Abstract
Consistent survival of life-supporting pig heart xenograft recipients beyond 90 days was recently reported using genetically modified pigs and a clinically applicable drug treatment regimen. If this remarkable achievement proves reproducible, published benchmarks for clinical translation of cardiac xenografts appear to be within reach. Key mechanistic insights are summarized here that informed recent pig design and therapeutic choices, which together appear likely to enable early clinical translation.
Keywords: cardiomyopathies; genetic engineering; heart failure; heart transplantation; heterografts; myocardial ischemia; transplantation, heterologous
Over the 53 years since the first clinical attempt,1 cardiac allotransplantation, the transfer of a heart from 1 human to another, has evolved from a pioneering, newsworthy, and largely unsuccessful therapeutic adventure to become a standard-of-care treatment for end-stage heart failure. As a byproduct of this success, the scarcity of hearts from human donors has become the primary constraint to expanded application.
Xenotransplantation, or transplantation between different species, has the promise of an unlimited supply of readily available, safe, and optimally functioning organs. To date, clinical translation has been thwarted by a several obstinate barriers: vigorous innate and adaptive immune responses; interspecies incompatibilities in molecular interactions involving the complement and coagulation pathways, among others; and the risk of causing adventitious infection.2,3 Progress in the understanding of each of these barriers is reviewed here in the context of 3 recent breakthroughs that have culminated in consistent and prolonged life-supporting pig heart graft function and prolonged survival in baboon orthotopic heart xenograft recipients, which represent a clear path toward clinical trials.4,5
IMMUNE BARRIERS
The consensus on developing swine strains designed as organ donors for humans was informed by considerations framed in Table 1. For wild-type pig organs perfused ex vivo with human blood or transplanted into a baboon or macaque monkey, endothelial injury occurs within minutes, driven largely by preformed natural antibodies in primate blood.6 More than 95% of human anti-pig antibodies are directed against 3 pig carbohydrates: Gal 1 to 3αGal (≈80%–90%),7 Neu5Gc (≈5%–15%),8 and β4Gal (1%–5%).9 These carbohydrates are absent in humans and old-world primates (baboons, macaque monkeys) but are expressed on the cell membranes of intestinal bacteria, pigs, and many other species. Binding of preformed natural anticarbohydrate antibodies triggers complement and coagulation cascade activation, endothelial activation and injury, and graft dysfunction (hyperacute rejection) within minutes or hours after perfusion of a pig organ with human blood or when transplanted into nonhuman primates (Figure 1 and Tables 2 and 3).10 Complement activation and coagulation pathway dysregulation are observed even when anti-pig antibody binding is minimized.2,3 In this context, a variety of phenomena, including organ xenograft dysfunction and recipient injury, evolve within days or weeks. Collectively called delayed xenograft rejection (DXR), these phenomena have been traced to molecular incompatibilities between species, particularly involving inefficient binding of human blood coagulation and complement pathway proteins to pig thromboregulatory (Figure 2) and complement regulatory molecules, respectively.
Table 1.
Considerations in Selecting a Source Species for Organ Xenografts
| Species | Advantages | Disadvantages |
|---|---|---|
| Primates | ||
| Ape | Concordant | Scarce, endangered, endogenous retrovirus |
| Chimpanzee, gorilla, orangutan | Ethical concerns, slow breeding | |
| Bonobo | Small size except mature adult male | |
| Gibbon | Small size | |
| Monkey | Concordant | Endogenous retrovirus |
| Baboon | Small size except mature adult male | |
| Macaque | Small size, B-virus susceptible | |
| Marmoset, capuchin, howler | Small size | |
| Large mammals bred in captivity | ||
| Bear | Discordant? Large size at maturity, slow breeding | |
| Great cat (lion, tiger) | Discordant? Slow breeding, endangered in wild | |
| Domesticated large mammals | ||
| Horse | Discordant, large size at maturity | |
| Cow | Discordant, large size at maturity | |
| Dog | Size (some breeds) | Discordant, ethical concerns |
| Sheep | Discordant, small size except adult male | |
| Pig | Rapid propagation | Discordant, porcine endogenous retrovirus |
| Commercial | Large size at maturity | |
| Mini-swine | Size-compatible | Slower growth to adult human size |
To be practical as an orthotopic heart replacement, the cardiac physiology must be compatible with humans in size and functional capacity. Organs from concordant species avoid the immunological barrier of preformed antibody and hyperacute rejection but are considered impractical because of infectious disease risks, compelling ethical concerns, and scarcity and slow natural breeding cycles that would constrain the impact even if successful.
Cloning and in vitro fertilization have been developed for most domesticated species, including swine, and are useful to rapidly propagate animals with desired characteristics for evaluation in informative models but are expensive and inefficient for commercial-scale production relative to natural breeding.
Among domesticated species, pigs were chosen as the best potential source of heart and other organ xenografts because their short gestation (3 months, 3 weeks, and 3 days), rapid growth to adult human size, and sexual maturity within 1 year compare favorably with other potentially size-compatible domesticated animals. These characteristics should facilitate efficient propagation by natural breeding once lines with genetic modification necessary to clinical success are defined. Genetic engineering for knockout of carbohydrate genes, for expression of protective transgenes, and to disable potentially pathogenic endogenous retroviruses has been developed and refined in swine specifically to enable clinical xenotransplantation. Hearts and other organs from mature naturally occurring mini-swine and pigs with knockout of the growth hormone receptor are similar to those of adult humans in size and functional capacity. Inbred genetically defined pig lines have been developed that may be useful to promote induction of donor-specific cross-species immunological tolerance and to minimize long-term recipient immunosuppression requirements.
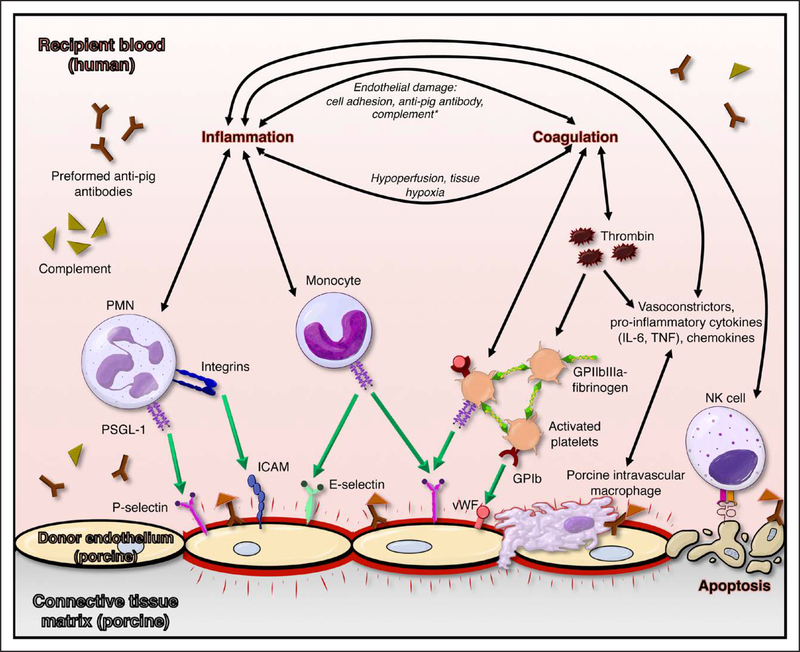
Figure 1. Mechanisms participating in porcine endothelial injury by human blood.
Preformed human anti-pig antibodies bind to porcine endothelium, triggering complement binding and Fc-receptor–mediated ligation of platelets and leukocytes and upregulation of adhesion molecules on both adherent formed blood elements and inflamed endothelium. Complement cascade activation (orange symbols), nonphysiological adhesion of human platelets to porcine endothelium, and absence of nonself signals (illustrated for natural killer cells) contribute to a prothrombotic, proinflammatory milieu that leads to loss of vascular barrier function and organ failure. GP indicates glycoprotein; ICAM, intercellular adhesion molecule; IL-6, interleukin-6; PMN, polymorphonuclear; PSGL-1, P-selectin glycoprotein ligand 1; TNF, tumor necrosis factor; and vWF, von Willebrand factor.
Table 2.
Mechanistic Barriers to Pig-to-Human Heart Xenografting
| Phenomenon | Kinetics | Mechanisms |
|---|---|---|
| Hyperacute rejection | Minutes to hours | Preformed antibody, complement, clot formation |
| Initial xenograft dysfunction | Minutes to hours | Immunological? Physiological? |
| Acute humoral rejection | Days | Preformed antibody rebound, elicited antibody |
| Weeks | Elicited immunity, dysregulated coagulation |
DXR indicates delayed xenograft rejection.
Genetic modifications to swine and mechanism-directed graft and recipient treatments have successfully prevented each of the phenomena encountered in pig-to-primate heart xenotransplantation models as described in the text. We expect that preclinical results in a life-supporting pig-to-baboon model4 predict safe clinical translation.
Chronic rejection will be observed in long-surviving organ xenografts if the immune response to the donor cannot be safely constrained by genetic modifications to the graft combined with immunomodulatory drug treatments. For organ xenografts, the source pig is genetically defined and known in advance, and the transplantation procedure can be timed to take advantage of recipient pretreatments designed to favor graft acceptance. Consequently, induction of cross-species tolerance—long-term graft acceptance without ongoing immunosuppressive treatment—is likely to be feasible, potentially offering advantages relative to conventional allotransplantation from deceased human donors. Patient selection criteria and clinical trial design considerations for the initial pilot studies in humans have been reviewed recently.10
Table 3.
Glossary of Heart Transplantation Procedures and Applications for Xenotransplantation
| Procedural Definitions | ||
|---|---|---|
| Technique | Description | Utility |
| Heterotopic | Vascularized, extra-anatomic | Evaluate biology and histology |
| Abdomen or neck location | Evaluate immunosuppression | |
| Right thorax, auxiliary parallel circuit | Provide partial hemodynamic support | |
| Orthotopic | Replace native heart | Evaluate full hemodynamic support |
The heterotopic (“other place,” not placed in its normal anatomic position) heart transplantation model was originally developed in rodents and later adapted to large animal models for preclinical allotransplantation and xenotransplantation research. The coronary arterial circulation to the unloaded, nonworking heart graft is supported by the recipient’s circulation, with coronary sinus blood returned via the donor pulmonary artery to the recipient inferior vena cava, effectively creating a parasitic arteriovenous shunt. The heterotopic technique permits efficient study of xenograft injury mechanisms and immunosuppressive drug efficacy. The auxiliary chest piggy-back heterotopic technique supports recipient circulation by pumping blood in parallel with and supplemental to the retained native heart and was used by Barnard in his first 2 clinical cases. Because of the operative complexity and a high incidence of pulmonary and thromboembolic complications, current clinical use is limited to patients with elevated pulmonary vascular resistance. Replacing the native heart with the graft, the orthotopic (“same place”) technique pioneered by Lower and Shumway is used in most clinical circumstances. Orthotopic transplantation allows rigorous preclinical evaluation of heart xenograft performance and the best available prediction of the clinical efficacy and safety of candidate therapeutic strategies.
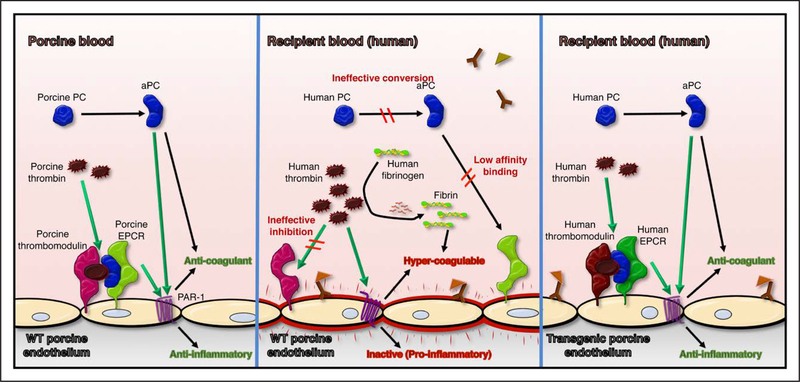
Figure 2. Dysregulated coagulation with porcine endothelium exposed to human blood.
Relative to physiological regulation of thrombosis (left), porcine endothelium exposed to human blood is activated by binding of anti-pig antibodies, creating a prothrombotic environment (middle). Physiologically inappropriate amplification of blood clotting is contributed to by ineffective neutralization of human thrombin by porcine thrombomodulin, inefficient conversion of protein C (PC) to activated PC (aPC) by thrombin-thrombomodulin complex, and low-affinity binding of human aPC to porcine endothelial protein C receptor (EPCR), which in turn leads to inefficient thrombin degradation and reduced cytoprotective signaling through endothelial cell proteinase-activated receptor 1 (PAR-1). These molecular incompatibilities between species are addressed by expression of human thromboregulatory proteins, including human thrombomodulin and human EPCR (right), as well as human tissue factor pathway inhibitor (not illustrated). WT indicates wild-type.
To prevent hyperacute rejection and DXR and to enable the use of pig organ xenografts in humans, 3 general genetic engineering strategies have been deployed to minimize antibody binding and to improve regulation of the complement and coagulation pathways across species. The first approach was enabled in the 1990s by advances in embryo microinjection and in vitro fertilization for large mammals, including pigs. Several groups demonstrated that surface expression of 1 of 3 human complement pathway regulatory proteins (hCPRPs), hCD46,11,12 hCD55,13,14 or hCD59,15 downmodulates complement-mediated cell injury and prolongs survival of pig cells and organs.12–19 Although hyperacute rejection was usually prevented, any single modification proved insufficient to prevent DXR with the immunosuppressive drugs conventionally used in allotransplantation15,16 or experimental immunosuppression based on costimulation pathway blockade.14,17–19 With a tolerogenic mixed-chimerism strategy, DXR was observed even in the absence of detectible elicited anti-pig antibody15,17–19 and with demonstrable donor-specific cellular immune unresponsiveness,19 suggesting that either residual effects of anti-pig antibody or other cross-species incompatibilities—perhaps via mechanisms not conventionally considered immune—were causing DXR.
To reduce anti-pig antibody binding to the xenograft, the 3 principal carbohydrate antigen targets have each been successfully removed from pigs by gene knockout (KO) (Figure 3): first of the Gal 1 to 3α galactosyl transferase gene (GalTKO)20,21 and more recently of cytidine monophospho-N-acetylneuraminic acid hydroxylase22 and β4 galactosyl transferase.23 The pig gene KO process was greatly facilitated first by transcription activator-like effector nuclease–directed mutagenesis or zinc finger nucleases and more recently by CRISPR-Cas9–based germline gene editing technology. Pigs with KO of all 3 genes, or triple knockouts (TKOs), have recently been produced by multiple groups.24–27 Organs from pigs with GalTKO, alone18,28 or with complement regulatory gene modifications,29,30 and TKO organs31 do not usually exhibit hyperacute rejection; they typically survive for days or weeks in immunosuppressed primates before exhibiting DXR.18,29–31
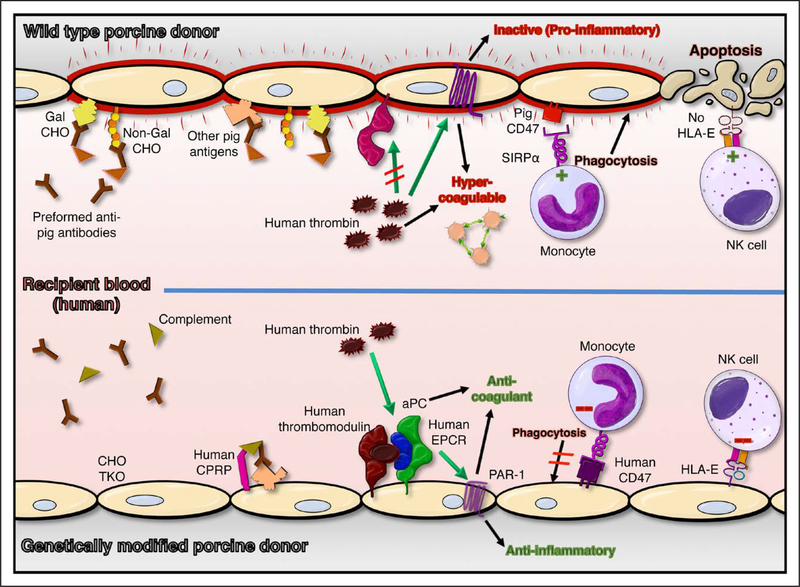
Figure 3. Genetic modifications designed to address xenograft injury mechanisms.
Examples of genetic modifications designed to prevent known xenograft injury (top) include Gal α1–3Gal (Gal) and 2 other carbohydrate (CHO) gene knockouts (TKO), and expression of human complement pathway regulatory proteins (hCPRPs), coagulation pathway regulatory proteins (eg, thrombomodulin [hTBM] and endothelial protein C receptor [hEPCR]), and self-recognition receptors (hCD47; human leukocyte antigen-E [HLA-E]; bottom). Absence of carbohydrate antigens (CHO TKO) and expression of human complement and coagulation pathway regulatory molecules reduce endothelial activation and injury and promote endothelial cytoprotective mechanisms. Expression of self-recognition receptors inhibits (red negative symbols, bottom) pathogenic mechanisms mediated by monocytes and natural killer (NK) cells that contribute to cross-species injury (green positive symbols, top). In addition to the pathways illustrated, hCD39, human tissue factor pathway inhibitor, hemoxygenase-1, and A20 are among the human genes included in some of the various multigene pig constructs that are currently under preclinical evaluation. aPC indicates activated protein C; PAR-1, proteinase-activated receptor 1; and SIRPα, signal regulatory protein-α.
Exceptionally, TKO kidney grafts survivals of >1 year have been reported in rhesus macaques preselected for very low levels of anti-pig antibodies and using experimental costimulation-based immunosuppression.32 If reproducible with an immunosuppressive regimen conventionally used for allotransplantation, this notable accomplishment could justify first-in-human xenograft trials. However, TKO kidney xenografts exhibit DXR and fail within days or weeks in recipients with anti-pig antibody levels that would not be associated with adverse early outcomes for clinical kidney allografts.33 Because the majority of humans would not meet this group’s definition of a negative cross-match, even if successful, this strategy would have a limited impact on the organ donor shortage.
PHYSIOLOGICAL BARRIERS
Meanwhile, several molecular incompatibilities between pig thromboregulatory molecules and human blood proteins have been described that are physiologically consequential.2,34–36 At the all-important human blood-porcine endothelial interface, porcine thromboregulatory molecules such as thrombomodulin (TBM), endothelial protein C receptor (EPCR), and thrombinactivatable fibrinolysis inhibitor interact inefficiently with various human coagulation pathway molecules. For example, ineffective binding of activated human protein C to porcine TBM interferes with neutralization of human thrombin, promoting physiologically inappropriate intravascular clot propagation. In addition, dysregulated adenosine metabolism consequent to low surface expression of CD39 on porcine ECs contributes to vasoconstriction and prothrombotic effects on platelets and endothelial cells (Figure 2). Dysregulated coagulation results from both physiologically inappropriate clot initiation and propagation combined with inefficient thromboregulation. Thrombodysregulation within the organ xenograft (thrombotic microangiopathy) and in the recipient’s circulation (consumptive coagulopathy) is likely caused, or amplified, by anti-pig antibody and associated complement cascade activation.2,34,36,37 Thrombodysregulation is sufficient to cause DXR despite intense immunosuppression15,16 in immunologically tolerized recipients of GalTKO organs19 and in recipients of GalTKO.hCPRP organs.36–38
Consequently, multiple groups have produced pigs that express ≥1 human coagulation pathway regulatory genes on the GalTKO.hCPRP background.36–39 Expression of human TBM (hTBM) by a GalTKO.hCD46 heart led to the first recent breakthrough when we showed that expression of hTBM was necessary and sufficient to prevent consumptive coagulopathy in the baboon and thrombotic microangiopathy in a GalTKO.hCD46 heterotopic pig heart xenograft.40 Non–life-supporting heterotopic heart xenografts continued to function with preserved myocardial histology for >2 years as long as immunosuppression with anti-CD40 monoclonal antibody–based costimulation pathway blockade was maintained. Aside from anti-CD40, all the other components of the immunosuppressive regimen—induction T-cell depletion with antithymocyte globulin, B-cell depletion with anti-CD20 antibody, tapered-dose steroids, and long-term treatment with mycophenolate mofetil—are routinely used clinically in allotransplantation. These results demonstrate that the previously intractable barrier of DXR was overcome for the first time.
Längin et al4 then evaluated the GalTKO.hCD46. hTBM heart phenotype and the Mohiuddin immunomodulatory regimen in a life-supporting orthotopic heart model. Critical to their breakthrough results, they showed that perfusing the explanted pig heart on an ex vivo device until just before revascularization consistently prevented initial xenograft dysfunction for GalTKO. hCD46.hTBM hearts in baboons. With minimization of xenograft ischemia, 9 consecutive orthotopic GalTKO. hCD46.hTBM heart xenograft recipients were successfully weaned from cardiopulmonary bypass with transient low-dose inotrope requirements, in contrast with high perioperative inotrope requirements and prevalent early mortality without ex vivo perfusion. Using either of 2 costimulation pathway blocking regimens adopted from Mohiuddin, they confirmed our observation that DXR was consistently prevented. However, of the first 4 of these 9 consecutive recipients, 3 died within 6 weeks of complications of massive myocardial hypertrophy, which was associated with increased expression of the mammalian target of rapamycin in the heart xenograft. In the subsequent 5 animals, a mammalian target of rapamycin inhibitor, temsirolimus, combined with an aggressive antihypertensive regimen prevented cardiac hypertrophy. One recipient died of a late technical complication; the other 4 survived to elective euthanasia at 3 (n=2) or 6 (n=2) months. Graft hypertrophy was observed by echocardiography in the 2 longest survivors after temsirolimus was stopped 3 weeks before euthanasia, supporting their hypothesis that mammalian target of rapamycin inhibition was pivotal to preventing xenograft hypertrophy. This result closely approximates the preclinical trial outcomes that an International Society for Heart and Lung Transplantation expert panel proposed in 2000 as a reasonable basis for initiating a clinical heart xenotransplantation trial.41
INFECTIOUS DISEASE BARRIERS
The third recent breakthrough was reported in 2016 when CRISPR-based gene editing was successfully used to simultaneously edit and functionally disable all 62 copies of the pig genes encoding for porcine endogenous retrovirus (PERV), first in cell lines and then to generate viable, fertile pigs.42 In the late 1990s, demonstration that PERV could productively infect specific permissive human cell lines43 raised the specter that pig-to-human xenografts might cause infection in the xenograft recipient and risk pandemic viral infection.44 Concerns about the spread of pig-derived pathogens to the community reflected, at that time, the spread of HIV, an exogenous retrovirus, and experience with swine serving as an intermediate host for human pathogens (influenza virus). Along with the immune barriers, infectious disease concerns and related regulatory barriers contributed to collapse of enthusiasm for xenotransplantation, and corporate investment evaporated in the early 2000s. Recent pandemic infection by another unrelated exogenous virus (severe acute respiratory syndrome coronavirus 2, likely from bats and Malayan Pangolin species) might raise similar questions. Studies of PERV have demonstrated that human infection was generally, but not exclusively, the result of viral recombination between 2 PERV strains (A and C) that produced a strain more capable of replication in specific human cell lines. Multiple innovative strategies were developed to inhibit PERV transmission (eg, using nontransmitting swine lacking PERV A or C, antiviral therapies with silencing or shRNAs). Given the general lack of transmission of PERV to normal human cells, it seems less likely that PERV will prove clinically important; should infection occur, antiretroviral drugs developed to treat HIV exhibit potent in vitro activity against PERV replication.45,46 However, the availability of PERV-deleted swine for clinical studies and the use of CRISPR-based technology to engineer further immunological modifications helped reawaken interest in the xenotransplant field by providing proof of principle that, if necessary, PERV could be eliminated from pig strains intended for clinical use. Although all species harbor coronaviruses, severe acute respiratory syndrome coronavirus 2 does not appear to have been derived from swine. Lists of potential human pathogens from swine allow routine testing and exclusion of these organisms from breeding colonies.46 In addition, molecular surveillance strategies can now be applied (eg, whole-genome sequencing on blood samples) that should allow routine detection of asymptomatic infections caused by PERV or any novel human pathogens from swine (xenozoonosis) and the microbiological investigation of the common infectious syndromes (eg, fever and neutropenia or pneumonia) of immunosuppressed transplant recipients.46–48 Together, these developments justify confidence that pig xenografts are likely to prove safe and that protocols including surveillance of donor herds, recipients, and their close contacts will define any remaining infectious risks for the informed xenograft recipient, their social contacts, and society at large.
THE PATH TO CLINICAL APPLICATION
What are the residual barriers to clinical translation? Reproducibility of the Längin group’s orthotopic heart results and demonstration of some longer-term recipient survivals would improve confidence that clinical translation is timely. However, evaluating whether pig heart xenograft survival will prove durable in humans will ultimately require a leap of faith on the part of patients, investigators, and regulatory authorities because the predictive value of the baboon model has never been tested. Meanwhile, current preclinical research seeks to better understand whether xeno-heart initial xenograft dysfunction is an immunological or biochemical phenomenon and, in either case, whether it might be addressed by additional genetic modifications to the pig. To that end, at least 4 groups have created pigs with multiple additional genetic modifications in various logical combinations. Revivicor pigs have up to 9 genetic modifications, typically including TKO, multiple human complement regulatory (hCD46, hCD55) and thromboregulatory molecules (hTBM, hEPCR, hCD39, and hTFPI), and molecules to inhibit phagocytosis (hCD47) or natural killer cell activation (HLA-E) (Figure 3).30,36,39 Tector’s group33 is testing whether TKO pigs that additionally lack swine histocompatibility antigens may exhibit reduced immunogenicity and thus enable use of conventional rather than experimental immunosuppressive drugs. Qihan Bio/eGenesis has used cutting-edge CRISPR.Cas9 gene editing techniques to create PERVKO pig versions with up to 12 additional xeno-targeted genetic modifications: TKO with expression of human complement regulatory (hCD46, hCD55, hCD59) and thromboregulatory molecules (hTBM, hCD39, hTFPI), as well as molecules to trigger protective self-recognition pathways (HLA-E, with β2μ; hCD47).26 Testing of cells and organs from pigs with various combinations of these genetic modifications is currently in progress by multiple groups.
As described in the World Health Organization’s guidance documents,49 a proposed first-in-human xenotransplantation trial will require protocol review and approval by both institutional and national regulatory bodies, as well as implementation of an infection monitoring program for trial subjects and their close contacts sufficient to safeguard public health. On the basis of the currently available evidence, regulatory authorities might determine that the Längin regimen—combining complex pig genetics with a previously unapproved anti-CD40/CD154 antibody and multiple off-label uses of drugs approved for other indications—is clinically acceptable for a first-in-human heart xenograft trial in carefully chosen candidates (Table 4). Indeed, the US Food and Drug Administration has publicly accepted the principle that a clinical xenotransplantation trial proposal would be reviewed, and could be approved, if the pig genotype design is based on established mechanisms and pharmacological choices and treatment strategies are supported by preclinical safety and efficacy data.50,51 On the other hand, a regulatory authority or institutional review board might request additional preclinical studies to justify the use of particular regimen components. In that case, the regulatory approval path would be simplified if pigs with extended genetic modifications are protected from initial xenograft dysfunction or allow substitution of conventional immunosuppression for as-yet-unapproved costimulation blocking approaches. In our estimation, such a transparent, thorough, and evidence-driven oversight process is more likely to delay than to prevent the initiation of a clinical trial of heart xenotransplantation. Such a public, transparent process will ultimately be essential to justify society’s confidence that the best interests of both study participants and the general public have been fully considered and fairly accounted. In that context, even an initial failure that informs later success would be viewed as a justifiable risk.
Table 4.
Initial Patient Selection Criteria for a Pig Heart Xenograft Trial*
| High immunological risk for heart allograft failure |
| Broadly reactive, high-titer antibody against HLA antigens |
| History of early onset and rapid progression of cardiac allograft vasculopathy after prior heart transplantation |
| Obstacles to VAD implantation |
| Structural |
| Aortic valve insufficiency |
| Ascending aortic aneurysmal disease |
| Aortic or mitral mechanical valve prosthesis |
| Congenital or acquired ventricular septal defect |
| Congenital or acquired single-ventricle physiology |
| Physiological |
| Restrictive or hypertrophic cardiomyopathy |
| Declining reversibility of PVR elevation |
| Severe biventricular failure with impending end-organ failure |
| Biventricular assist or total artificial heart candidate |
HLA indicates human leukocyte antigen; PVR, pulmonary vascular resistance; and VAD, ventricular assist device.
Patients often exhibit multiple relative or absolute contraindications to receiving a heart allograft or mechanical support therapeutic options. Patients with these characteristics face a high likelihood of adverse outcomes or are not offered currently available therapies and would be appropriate to consider as candidates for an initial clinical trial of heart xenotransplantation.
CONCLUSIONS
Consistent orthotopic heart xenograft recipient survival to 180 days represents a major advance toward the International Society for Heart and Lung Transplantation’s benchmark for clinical trial justification.41 Striking recent progress to avoid thrombotic microangiopathy and consumptive coagulopathy has derived from addressing the pathophysiology of these phenomena, primarily by genetic engineering of the pig to reduce graft antigenicity (GalTKO) and to correct molecular incompatibilities in cross-species regulation of complement (hCD46) and coagulation pathway (hTBM) activation. This triple-transgenic pig phenotype appears to be sufficient to protect the heart xenograft from residual effects associated with preformed natural antibody, whereas emergence of elicited antibody against pig proteins or other antigens and amplification of preexisting antibody are effectively controlled by anti-CD40/CD154–based costimulation pathway blockade. A role for more extensive genetic modifications to reduce or potentially eliminate various components of the other regimens has not yet been explored, but preliminary results suggest that more complicated genetics may not be necessary for a heart xenograft to provide a safe, effective alternative to an allograft.4,31
Barnard’s1 pioneering heart allotransplantation efforts yielded only occasional long-term survivors, and the procedure was quickly abandoned by many institutions. Persistent, thoughtful clinical experimentation in brave patients, led by many early medical and surgical teams, systematically addressed the many problems encountered,52–56 and gradually yielded incremental progress until the advent of calcineurin-based immunosuppression enabled consistent success at a few pioneering centers57 and then safe dissemination. Similarly, the first clinical heart xenotransplantation trials will necessarily venture into uncharted territory and may reveal obstacles not predicted by the preclinical models. From the remarkable advances described here, we predict that clinical heart xenotransplantation trials will soon begin as the appropriate next step toward cardiac xenotransplantation finally realizing its full therapeutic potential.
Acknowledgments
Disclosures
Drs Pierson and Azimzadeh (U19 AI090959, R01 AI153612), Dr Madsen (R01 AI153612), and Dr Fishman (PO1-5P01AI45897) are funded by the National Institutes of Health for work in the xenotransplantation field and have received key research reagents, research grants, and other support from United Therapeutics (doing business as Revivicor and Lung Biotechnology PBC) and/or eGenesis. Dr Pierson serves as chair of the Ethics Committee of the International Xenotransplantation Association, a section of the Transplantation Society. Dr Azimzadeh is the President of the International Xenotransplantation Association. Dr Fishman has served as a consultant for eGenesis and United Therapeutics.
Contributor Information
Richard N. Pierson, III, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston; Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
Jay A. Fishman, Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
Gregory D. Lewis, Division of Cardiology, Department of Medicine, Massachusetts General Hospital and Harvard University, Boston.
David A. D’Alessandro, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston.
Margaret R. Connolly, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston; Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
Lars Burdorf, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston; Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
Joren C. Madsen, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston; Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
Agnes M. Azimzadeh, Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital and Harvard University, Boston; Center for Transplantation Sciences, Massachusetts General Hospital and Harvard University, Boston.
REFERENCES
- 1.Barnard CN. Human cardiac transplantation: an evaluation of the first two operations performed at the Groote Schuur Hospital, Cape Town. Am J Cardiol. 1968;22: 584–596. doi: 10.1016/0002-9149(68)90166-5 [DOI] [PubMed] [Google Scholar]
- 2.Pierson RN 3rd, Dorling A, Ayares D, Rees MA, Seebach JD, Fishman JA, Hering BJ, Cooper DK. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DK, Satyananda V, Ekser B, an der Windt DJ, Hara H, Ezzelarab MB, Schuurman HJ. Progress in pig-to-nonhuman primate transplantation models (1998–2013): a comprehensive review of the literature. Xenotransplantation. 2014;21:397–419. doi: 10.1111/xen.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. doi: 10.1038/s41586-018-0765-z [DOI] [PubMed] [Google Scholar]
- 5.Pierson RN 3rd. A major advance toward clinical cardiac xenotransplantation [published online June 13, 2019]. J Thorac Cardiovasc Surg. 2019;S0022–5223(19)31024–4. doi: 10.1016/j.jtcvs.2019.04.087 [DOI] [PubMed] [Google Scholar]
- 6.Cooper DK, Human PA, Lexer G, Rose AG, Rees J, Keraan M, Du Toit E. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J Heart Transplant. 1988;7:238–246. [PubMed] [Google Scholar]
- 7.Cooper DK, Good AH, Koren E, Oriol R, Malcolm AJ, Ippolito RM, Neethling FA, Ye Y, Romano E, Zuhdi N. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. doi: 10.1016/0966-3274(93)90047-c [DOI] [PubMed] [Google Scholar]
- 8.Basnet NB, Ide K, Tahara H, Tanaka Y, Ohdan H. Deficiency of N-glycolylneuraminic acid and Galα1–3Galβ1–4GlcNAc epitopes in xenogeneic cells attenuates cytotoxicity of human natural antibodies. Xenotransplantation. 2010;17:440–448. doi: 10.1111/j.1399-3089.2010.00610.x [DOI] [PubMed] [Google Scholar]
- 9.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pierson RN 3rd, Burdorf L, Madsen JC, Lewis GD, D’Alessandro DA. Pig-to-human heart transplantation: who goes first [published online April 17, 2020]? Am J Transplant. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.15916. doi: 10.1111/ajt.15916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams DH, Kadner A, Chen RH, Farivar RS. Human membrane cofactor protein (MCP, CD 46) protects transgenic pig hearts from hyperacute rejection in primates. Xenotransplantation. 2001;8:36–40. doi: 10.1046/j.0908-665x.2000.00085.x [DOI] [PubMed] [Google Scholar]
- 12.Loveland BE, Milland J, Kyriakou P, Thorley BR, Christiansen D, Lanteri MB, Regensburg M, Duffield M, French AJ, Williams L, et al. Characterization of a CD46 transgenic pig and protection of transgenic kidneys against hyperacute rejection in non-immunosuppressed baboons. Xenotransplantation. 2004;11:171–183. doi: 10.1046/j.1399-3089.2003.00103.x [DOI] [PubMed] [Google Scholar]
- 13.Schmoeckel M, Nollert G, Shahmohammadi M, Young VK, Chavez G, Kasper-König W, White DJ, Müller-Höcker J, Arendt RM, Wilbert-Lampen U, et al. Prevention of hyperacute rejection by human decay accelerating factor in xenogeneic perfused working hearts. Transplantation. 1996;62:729–734. doi: 10.1097/00007890-199609270-00005 [DOI] [PubMed] [Google Scholar]
- 14.Bühler L, Yamada K, Alwayn I, Kitamura H, Basker M, Barth RN, Appel J, Awwad M, Thall A, White-Scharf ME, et al. Miniature swine and hDAF pig kidney transplantation in baboons treated with a nonmyeloablative regimen and CD154 blockade. Transplant Proc. 2001;33:716. doi: 10.1016/s0041-1345(00)02220-x [DOI] [PubMed] [Google Scholar]
- 15.Kroshus TJ, Bolman RM 3rd, Dalmasso AP, Rollins SA, Guilmette ER, Williams BL, Squinto SP, Fodor WL. Expression of human CD59 in transgenic pig organs enhances organ survival in an ex vivo xenogeneic perfusion model. Transplantation. 1996;61:1513–1521. doi: 10.1097/00007890-199605270-00018 [DOI] [PubMed] [Google Scholar]
- 16.Cozzi E, Vial C, Ostlie D, Farah B, Chavez G, Smith KG, Bradley JR, Thiru S, Davies HF, Wallwork J, et al. Maintenance triple immunosuppression with cyclosporin A, mycophenolate sodium and steroids allows prolonged survival of primate recipients of hDAF porcine renal xenografts. Xenotransplantation. 2003;10:300–310. doi: 10.1034/j.1399-3089.2003.02014.x [DOI] [PubMed] [Google Scholar]
- 17.Kuwaki K, Knosalla C, Dor FJ, Gollackner B, Tseng YL, Houser S, Mueller N, Prabharasuth D, Alt A, Moran K, et al. Suppression of natural and elicited antibodies in pig-to-baboon heart transplantation using a human anti-human CD154 mAb-based regimen. Am J Transplant. 2004;4:363–372. doi: 10.1111/j.1600-6143.2004.00353.x [DOI] [PubMed] [Google Scholar]
- 18.Shimizu A, Yamada K, Yamamoto S, Lavelle JM, Barth RN, Robson SC, Sachs DH, Colvin RB. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148 [DOI] [PubMed] [Google Scholar]
- 19.Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, O’Malley P, Nobori S, Vagefi PA, Patience C, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172 [DOI] [PubMed] [Google Scholar]
- 20.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, Burlak C, Wang ZY, Reyes LM, Ivary B, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α−1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019 [DOI] [PubMed] [Google Scholar]
- 23.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine β1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21:543–554. doi: 10.1111/xen.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation. 2015;22:194–202. doi: 10.1111/xen.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang RG, Ruan M, Zhang RJ, Chen L, Li XX, Fang B, Li C, Ren XY, Liu JY, Xiong Q, et al. Antigenicity of tissues and organs from GGTA1/CMAH/β4GalNT2 triple gene knockout pigs. J Biomed Res. 2018;33:235–243. doi: 10.7555/JBR.32.20180018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yue Y, Kan Y, Xu W, Zhao H-Y, Zhou Y, Song X, Wu J, Xiong J, Goswami D, Yang M, et al. Extensive mammalian germline genome engineering. BioRxiv. 10.1101/2019.12.17.876862. [DOI] [Google Scholar]
- 27.Fischer K, Rieblinger B, Hein R, Sfriso R, Zuber J, Fischer A, Klinger B, Liang W, Flisikowski K, Kurome M, et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation. 2020;27:e12560. doi: 10.1111/xen.12560 [DOI] [PubMed] [Google Scholar]
- 28.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azimzadeh AM, Kelishadi SS, Ezzelarab MB, Singh AK, Stoddard T, Iwase H, Zhang T, Burdorf L, Sievert E, Avon C, et al. Early graft failure of GalTKO pig organs in baboons is reduced by expression of a human complement pathway-regulatory protein. Xenotransplantation. 2015;22:310–316. doi: 10.1111/xen.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, Liu H, Long C, Wang Y, Cassano A, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24:10.1111/xen.12293. doi: 10.1111/xen.12293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML 3rd, Lewis BG, Eckhaus M, Reimann KA, Klymiuk N, Wolf E, et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14:488–489. doi: 10.1111/ajt.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams AB, Kim SC, Martens GR, Ladowski JM, Estrada JL, Reyes LM, Breeden C, Stephenson A, Eckhoff DE, Tector M, et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann Surg. 2018;268:564–573. doi: 10.1097/SLA.0000000000002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martens GR, Reyes LM, Li P, Butler JR, Ladowski JM, Estrada JL, Sidner RA, Eckhoff DE, Tector M, Tector AJ. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation. 2017;101:e86–e92. doi: 10.1097/TP.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009;21:75–80. doi: 10.1016/j.trim.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu G, Pfeiffer S, Schröder C, Zhang T, Nguyen BN, Kelishadi S, Atkinson JB, Schuurman HJ, White DJ, Azimzadeh AM, et al. Coagulation cascade activation triggers early failure of pig hearts expressing human complement regulatory genes. Xenotransplantation. 2007;14:34–47. doi: 10.1111/j.1399-3089.2006.00362.x [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Cooper DKC, Burdorf L, Wang Y, Iwase H. Overcoming coagulation dysregulation in pig solid organ transplantation in nonhuman primates: recent progress. Transplantation. 2018;102:1050–1058. doi: 10.1097/TP.0000000000002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Chan JL, DiChiacchio L, Hardy NL, Corcoran PC, Lewis BGT, Thomas ML, Burke AP, Ayares D, Horvath KA, et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation. 2019;26:e12465. doi: 10.1111/xen.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23(pt B):296–300. doi: 10.1016/j.ijsu.2015.07.682 [DOI] [PubMed] [Google Scholar]
- 39.Harris DG, Quinn KJ, French BM, Schwartz E, Kang E, Dahi S, Phelps CJ, Ayares DL, Burdorf L, Azimzadeh AM, et al. Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22:102–111. doi: 10.1111/xen.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohiuddin MM, Singh AK, Corcoran PC, Thomas ML 3rd, Clark T, Lewis BG, Hoyt RF, Eckhaus M, Pierson RN 3rd, Belli AJ, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi: 10.1038/ncomms11138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper DK, Keogh AM, Brink J, Corris PA, Klepetko W, Pierson RN, Schmoeckel M, Shirakura R, Warner Stevenson L; Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation. Report of the Xenotransplantation Advisory Committee of the International Society for Heart and Lung Transplantation: the present status of xenotransplantation and its potential role in the treatment of end-stage cardiac and pulmonary diseases. J Heart Lung Transplant. 2000;19:1125–1165. doi: 10.1016/s1053-2498(00)00224-2 [DOI] [PubMed] [Google Scholar]
- 42.Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E, et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357:1303–1307. doi: 10.1126/science.aan4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patience C, Takeuchi Y, Weiss RA. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282 [DOI] [PubMed] [Google Scholar]
- 44.Bach FH, Fishman JA, Daniels N, Proimos J, Anderson B, Carpenter CB, Forrow L, Robson SC, Fineberg HV. Uncertainty in xenotransplantation: individual benefit versus collective risk. Nat Med. 1998;4:141–144. doi: 10.1038/nm0298-141 [DOI] [PubMed] [Google Scholar]
- 45.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, Chapman LE, Lockey C, Onions D, Otto E. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue: the XEN 111 Study Group. Science. 1999;285:1236–1241. doi: 10.1126/science.285.5431.1236 [DOI] [PubMed] [Google Scholar]
- 46.Fishman JA. Infectious disease risks in xenotransplantation. Am J Transplant. 2018;18:1857–1864. doi: 10.1111/ajt.14725 [DOI] [PubMed] [Google Scholar]
- 47.Krüger L, Kristiansen Y, Reuber E, Möller L, Laue M, Reimer C, Denner J. A comprehensive strategy for screening for xenotransplantation-relevant viruses in a second isolated population of Göttingen minipigs. Viruses. 2019;12:E38. doi: 10.3390/v12010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denner J Can Antiretroviral drugs be used to treat porcine endogenous retrovirus (PERV) infection after xenotransplantation? Viruses. 2017;9:E213. doi: 10.3390/v9080213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization. Second WHO global consultation on regulatory requirements for xenotransplantation clinical trials. 2011. https://www.who.int/transplantation/xeno/report2nd_global_consultation_xtx.pdf?ua=1. Accessed January 1, 2019.
- 50.Cooper DKC, Cowan P, Fishman JA, Hering BJ, Mohiuddin MM, Pierson RN 3rd, Sachs DH, Schuurman HJ, Dennis JU, Tönjes RR. Joint FDA IXA Symposium, September 20, 2017 [published online November 28, 2017]. Xenotransplantation. 2017;24:10.1111/xen.12365. doi: 10.1111/xen.12365 [DOI] [PubMed] [Google Scholar]
- 51.Mueller NJ, Kuwaki K, Dor FJ, Knosalla C, Gollackner B, Wilkinson RA, Sachs DH, Cooper DK, Fishman JA. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78:1449–1453. doi: 10.1097/01.tp.0000141361.68446.1f [DOI] [PubMed] [Google Scholar]
- 52.Rider AK, Copeland JG, Hunt SA, Mason J, Specter MJ, Winkle RA, Bieber CP, Billingham ME, Dong E Jr, Griepp RB, et al. The status of cardiac transplantation, 1975. Circulation. 1975;52:531–539. doi: 10.1161/01.cir.52.4.531 [DOI] [PubMed] [Google Scholar]
- 53.Cabrol C, Cabrol A, Guiraudon G, Zafy A, Le Picard P, Mattei S, Luciani J. Perfusion of the graft during heart transplantation. Laval Med. 1970;41:205–208. [PubMed] [Google Scholar]
- 54.Sagar KB, Hastillo A, Wolfgang TC, Lower RR, Hess ML. Left ventricular mass by M-mode echocardiography in cardiac transplant patients with acute rejection. Circulation. 1981;64(pt 2):II217–II220. [PubMed] [Google Scholar]
- 55.Romhilt DW, Doyle M, Sagar KB, Hastillo A, Wolfgang TC, Lower RR, Hess ML. Prevalence and significance of arrhythmias in long-term survivors of cardiac transplantation. Circulation. 1982;66(pt 2):I 219–I222. [PubMed] [Google Scholar]
- 56.Barnard CN, Losman JG, Curcio CA, Sanchez HE, Wolpowitz A, Barnard MS. The advantage of heterotopic cardiac transplantation over orthotopic cardiac transplantation in the management of severe acute rejection. J Thorac Cardiovasc Surg. 1977;74:918–924. [PubMed] [Google Scholar]
- 57.Barnhart GR, Hastillo A, Goldman MH, Szentpetery S, Wolfgang TC, Mohanakumar T, Katz MR, Rider S, Hanrahan J, Lower RR. A prospective randomized trial of pretransfusion/azathioprine/prednisone versus cyclosporine/prednisone immunosuppression in cardiac transplant recipients: preliminary results. Circulation. 1985;72(pt 2):II227–II230. [PubMed] [Google Scholar]