal of Applied Physiology. 2008. July;105(1):180–6. [DOI] [PubMed] [Google Scholar]
- 46.Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Vol. 28, Osteoporosis International. Springer London; 2017. p. 1817–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chilibeck P, Kaviani M, Candow D, Zello GA. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: a meta-analysis. Open Access Journal of Sports Medicine. 2017. November;Volume 8:213–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pradon D, Roche N, Enette L, Zory R. Relationship between lower limb muscle strength and 6-minute walk test performance in stroke patients. Journal of rehabilitation medicine [Internet]. 2013. January [cited 2020 Jan 9];45(1):105–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23095981 [DOI] [PubMed] [Google Scholar]
- 49.Stoquart GG, Detrembleur C, Nielens H, Lejeune TM. Efficiency of work production by spastic muscles. Gait & posture [Internet]. 2005. December [cited 2020 Jan 9];22(4):331–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16274915 [DOI] [PubMed] [Google Scholar]
- 50.Bohannon RW. Test-retest reliability of hand-held dynamometry during a single session of strength assessment. Physical therapy [Internet]. 1986. February [cited 2020 Jan 9];66(2):206–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/3945674 [DOI] [PubMed] [Google Scholar]
- 51.Ryan AS, Ivey FM, Serra MC, Hartstein J, Hafer-Macko CE. Sarcopenia and Physical Function in Middle-Aged and Older Stroke Survivors. Archives of physical medicine and rehabilitation [Internet]. 2017. [cited 2020 Jan 9];98(3):495–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27530769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiraishi A, Yoshimura Y, Wakabayashi H, Tsuji Y. Prevalence of stroke-related sarcopenia and its association with poor oral status in post-acute stroke patients: Implications for oral sarcopenia. Clinical nutrition (Edinburgh, Scotland) [Internet]. 2018. [cited 2020 Jan 9];37(1):204–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28017450 [DOI] [PubMed] [Google Scholar]
- 53.Limpawattana P, Kotruchin P, Pongchaiyakul C. Sarcopenia in Asia. Osteoporosis and Sarcopenia [Internet]. 2015. December 1 [cited 2020 Jan 13];1(2):92–7. Available from: https://www.sciencedirect.com/science/article/pii/S2405525515300212#bib14 [Google Scholar]
- 54.Nozoe M, Kanai M, Kubo H, Yamamoto M, Shimada S, Mase K. Prestroke sarcopenia and functional outcomes in elderly patients who have had an acute stroke: A prospective cohort study. Nutrition (Burbank, Los Angeles County, Calif) [Internet]. 2019. October [cited 2020 Jan 9];66:44–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31207438 [DOI] [PubMed] [Google Scholar]
- 55.Jang Y, Im S, Han Y, Koo H, Sohn D, Park G-Y. Can initial sarcopenia affect poststroke rehabilitation outcome? Journal of Clinical Neuroscience [Internet]. 2019. September 5 [cited 2020 Jan 9]; Available from: https://www.sciencedirect.com/science/article/pii/S0967586819313232 [DOI] [PubMed]
- 56.Nozoe M, Kanai M, Kubo H, Yamamoto M, Shimada S, Mase K. Prestroke Sarcopenia and Stroke Severity in Elderly Patients with Acute Stroke. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association [Internet]. 2019. August [cited 2020 Jan 9];28(8):2228–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31129104 [DOI] [PubMed] [Google Scholar]
- 57.Yoshimura Y, Wakabayashi H, Bise T, Nagano F, Shimazu S, Shiraishi A, et al. Sarcopenia is associated with worse recovery of physical function and dysphagia and a lower rate of home discharge in Japanese hospitalized adults undergoing convalescent rehabilitation. Nutrition. 2019. May 1;61:111–8. [DOI] [PubMed] [Google Scholar]
- 58.Matsushita T, Nishioka S, Taguchi S, Yamanouchi A. Sarcopenia as a predictor of activities of daily living capability in stroke patients undergoing rehabilitation. Geriatrics & Gerontology International. 2019. October 7; [DOI] [PubMed]
- 59.Park JG, Lee KW, Kim SB, Lee JH, Kim YH. Effect of Decreased Skeletal Muscle Index and Hand Grip Strength on Functional Recovery in Subacute Ambulatory Stroke Patients. Annals of Rehabilitation Medicine. 2019. October 31;43(5):535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Zehr EP. Training-induced neural plasticity and strength are amplified after stroke. Exercise and Sport Sciences Reviews. 2019. October 1;47(4):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akazawa N, Harada K, Okawa N, Tamura K, Moriyama H. Muscle mass and intramuscular fat of the quadriceps are related to muscle strength in non-ambulatory chronic stroke survivors: A cross-sectional study. PLoS ONE. 2018. August 1;13(8). [DOI] [PMC free article] [PubMed] [Google Scholar]


