Abstract
Radiopharmaceutical therapy or targeted radionuclide therapy (TRT) is a well-established class of cancer therapeutics that includes a growing number of FDA-approved drugs and a promising pipeline of experimental therapeutics. Radiobiology is fundamental to a mechanistic understanding of the therapeutic capacity of these agents and their potential toxicities. However, the field of radiobiology has historically focused on external beam radiation. Critical differences exist between TRT and external beam radiotherapy with respect to dosimetry, dose rate, linear energy transfer, duration of treatment delivery, fractionation, range, and target volume. These distinctions simultaneously make it difficult to extrapolate from the radiobiology of external beam radiation to that of TRT and pose considerable challenges for preclinical and clinical studies investigating TRT. Here, we discuss these challenges and explore the current understanding of the radiobiology of radiopharmaceuticals.
General Radiobiological Properties of Radiopharmaceuticals
Radiopharmaceutical therapy or targeted radionuclide therapy (TRT) differs from conventional external beam radiation therapy (EBRT) in several important ways.1 EBRT is generally given with low linear energy transfer (LET) photons and electrons (0.2 keV/um), at a high dose rate (eg, 1-2 Gy/min), with well-defined dosimetry, often with a fairly homogeneous dose distribution in the targeted field, and commonly as part of a fractionated treatment regimen. In contrast, TRT uses radionuclides that are conjugated to a carrier, such as an antibody, peptide, or ligand. The specific radionuclide used has a physical half-life, and unique activity decay spectrum that may consist of alpha or beta particles, auger electrons and gamma emissions of varying energies. Dose deposition in the targeted tumor is heterogeneous secondary to the emission properties of the radionuclide utilized and characteristics of the tumor, such as size and the distribution/density of the target of the TRT carrier. In addition, the effective half-life of the radiopharmaceutical, determined by both the biological half-life of the carrier and the physical half-life of the radionuclide, is an important determinant of tumor uptake and retention. The radiation exposure from TRT is continuous, with an exponential decay over hours to days/weeks, with a low absorbed dose rate, usually less than 0.5 Gy/h.1 The biological response to continuous, exponentially decreasing low dose rate radiation is markedly different from the response to EBRT (high dose rate, short exposure time) in terms of a variety of factors including effects on cell cycle, proliferation, signal transduction pathways, inflammation, and adaptive/compensatory responses, with the relative importance dependent upon the dose rate, total dose and tissue irradiated.2 Dosimetry (Medical Internal Radiation Dose) with TRT is also much less well defined than for EBRT.1
Basic Principles of Radiobiology Applied to TRT
The biological effect of TRT is determined by a large number of complex and interacting factors, including direct tumor and normal organ damage, and indirect/bystander and abscopal effects (Fig. 1), which will be discussed below. In aggregate, these effects ultimately determine both acute and late effects of radiation in tumors and normal tissues.3 Many of the basic radiobiological principles of EBRT also apply to TRT.4,5 The LET of the radionuclide determines the depth of penetration in tumor/tissue6 as well as the density of ionization or energy transferred per unit depth travelled. For example, beta and gamma emissions have a LET of 0.2 keV/um and a depth of penetration of 12 mm or more in the case of gamma emissions. In contrast, Auger electrons have a LET of 4-25 keV/um, with a range of less than 1 μm, and alpha particles have a LET of 50-230 keV/um and a depth of penetration of 50-100 μm. Most radionuclides emit multiple types of radiation as they decay. High LET is associated with increased biological effectiveness, low oxygen enhancement ratio, and relatively low cell cycle dependent radiosensitivity. The biologically effect dose (BED) is the product of the relative effectiveness and total dose (D). BED is based on linear quadratic dose terms and was developed as a means to correlate biological responses to fractionated regimens, with a dose-related quantity. For very low doses/fraction (eg, protracted irradiation from a radionuclide), there are concerns about the validity of the linear quadratic model, which does not adequately take into account proliferation, repair of sublethal damage, or adaptive responses during treatment at low dose rates (LDR). In order to make the BED more relevant to TRT, the relative effectiveness for radionuclides should be modified for these LDR.
Figure 1.
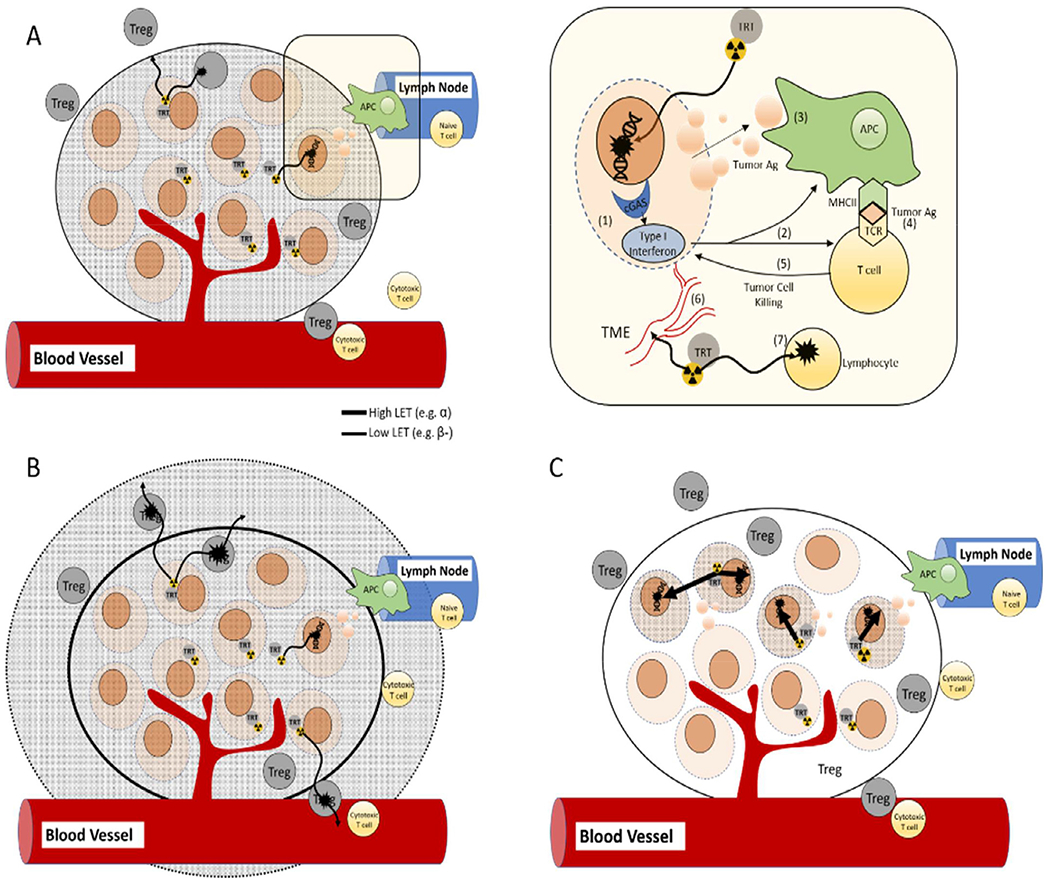
The radiobiological effects of TRT on tumor cells and the tumor-immune microenvironment are multifaceted and change with the type, range, rate, and dose of radiation emitted from a given radionuclide. In tumor cells (upper right), DNA damage (1) is thought to be the primary mechanism of TRT-induced cell death. In addition, lower doses of radiation from TRT may elicit sublethal DNA damage that is detected by the sensor cGAS and leads to the production of type I interferon. (2) Type I interferon may help to prime tumor antigen (TA)-specific T cells by inducing antigen uptake and maturation of antigen presenting cells (APC) such as dendritic cells. (3) Cellular debris is phagocytosed by APC’s and (4) presented to T cells. (5) T cells mount an adaptive response to the tumor antigen which can lead to local and distant tumor cell killing. (6) Radiation also impacts the tumor microenvironment by increasing leukocyte infiltration into tumor tissue by changing vessel structure (ie, normalization), increased adhesion molecule expression on endothelium, and by triggering the expression and release of chemokines and cytokines. (7) Radiation from TRT may also kill or alter the function of immune cells in the tumor microenvironment. The radiobiological effects of dose rate and LET associated with TRT on tumor cells and the tumor microenvironment are poorly understood. A-C illustrate the dosimetric envelope for (A) medium range (millimeter), (B) long range (centimeter), and (C) short range (micrometer) radiation emitted from a tumor cell selective TRT in the tumor microenvironment. Figure graciously provided by Dr. Bryan Bednarz, University of Wisconsin.
Biological Effects of Dose Heterogeneity
The dose distribution with TRT is very heterogeneous compared to that typically achieved with EBRT, with relatively hot and cold areas. This is evident from the use of theranostic imaging and personalized dosimetry, with TRT commonly delivering a heterogeneous radiation dose across a tumor, with variation compounded when comparing across tumor sites, patients and disease histologies. This dose heterogeneity or nonuniformity occurs at both the tumor/tissue and subcellular level and is biologically meaningful. For example, a given effect requires a higher dose when the dose distribution is nonuniform.5 The magnitude of the difference in dose required for a calculated survival fraction is greater for high LET alpha particles than for low LET beta particles.5,7,8 Dose heterogeneity in normal tissues, such as the kidney, may result in increased damage within a specific tissue region, and thereby contribute to toxicity (eg, renal toxicity).
Modeling of tumor dose heterogeneity poses a challenge to preclinical and clinical studies on the radiobiology of radiopharmaceuticals. The dose heterogeneity from TRT is affected by both the targeting vector (eg, specificity, affinity, and stability) and the physical properties of the radionuclide,9 as well as tumor perfusion and permeability.10,11 The dynamics and effects of dose gradients during TRT are complex, and are affected by a number of factors including location in the tumor, proximity to sensitive normal tissues, vascularity, and hypoxia12 (Fig. 1). Ex vivo biodistribution studies can be used in preclinical models to estimate dose distribution as a function of time, but this is not feasible in clinical settings where imaging-based dosimetry approaches are critical. The use of SPECT/CT or PET/CT of theranostic agents, paired with advanced dosimetry calculation can illuminate patient-specific and tumor-specific dose heterogeneity in preclinical and clinical settings.13 Additional imaging strategies such as MRI with time-resolved contrast kinetics may resolve dynamic changes in tumor perfusion and permeability and these have been correlated with tumor response to TRT.10
The biological consequences of heterogeneous tumor dose distribution following TRT have not been fully elucidated. For many TRT applications, it is unknown whether a minimum threshold dose, mean dose, or maximum dose is most important for predicting tumor control. Furthermore, it is generally unknown whether such parameters equally apply to all tumor volumes including radiographically occult sites and isolated or circulating tumor cells, which are not typically accounted for in dosimetry. Intriguingly, nonuniform TRT distribution across a single tumor volume may be advantageous and preclinical studies suggest that a bystander effect of radiation may augment tumor control probability in settings of dose heterogeneity.14,15 On the other hand, heterogeneity in micro-dosimetry may highlight advantages and disadvantages of distinct radionuclides. Those emitting short-range radiation (eg, alpha particles and Auger electrons) may be optimal for triggering target cell death and radiation-dependent phenotypic changes in target cells because these radionuclides effectively deliver dose to the cell taking up TRT. However, such radionuclides may be ineffective in modulating the tumor microenvironment or local immune response due to limited delivery of radiation to tumor stroma. Conversely, radionuclides emitting longer-range radiation (eg, beta particles and gamma rays) rely on “crossfire” radiation from neighboring cells to deliver full dose at target cells. This may limit the effect of such agents against isolated or circulating tumor cells while ensuring dose delivery to tumor stroma. Additional studies are needed to determine how such dose heterogeneity from TRT may affect clinical and radiobiologic endpoints.
Direct Versus Indirect/Bystander Effects
TRT can have direct cytotoxic effects as well as indirect or bystander effects, the relative contribution of each being dependent in part on the radiobiological characteristics of the TRT, such as absorbed dose rate and LET, and may involve different signaling pathways. Bystander effects occur in adjacent nonirradiated cells, that are similar to that observed in irradiated cells. These may include mutations, clastogenic effects, cell death, apoptosis and cellular transformation, and occur primarily at doses less than 1 Gy or at LDR.1,16 These have been studied much less with radionuclide therapy than EBRT, with the heterogeneity, kinetics and dose/dose rate gradient of TRT adding to the complexity.12 With bystander effects, irradiated cells communicate with nonirradiated cells through gap junctions.5,17 Many different cell types may be involved, including tumor cells, fibroblasts and endothelial cells. Mediators of bystander effects may include secreted/shed soluble factors and stress mediators, apoptotic factors, inflammatory response cytokines, reactive oxygen (ROS) and nitrogen species, calcium and extracellular DNA.16,18,19 Scavengers of ROS can abolish the bystander response.20 Intercellular communication can be bidirectional,12,21 and under some circumstances result in protection rather than damage. The extracellular matrix and tumor immune microenvironment may be affected and signaling pathways other than those involved in direct cell killing may be involved. For example, direct effects on DNA activate different signaling pathways than indirect oxidative stress-induced bystander effects on DNA.22 While there is a dose rate effect, there is no clear dose response relationship for the bystander effect.1
Dose and Dose Rate Effects
The typical dose rate (DR) from TRT is 0.01-1.0 Gy/h. Dose effects include type of radiation-induced damage, gene expression and mode of cell death. The cumulative total absorbed dose in tumor or normal tissue is the sum of dose from direct irradiation, crossfire, and nonspecific dose deposition from circulation of the radiopharmaceutical. There is a positive correlation between tumor absorbed dose and tumor cell killing, although this relationship is not strong for lymphoma. Importantly, the dose response relationship in TRT is less well defined than with EBRT.
Radiopharmaceuticals deliver radiation over protracted time with a continuously varying and exponentially decreasing DR.23 The energy and dose-deposition pattern as a function of diameter or depth varies for specific radionuclides.6 The absorbed DR in TRT is determined by a number of factors including the T1/2 of the radionuclide, specific activity, biologic T1/2 of the carrier (eg, antibody, ligand, and peptide) as determined by transit, uptake and clearance times, and by repair of sublethal damage.1 As expected, at LDR, the radiation is more sparse, and generally less damaging that high DR (HDR) radiation, resulting in repairable sublethal damage.
As above, generally the efficacy of LDR is less than that of HDR irradiation, with LDR irradiation being delivered over a longer period of time than HDR irradiation.5 Although one would therefore predict that LDR radiation is less effective than EBRT/Gy, this is not always true.1 For example, synchronization of cells in the radiosensitive part of the cell cycle (eg, G2/M) with continuous irradiation, defects in detection of low levels of DNA damage, and the relative contribution of nontargeted effects can play a role in determining the efficacy of LDR.1 There is also the phenomena of an inverse DR effect24,25 or low dose hyper-radiosensitivity,12 usually observed at very low doses (eg, 0.01-10 Gy, but typically less than or equal to 1 Gy) and very low DR such as 0.003-0.3 Gy/min. This has been shown in vitro with a variety of cell types as well as in vivo animal models, and is more common in leukemia and lymphoma models.26–28
Adaptive/Compensatory Responses
Adaptive or compensatory responses can diminish the effect of radiation over time and may be evidenced by a decrease in the magnitude of radiation-induced damage when a high dose of radiation follows a low dose.12 DR patterns from radionuclides are characterized by an initial exponential increase in the DR, followed by an exponential decrease in DR over time. Howell et al23 studied the effect of changing DR during the uptake phase of a radiopharmaceutical. With slower uptake of a radiopharmaceutical, the initial rate of increase in DR is lower, and there is a flattening of the shoulder on the survival curve. This means that the biological effect is less, secondary to an adaptive response from the initial phase of the exposure. This has been confirmed by others29 as well in preclinical animal studies,30 where slow effective uptake of radiopharmaceuticals by tumors was less effective than fast uptake at inhibiting xenograft tumor growth. Possible mechanisms include induction of DNA repair enzymes DNA double strand breaks with blunt or staggered ends (that can induce an adaptive response)31 and changes in cell cycle distribution.23 Of note, this effect may not be as relevant to high LET (eg, alpha) radiation.32 Therefore, absorbed dose may not accurately predict the surviving fraction. Current models may overestimate BED at low DR and underestimate them at high DR, so that rate of uptake, DR and maximal DR should be factored into predictive models.23
Effect of Fractionation
It has long been known that prolongation of the overall treatment time spares early reactions, with little sparing effect on late reactions. The rationale for fractionation is to allow for repair of sublethal damage in normal tissues, reoxygenate hypoxic cells in tumors, and redistribute tumor cells in the cell cycle into more radiosensitive phases and/or promote proliferation of relatively radioresistant cancer stem cells.4 Compared with EBRT, there is very limited data on fractionation with TRT. For example, in a study of 177-Lu-octreotate in 16 patients, all cycles of TRT contributed approximately equally to the absorbed dose in kidneys, but in tumor, there was a decrease with each subsequent cycle (eg, 35% cumulative dose after the first treatment vs 15% of the cumulative dose after the 4th dose). Presumably this was due, in part, to saturation or down regulation of the targeted peptide receptors, and also possibly to an adaptive response.33 This is consistent with clinical outcomes reported by Van Essen et al,34 and suggests that the use of lower number of cycles with higher activities may be advantageous.35 When factoring in BED as a function of absorbed dose to kidneys for either 90-Y or 177-Lu based TRT, with fractionation the kidneys are able to tolerate higher total absorbed doses, presumably due to repair between cycles.36
Effects of TRT on Immunomodulation of the Tumor Microenvironment
Even though TRT has limited therapeutic range, they can elicit immunomodulatory effects on tumor cells as well as the tumor microenvironment (Fig. 1). Tumor cell death triggered by radiation is immunogenic and characterized by pro-inflammatory translocation of calreticulin to the plasma membrane and extracellular release of HMGB1 and ATP.37–40 Such immunogenic cell death can be synergistic with immune checkpoint inhibitors and cancer immunotherapy. On the other hand, cells receiving a sub-lethal dose of radiation undergo phenotypic changes that may alter their susceptibility to immune response. Doses as low as 2-4 Gy have been shown to increase expression of MHC-I on the surface of cancer cells and this has been observed following alpha-emitting TRT.40 Radiation also activates a type I interferon response in surviving cells via the cGAS/STING pathway41 and this is critical to the cooperative interaction of radiation with certain immunotherapies.42
TRT affects components of the tumor microenvironment and this may have positive and negative influences on antitumor immunity. Radiation increases expression of vascular and matrix adhesion proteins and induces production of inflammatory cytokines.43,44 These effects are observed at low doses (between 2 and 5 Gy) and at LDR.45 Radiation also modifies the composition of tumor infiltrating immune cells through rapid effect and direct cytotoxic effect on radiation-sensitive tumor-infiltrating lymphocytic lineages.46 This is observed with low doses (1-4 Gy) and at LDR,45 suggesting that TRT may create a window of opportunity by temporarily depleting exhausted, anergic, and suppressive lymphocytes from the tumor microenvironment and enabling subsequent reconstitution with a more favorable infiltrate. Although research on radiation and cancer immunotherapy has been mostly focused on the adaptive immune system, radiation also has a strong effect on innate immune cells.47,48 For example, irradiation can modulate the phenotype of tumor associated macrophages49 as well as natural killer (NK) cells. NK cells are an important component of the innate immune system and there is growing interest in NK-mediated cancer immunotherapy. Radiation can induce NKG2D ligand, a key NK cell activating ligand, overexpressed in cancer cells,50 and several preclinical studies show that radiation can potentiate NK cell-based cancer immunotherapy.51–53
While radiation consistently induces a local inflammatory response, focal radiation of a single tumor site rarely leads to a sustained and systemic antitumor immune response.54 This may reflect a negative impact of distant tumor sites on the priming and propagation of systemic antitumor immunity from a focal site. While focal radiotherapy can dramatically alter the immune landscape in a targeted tumor, it does not alter the landscape within all tumors. Immune suppressive cells and pathways in these nonradiated tumors may quench the activation of effector immune cells emerging from the radiated site or may circulate between tumor sites and reconstitute a suppressive tumor microenvironment at the radiated site.55 Consequently, to engage radiation in effectively priming and propagating antitumor immunity, it may be beneficial to deliver immunomodulatory radiation to the collective tumor microenvironment at all tumor sites. TRTs enable effective delivery of radiation to all tumor sites in settings of metastatic disease without triggering systemic lymphopenia. Combining TRT with immunotherapies may overcome mechanisms that currently limit the effectiveness of these agents as monotherapies. Further studies are warranted to evaluate whether TRT may prime and propagate a more effective response to immunotherapies by delivering immunomodulatory radiation to all tumor microenvironments.
Combination Therapies With TRT
It is well recognized that many therapeutic agents can potentiate the effects of radiation.56 These agents include classic radiosensitizers, chemotherapeutic agents, biologically targeted agents, and recently developed nanoparticles that are made from high Z materials.57 The mechanisms of synergy between these agents and radiation have been studied extensively and beyond the scope of this review. Although most of these studies utilized external beam radiotherapy, given the fact that the mechanisms of action between x-ray treatment and radioisotopes are the same, the data from these reports can be directly applied to TRT as well.
Several preclinical studies have examined combination therapy with TRT. Because TRT is a systemic agent just like other therapeutics, these studies were focused on combining TRT with chemotherapeutics into a single combined agent using nanotechnology. One of the earliest studies engineered a folate-targeted nanoparticle that contained both yttrium-90 and paclitaxel.58 The combination agent was studied in a mouse model of ovarian cancer peritoneal metastasis. The investigators demonstrated that combination treatment with both TRT and paclitaxel improved therapeutic efficacy. Another such study examined the combination of I-131 with paclitaxel as cancer treatment in a mouse model of breast cancer.59 The investigators formulated I-131 labeled albumin-based nanoparticles containing paclitaxel. They showed the combination of I-131 and paclitaxel is significantly more effective than either therapy alone.
Clinical experience combining TRT and other agents have been limited, largely due to the limited number of clinical TRT available. Both 131I-tositumomab and 90Y-ibritumomab tiuxetan were studied in the context of chemotherapy for lymphomas but they are not given concurrently.60 In a case report, 131I-MIBG was combined with sunitinib to treat a patient with metastatic paraganglioma with an excellent response.61 The largest clinical experience of combination therapy is with radium-223 in prostate cancer. In a large randomized phase III trial, abiraterone was compared to abiraterone with radium-223 in metastatic castration resistant prostate cancer patients.62 The study randomized 806 patients and the addition of radium-223 did not improve symptomatic skeletal event-free survival. Radium-223 is also being studied in combination with docetaxel in prostate cancer patients. Morris et al. recently reported from a phase I dose escalation and randomized phase IIa trial that the combination was safe and well tolerated.63 A large randomized phase III trial comparing docetaxel to docetaxel with radium-223 is ongoing.
Another promising combination approach with TRT is to combine TRT with cancer immunotherapy. Radiation has been shown to stimulate the immune system and improve cancer immunotherapy response.64 Before cancer immunotherapy achieved clinical success, there were already many preclinical studies demonstrating the synergistic effects between radiation and cancer immunotherapy. Much of the work was done by Formenti and Demaria and colleagues who showed radiation can improve the response of immune checkpoint inhibitors in mouse models of cancer.65,66 Although we do not have a complete understanding of the interplay between radiation and immunotherapy, the immunomodulatory mechanisms of radiation enable the development of an antigenic “cascade” (expansion of T cell clones that are reactive against a variety of tumor antigens).67 This is critical to the success of immunotherapy. Strongly immunogenic antigens are usually lost during tumor development and progression as tumors evade the immune system.68,69 Effective immunotherapy commonly relies on the immune system to recognize a multitude of tumor antigens. The antigenic cascade primed by radiotherapy may therefore promote response to immunotherapy.70,71 Radiation can also induce the release of immune modulating molecules called damage-associated molecular patterns that can further enhance immunotherapy. For example, radiotherapy can cause the release of alarmins.72 One particular alarmin released by radiotherapy, HMGB1, is known to induce maturation of dendritic cells to promote a cytotoxic T-lymphocyte response through cross presentation of tumor antigens.39,73 The mechanisms of synergy between radiation and immune checkpoints were best demonstrated by Minn and colleagues, who showed that radiation enhances the diversity of the T cell receptor repertoire, anti-CTLA-4 increases T cell expansion, and anti-PD-1 prevents T cell exhaustion.74
Combination TRT and cancer immunotherapy has been studied preclinically. Chao et al. examined the combination of I-131 and anti-CTLA-4 and showed that I-131 improved response and prevented tumor recurrence in mouse model’s cancer.75 Clinical experience with combination radiation and immunotherapy has not been robust. There is evidence that radiotherapy can improve outcomes in several retrospective studies, including a recent report by Lee and colleagues that previous treatment with radiotherapy in patients with advanced NSCLC results in longer progression-free survival and overall survival with pembrolizumab treatment than that seen in patients who did not have previous radiotherapy.76 However, many randomized trials have been conducted to evaluate the combination of radiation and cancer immunotherapy and there has been limited reports of positive data. Thus, further understanding of radiation’s effect on the immune response is needed to improve clinical investigations and these combination studies.
Important Questions for the Future
‘Ongoing work on the radiobiology of TRT will help to better elucidate the underlying biology and facilitate optimization of treatment regimens with theranostic agents. There are a number of important questions to address in the future,12 including (1) what TRT dose/fractionation regimens are associated with damaging vs protective bystander effects, as well as abscopal effects, (2) what is the contribution of predosing on the development of radioresistance using fractionated regimens, and (3) what level of activity/dose/dose rate is required for inverse dose rate effects and hyper-radiosensitivity? Ideally, TRT should use radiopharmaceuticals with the energy, half-life and activity optimized to induce hyper-radiosensitization, and suppress radiation-induced adaptive responses, in order to maximize tumor cell killing without activating radioresistance responses.
Footnotes
Conflicts of Interest: ZSM declares the following potential conflicts of interest: Member of the Scientific Advisory Boards for Archeus Technologies and Seneca Therapeutics. Patents related to NM600 and combination of targeted radionuclide therapy with immunotherapies.
References
- 1.Pouget J-P, Lozza C, Deshayes E, et al. : Introduction to radiobiology of targeted radionuclide therapy. Front Med 2:12, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joiner M, Kogel A: Basic Clinical Radiobiology. 4th ed. London: Hodder Arnold, 375, 2009 [Google Scholar]
- 3.Ahmed KA, Caudell JJ, El-Haddad G, et al. : Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol 95:1399–1404, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marques IA, Neves AR, Abrantes AM, et al. : Targeted alpha therapy using radium-223: From physics to biological effects. Cancer Treat Rev 68:47–54, 2018 [DOI] [PubMed] [Google Scholar]
- 5.Kassis AI: Therapeutic radionuclides: Biophysical and radiobiologic principles. Semin Nucl Med 38:358–366, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bavelaar BM, Lee BQ, Gill MR, et al. : Subcellular targeting of theranostic radionuclides. Front Pharmacol 9:996, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humm JL, Cobb LM: Nonuniformity of tumor dose in radioimmunotherapy. J Nucl Med Off Publ Soc Nucl Med 31:75–83, 1990 [PubMed] [Google Scholar]
- 8.Humm JL, Chin LM, Cobb L, et al. : Microdosimetry in radioimmunotherapy. Radiat Prot Dosimetry 31:433–436, 1990 [Google Scholar]
- 9.Flynn AA, Pedley RB, Green AJ, et al. : Antibody and radionuclide characteristics and the enhancement of the effectiveness of radioimmunotherapy by selective dose delivery to radiosensitive areas of tumour. Int J Radiat Biol 78:407–415, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Haeck J, Bol K, Bison S, et al. : Optimized time-resolved imaging of contrast kinetics (TRICKS) in dynamic contrast-enhanced MRI after peptide receptor radionuclide therapy in small animal tumor models. Contrast Media Mol Imaging 10:413–420, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Bussink J, Kaanders JHAM, van der Kogel AJ: Tumor hypoxia at the micro-regional level: Clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol J Eur Soc Ther Radiol Oncol 67:3–15, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Kumar C, Shetake N, Desai S, et al. : Relevance of radiobiological concepts in radionuclide therapy of cancer. Int J Radiat Biol 92:173–186, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Besemer AE, Yang YM, Grudzinski JJ, et al. : Development and validation of RAPID: A patient-specific Monte Carlo three-dimensional internal dosimetry platform. Cancer Biother Radiopharm 33:155–165, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahon SJ, Butterworth KT, Trainor C, et al. : A kinetic-based model of radiation-induced intercellular signalling. PloS One 8:e54526, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balderson MJ, Kirkby C: Potential implications on TCP for external beam prostate cancer treatment when considering the bystander effect in partial exposure scenarios. Int J Radiat Biol 90:133–141, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Hamada N, Maeda M, Otsuka K, et al. : Signaling pathways underpinning the manifestations of ionizing radiation-induced bystander effects. Curr Mol Pharmacol 4:79–95, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Azzam EI, de Toledo SM, Little JB: Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A 98:473–478, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prise KM, O’Sullivan JM: Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9:351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ridder M, Jiang H, Van Esch G, et al. : IFN-gamma+ CD8+ T lymphocytes: Possible link between immune and radiation responses in tumor-relevant hypoxia. Int J Radiat Oncol Biol Phys 71:647–651, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Havaki S, Kotsinas A, Chronopoulos E, et al. : The role of oxidative DNA damage in radiation induced bystander effect. Cancer Lett 356:43–51, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Desai NB, Laine AM, Timmerman RD: Stereotactic ablative body radio-therapy (SAbR) for oligometastatic cancer. Br J Radiol [Internet] 90, 2019. [cited 2019 May 30]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5685107/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burdak-Rothkamm S, Rothkamm K, Prise KM: ATM acts downstream of ATR in the DNA damage response signaling of bystander cells. Cancer Res 68:7059–7065, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solanki JH, Tritt T, Pasternack JB, et al. : Cellular response to exponentially increasing and decreasing dose rates: Implications for treatment planning in targeted radionuclide therapy. Radiat Res 188:221–234, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knox S, Levy R, Miller A, et al. : Determinants of the antitumor effect of radiolabeled monoclonal antibodies. Cancer Res 50:4935–4940, 1990 [PubMed] [Google Scholar]
- 25.Mitchell J, Bedford J, Bailey S: Dose-rate effects on the cell cycle and survival of S3 HeLa and V79 cells. Radiat Res 79:520–536, 1979 [PubMed] [Google Scholar]
- 26.Ren R, He M, Dong C, et al. : Dose response of micronuclei induced by combination radiation of α-particles and γ-rays in human lymphoblast cells. Mutat Res 741–742:51–56, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Kumar C, Jayakumar S, Pandey BN, et al. : Cellular and molecular effects of beta radiation from I-131 on human tumor cells a comparison with gamma radiation [Internet]. Curr Radiopharm. [cited 2020. February 7]. Available from: http://www.eurekaselect.com/123431/article [DOI] [PubMed]
- 28.Friesen C, Lubatschofski A, Kotzerke J, et al. : Beta-irradiation used for systemic radioimmunotherapy induces apoptosis and activates apoptosis pathways in leukaemia cells. Eur J Nuc Med Mol Imag 30:1251–1261, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Buonanno M, Randers-Pehrson G, Smilenov L, et al. : A mouse ear model for bystander studies induced by microbeam irradiation. Radiat Res 184:219–225, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behr TM, Memtsoudis S, Sharkey RM, et al. : Experimental studies on the role of antibody fragments in cancer radio-immunotherapy: Influence of radiation dose and dose rate on toxicity and anti-tumor efficacy. Int J Cancer 77:787–795, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Wolff S: The adaptive response in radiobiology: Evolving insights and implications. Environ Health Perspect 106(Suppl 1):277–283, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi X, Mothersill C, Seymour C: No adaptive response is induced by chronic low-dose radiation from Ra-226 in the CHSE/F fish embryonic cell line and the HaCaT human epithelial cell line. Environ Res 151:537–546, 2016 [DOI] [PubMed] [Google Scholar]
- 33.Garkavij M, Nickel M, Sjögreen-Gleisner K, et al. : 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: Analysis of dosimetry with impact on future therapeutic strategy. Cancer 116(4 Suppl):1084–1092, 2010 [DOI] [PubMed] [Google Scholar]
- 34.van Essen M, Krenning EP, Kam BLR, et al. : Salvage therapy with (177) Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med Off Publ Soc Nucl Med 51:383–390, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Cremonesi M, Ferrari M, Di Dia A, et al. : Recent issues on dosimetry and radiobiology for peptide receptor radionuclide therapy. Q J Nucl Med Mol Imaging Off Publ Ital Assoc Nucl Med AIMN Int Assoc Radiopharmacol IAR Sect Soc Of 55:155–167, 2011 [PubMed] [Google Scholar]
- 36.Eberlein U, Cremonesi M, Lassmann M: Individualized dosimetry for theranostics: Necessary, nice to have, or counterproductive? J Nucl Med 58(Supplement 2):97S–103S, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Panaretakis T, Kepp O, Brockmeier U, et al. : Mechanisms of pre-apopto-tic calreticulin exposure in immunogenic cell death. EMBO J 28:578–590, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golden EB, Frances D, Pellicciotta I, et al. : Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 3:e28518, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apetoh L, Ghiringhelli F, Tesniere A, et al. : Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13:1050–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Malamas AS, Gameiro SR, Knudson KM, et al. : Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget 7:86937–86947, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harding SM, Benci JL, Irianto J, et al. : Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548:466–470, 2017. 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. : DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun [Internet] 8, 2017. [cited 2020 Feb 18]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5472757/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Maggio FM, Minafra L, Forte GI, et al. : Portrait of inflammatory response to ionizing radiation treatment. J Inflamm Lond Engl 12:14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Ruiz ME, Garasa S, Rodriguez I, et al. : Intercellular adhesion molecule-1 and vascular cell adhesion molecule are induced by ionizing radiation on lymphatic endothelium. Int J Radiat Oncol Biol Phys 97:389–400, 2017. 01 [DOI] [PubMed] [Google Scholar]
- 45.Liu S-Z: Nonlinear dose-response relationship in the immune system following exposure to ionizing radiation: Mechanisms and implications. Nonlinearity Biol Toxicol Med 1:71–92, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura N, Kusunoki Y, Akiyama M: Radiosensitivity of CD4 or CD8 positive human T-lymphocytes by an in vitro colony formation assay. Radiat Res 123:224–227, 1990 [PubMed] [Google Scholar]
- 47.Vatner RE, Formenti SC: Myeloid-derived cells in tumors: Effects of radiation. Semin Radiat Oncol 25:18–27, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Wennerberg E, Lhuillier C, Vanpouille-Box C, et al. : Barriers to radiation-induced in situ tumor vaccination. Front Immunol 8:229, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teresa Pinto A, Laranjeiro Pinto M, Patrícia Cardoso A, et al. : Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep 6:18765, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen MJ, Xu LJ, Yang L, et al. : Radiation alters PD-L1/NKG2D ligand levels in lung cancer cells and leads to immune escape from NK cell cytotoxicity via IL-6-MEK/Erk signaling pathway. Oncotarget 8:80506–80520, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon MS, Pham CT, Phan M-TT, et al. : Irradiation of breast cancer cells enhances CXCL16 ligand expression and induces the migration of natural killer cells expressing the CXCR6 receptor. Cytotherapy 18:1532–1542,2016 [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Kong Q, Wang G, et al. : Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer Biother Radiopharm 29:428–434, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KW, Jeong J-U, Lee K-H, et al. : Combined NK cell therapy and radiation therapy exhibit long-term therapeutic and antimetastatic effects in a human triple negative breast cancer model. Int J Radiat Oncol Biol Phys 2019 [DOI] [PubMed] [Google Scholar]
- 54.Abuodeh Y, Venkat P, Kim S: Systematic review of case reports on the abscopal effect. Curr Probl Cancer 40:25–37, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Morris ZS, Guy EI, Werner LR, et al. : Tumor-specific inhibition of in situ vaccination by distant untreated tumor sites. Cancer Immunol Res 6:825–834, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson GD, Bentzen SM, Harari PM: Biologic basis for combining drugs with radiation. Semin Radiat Oncol 16:2–9, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Wang H, Mu X, He H, et al. : Cancer radiosensitizers. Trends Pharmacol Sci 39:24–48,2018 [DOI] [PubMed] [Google Scholar]
- 58.Werner ME, Karve S, Sukumar R, et al. : Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials 32:8548–8554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tian L, Chen Q, Yi X, et al. : Radionuclide I-131 labeled albumin-paclitaxel nanoparticles for synergistic combined chemo-radioisotope therapy of cancer. Theranostics 7:614–623, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pouget J- P, Navarro-Teulon I, Bardiés M, et al. : Clinical radioimmunotherapy—the role of radiobiology. Nat Rev Clin Oncol 8:720–734, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Makis W, McCann K, McEwan AJB, et al. : Combined treatment with 131I-MIBG and sunitinib induces remission in a patient with metastatic paraganglioma due to hereditary paraganglioma-pheochromocytoma syndrome from an SDHB mutation. Clin Nucl Med 41:204–206, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Smith M, Parker C, Saad F, et al. : Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:408–419,2019 [DOI] [PubMed] [Google Scholar]
- 63.Morris MJ, Loriot Y, Sweeney CJ, et al. : Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: A phase 1 dose escalation/randomised phase 2a trial. Eur J Cancer Oxf Engl 1990 114:107–116, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shabason JE, Minn AJ: Radiation and immune checkpoint blockade: From bench to clinic. Semin Radiat Oncol 27:289–298, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Formenti SC, Demaria S: Systemic effects of local radiotherapy. Lancet Oncol 10:718–726, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demaria S, Bhardwaj N, McBride WH, et al. : Combining radiotherapy and immunotherapy: A revived partnership. Int J Radiat Oncol Biol Phys 63:655–666, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lurquin C, Lethé B, De Plaen E, et al. : Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med 201:249–257, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dunn GP, Koebel CM, Schreiber RD: Interferons, immunity and cancer immunoediting. Nat Rev Immunol 6:836–848, 2006 [DOI] [PubMed] [Google Scholar]
- 69.DuPage M, Mazumdar C, Schmidt LM, et al. : Expression of tumour-specific antigens underlies cancer immunoediting. Nature 482:405–409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulley JL, Arlen PM, Bastian A, et al. : Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res Off J Am Assoc Cancer Res 11:3353–3362, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Hodge JW, Sharp HJ, Gameiro SR: Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm 27:12–22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chan JK, Roth J, Oppenheim JJ, et al. : Alarmins: Awaiting a clinical response. J Clin Invest 122:2711–2719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Curtin JF, Liu N, Candolfi M, et al. : HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 6:e10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Twyman-Saint\sVictor C, Rech AJ, Maity A, et al. : Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520:373–377, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao Y, Xu L, Liang C, et al. : Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng 2:611–621, 2018 [DOI] [PubMed] [Google Scholar]
- 76.Shaverdian N, Lisberg AE, Bornazyan K, et al. : Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895–903, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


